Research on the Response of Arbuscular Mycorrhizae Fungi to Grape Growth Under High Temperature Stress
Abstract
1. Introduction
2. Results and Analysis
2.1. Effects of AMF on Grape Growth
2.2. Mycorrhizal Growth of Grape Roots
2.3. Effects of AMF on Grape Root Growth
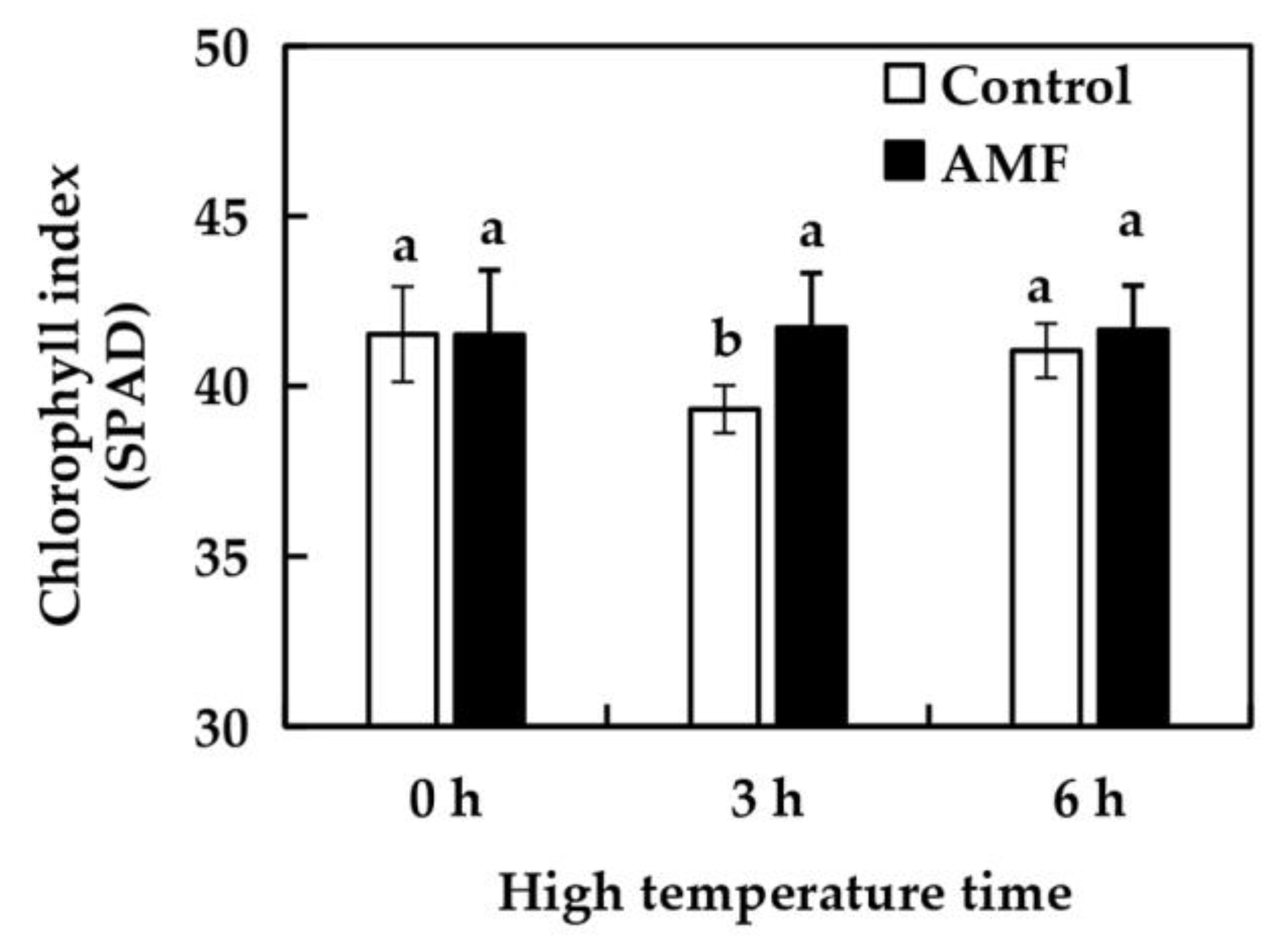
2.4. Effects of AMF on Grape Chlorophyll Index Under High Temperature Stress
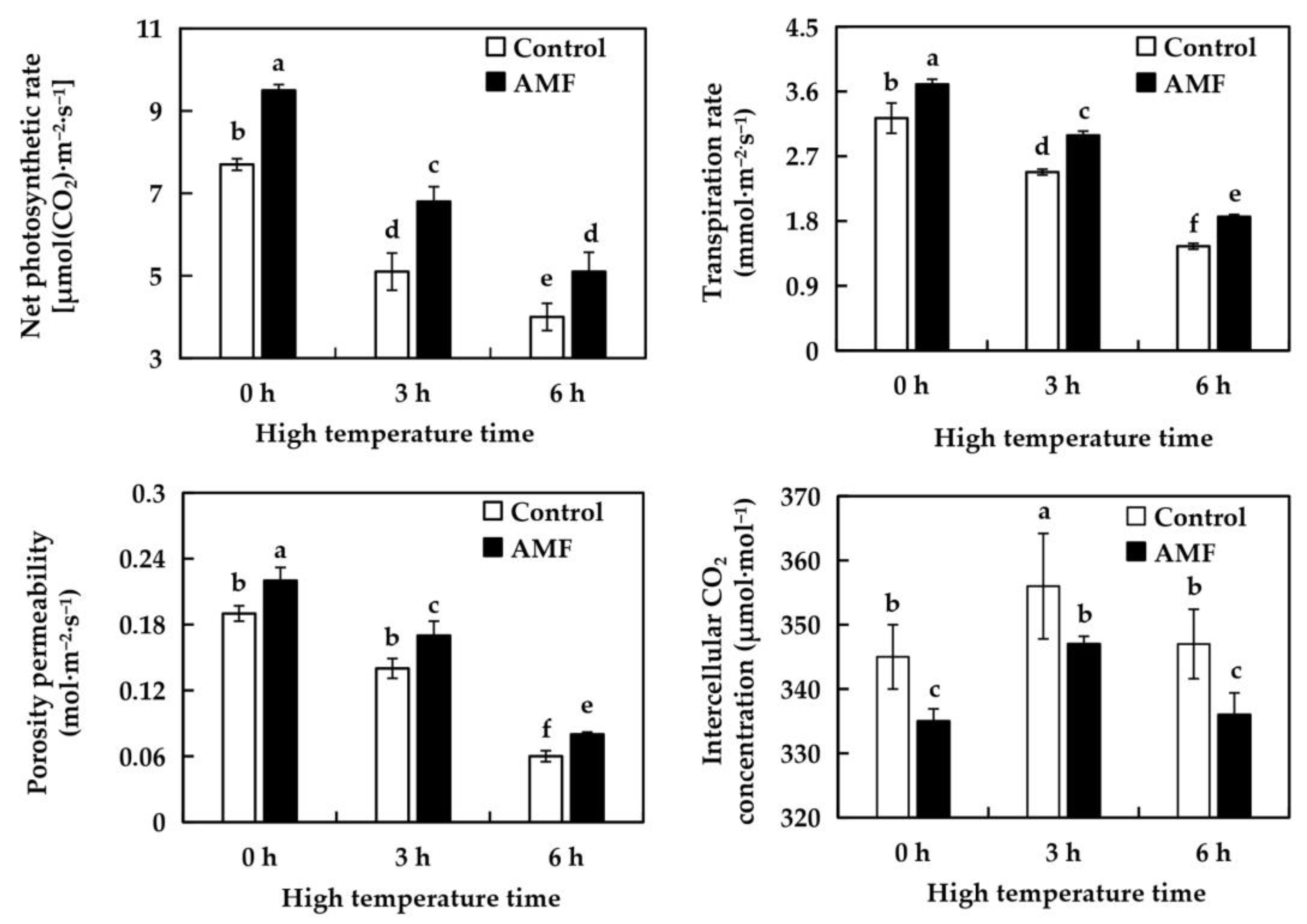
2.5. Effects of AMF on Photosynthetic Characteristics of Grape Under High Temperature Stress
2.6. Effects of AMF on MDA Content in Grape Leaves Under High Temperature Stress
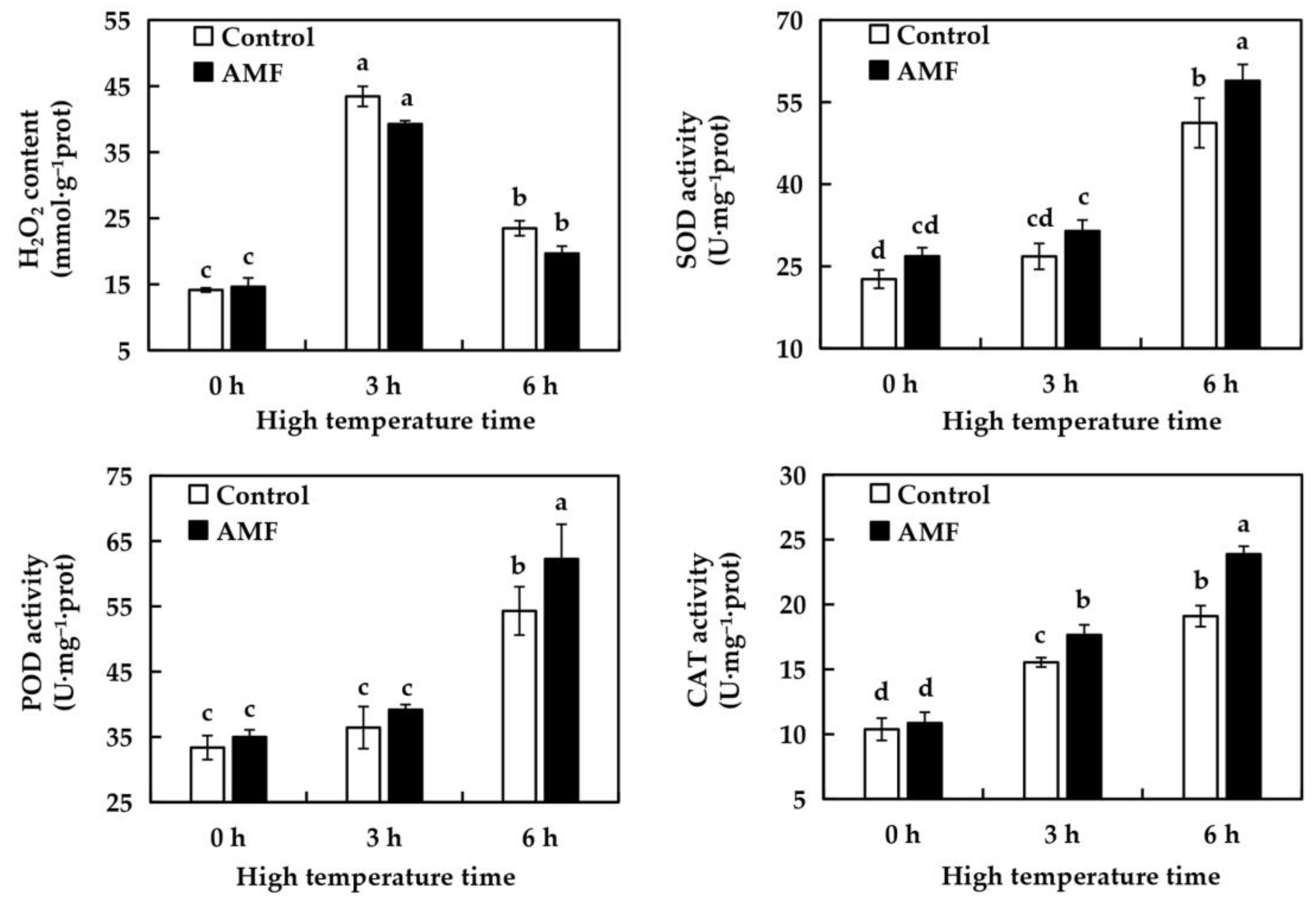
2.7. Effects of AMF on H2O2 and Antioxidant Enzyme Activities of Grape Leaves Under High Temperature Stress
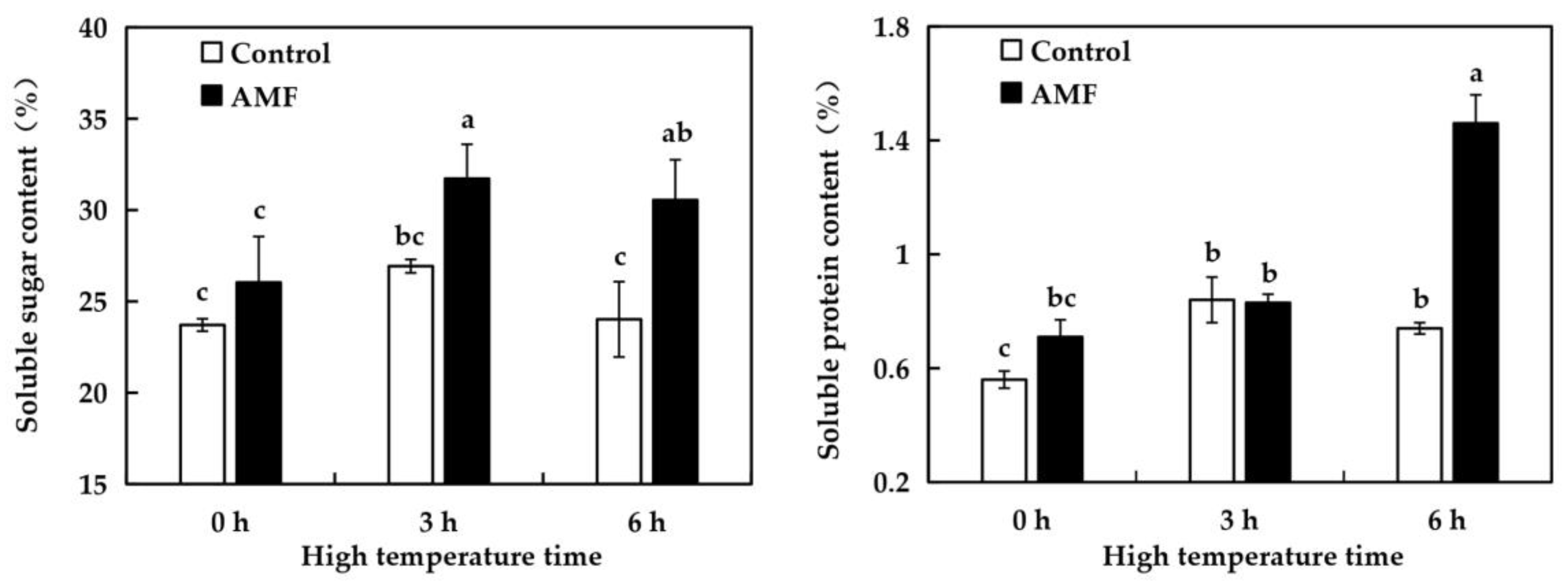
2.8. Effects of AMF on the Content of Osmotic Regulatory Substances in Grape Leaves Under High Temperature Stress
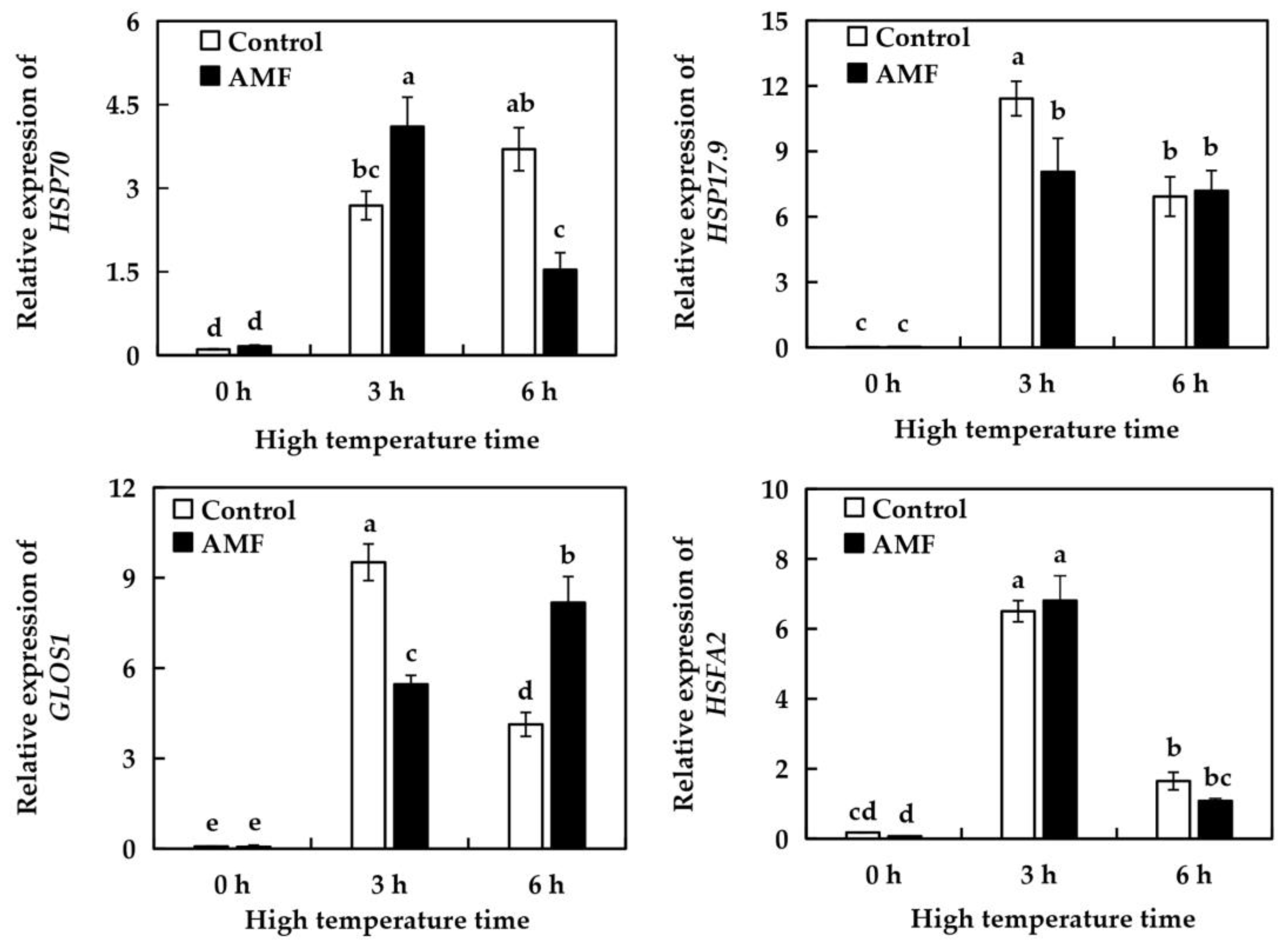
2.9. Effect of AMF on the Relative Expression of High Temperature Resistance Genes in Grape Under High Temperature Stress
3. Discussion
4. Materials and Methods
4.1. Summary of Test Materials and Test Sites
4.2. Experimental Design
4.3. Test Methods
4.3.1. Plant Growth and Development Status
4.3.2. Leaf Chlorophyll Index
4.3.3. Leaf Photosynthetic Parameters
4.3.4. Root System Configuration
4.3.5. Mycorrhizal Growth
4.3.6. Determination of MDA Content in Leaves
4.3.7. Determination of H2O2 and Antioxidant Enzyme Activities in Leaves
4.3.8. Determination of Leaf Permeation Regulation Substances
4.3.9. Analysis of High Temperature Related Gene Expression
4.3.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.N.; Wang, L.N.; Ye, J.B.; X, F. Fruit quality of Vitis vinifera: How plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2022, 305, 111404. [Google Scholar] [CrossRef]
- Prathiksha, M.; Hegde, K. A Review on Vitis vinifera L.: The Grape. Int. J. Pharm. Sci. Rev. Res. 2022, 74, 142–145. [Google Scholar] [CrossRef]
- Eswari, A. Diversity of Arbuscular Mycorrhizae Fungi from Orchard Ecosystem. OMICS Int. 2014, 5, 1000230. [Google Scholar] [CrossRef]
- Brundrett, M. Diversity and classification of mycorrhizal associations. Biol. Rev. Camb. Philos. Soc. 2004, 79, 473–495. [Google Scholar] [CrossRef]
- Li, C.Y.; Chen, X.; Yang, J.Y.; Li, J.; Wang, R.Y.; Xu, H.Y.; Zhang, F.J. Keystone root bacteria in Ambrosia artemisiifolia promote invasive growth by increasing the colonization rate of Funneliformis mosseae. Microbiol. Res. 2025, 293, 128081. [Google Scholar] [CrossRef] [PubMed]
- Prisa, D. Mycorrhizal symbioses and plant interactions. Karbala Int. J. Mod. Sci. 2023, 9, 194–207. [Google Scholar] [CrossRef]
- Hall, A.E. Consideration of Crop Responses to Environment in Plant Breeding; Springer: Ames, IA, USA, 2001; pp. 215–226. [Google Scholar]
- Suzuki, N. Temperature Stress and Responses in Plants. Int. J. Mol. Sci. 2019, 20, 2001. [Google Scholar] [CrossRef]
- Gaofeng, L.; Yudong, X.; Tongkun, L.; Shaojun, D.; Xilin, H. The DNA Methylome and Association of Differentially Methylated Regions with Differential Gene Expression during Heat Stress in Brassica rapa. Int. J. Mol. Sci. 2018, 19, 1414. [Google Scholar] [CrossRef]
- Das, R.; Devi, S.H.; Das, S.; Mollier, M. Understanding the Mechanism of high temperature Stress Effect and Tolerance in Wheat. In Thermotolerance in Crop Plants; Springer: Singapore, 2022; pp. 105–127. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Howell, G.S. Sustainable grape productivity and the growth-yield relationship: A review. Am. J. Enol. Vitic. 2001, 52, 165–174. [Google Scholar] [CrossRef]
- Caprio, J.M.; Quamme, H.A. Weather conditions associated with grape pro-duction in the Okanagan Valley of British Columbia and potential impact of climate change. Can. J. Plant Sci. 2002, 82, 755–763. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, B.W.; Kim, J. Signaling Peptides Regulating Abiotic Stress Responses in Plants. Front. Plant Sci. 2021, 12, 704490. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, A.K. Global transcriptome analysis of heat stress response of grape variety ‘Fantasy Seedless’ under different irrigation regimens. Vitis 2021, 60, 143–151. [Google Scholar] [CrossRef]
- Sepulveda, G.; Kliewer, W.M. Effect of high temperature on grapevines(Vitis vinifera L.) II. Distribution of soluble sugars. Am. J. Enol. Vitic. 1986, 37, 20–25. [Google Scholar] [CrossRef]
- Schultz, H.R.; Jones, G.V. Climate Induced Historic and Future Changes in Viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, B.H.; Yan, H.; Rui, Y.; Yuan, W. Effects of short-term heat stress on PSII and subsequent recovery for senescent leaves of Vitis vinifera L. cv. Red Globe. J. Integr. Agric. 2018, 17, 2683–2693. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; Jiang, A.L.; Tian, Y. Influence of heat stress on the expression of related genes and proteins in grapevines. Sci. Agric. Sin. 2017, 50, 1674–1683. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. Off. J. Eur. Soc. Photobiol. 2018, 180, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Haugen, L.M.; Smith, S.E. The effect of high temperatureand fallow period on infection of mung bean and cashewroots by the vesicular-arbuscular mycorrhizal fungus Glo-mus intraradices. Plant Soil 1992, 145, 71–80. [Google Scholar] [CrossRef]
- Martin, C.A.; Stutz, J.C. Interactive effects of temperatureand arbuscular mycorrhizal fungi on growth, Puptake and root respiration of Capsicum annuum L. Mycorrhiza 2004, 14, 241–244. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The relationship between chlorophyll content and rate of photosynthesis in soybeans. Rev. Can. De Phytotechnol. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Kim, S.H.; Gitz, D.C.; Sicher, R.C.; Baker, J.T.; Timlin, D.J.; Reddy, V.R. Temperature dependence of growth, development, and photosynthesis in maize under elevated CO2. Environ. Exp. Bot. 2007, 61, 224–236. [Google Scholar] [CrossRef]
- Beltrano, J.; Ronco, M.G. Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: Effect on growth and cell membrane stability. Braz. J. Plant Physiol. 2008, 20, 29–37. [Google Scholar] [CrossRef]
- Elahi, F.E.; Mridha, M.A.U.; Aminuzzaman, F.M. Influence of AMF inoculation on growth, nutrient uptake, arsenic toxicity and chlorophyll content of eggplant grown in arsenic amended soil. Adv. Nat. Appl. Sci. 2010, 4, 184–192. [Google Scholar]
- Sun, J.Q.; Liu, R.J.; Li, M. Advances in the study of increasing plant stress resistance and mechanisms by arbuscular mycorrhizal fungi. J. Plant Physiol. 2012, 48, 845–852. [Google Scholar]
- Matsubara, Y.I.; Hirano, I.; Sassa, D.; Koshikawa, K. Alleviation of High Temperature Stress in Strawberry Plants Infected with Arbuscular Mycorrhizal Fungi. Environ. Control Biol. 2004, 2, 105–111. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance andphotosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cuhivars. J. Agric. Sci. Technol. 2013, 15, 593–602. [Google Scholar]
- Savicka, M.; Kute, N. Eff ects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Zhou, H.H.; Ge, C.; Huang, X.B.; Zhang, L.; Lu, C.H.; Zhang, Y.P.; Wang, P. Inoculation with AMF Promotes the Photosynthetic and Antioxidant Capacities of Ponkan leaves under High Temperature and Drought Stress. Subtrop. Plant Sci. 2024, 53, 389–398. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Xu, H. Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2010, 20, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Tang, L.; Lu, L.; Zhang, L.Y.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015, 94, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Kalyar, T.; Rauf, S.; Da Silva, J.A.T.; Shahzad, M. Handling sunflower (Helianthus annuus L.) populations under heat stress. Arch. Agron. Amp Soil Sci. 2014, 60, 655–672. [Google Scholar] [CrossRef]
- Li, Y.; Matsubara, Y.; Miyawaki, C.; Liu, Y. Temperature stress tolerance and increase in antioxidative enzyme activities in mycorrhizal strawberry plants. Acta Hortic. 2008, 774, 391–396. [Google Scholar] [CrossRef]
- Shu, J.; Fu, J.T.; Nazamid; Mitsuru, M.; Toshisuke, T.; Nobuyuki, H.; Tztsuzo, T. Purification and Properties of Polyphenol Oxidase from Cabbage (Brassica oleracea L.). J. Agric. Food Chem. 1995, 43, 1138–1142. [Google Scholar] [CrossRef]
- Cansev, A. Physiological effects of high temperature treatments on leaves of olive cv. gemlik. Plant Arch. 2012, 12, 521–525. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.S.; Zhang, R.; Guo, S.X. The influence of arbuscular mycorrhizal fungi on the heat tolerance of lilies under high temperature stress. J. Qingdao Agric. Univ. (Nat. Sci. Ed.) 2018, 35, 258–264. (In China) [Google Scholar]
- Xing, H.S.; Sun, P.F.; Li, F.; Li, W.; Guo, S.X. Effects of arbuscular mycorrhizal fungi on heat tolerance of Lavandula angustifolia. J. Mycol. 2019, 38, 698–706. [Google Scholar] [CrossRef]
- Hadiarto, T.; Tran, L.-S.P. Progress studies of drought responsive genes in rice. Plant Cell Rep. 2011, 30, 297–310. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]


| Treatment | Shoot Length/cm | Shoot Thickness/mm | Leaf Area/cm2 | Fresh Weight Above Ground/g | Fresh Underground Weight/g |
|---|---|---|---|---|---|
| Control | 47.9 ± 4.7 | 4.3 ± 0.4 | 128.8 ± 12.5 | 32.2 ± 1.9 | 88.8 ± 2.7 |
| AMF | 74.7 ± 5.8 ** | 5.1 ± 0.2 ** | 172.9 ± 20.6 ** | 39.9 ± 1.7 ** | 133.6 ± 4.1 ** |
| Treatment | Mycorrhizal Infection Rate/% | Soil Spore Density/Spores·g−1 | Soil Mycelium Length/cm·g−1 |
|---|---|---|---|
| Control | - | - | - |
| AMF | 20.78 | 6.3 | 6.56 |
| Treatment | Total Root Length/cm | Projected Area/cm2 | Surface Area/cm2 | Volume/cm3 | Mean Diameter/mm |
|---|---|---|---|---|---|
| Control | 225.9 ± 19.3 | 14.2 ± 0.2 | 15.9 ± 0.6 | 22.9 ± 1.7 | 2.0 ± 0.2 |
| AMF | 251.0 ± 14.5 * | 14.8 ± 0.3 ** | 17.7 ± 0.8 * | 28.89 ± 3.3 ** | 3.5 ± 0.4 ** |
| Name | Sequence | NCBI No. |
|---|---|---|
| VvGOLS1-qF | TGATTACAGCAGCGTTTTGCC | VIT_07s0005g01970 |
| VvGOLS1-qR | CGAGAGTACTGGCCTCTTCTAG | |
| VvHSFA2-qF | AGGCGGCTGGGACAATGAATC | VIT_04s0008g01110 |
| VvHSFA2-qR | ATCCTCCACCTCCACATCAGTTTC | |
| VvHSP70-qF | CGGAGAAATGCGGCTGATA | TC38947 |
| VvHSP70- qR | TCCCTTTACTTCCACCGCTAGA | |
| VvHSP17.9-qF | CGTCAAGGAGTACCCCAATTC | XM_002280644 |
| VvHSP17.9-qR | AACTTCCCCACCCTCCTCT | |
| VvACTIN-qF | CTTGCATCCCTCAGCACCTT | EC969944 |
| VvACTIN-qR | TCCTGTGGACAATGGATGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, P.; Zhang, H.; Xi, X.; Yin, X.; Sun, P.; Zha, Q.; Zhang, D. Research on the Response of Arbuscular Mycorrhizae Fungi to Grape Growth Under High Temperature Stress. Int. J. Mol. Sci. 2025, 26, 6165. https://doi.org/10.3390/ijms26136165
Jian P, Zhang H, Xi X, Yin X, Sun P, Zha Q, Zhang D. Research on the Response of Arbuscular Mycorrhizae Fungi to Grape Growth Under High Temperature Stress. International Journal of Molecular Sciences. 2025; 26(13):6165. https://doi.org/10.3390/ijms26136165
Chicago/Turabian StyleJian, Panyu, He Zhang, Xiaojun Xi, Xiangjing Yin, Pengpeng Sun, Qian Zha, and Dejian Zhang. 2025. "Research on the Response of Arbuscular Mycorrhizae Fungi to Grape Growth Under High Temperature Stress" International Journal of Molecular Sciences 26, no. 13: 6165. https://doi.org/10.3390/ijms26136165
APA StyleJian, P., Zhang, H., Xi, X., Yin, X., Sun, P., Zha, Q., & Zhang, D. (2025). Research on the Response of Arbuscular Mycorrhizae Fungi to Grape Growth Under High Temperature Stress. International Journal of Molecular Sciences, 26(13), 6165. https://doi.org/10.3390/ijms26136165






