A New Adjuvant Treatment for Glioblastoma Using Aprepitant, Vortioxetine, Roflumilast and Olanzapine: The AVRO Regimen
Abstract
1. Introduction
2. Aprepitant
| Cancer Type | Aprepitant Effects, References |
|---|---|
| ALL | apoptosis, cytostatic, additive with doxorubicin [49,50] |
| AML | inhibited in vitro and xenograft growth [51,52,53,54] |
| APL | growth inhibition and additive with vincristine [55,56] |
| breast | inhibited in vitro and xenograft growth [30,36,57,58,59,60,61,62,63] |
| cervical | inhibited growth in vitro [37,64] |
| cholangio | inhibited growth, in vitro and xenograft [65,66] |
| CML | apoptosis and decreased colony formation [52,54] |
| colon | inhibited exosome release, xenograft growth [59,67,68,69,70] |
| esophageal | inhibited growth in vitro and xenograft [71,72] |
| glioblastoma | xenograft and potential clinical growth inhibition [10,25,26,27,28,30,31,32,33,34,35,73] |
| hepatoblastoma | stem cell inhibition by Wnt suppression [74,75] |
| hepatocellular | in vitro cytotoxicity and xenograft inhibition [76] |
| melanoma | in vitro growth inhibition [77] |
| myeloma | inhibited metabolism and growth in vitro [56,78] |
| neuroblastoma | xenograft growth inhibition [47,79] |
| NSCLC | NK-1 stimulated aprepitant inhibited growth in vitro [80,81] |
| osteosarcoma | inhibited growth in vitro [82,83] |
| ovarian | inhibited growth, synergy with doxorubicin in vitro [39,40] |
| rhabdoid | growth inhibition and apoptosis in vitro [84] |
| pancreatic | inhibited growth and motilityin vitro [85,86] |
| prostate | inhibited growth in vitro [87,88,89,90,91] |
| SCLC | NK-1 stimulated aprepitant inhibited growth in vitro [41] |
3. Vortioxetine
4. Roflumilast and PDE4
5. Olanzapine, D2 Dopamine Receptors, cAMP, and GB
| Drug | 2020 to 2024 | Prior to 2020 |
|---|---|---|
| aripiprazole | --- | [206,207] |
| asenapine | [208] | --- |
| brexpiprazole | --- | [209,210] |
| cariprazine | --- | --- |
| chlorpromazine | [141,211,212,213,214,215] | [216,217,218,219,220,221] |
| clozapine | --- | [222] |
| droperidol | --- | --- |
| fluphenazine | [141] | [219,220,223] |
| fluspirilene | [129,223,224] | [225] |
| haloperidol | [226,227,228,229,230,231] | [231,232] |
| iloperidone | [233] | --- |
| levomepromazine | [230] | --- |
| loxapine | --- | [219] |
| lurasidone | [234] | --- |
| metoclopramide | --- | [235,236,237] |
| molindone | --- | [219] |
| olanzapine | [238] | [239,240,241] |
| paliperidone | [242] | --- |
| penfluridol | [129] | [220,243,244,245] |
| perphenazine | [246,247] | [130,248,249,250] |
| pimozide | [129,203,229,251,252,253,254,255] | [256,257,258,259,260] |
| prochlorperazine | [247] | [248] |
| quetiapine | [34,261] | [262,263] |
| risperidone | [242,264,265] | [227,266] |
| thioridazine | [229,267] | [268,269,270,271] |
| thiothixene | --- | --- |
| trifluoperazine | [272,273,274,275] | [216,276,277] |
- Agonism at D5 increases, agonism at D2 decreases cAMP production. A greater D5 to D2 ratio is associated with longer survival in GB.
- D2 agonism decreases intracellular cAMP and enhances GB growth.
- D2-blocking drugs such as olanzapine diminish the D2 mediated inhibition of adenylate cyclase. This is the primary theory on how antipsychotic drugs decrease GB growth.
- Several different drugs (rolipram, forskolin, and dibutyryl cAMP) that increase the intracellular cAMP level via different MOAs slow GB growth both in vitro and in rodent graft studies.
- Rolipram, apremilast, and roflumilast are related PDE4 inhibitors. Ten studies demonstrated the ability of rolipram to inhibit GB growth.
6. Discussion
7. Caveats
- The 12 studies in Table 2 showing GB growth inhibition by the antipsychotic drug chlorpromazine prompted a 2023 clinical study by Pace et al. [325]. There was no clinical benefit from adding chlorpromazine 50 mg/day to GB’s standard of care [325]. Several problems limit the significance of this study. (i) As reviewed elsewhere, a multidrug regimen is needed to retard GB growth (in the absence of a silver bullet) [2,3,4,5,6,7,8,9]. (ii) The dose of chlorpromazine was too low to occupy and inhibit a significant percentage of the D2 receptors in the brain or tumor. (iii) As an inverse agonist at D1 [326], chlorpromazine would not be the ideal D2-blocking drug. Inverse agonism at D1 functionally lowers basal intracellular cAMP, the opposite of what we are aiming for.
- The database for D2 blockade resulting in GB growth arrest or cell death includes studies ascribing this to autophagy inhibition, while other studies ascribe this to autophagy stimulation. How do we resolve this apparent contradiction?
- Are we dealing with the metaphor of “blind men examining an elephant”? Are the many different MOAs ascribed to the inhibition of GB growth by antipsychotic drugs just the downstream consequences of a single D2 receptor event?
- Some of the studies supporting GB inhibition by the AVRO drugs reported low microM range IC50 values. Ideally, we seek candidate drugs with low nM IC50 inhibition.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- van Dijck, J.T.J.M.; Ardon, H.; Balvers, R.K.; Bos, E.M.; Bosscher, L.; Brouwers, H.B.; Ho, V.K.Y.; Hovinga, K.; Kwee, L.; ter Laan, M.; et al. Survival prediction in glioblastoma: 10-year follow-up from the Dutch Neurosurgery Quality Registry. J. Neurooncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hainfellner, A.; Borkovec, M.; Seebrecht, L.; Neuhauser, M.; Roetzer-Pejrimovsky, T.; Greutter, L.; Surböck, B.; Hager-Seifert, A.; Hof, D.G.-V.; Urbanic-Purkart, T.; et al. Glioblastoma in the real-world setting: Patterns of care and outcome in the Austrian population. J. Neurooncol. 2024, 170, 407–418. [Google Scholar] [CrossRef]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Hwangbo, H.; Patterson, S.C.; Dai, A.; Plana, D.; Palmer, A.C. Additivity predicts the efficacy of most approved combination therapies for advanced cancer. Nat. Cancer 2023, 4, 1693–1704. [Google Scholar] [CrossRef]
- Kast, R.E.; Boockvar, J.A.; Brüning, A.; Cappello, F.; Chang, W.-W.; Cvek, B.; Dou, Q.P.; Duenas-Gonzalez, A.; Efferth, T.; Focosi, D.; et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 2013, 4, 502–530. [Google Scholar] [CrossRef]
- Duenas-Gonzalez, A.; Gonzalez-Fierro, A.; Bornstein-Quevedo, L.; Gutierrez-Delgado, F.; Kast, R.E.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Candelaria, M.; Romo-Pérez, A.; Correa-Basurto, J.; et al. Multitargeted polypharmacotherapy for cancer treatment. theoretical concepts and proposals. Expert Rev. Anticancer Ther. 2024, 24, 665–677. [Google Scholar] [CrossRef]
- Johanssen, T.; McVeigh, L.; Erridge, S.; Higgins, G.; Straehla, J.; Frame, M.; Aittokallio, T.; Carragher, N.O.; Ebner, D. Glioblastoma and the search for non-hypothesis driven combination therapeutics in academia. Front. Oncol. 2023, 12, 1075559. [Google Scholar] [CrossRef]
- Halatsch, M.E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. NeuroOncol Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef]
- Qiu, T.; Men, P.; Xu, X.; Zhai, S.; Cui, X. Antiemetic regimen with aprepitant in the prevention of chemotherapy-induced nausea and vomiting: An updated systematic review and meta-analysis. Medicine 2020, 99, e21559. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.S.; Schmoll, H.J.; Jahn, F.; Carides, A.D.; Webb, R.T. Review of the efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in a range of tumor types. Cancer Treat. Rev. 2013, 39, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Dando, T.M.; Perry, C.M. Aprepitant: A review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 2004, 64, 777–794. [Google Scholar] [CrossRef]
- Hargreaves, R.; Ferreira, J.C.; Hughes, D.; Brands, J.; Hale, J.; Mattson, B.; Mills, S. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann. N. Y. Acad. Sci. 2011, 1222, 40–48. [Google Scholar] [CrossRef]
- Stoutenburg, J.P.; Raftopoulos, H. Antiemetic studies on the NK1 receptor antagonist aprepitant. J. Natl. Compr. Cancer Netw. 2004, 2, 491–497. [Google Scholar] [CrossRef]
- Higa, G.M.; Auber, M.L.; Altaha, R.; Kurian, S.; Hobbs, G. Concordance between substance P levels and antiemetic guidelines. J. Support Oncol. 2009, 7, 138–142. [Google Scholar] [CrossRef]
- Park, H.S.; Won, H.S.; An, H.J.; Cho, S.S.; Kim, H.H.; Sun, S.; Ko, Y.H.; Shim, B.Y. Elevated serum substance P level as a predictive marker for moderately emetogenic chemotherapy induced nausea and vomiting: A prospective cohort study. Cancer Med. 2021, 10, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.; Habib, A.S. A pharmacological overview of aprepitant for the prevention of postoperative nausea and vomiting. Expert Rev. Clin. Pharmacol. 2023, 16, 491–505. [Google Scholar] [CrossRef]
- Sanchez, R.I.; Wang, R.W.; Newton, D.J.; Bakhtiar, R.; Lu, P.; Chiu, S.H.; Evans, D.C.; Huskey, S.E. Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab. Dispos. 2004, 32, 1287–1292. [Google Scholar] [CrossRef]
- Łazarczyk, M.; Matyja, E.; Lipkowski, A. Substance P and its receptors—A potential target for novel medicines in malignant brain tumour therapies (mini-review). Folia Neuropathol. 2007, 45, 99–107. [Google Scholar]
- Gitter, B.D.; Regoli, D.; Howbert, J.J.; Glasebrook, A.L.; Waters, D.C. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J. Neuroimmunol. 1994, 51, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.; Manzini, S. Substance P induces secretion of immunomodulatory cytokines by human astrocytoma cells. J. Neuroimmunol. 1998, 81, 127–137. [Google Scholar] [CrossRef]
- Palma, C.; Maggi, C.A. The role of tachykinins via NK1 receptors in progression of human gliomas. Life Sci. 2000, 67, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M. Radiotherapy Plus the Neurokinin-1 Receptor Antagonist Aprepitant: A Potent Therapeutic Strategy for the Treatment of Diffuse Intrinsic Pontine Glioma. Cancers 2025, 17, 520. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Mirzavi, F.; Hashemy, S.I.; Ghadiri, M.K.; Stummer, W.; Gorji, A. The in vitro anti-cancer synergy of neurokinin-1 receptor antagonist, aprepitant, and 5-aminolevulinic acid in glioblastoma. Biofactors 2023, 49, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Darban, R.A.; Javid, H.; Hashemy, S.I. The Therapeutic Potential of Aprepitant in Glioblastoma Cancer Cells through Redox Modification. Biomed Res. Int. 2022, 2022, 8540403. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, Y.J.; Jung, H.J. Discovery of a New CaMKII-Targeted Synthetic Lethal Therapy against Glioblastoma Stem-like Cells. Cancers 2022, 14, 1315. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Dwucet, A.; Schmidt, C.J.; Mühlnickel, J.; Heiland, T.; Zeiler, K.; Siegelin, M.D.; Kast, R.E.; Karpel-Massler, G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals 2021, 14, 1241. [Google Scholar] [CrossRef]
- Ghahremani, F.; Sabbaghzade, R.; Ebrahimi, S.; Javid, H.; Ghahremani, J.; Hashemy, S.I. Pathogenic role of the SP/NK1R system in GBM cells through inhibiting the thioredoxin system. Iran. J. Basic. Med. Sci. 2021, 24, 499–505. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. Biomed Res. Int. 2022, 2022, 6291504. [Google Scholar] [CrossRef]
- Mehrabani, N.; Kakhki, M.R.V.; Javid, H.; Ebrahimi, S.; Hashemy, S.I.; Bronstein, J. The SP/NK1R System-Mediated ROS Generation in GBM Cells through Inhibiting Glutaredoxin Protein. Neurol. Res. Int. 2021, 2021, 9966000. [Google Scholar] [CrossRef] [PubMed]
- Korfi, F.; Javid, H.; Darban, R.A.; Hashemy, S.I. The Effect of SP/NK1R on the Expression and Activity of Catalase and Superoxide Dismutase in Glioblastoma Cancer Cells. Biochem. Res. Int. 2021, 2021, 6620708. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Ramiro, S.; Lladó, S.; Toro, S.; Coveñas, R.; Muñoz, M. Antitumor action of temozolomide, ritonavir and aprepitant against human glioma cells. J. Neurooncol. 2016, 126, 425–431. [Google Scholar] [CrossRef]
- Skaga, E.; Skaga, I.Ø.; Grieg, Z.; Sandberg, C.J.; Langmoen, I.A.; Vik-Mo, E.O. The efficacy of a coordinated pharmacological blockade in glioblastoma stem cells with nine repurposed drugs using the CUSP9 strategy. J. Cancer Res. Clin. Oncol. 2019, 145, 1495–1507. [Google Scholar] [CrossRef]
- Cao, Q.; Hajosch, A.; Kast, R.E.; Loehmann, C.; Hlavac, M.; Fischer-Posovszky, P.; Strobel, H.; Westhoff, M.-A.; Siegelin, M.D.; Wirtz, C.R.; et al. Tumor Treating Fields (TTFields) combined with the drug repurposing approach, C.U.SP9v3 induce metabolic reprogramming and synergistic anti-glioblastoma activity in vitro. Br. J. Cancer 2024, 130, 1365–1376. [Google Scholar] [CrossRef]
- Gutierrez, S.; Boada, M.D. NK1 receptor blockade disrupts microtumor growth and aggregation in a three-dimensional murine breast cancer model. Neuropeptides 2025, 109, 102479. [Google Scholar] [CrossRef]
- Mozafari, M.; Ebrahimi, S.; Darban, R.A.; Hashemy, S.I. Potential in vitro therapeutic effects of targeting SP/NK1R system in cervical cancer. Mol. Biol. Rep. 2022, 49, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, M.; Firoozrai, M.; Javid, H.; Hashemy, S.I. The substance P/neurokinin-1 receptor signaling pathway mediates metastasis in human colorectal SW480 cancer cells. Mol. Biol. Rep. 2022, 49, 4893–4900. [Google Scholar] [CrossRef]
- AlAlikhan, A.; Ghahremanloo, A.; Javid, H.; Ebrahimi, S.; Hashemy, S.I. The Effect of Blocking Neurokinin-1 Receptor by Aprepitant on the Inflammatory and Apoptosis Pathways in Human Ovarian Cancer Cells. Cell Biochem. Biophys. 2022, 80, 819–827. [Google Scholar] [CrossRef]
- Razmgah, M.M.; Ghahremanloo, A.; Javid, H.; AlAlikhan, A.; Afshari, A.-R.; Hashemy, S.I. The effect of substance P and its specific antagonist (aprepitant) on the expression of MMP-2, MMP-9, VEGF, and VEGFR in ovarian cancer cells. Mol. Biol. Rep. 2022, 49, 9307–9314. [Google Scholar] [CrossRef]
- Munoz, M.; Recio, S.; Rosso, M.; Redondo, M.; Covenas, R. The antiproliferative action of [D-Arg(1), D-Phe(5), D-Trp(7,9), LEU(11)] substance P analogue antagonist against small-cell- and non-small-cell lung cancer cells could be due to the pharmacological profile of its tachykinin receptor antagonist. J. Physiol. Pharmacol. 2015, 66, 421–426. [Google Scholar] [PubMed]
- Luo, W.; Sharif, T.R.; Sharif, M. Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signaling pathway. Cancer Res. 1996, 56, 4983–4991. [Google Scholar]
- Mukerji, I.; Ramkissoon, S.H.; Reddy, K.K.; Rameshwar, P. Autocrine proliferation of neuroblastoma cells is partly mediated through neurokinin receptors: Relevance to bone marrow metastasis. J. Neurooncol. 2005, 71, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Rosso, M.; Gonzalez-Ortega, A.; Coveñas, R. The NK-1 receptor antagonist L732,138 induces apoptosis and counteracts substance P-related mitogenesis in human melanoma cell lines. Cancers 2010, 2, 611–623. [Google Scholar] [CrossRef]
- Rosso, M.; Robles-Frías, M.J.; Coveñas, R.; Salinas-Martín, M.V.; Muñoz, M. The NK-1 receptor is expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer cell lines. Tumour Biol. 2008, 29, 245–254. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Aguilar, F.J.; A González-Moles, M.; Redondo, M.; Esteban, F. NK-1 receptor antagonists induce apoptosis and counteract substance P-related mitogenesis in human laryngeal cancer cell line HEp-2. Investig. New Drugs 2008, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M.; Pérez, A.; Coveñas, R.; Rosso, R.; Zamarriego, C.; Piruat, J. The NK1 receptor is involved in the antitumoural action of L-733,060 and in the mitogenic action of substance P on neuroblastoma and glioma cell lines. Neuropeptides 2005, 39, 427–432. [Google Scholar] [CrossRef]
- Lang, K.; Drell, T.L.; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S.; Entschladen, F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer. 2004, 112, 231–238. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Investig. New Drugs. 2012, 30, 529–540. [Google Scholar] [CrossRef]
- Bayati, S.; Bashash, D.; Ahmadian, S.; Safaroghli-Azar, A.; Alimoghaddam, K.; Ghavamzadeh, A.; Ghaffari, S.H. Inhibition of tachykinin NK1 receptor using aprepitant induces apoptotic cell death and G1 arrest through Akt/p53 axis in pre-B acute lymphoblastic leukemia cells. Eur. J. Pharmacol. 2016, 791, 274–283. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, X.; Huang, F.; Shao, G.; Meng, Y.; Wang, L.; Wang, T.; Jia, X.; Yang, T.; Wang, X.; et al. Aprepitant Sensitizes Acute Myeloid Leukemia Cells to the Cytotoxic Effects of Cytosine Arabinoside in vitro and in vivo. Drug Des. Devel. Ther. 2020, 14, 2413–2422. [Google Scholar] [CrossRef]
- Dikmen, M.; Gökhaner, G.; Cantürk, Z. Evaluation of the antileukemic effects of neurokinin-1 receptor antagonists, aprepitant, and L-733,060, in chronic and acute myeloid leukemic cells. Anticancer Drugs 2019, 30, e0769. [Google Scholar] [CrossRef]
- Molinos-Quintana, A.; Trujillo-Hacha, P.; Piruat, J.I.; Bejarano-García, J.A.; García-Guerrero, E.; Pérez-Simón, J.A.; Muñoz, M. Human acute myeloid leukemia cells express Neurokinin-1 receptor, which is involved in the antileukemic effect of Neurokinin-1 receptor antagonists. Investig. New Drugs 2019, 37, 17–26. [Google Scholar] [CrossRef]
- Ge, C.; Huang, H.; Huang, F.; Yang, T.; Zhang, T.; Wu, H.; Zhou, H.; Chen, Q.; Shi, Y.; Sun, Y.; et al. Neurokinin-1 receptor is an effective target for treating leukemia by inducing oxidative stress through mitochondrial calcium overload. Proc. Natl. Acad. Sci. USA 2019, 116, 19635–19645. [Google Scholar] [CrossRef]
- Bashash, D.; Safaroghli-Azar, A.; Bayati, S.; Razani, E.; Pourbagheri-Sigaroodi, A.; Gharehbaghian, A.; Momeny, M.; Sanjadi, M.; Rezaie-Tavirani, M.; Ghaffari, S.H. Neurokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-κB axis: Shedding new light on resistance to Aprepitant. Int. J. Biochem. Cell Biol. 2018, 103, 105–114. [Google Scholar] [CrossRef]
- Bayati, S.; Razani, E.; Bashash, D.; Safaroghli-Azar, A.; Safa, M.; Ghaffari, S.H. Antileukemic effects of neurokinin-1 receptor inhibition on hematologic malignant cells: A novel therapeutic potential for aprepitant. Anticancer Drugs 2018, 29, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, V.; Keller, I.; Seltzer, E.S.; Ostendorf, B.N.; Kerner, Z.; Tavazoie, S.F. Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis. Nature 2024, 633, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Jafarinezhad, S.; Darban, R.A.; Javid, H.; Hashemy, S.I. The SP/NK1R system promotes the proliferation of breast cancer cells through NF-κB-mediated inflammatory responses. Cell Biochem. Biophys. 2023, 81, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, J.; Perry, S.; Jiang, Q.; Shapiro, D.J. How FDA-approved drug, Aprepitant, induces immunogenic death of cancer cells. Cancer Res. 2024, 84 (Suppl. S6), 5995. [Google Scholar] [CrossRef]
- Nizam, E.; Erin, N. Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma cells; Effects independent of Substance P. Biomed. Pharmacother. 2018, 108, 263–270. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef]
- Rodriguez, E.; Pei, G.; Kim, S.T.; German, A.; Robinson, P. Substance P Antagonism as a Novel Therapeutic Option to Enhance Efficacy of Cisplatin in Triple Negative Breast Cancer and Protect PC12 Cells against Cisplatin-Induced Oxidative Stress and Apoptosis. Cancers 2021, 13, 3871. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanloo, A.; Erfani, B.; Asgharzadeh, F.; Mansoori, S.; Gheybi, F.; Hashemy, S.I. Reducing toxicity and enhancing efficacy of doxorubicin by liposomal doxorubicin and aprepitant in breast cancer. Sci. Rep. 2025, 15, 9798. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yuan, S.; Ma, J.; Liu, H.; Huang, L.; Zhang, F. Neurokinin-1 receptor is highly expressed in cervical cancer and its antagonist induces cervical cancer cell apoptosis. Eur. J. Histochem. 2023, 67, 3570. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, X.; Wang, Y.; Wu, X.; Zhou, P.; Miao, L.; Deng, X. Neurokinin-1 receptor antagonist aprepitant regulates autophagy and apoptosis via ROS/JNK in intrahepatic cholangiocarcinoma. Liver Int. 2024, 44, 1651–1667. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Zhou, W.; Wang, Y.; Wang, X.; Ge, X.; Wang, F.; Zhou, F.; Deng, X.; Miao, L. Aprepitant inhibits the development and metastasis of gallbladder cancer via ROS. and MAPK activation. BMC Cancer 2023, 23, 471. [Google Scholar] [CrossRef]
- Alalikhan, A.; Ebrahimi, S.; Aliee, A.; Mirzavi, F.; Hashemy, S.I. The combined anti-tumor effects of 5-fluorouracil and neurokinin receptor inhibitor, aprepitant, against colorectal cancer: In vitro and in vivo study. Med. Oncol. 2024, 41, 70. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Meng, Y.; Ma, J.; Zhang, Q.; Shao, G.; Wang, L.; Cheng, X.; Hong, X.; Wang, Y.; et al. A Novel Mechanism of Endoplasmic Reticulum Stress- and c-Myc-Degradation-Mediated Therapeutic Benefits of Antineurokinin-1 Receptor Drugs in Colorectal Cancer. Adv Sci. 2021, 8, e2101936. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Javid, H.; Afshari, A.R.; Hashemy, S.I. Investigation of the Role of Neurokinin-1 Receptor Inhibition Using Aprepitant in the Apoptotic Cell Death through PI3K/Akt/NF-κB Signal Transduction Pathways in Colon Cancer Cells. Biomed Res. Int. 2021, 2021, 1383878. [Google Scholar] [CrossRef]
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160. [Google Scholar] [CrossRef]
- Zheng, Y.; Sang, M.; Liu, F.; Gu, L.; Li, J.; Wu, Y.; Shan, B. Aprepitant inhibits the progression of esophageal squamous cancer by blocking the truncated neurokinin-1 receptor. Oncol. Rep. 2023, 50, 131. [Google Scholar] [CrossRef] [PubMed]
- Javid, H.; Afshari, A.R.; Zahedi Avval, F.; Asadi, J.; Hashemy, S.I. Aprepitant Promotes Caspase-Dependent Apoptotic Cell Death and G2/M. Arrest through PI3K/Akt/NF-κB Axis in Cancer Stem-Like Esophageal Squamous Cell Carcinoma Spheres. Biomed Res. Int. 2021, 2021, 8808214. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Javid, H.; Iranpour, S.; Darban, R.A.; Hashemy, S.I. Unveiling the Promising Role of Substance P/Neurokinin 1 Receptor in Cancer Cell Proliferation and Cell Cycle Regulation in Human Malignancies. Curr. Med. Chem. 2024, 31. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustín Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef]
- Ilmer, M.; Garnier, A.; Vykoukal, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M. Targeting the Neurokinin-1 Receptor Compromises Canonical Wnt Signaling in Hepatoblastoma. Mol. Cancer Ther. 2015, 14, 2712–2721. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. G.A.&HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. Int. J. Nanomed. 2022, 17, 2559–2575. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Robles-Frias, M.J.; Salinas-Martín, M.V.; Rosso, R.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab. Investig. 2010, 90, 1259–1269. [Google Scholar] [CrossRef]
- Razani, E.; Bashash, D. Cytotoxic and apoptotic effects of neurokinin-1 receptor (NK1R) antagonist on multiple myeloma cells. Sci. J. Kurd. Univ. Med. Sci. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Henssen, A.G.; Odersky, A.; Szymansky, A.; Seiler, M.; Althoff, K.; Beckers, A.; Speleman, F.; Schäfers, S.; De Preter, K.; Astrahanseff, K.; et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget 2017, 8, 430–443. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, L.; Hu, W.Q.; Hu, M.N.; Tao, Y.; Hu, H.; Miao, X.K.; Yang, W.L.; Zhu, Q.; Mou, L.Y. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis. 2022, 13, 41. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Rosso, M.; Robles-Frias, M.J.; Carranza, A.; Salinas-Martín, M.V.; Coveñas, R. The substance P/neurokinin-1 receptor system in lung cancer: Focus on the antitumor action of neurokinin-1 receptor antagonists. Peptides 2012, 38, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, M.A.; Ebrahimi, S.; Alalikhan, A.; Hashemi, S.F.; Hashemy, S.I. The Potential In Vitro Inhibitory Effects of Neurokinin-1 Receptor (NK-1R) Antagonist, Aprepitant, in Osteosarcoma Cell Migration and Metastasis. Biomed Res. Int. 2022, 2022, 8082608. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef]
- Kolorz, J.; Demir, S.; Gottschlich, A.; Beirith, I.; Ilmer, M.; Lüthy, D.; Walz, C.; Dorostkar, M.M.; Magg, T.; Hauck, F.; et al. The Neurokinin-1 Receptor Is a Target in Pediatric Rhabdoid Tumors. Curr. Oncol. 2021, 29, 94–110. [Google Scholar] [CrossRef]
- Beirith, I.; Renz, B.W.; Mudusetti, S.; Ring, N.S.; Kolorz, J.; Koch, D.; Bazhin, A.V.; Berger, M.; Wang, J.; Angele, M.K.; et al. Identification of the Neurokinin-1 Receptor as Targetable Stratification Factor for Drug Repurposing in Pancreatic Cancer. Cancers 2021, 13, 2703. [Google Scholar] [CrossRef]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, J.; Zhang, L.; Liu, H.; Jiang, B.; Liang, Y.; Zhang, Y. Comprehensive analysis of the progression mechanisms of CRPC and its inhibitor discovery based on machine learning algorithms. Front. Genet. 2023, 14, 1184704. [Google Scholar] [CrossRef]
- Akbari, S.; Assaran Darban, R.; Javid, H.; Esparham, A.; Hashemy, S.I. The anti-tumoral role of Hesperidin and Aprepitant on prostate cancer cells through redox modifications. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Erfani, B.; Alalikhan, A.; Ghorbani, H.; Farzadnia, M.; Afshari, A.R.; Mashkani, B.; Hashemy, S.I. The In Vitro Pro-inflammatory Functions of the SP/NK1R System in Prostate Cancer: A Focus on Nuclear Factor-Kappa B (NF-κB) and Its Pro-inflammatory Target Genes. Appl. Biochem. Biotechnol. 2023, 195, 7796–7807. [Google Scholar] [CrossRef]
- Shandiz, S.Z.; Darban, R.A.; Javid, H.; Ghahremanloo, A.; Hashemy, S.I. The effect of SP/NK1R on expression and activity of glutaredoxin and thioredoxin proteins in prostate cancer cells. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 5875–5882. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Li, J.-Y.; Li, L.; Hu, W.-Q.; Tao, Y.; Gao, W.-Y.; Ye, Z.-N.; Jia, H.-Y.; Wang, J.-N.; Miao, X.-K.; et al. Neurokinin-1 receptor drives PKCɑ-AURKA/N-Myc signaling to facilitate the neuroendocrine progression of prostate cancer. Cell Death Dis. 2023, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.D.; Covenas, R. Association of Neurokinin-1 Receptor Signaling Pathways with Cancer. Curr. Med. Chem. 2024, 31, 6460–6486. [Google Scholar] [CrossRef]
- Coveñas, R.; Muñoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef] [PubMed]
- Javid, H.; Mohammadi, F.; Zahiri, E.; Hashemy, S.I. The emerging role of substance P/neurokinin-1 receptor signaling pathways in growth and development of tumor cells. J. Physiol. Biochem. 2019, 75, 415–421. [Google Scholar] [CrossRef]
- García-Aranda, M.; Téllez, T.; McKenna, L.; Redondo, M. Neurokinin-1 Receptor (NK-1R) Antagonists as a New Strategy to Overcome Cancer Resistance. Cancers 2022, 14, 2255. [Google Scholar] [CrossRef] [PubMed]
- De Mehboob, R.; Kurdi, M.; Baeesa, S.; Fawzy Halawa, T.; Tanvir, I.; Maghrabi, Y.; Hakamy, S.; Saeedi, R.; Moshref, R.; Nasief, H.; et al. Immunolocalization of neurokinin 1 receptor in WHO grade 4 astrocytomas, oral squamous cell and urothelial carcinoma. Folia Neuropathol. 2022, 60, 165–176. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, R.; Dong, D.; Liu, R.; Zhang, L.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; et al. TGFβ regulates NK1R-Tr to affect the proliferation and apoptosis of breast cancer cells. Life Sci. 2020, 256, 117674. [Google Scholar] [CrossRef]
- Coveñas, R.; Rodríguez, F.D.; Robinson, P.; Muñoz, M. The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant. Int. J. Mol. Sci. 2023, 24, 15936. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 30, 1798–1812. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Young, A.; Bennett, C.J.; Hu, X.; Connor, C.O.; Cernak, I.; Nimmo, A.J. Neuropeptide release influences brain edema formation after diffuse traumatic brain injury. Acta Neurochir. Suppl. 2003, 86, 257–260. [Google Scholar] [CrossRef]
- Turner, R.J.; Helps, S.C.; Thornton, E.; Vink, R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res. 2011, 1393, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Harford-Wright, E.; Lewis, K.M.; Ghabriel, M.N.; Vink, R. Treatment with the NK1 antagonist emend reduces blood brain barrier dysfunction and edema formation in an experimental model of brain tumors. PLoS ONE 2014, 9, e97002. [Google Scholar] [CrossRef]
- Harford-Wright, E.; Lewis, K.M.; Vink, R. The potential for substance P antagonists as anti-cancer agents in brain tumours. Recent Pat. CNS Drug Discov. 2013, 8, 13–23. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Marian, O.C.; Bilecki, I.M.; Elms, L.E.; Yassi, N.; Hood, R.J.; Coller, J.K.; Stuckey, S.M.; Kimberly, W.T.; Farr, T.D.; et al. NK1 tachykinin receptor antagonist treatment reduces cerebral edema and intracranial pressure in an ovine model of ischemic stroke. J. Cereb. Blood Flow. Metab. 2024, 44, 1759–1773. [Google Scholar] [CrossRef]
- Gabrielian, L.; Helps, S.C.; Thornton, E.; Turner, R.J.; Leonard, A.V.; Vink, R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir. Suppl. 2013, 118, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Zee, E.D.; Schomberg, S.; Carpenter, T.C. Hypoxia upregulates lung microvascular neurokinin-1 receptor expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L102–L110. [Google Scholar] [CrossRef][Green Version]
- Ostwal, V.; Ramaswamy, A.; Mandavkar, S.; Bhargava, P.; Naughane, D.; Sunn, S.F.; Srinivas, S.; Kapoor, A.; Mishra, B.K.; Gupta, A.; et al. Olanzapine as Antiemetic Prophylaxis in Moderately Emetogenic Chemotherapy: A Phase 3 Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2426076. [Google Scholar] [CrossRef]
- Asadi, M.; Mirdoosti, S.M.; Majidi, S.; Boroumand, N.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum substance P level and tissue distribution of NK-1 receptor in papillary thyroid cancer. Middle East J. Cancer 2021, 12, 491–498. [Google Scholar]
- Lorestani, S.; Ghahremanloo, A.; Jangjoo, A.; Abedi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in colorectal cancer. Mol. Biol. Rep. 2020, 47, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Șerban, R.E.; Boldeanu, M.V.; Florescu, D.N.; Ionescu, M.; Șerbănescu, M.S.; Boldeanu, L.; Florescu, M.M.; Stepan, M.D.; Obleagă, V.C.; Constantin, C.; et al. Comparison between Substance P and Calcitonin Gene-Related Peptide and Their Receptors in Colorectal Adenocarcinoma. J. Clin. Med. 2024, 13, 5616. [Google Scholar] [CrossRef]
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262. [Google Scholar] [CrossRef]
- Zahiri, E.; Ghorbani, H.; Moradi, A.; Mehrad-Majd, H.; Mohammadi, F.; Sharifi Sistani, N.; Hashemy, S.I. Prognostic Significance of Substance P and Neurokinin-1 Receptor in Bladder Cancer. Rep. Biochem. Mol. Biol. 2022, 11, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, M.; Boroumand, N.; Mehrabi Bahar, M.; Jafarian, A.H.; Asadi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in breast cancer. Mol. Biol. Rep. 2019, 46, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Sharma, J.; Sikarwar, P.; Kakkar, A.K. Dipeptidyl peptidase 4 (DPP-4) inhibitors and the risk of lung cancer: Current evidence and future directions. Expert Rev. Clin. Pharmacol. 2023, 16, 39–47. [Google Scholar] [CrossRef]
- Scholzen, T.E.; Luger, T.A. Neutral endopeptidase and angiotensin-converting enzyme -- key enzymes terminating the action of neuroendocrine mediators. Exp. Dermatol. 2004, 13 (Suppl. S4), 22–26. [Google Scholar] [CrossRef]
- Choi, J.G.; Choi, S.R.; Kang, D.W.; Shin, H.J.; Lee, M.; Hwang, J.; Kim, H.W. Inhibition of angiotensin converting enzyme increases PKCβI isoform expression via activation of substance P and bradykinin receptors in cultured astrocytes of mice. J. Vet. Sci. 2023, 24, e26. [Google Scholar] [CrossRef]
- Choi, J.G.; Choi, S.R.; Kang, D.W.; Kim, J.; Park, J.B.; Kim, H.W. Inhibition of angiotensin converting enzyme induces mechanical allodynia through increasing substance P expression in mice. Neurochem. Int. 2021, 146, 105020. [Google Scholar] [CrossRef]
- Wilson, J.R.; Kerman, S.J.; A Hubers, S.; Yu, C.; Nian, H.; Grouzmann, E.; Eugster, P.J.; Mayfield, D.S.; Brown, N.J. Dipeptidyl Peptidase 4 Inhibition Increases Postprandial Norepinephrine via Substance P (NK1 Receptor) During RAAS Inhibition. J. Endocr. Soc. 2019, 3, 1784–1798. [Google Scholar] [CrossRef]
- Bergström, M.; Hargreaves, R.J.; Burns, H.D.; Goldberg, M.R.; Sciberras, D.; Reines, S.A.; Petty, K.J.; Ögren, M.; Antoni, G.; Långström, B.; et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol. Psychiatry 2004, 55, 1007–1012. [Google Scholar] [CrossRef]
- Spitsin, S.; Tebas, P.; Barrett, J.S.; Pappa, V.; Kim, D.; Taylor, D.; Evans, D.L.; Douglas, S.D. Antiinflammatory effects of aprepitant coadministration with cART regimen containing ritonavir in HIV-infected adults. JCI Insight 2017, 2, e95893. [Google Scholar] [CrossRef]
- Krupa, A.J.; Wojtasik-Bakalarz, K.; Siwek, M. Vortioxetine—Pharmacological properties and use in mood disorders. The current state of knowledge. Psychiatr. Pol. 2023, 57, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Mørk, A.; Pehrson, A.; Brennum, L.T.; Nielsen, S.M.; Zhong, H.; Lassen, A.B.; Miller, S.; Westrich, L.; Boyle, N.J.; Sánchez, C.; et al. Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J. Pharmacol. Exp. Ther. 2012, 340, 666–675. [Google Scholar] [CrossRef]
- Berardelli, I.; Rogante, E.; Formica, F.; Iannazzo, R.; Mammoliti, A.V.; Riccioni, R.; Veizi, S.; McIntyre, R.S.; Pompili, M. The efficacy of vortioxetine in the acute treatment of major depressive disorder: A systematic review and meta-analysis. J. Psychopharmacol. 2025, 39, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Glioblastoma chemotherapy adjunct via potent serotonin receptor-7 inhibition using currently marketed high-affinity antipsychotic medicines. Br. J. Pharmacol. 2010, 161, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mahé, C.; Bernhard, M.; Bobirnac, I.; Keser, C.; Loetscher, E.; Feuerbach, D.; Dev, K.K.; Schoeffter, P. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br. J. Pharmacol. 2004, 143, 404–410. [Google Scholar] [CrossRef]
- Elmaci, I.; Altinoz, M.A. Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit. Rev. Oncol. Hematol. 2018, 128, 96–109. [Google Scholar] [CrossRef]
- Varalda, M.; Antona, A.; Bettio, V.; Roy, K.; Vachamaram, A.; Yellenki, V.; Massarotti, A.; Baldanzi, G.; Capello, D. Psychotropic Drugs Show Anticancer Activity by Disrupting Mitochondrial and Lysosomal Function. Front. Oncol. 2020, 10, 562196. [Google Scholar] [CrossRef]
- Tzadok, S.; Beery, E.; Israeli, M.; Uziel, O.; Lahav, M.; Fenig, E.; Gil-Ad, I.; Weizman, A.; Nordenberg, J. In vitro novel combinations of psychotropics and anti-cancer modalities in U87 human glioblastoma cells. Int. J. Oncol. 2010, 37, 1043–1051. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Gil-Ad, I.; Zeldich, E.; Dayag, M.; Weizman, A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: Evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J. Mol. Neurosci. 2005, 27, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Stepulak, A.; Rzeski, W.; Sifringer, M.; Brocke, K.; Gratopp, A.; Kupisz, K.; Turski, L.; Ikonomidou, C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol. Ther. 2008, 7, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Najafi, S.H.; Shafiee, F.; Hassanzadeh, S.; Farzipour, S.; Ghasemi, A.; Asgarian-Omran, H. Fluoxetine as an antidepressant medicine improves the effects of ionizing radiation for the treatment of glioma. J. Bioenerg. Biomembr. 2020, 52, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Yang, S.T.; Lin, Y.K.; Lin, J.W.; Lee, Y.H.; Wang, J.Y.; Hu, C.J.; Lin, E.Y.; Chen, S.M.; Then, C.K.; et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking, A.M.PAR-mediated calcium-dependent apoptosis. Oncotarget 2015, 6, 5088–5101. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.R.; Chen, W.; Chen, M.H.; Wang, H.; Wang, X.D.; Sun, L.L.; Wang, F.Z.; Wang, D.C. Fluoxetine synergizes with temozolomide to induce the, C.H.OP-dependent endoplasmic reticulum stress-related apoptosis pathway in glioma cells. Oncol. Rep. 2016, 36, 676–684. [Google Scholar] [CrossRef]
- Song, T.; Li, H.; Tian, Z.; Xu, C.; Liu, J.; Guo, Y. Disruption of NF-κB signaling by fluoxetine attenuates MGMT expression in glioma cells. Onco. Targets Ther. 2015, 8, 2199–2208. [Google Scholar] [CrossRef]
- Sarker, A.; Aziz, A.; Hossen, B.; Mollah, M.H.; Amin, A.; Mollah, N.H. Discovery of key molecular signatures for diagnosis and therapies of glioblastoma by combining supervised and unsupervised learning approaches. Sci. Rep. 2024, 14, 27545. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Chantzi, E.; Hammerling, U.; Gustafsson, M.G. Exhaustive in vitro evaluation of the 9-drug cocktail CUSP9 for treatment of glioblastoma. Comput. Biol. Med. 2024, 178, 108748. [Google Scholar] [CrossRef]
- Duarte, D.; Nunes, M.; Ricardo, S.; Vale, N. Combination of Antimalarial and CNS Drugs with Antineoplastic Agents in MCF-7 Breast and HT-29 Colon Cancer Cells: Biosafety Evaluation and Mechanism of Action. Biomolecules 2022, 12, 1490. [Google Scholar] [CrossRef]
- Jacob, J.R.; Palanichamy, K.; Chakravarti, A. Antipsychotics possess anti-glioblastoma activity by disrupting lysosomal function and inhibiting oncogenic signaling by stabilizing PTEN. Cell Death Dis. 2024, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, N.; Otani, Y.; Uneda, A.; Ishida, J.; Suruga, Y.; Matsumoto, Y.; Fujimura, A.; Fujii, K.; Matsui, H.; Kurozumi, K.; et al. New Anti-Angiogenic Therapy for Glioblastoma with the Anti-Depressant Sertraline. Cancer Med. 2024, 13, e70288. [Google Scholar] [CrossRef]
- Petrosyan, E.; Fares, J.; Cordero, A.; Rashidi, A.; Arrieta, V.A.; Kanojia, D.; Lesniak, M.S. Repurposing autophagy regulators in brain tumors. Int. J. Cancer 2022, 151, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Baskaran, S.; Johansson, P.; Padhan, N.; Matuszewski, D.; Green, L.C.; Elfineh, L.; Wee, S.; Häggblad, M.; Martens, U.; et al. Case-specific potentiation of glioblastoma drugs by pterostilbene. Oncotarget 2016, 7, 73200–73215. [Google Scholar] [CrossRef]
- Schmidt, L.; Kling, T.; Monsefi, N.; Olsson, M.; Hansson, C.; Baskaran, S.; Lundgren, B.; Martens, U.; Häggblad, M.; Westermark, B.; et al. Comparative drug pair screening across multiple glioblastoma cell lines reveals novel drug-drug interactions. Neuro Oncol. 2013, 15, 1469–1478. [Google Scholar] [CrossRef]
- Bielecka-Wajdman, A.M.; Lesiak, M.; Ludyga, T.; Sieroń, A.; Obuchowicz, E. Reversing glioma malignancy: A new look at the role of antidepressant drugs as adjuvant therapy for glioblastoma multiforme. Cancer Chemother. Pharmacol. 2017, 79, 1249–1256. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Wu, W.; Li, W.; Zhao, L.; Li, A.; Lu, X.; He, X.; Dong, Z.; Liu, K.; et al. Vortioxetine hydrobromide inhibits the growth of gastric cancer cells in vivo and in vitro by targeting JAK2 and SRC. Oncogenesis 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.B.; Wang, T.T.; Zhu, H.L.; Wang, H.K.; Sun, W.; Zhao, L.F. Vortioxetine induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway. FEBS Open Bio 2020, 10, 2157–2165. [Google Scholar] [CrossRef]
- Lee, S.; Weiss, T.; Bühler, M.; Mena, J.; Lottenbach, Z.; Wegmann, R.; Sun, M.; Bihl, M.; Augustynek, B.; Baumann, S.P.; et al. High-throughput identification of repurposable neuroactive drugs with potent anti-glioblastoma activity. Nat. Med. 2024, 30, 3196–3208. [Google Scholar] [CrossRef]
- Devare, M.N.; Kaeberlein, M. An anti-depressant drug vortioxetine suppresses malignant glioblastoma cell growth. MicroPubl Biol. 2024, 2024. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhang, D.M.; Huang, Z.Z.; Cheng, J.; Zhang, C.; Lin, N.M.; Li, Y. Vortioxetine exhibits anti-glioblastoma activity via the PI3K-Akt signaling pathway. Iran. J. Basic. Med. Sci. 2025, 28, 401–408. [Google Scholar] [CrossRef]
- Zhuo, C.; Li, C.; Zhang, Q.; Yang, L.; Zhang, Y.; Chen, X.; Ma, X.; Li, R.; Wang, L.; Tian, H. Molecular targets of vortioxetine mediating glioblastoma suppression revealed by gene and protein network analyses and molecular docking simulations. Int. J. Neuropsychopharmacol. 2025, 28, pyaf029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Siebzehnrubl, D.; Weller, M.; Weiss, T.; Siebzehnrubl, F.A.; Newland, B. Vortioxetine: A Potential Drug for Repurposing for Glioblastoma Treatment via a Microsphere Local Delivery System. ACS Biomater. Sci. Eng. 2025, 11, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Eskelund, A.; Li, Y.; Budac, D.P.; Müller, H.K.; Gulinello, M.; Sanchez, C.; Wegener, G. Drugs with antidepressant properties affect tryptophan metabolites differently in rodent models with depression-like behavior. J. Neurochem. 2017, 142, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Teo, C.; McDonald, K.L.; Zinger, A.; Bustamante, S.; Lim, C.K.; Sundaram, G.; Braidy, N.; Brew, B.J.; Guillemin, G.J. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS ONE 2014, 9, e112945. [Google Scholar] [CrossRef]
- Sahm, F.; Oezen, I.; Opitz, C.A.; Radlwimmer, B.; von Deimling, A.; Ahrendt, T.; Adams, S.; Bode, H.B.; Guillemin, G.J.; Wick, W.; et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidativestress. Cancer Res. 2013, 73, 3225–3234. [Google Scholar] [CrossRef]
- Bhat, A.; Tan, V.; Heng, B.; Lovejoy, D.B.; Sakharkar, M.K.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J. Roflumilast, a cAMP-Specific Phosphodiesterase-4 Inhibitor, Reduces Oxidative Stress and Improves Synapse Functions in Human Cortical Neurons Exposed to the Excitotoxin Quinolinic Acid. ACS Chem. Neurosci. 2020, 11, 4405–4415. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Sordillo, L.A.; Helson, L. The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res. 2017, 37, 2159–2171. [Google Scholar] [CrossRef]
- Krupa, M.M.; Pienkowski, T.; Tankiewicz-Kwedlo, A.; Lyson, T. Targeting the kynurenine pathway in gliomas: Insights into pathogenesis, therapeutic targets, and clinical advances. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189343. [Google Scholar] [CrossRef]
- Jane, E.P.; Premkumar, D.R.; Thambireddy, S.; Golbourn, B.; Agnihotri, S.; Bertrand, K.C.; Mack, S.C.; Myers, M.I.; Chattopadhyay, A.; Taylor, D.L.; et al. Targeting NAD+ Biosynthesis Overcomes Panobinostat and Bortezomib-Induced Malignant Glioma Resistance. Mol. Cancer Res. 2020, 18, 1004–1017. [Google Scholar] [CrossRef]
- Kesarwani, P.; Kant, S.; Zhao, Y.; Prabhu, A.; Buelow, K.L.; Miller, C.R.; Chinnaiyan, P. Quinolinate promotes macrophage-induced immune tolerance in glioblastoma through the NMDAR/PPARγ signaling axis. Nat. Commun. 2023, 14, 1459. [Google Scholar] [CrossRef] [PubMed]
- Field, S.K. Roflumilast: An oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin. Investig. Drugs 2008, 17, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Giembycz, M.A.; Field, S.K. Roflumilast: First phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther. 2010, 4, 147-q58. [Google Scholar] [CrossRef]
- Lahu, G.; Nassr, N.; Hünnemeyer, A. Pharmacokinetic evaluation of roflumilast. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1577–1591. [Google Scholar] [CrossRef]
- Rabe, K.F. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br. J. Pharmacol. 2011, 163, 53–67. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, X.; Yang, M.; Cai, Q.; Shao, N. Potential treatment benefits and safety of roflumilast in COPD: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef]
- Sengupta, R.; Sun, T.; Warrington, N.M.; Rubin, J.B. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol. Sci. 2011, 32, 337–344. [Google Scholar] [CrossRef]
- Kast, R.E. Paths for Improving Bevacizumab Available in 2018: The ADZT Regimen for Better Glioblastoma Treatment. Med. Sci. 2018, 6, 84. [Google Scholar] [CrossRef]
- Dixit, D.; Prager, B.C.; Gimple, R.C.; Miller, T.E.; Wu, Q.; Yomtoubian, S.; Kidwell, R.L.; Lv, D.; Zhao, L.; Qiu, Z.; et al. Glioblastoma stem cells reprogram chromatin in vivo to generate selective therapeutic dependencies on DPY30 and phosphodiesterases. Sci. Transl. Med. 2022, 14, eabf3917. [Google Scholar] [CrossRef]
- Ou, M.; Cho, H.Y.; Fu, J.; Thein, T.Z.; Wang, W.; Swenson, S.D.; Minea, R.O.; Stathopoulos, A.; Schönthal, A.H.; Hofman, F.M.; et al. Inhibition of autophagy and induction of glioblastoma cell death by NEO214, a perillyl alcohol-rolipram conjugate. Autophagy 2023, 19, 3169–3188. [Google Scholar] [CrossRef] [PubMed]
- Goldhoff, P.; Warrington, N.M.; Limbrick, D.D., Jr.; Hope, A.; Woerner, B.M.; Jackson, E.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Targeted Inhibition of Cyclic AMP Phosphodiesterase-4 Promotes Brain Tumor Regression. Clin. Cancer Res. 2008, 14, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Vousooghi, N.; Kapourchali, F.R.; Hadjighasem, M.; Hayat, P.; Amini, N.; Joghataei, M.T. Rolipram potentiates bevacizumab-induced cell death in human glioblastoma stem-like cells. Life Sci. 2017, 173, 11–19. [Google Scholar] [CrossRef]
- Chen, T.C.; Wadsten, P.; Su, S.; Rawlinson, N.; Hofman, F.M.; Hill, C.K.; Schönthal, A.H. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol. Ther. 2002, 1, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Biltekin, S.N.; Yurttas, L.; Berk, B.; Özhan, Y.; Sipahi, H.; Gao, Z.G.; Jacobson, K.A.; Demirayak, Ş. Novel cyanothiouracil and cyanothiocytosine derivatives as concentration dependent selective inhibitors of U87MG glioblastomas: Adenosine receptor binding and potent PDE4 inhibition. Eur. J. Med. Chem. 2021, 212, 113125. [Google Scholar] [CrossRef]
- Ramezani, S.; Vousooghi, N.; Ramezani Kapourchali, F.; Yousefzadeh-Chabok, S.; Reihanian, Z.; Alizadeh, A.M.; Khodayari, S.; Khodayari, H. Rolipram optimizes therapeutic effect of bevacizumab by enhancing proapoptotic, antiproliferative signals in a glioblastoma heterotopic model. Life Sci. 2019, 239, 116880. [Google Scholar] [CrossRef]
- Yang, L.; Jackson, E.; Woerner, B.M.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res. 2007, 67, 651–658. [Google Scholar] [CrossRef]
- Moon, E.Y.; Lee, G.H.; Lee, M.S.; Kim, H.M.; Lee, J.W. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci. 2012, 90, 373–380. [Google Scholar] [CrossRef]
- Kang, T.W.; Choi, S.W.; Yang, S.R.; Shin, T.H.; Kim, H.S.; Yu, K.R.; Hong, I.S.; Ro, S.; Cho, J.M.; Kang, K.S. Growth arrest and forced differentiation of human primary glioblastoma multiforme by a novel small molecule. Sci Rep. 2014, 4, 5546. [Google Scholar] [CrossRef][Green Version]
- Awad, J.A.; Johnson, R.A.; Jakobs, K.H.; Schultz, G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J. Biol. Chem. 1983, 258, 2960–2965. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Papa, D.; Hübner, M.; Mou, T.C.; Lushington, G.H.; Seifert, R. Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J. Pharmacol. Exp. Ther. 2008, 325, 27–36. [Google Scholar] [CrossRef]
- Qi, C.; Lavriha, P.; Mehta, V.; Khanppnavar, B.; Mohammed, I.; Li, Y.; Lazaratos, M.; Schaefer, J.V.; Dreier, B.; Plückthun, A.; et al. Structural basis of adenylyl cyclase 9 activation. Nat. Commun. 2022, 13, 1045. [Google Scholar] [CrossRef]
- Li, F.; Zhou, K.; Gao, L.; Zhang, B.; Li, W.; Yan, W.; Song, X.; Yu, H.; Wang, S.; Yu, N.; et al. Radiation induces the generation of cancer stem cells: A novel mechanism for cancer radioresistance. Oncol. Lett. 2016, 12, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, R.; Li, D.; Sun, J. Induced cancer stem cells generated by radiochemotherapy and their therapeutic implications. Oncotarget 2017, 8, 17301–17312. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Pisco, A.O.; Huang, S. Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me’. Br. J. Cancer 2015, 112, 1725–1732. [Google Scholar] [CrossRef]
- He, L.; Azizad, D.; Bhat, K.; Ioannidis, A.; Hoffmann, C.J.; Arambula, E.; Eghbali, M.; Bhaduri, A.; Kornblum, H.I.; Pajonk, F. Radiation-induced cellular plasticity primes glioblastoma for forskolin-mediated differentiation. Proc. Natl. Acad. Sci. USA 2025, 122, e2415557122. [Google Scholar] [CrossRef]
- Sharma, S.K.; Raj, A.B. Transient increase in intracellular concentration of adenosine 3′:5′-cyclic monophosphate results in morphological and biochemical differentiation of C6 glioma cells in culture. J. Neurosci. Res. 1987, 17, 135–141. [Google Scholar] [CrossRef]
- Daniel, P.M.; Filiz, G.; Mantamadiotis, T. Sensitivity of GBM cells to cAMP agonist mediated apoptosis correlates with CD44 expression and agonist resistance with MAPK signaling. Cell Death Dis. 2016, 7, e2494. [Google Scholar] [CrossRef]
- Black, P.M.; Kornblith, P.L.; Davison, P.F.; Liszczak, T.M.; Merk, L.P.; Smith, B.H.; McKeever, P.E.; Quindlen, E.A. Immunological, biochemical, ultrastructural, and electrophysiological characteristics of a human glioblastoma-derived cell culture line. J. Neurosurg. 1982, 56, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Luan, Y.; Cai, J.; Wu, S.; Mai, J.; Gu, J.; Zhang, H.; Li, K.; Lin, Y.; Xiao, X.; et al. The Anti-Warburg Effect Elicited by the cAMP-PGC1α Pathway Drives Differentiation of Glioblastoma Cells into Astrocytes. Cell Rep. 2017, 18, 468–481. [Google Scholar] [CrossRef]
- Wartchow, K.M.; Schmid, B.; Tripal, P.; Stadlbauer, A.; Buchfelder, M.; Gonçalves, C.-A.; Kleindienst, A. Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy. Cells 2021, 10, 556. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Liu, X.; Sai, K.; Mai, J.; Xing, F.; Chen, Z.; Yang, X.; Lu, W.; Guo, C.; et al. IL-6 derived from therapy-induced senescence facilitates the glycolytic phenotype in glioblastoma cells. Am. J. Cancer Res. 2021, 11, 458–478. [Google Scholar] [PubMed]
- Zhang, H.; Liu, Y.; Liu, J.; Chen, J.; Wang, J.; Hua, H.; Jiang, Y. cAMP-PKA/EPAC signaling and cancer: The interplay in tumor microenvironment. J. Hematol. Oncol. 2024, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Fuxe, K.; Tarakanov, A.; Romero Fernandez, W.; Ferraro, L.; Tanganelli, S.; Filip, M.; Agnati, L.F.; Garriga, P.; Diaz-Cabiale, Z.; Borroto-Escuela, D.O. Diversity and Bias through Receptor Receptor Interactions in GPCR Heteroreceptor Complexes. Focus on Examples from Dopamine D2 Receptor Heteromerization. Front. Endocrinol. 2014, 5, 71. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Parhar, I. Emerging therapeutic potential of antipsychotic drugs in the management of human glioma: A comprehensive review. Oncotarget 2019, 10, 3952–3977. [Google Scholar] [CrossRef]
- Caragher, S.P.; Shireman, J.M.; Huang, M.; Miska, J.; Atashi, F.; Baisiwala, S.; Hong Park, C.; Saathoff, M.R.; Warnke, L.; Xiao, T.; et al. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J. Neurosci. 2019, 39, 1982–1993. [Google Scholar] [CrossRef]
- Lee, J.K.; Nam, D.H.; Lee, J. Repurposing antipsychotics as glioblastoma therapeutics: Potentials and challenges. Oncol. Lett. 2016, 11, 1281–1286. [Google Scholar] [CrossRef]
- Rundle-Thiele, D.; Head, R.; Cosgrove, L.; Martin, J.H. Repurposing some older drugs that cross the blood-brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br. J. Clin. Pharmacol. 2016, 81, 199–209. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, K.; Qi, J.; Zhang, Y.; Wang, X.; Zhang, L.; Zhou, Y.; Gu, L.; Yu, R.; et al. Chronic stress accelerates glioblastoma progression via DRD2/ERK/β-catenin axis and Dopamine/ERK/TH positive feedback loop. J. Exp. Clin. Cancer Res. 2023, 42, 161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, S.; Kozono, D.; Ng, K.; Futalan, D.; Shen, Y.; Akers, J.C.; Steed, T.; Kushwaha, D.; Schlabach, M.; et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget 2014, 5, 882–893. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Koga, T.; Ma, J.; Dhawan, S.; Suzuki, Y.; Furnari, F.; Prabhu, V.V.; E Allen, J.; Chen, C.C. Epidermal growth factor receptor as a molecular determinant of glioblastoma response to dopamine receptor D2 inhibitors. Neuro-Oncol. 2021, 23, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yoo, B.C.; Yang, W.S.; Han, S.Y.; Jeong, D.; Song, J.M.; Kim, K.H.; Aravinthan, A.; Kim, J.H.; Kim, J.-H.; et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget 2017, 9, 5979–5992. [Google Scholar] [CrossRef]
- Forno, F.; Maatuf, Y.; Boukeileh, S.; Dipta, P.; Mahameed, M.; Darawshi, O.; Ferreira, V.; Rada, P.; García-Martinez, I.; Gross, E.; et al. Aripiprazole Cytotoxicity Coincides with Activation of the Unfolded Protein Response in Human Hepatic Cells. J. Pharmacol. Exp. Ther. 2020, 374, 452–461. [Google Scholar] [CrossRef]
- Benítez-García, C.; Martínez-García, D.; Kotev, M.; Pérez-Hernández, M.; Westermaier, Y.; Díaz, L.; Korrodi-Gregório, L.; Fontova, P.; Torres, A.A.; Pérez-Tomás, R.; et al. Identification of the atypical antipsychotic Asenapine as a direct survivin inhibitor with anticancer properties and sensitizing effects to conventional therapies. Biomed. Pharmacother. 2025, 182, 117756. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamamoto, M.; Togashi, K.; Sanomachi, T.; Sugai, A.; Seino, S.; Yoshioka, T.; Kitanaka, C.; Okada, M. In vitro and in vivo anti-tumor effects of brexpiprazole, a newly-developed serotonin-dopamine activity modulator with an improved safety profile. Oncotarget 2019, 10, 3547–3558. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamamoto, M.; Sanomachi, T.; Togashi, K.; Sugai, A.; Seino, S.; Yoshioka, T.; Kitanaka, C.; Okada, M. Brexpiprazole, a Serotonin-Dopamine Activity Modulator, Can Sensitize Glioma Stem Cells to Osimertinib, a Third-Generation EGFR-TKI, via Survivin Reduction. Cancers 2019, 11, 947. [Google Scholar] [CrossRef]
- Matarrese, P.; Signore, M.; Ascione, B.; Fanelli, G.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine overcomes temozolomide resistance in glioblastoma by inhibiting Cx43 and essential DNA repair pathways. J. Transl. Med. 2024, 22, 667. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, C.; Matteoni, S.; Matarrese, P.; Signore, M.; Ascione, B.; Iessi, E.; Gurtner, A.; Sacconi, A.; Ricci-Vitiani, L.; Pallini, R.; et al. Chlorpromazine affects glioblastoma bioenergetics by interfering with pyruvate kinase M2. Cell Death Dis. 2023, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Lin, Y.S.; Liang, W.Z. The Impact of the Antipsychotic Medication Chlorpromazine on Cytotoxicity through Ca2+ Signaling Pathway in Glial Cell Models. Neurotox. Res. 2022, 40, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 347. [Google Scholar] [CrossRef]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Buccarelli, M.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Anticancer Properties of the Antipsychotic Drug Chlorpromazine and Its Synergism with Temozolomide in Restraining Human Glioblastoma Proliferation In Vitro. Front. Oncol. 2021, 11, 635472. [Google Scholar] [CrossRef]
- Pinheiro, T.; Otrocka, M.; Seashore-Ludlow, B.; Rraklli, V.; Holmberg, J.; Forsberg-Nilsson, K.; Simon, A.; Kirkham, M. A chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth. Biochem. Biophys. Res. Commun. 2017, 494, 477–483. [Google Scholar] [CrossRef]
- Oliva, C.R.; Zhang, W.; Langford, C.; Suto, M.J.; Griguer, C.E. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget 2017, 8, 37568–37583. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Matteoni, S.; Persico, M.; Villani, V.; Paggi, M.G. Repurposing chlorpromazine in the treatment of glioblastoma multiforme: Analysis of literature and forthcoming steps. J. Exp. Clin. Cancer Res. 2020, 39, 26. [Google Scholar] [CrossRef]
- Munyon, W.H.; Salo, R.; Briones, D.F. Cytotoxic effects of neuroleptic drugs. Psychopharmacology 1987, 91, 182–188. [Google Scholar] [CrossRef]
- Hait, W.N.; Gesmonde, J.F.; Lazo, J.S. Effect of anti-calmodulin drugs on the growth and sensitivity of C6 rat glioma cells to bleomycin. Anticancer Res. 1994, 14, 1711–1721. [Google Scholar]
- Aas, A.T.; Brun, A.; Pero, R.W.; Salford, L.G. Chlorpromazine in combination with nitrosourea inhibits experimental glioma growth. Br. J. Neurosurg. 1994, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Choi, B.H.; Ko, J.; Kim, S.H.; Kim, Y.S.; Lee, Y.H. Clozapine, a neuroleptic agent, inhibits Akt by counteracting Ca2+/calmodulin in PTEN-negative U-87MG human glioblastoma cells. Cell Signal. 2006, 18, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Pont, L.M.E.B.; Balvers, R.K.; Kloezeman, J.J.; Nowicki, M.O.; van den Bossche, W.; Kremer, A.; Wakimoto, H.; van den Hoogen, B.G.; Leenstra, S.; Dirven, C.M.F.; et al. In vitro screening of clinical drugs identifies sensitizers of oncolytic viral therapy in glioblastoma stem-like cells. Gene Ther. 2015, 22, 947–959. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, W.; Zhang, Z.; Sun, W.; Cui, G.; Chen, H.; Wang, H. Fluspirilene exerts an anti-glioblastoma effect through suppression of the FOXM1-KIF20A axis. Neoplasma 2024, 71, 333–346. [Google Scholar] [CrossRef]
- Dong, Y.; Furuta, T.; Sabit, H.; Kitabayashi, T.; Jiapaer, S.; Kobayashi, M.; Ino, Y.; Todo, T.; Teng, L.; Hirao, A.; et al. Identification of antipsychotic drug fluspirilene as a potential anti-glioma stem cell drug. Oncotarget 2017, 8, 111728–111741. [Google Scholar] [CrossRef]
- Shi, L.; Chen, H.; Chen, K.; Zhong, C.; Song, C.; Huang, Y.; Wang, T.; Chen, L.; Li, C.; Huang, A.; et al. The DRD2 Antagonist Haloperidol Mediates Autophagy-Induced Ferroptosis to Increase Temozolomide Sensitivity by Promoting Endoplasmic Reticulum Stress in Glioblastoma. Clin. Cancer Res. 2023, 29, 3172–3188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, X.; Gao, L.; Liu, X.; Li, J.; Huang, X.; Zeng, T. Synergistic Suppression of Glioblastoma Cell Growth by Combined Application of Temozolomide and Dopamine D2 Receptor Antagonists. World Neurosurg. 2019, 128, e468–e477. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines 2020, 8, 595. [Google Scholar] [CrossRef]
- Weissenrieder, J.S.; Reed, J.L.; Green, M.V.; Moldovan, G.L.; Koubek, E.J.; Neighbors, J.D.; Hohl, R.J. The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells. Pharmacology 2020, 105, 19–27. [Google Scholar] [CrossRef]
- Doello, K.; Mesas, C.; Quiñonero, F.; Rama, A.R.; Vélez, C.; Perazzoli, G.; Ortiz, R. Antitumor Effect of Traditional Drugs for Neurological Disorders: Preliminary Studies in Neural Tumor Cell Lines. Neurotox. Res. 2022, 40, 1645–1652. [Google Scholar] [CrossRef]
- Gont, A.; Daneshmand, M.; Woulfe, J.; Lavictoire, S.J.; Lorimer, I.A. PREX1 integrates G protein-coupled receptor and phosphoinositide 3-kinase signaling to promote glioblastoma invasion. Oncotarget 2017, 8, 8559–8573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mégalizzi, V.; Decaestecker, C.; Debeir, O.; Spiegl-Kreinecker, S.; Berger, W.; Lefranc, F.; Kast, R.E.; Kiss, R. Screening of anti-glioma effects induced by sigma-1 receptor ligands: Potential new use for old anti-psychiatric medicines. Eur. J. Cancer 2009, 45, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Raza, I.; Ujjan, B.; Wasim, B.; Khan, L.; Naeem, N.; Enam, S.A.; Hanif, F. Iloperidone and Temozolomide Synergistically Inhibit Growth, Migration and Enhance Apoptosis in Glioblastoma Cells. Biomedicines 2024, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yamamoto, M.; Sanomachi, T.; Togashi, K.; Seino, S.; Sugai, A.; Yoshioka, T.; Okada, M.; Kitanaka, C. Lurasidone Sensitizes Cancer Cells to Osimertinib by Inducing Autophagy and Reduction of Survivin. Anticancer Res. 2021, 41, 4321–4331. [Google Scholar] [CrossRef]
- Hua, J.; Olsson, A.R.; Pero, R.W. Neutral metoclopramide sensitizes cytotoxicity induced by ionizing radiation in, S.C.ID mice xenografted with a human brain astrocytoma. Int. J. Cancer 1997, 73, 871–874. [Google Scholar] [CrossRef]
- Olsson, A.; Sheng, Y.; Kjellén, E.; Pero, R.W. In vivo tumor measurement of DNA damage, DNA repair and NAD pools as indicators of radiosensitization by metoclopramide. Carcinogenesis 1995, 16, 1029–1035. [Google Scholar] [CrossRef]
- Salford, L.G.; Pero, R.W.; Aas, A.T.; Brun, A. Metoclopramide as a sensitizer of 1,3-bis(2-chloroethyl)-1-nitrosourea treatment of brain tumors in the rat. Anticancer Drugs 1992, 3, 267–272. [Google Scholar] [CrossRef]
- Allahgholipour, S.Z.; Farzipour, S.; Ghasemi, A.; Asgarian-Omran, H.; Hosseinimehr, S.J. The Radiosensitizing Effect of Olanzapine as an Antipsychotic Medication on Glioblastoma Cell. Curr. Radiopharm. 2022, 15, 50–55. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Kast, R.E.; Westhoff, M.-A.; Dwucet, A.; Welscher, N.; Nonnenmacher, L.; Hlavac, M.; Siegelin, M.D.; Wirtz, C.R.; Debatin, K.M.; et al. Olanzapine inhibits proliferation, migration and anchorage-independent growth in human glioblastoma cell lines and enhances temozolomide’s antiproliferative effect. J. Neurooncol. 2015, 122, 21–33. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, S.Q.; Chen, X.H.; Liu, R.S.; Liang, Z.Q. Autophagy involvement in olanzapine mediated cytotoxic effects in human glioma cells. Asian Pac. J. Cancer Prev. 2014, 15, 8107–8113. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.F.; Liu, R.S.; Xiong, Y.J.; Shen, X.; Wang, Y.; Liang, Z.Q. Olanzapine induced autophagy through suppression of NF-κB activation in human glioma cells. CNS Neurosci. Ther. 2019, 25, 911–921. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Huang, B.-R.; Lin, C.-J.; Shen, C.-K.; Lai, S.-W.; Chen, C.-W.; Lin, H.-J.; Lin, C.-H.; Hsieh, Y.-C.; Lu, D.-Y. Paliperidone Inhibits Glioblastoma Growth in Mouse Brain Tumor Model and Reduces PD-L1 Expression. Cancers 2021, 13, 4357. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Wright, S.; Srivastava, S.K. Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth. Oncotarget 2017, 8, 47632–47641. [Google Scholar] [CrossRef]
- Ranjan, A.; Srivastava, S.K. Penfluridol suppresses glioblastoma tumor growth by Akt mediated inhibition of GLI1. Oncotarget 2017, 8, 32960–32976. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chong, K.; Ryu, B.-K.; Park, K.-J.; Yu, M.O.; Lee, J.; Chung, S.; Choi, S.; Park, M.-J.; Chung, Y.-G.; et al. Repurposing Penfluridol in Combination with Temozolomide for the Treatment of Glioblastoma. Cancers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Hong, J.P.; Chjoi, R.J.; Shim, J.K.; Kim, K.; Kim, R.N.; Cho, H.J.; Kim, S.J.; Kim, S.; Kim, N.H.; Park, H.H.; et al. Synergistic combination of perphenazine and temozolomide suppresses patient-derived glioblastoma tumorspheres. Neuro Oncol. 2024, 27, 654–667. [Google Scholar] [CrossRef]
- Otręba, M.; Buszman, E. Perphenazine and prochlorperazine induce concentration dependent loss in human glioblastoma cells viability. Pharmazies 2018, 73, 19–21. [Google Scholar] [CrossRef]
- Otręba, M.; Stojko, J.; Kabała-Dzik, A.; Rzepecka-Stojko, A. Perphenazine and prochlorperazine decrease glioblastoma U-87 MG cell migration and invasion: Analysis of the ABCB1 and ABCG2 transporters, E-cadherin, α-tubulin and integrins (α3, α5, and β1) levels. Oncol. Lett. 2022, 23, 182. [Google Scholar] [CrossRef]
- Palanichamy, K.; Kanji, S.; Gordon, N.; Thirumoorthy, K.; Jacob, J.R.; Litzenberg, K.T.; Patel, D.; Chakravarti, A. NNMT Silencing Activates Tumor Suppressor PP2A, Inactivates Oncogenic STKs, and Inhibits Tumor Forming Ability. Clin. Cancer Res. 2017, 23, 2325–2334. [Google Scholar] [CrossRef]
- Jeon, H.-M.; Oh, Y.T.; Shin, Y.J.; Chang, N.; Kim, D.; Woo, D.; Yeup, Y.; Joo, K.M.; Jo, H.; Yang, H.; et al. Dopamine receptor D2 regulates glioblastoma survival and death through MET and death receptor 4/5. Neoplasia 2023, 39, 100894. [Google Scholar] [CrossRef]
- Zhong, Y.; Geng, F.; Mazik, L.; Yin, X.; Becker, A.P.; Mohammed, S.; Su, H.; Xing, E.; Kou, Y.; Chiang, C.-Y.; et al. Combinatorial targeting of glutamine metabolism and lysosomal-based lipid metabolism effectively suppresses glioblastoma. Cell Rep. Med. 2024, 5, 101706. [Google Scholar] [CrossRef] [PubMed]
- Remy, J.; Linder, B.; Weirauch, U.; Day, B.W.; Stringer, B.W.; Herold-Mende, C.; Aigner, A.; Krohn, K.; Kögel, D. STAT3 Enhances Sensitivity of Glioblastoma to Drug-Induced Autophagy-Dependent Cell Death. Cancers 2022, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Tulip, I.J.; Kim, S.O.; Kim, E.J.; Kim, J.; Lee, J.Y.; Kim, H.; Kim, S.C. Combined inhibition of STAT and Notch signalling effectively suppresses tumourigenesis by inducing apoptosis and inhibiting proliferation, migration and invasion in glioblastoma cells. Anim. Cells Syst. 2021, 25, 161–170. [Google Scholar] [CrossRef]
- Hong, J.-H.; Kang, S.; Sa, J.K.; Park, G.; Oh, Y.T.; Kim, T.H.; Yin, J.; Kim, S.S.; D’Angelo, F.; Koo, H.; et al. Modulation of Nogo receptor 1 expression orchestrates myelin-associated infiltration of glioblastoma. Brain 2021, 144, 636–654. [Google Scholar] [CrossRef]
- Meyer, N.; Henkel, L.; Linder, B.; Zielke, S.; Tascher, G.; Trautmann, S.; Geisslinger, G.; Münch, C.; Fulda, S.; Tegeder, I.; et al. Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy 2021, 17, 3424–3443. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.; Meyer, N.; Mari, M.; Abou-El-Ardat, K.; Reggiori, F.; van Wijk, S.J.L.; Kögel, D.; Fulda, S. Loperamide, pimozide, and STF-62247 trigger autophagy-dependent cell death in glioblastoma cells. Cell Death Dis. 2018, 9, 994. [Google Scholar] [CrossRef]
- Rath, B.H.; Camphausen, K.; Tofilon, P.J. Glioblastoma radiosensitization by pimozide. Transl. Cancer Res. 2016, 5 (Suppl. S6), S1029–S1032. [Google Scholar] [CrossRef]
- Sachdeva, R.; Wu, M.; Smiljanic, S.; Kaskun, O.; Ghannad-Zadeh, K.; Celebre, A.; Isaev, K.; Morrissy, A.S.; Guan, J.; Tong, J.; et al. ID1 Is Critical for Tumorigenesis and Regulates Chemoresistance in Glioblastoma. Cancer Res. 2019, 79, 4057–4071. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Kaushik, I.; Srivastava, S.K. Pimozide Suppresses the Growth of Brain Tumors by Targeting STAT3-Mediated Autophagy. Cells 2020, 9, 2141. [Google Scholar] [CrossRef]
- Roos, A.; Dhruv, H.D.; Peng, S.; Inge, L.J.; Tuncali, S.; Pineda, M.; Millard, N.; Mayo, Z.; Eschbacher, J.M.; Loftus, J.C.; et al. EGFRvIII–Stat5 Signaling Enhances Glioblastoma Cell Migration and Survival. Mol. Cancer Res. 2018, 16, 1185–1195. [Google Scholar] [CrossRef]
- Bhat, K.; Saki, M.; Cheng, F.; He, L.; Zhang, L.; Ioannidis, A.; Nathanson, D.; Tsang, J.; Bensinger, S.J.; Nghiemphu, P.L.; et al. Dopamine Receptor Antagonists, Radiation, and Cholesterol Biosynthesis in Mouse Models of Glioblastoma. J. Natl. Cancer Inst. 2021, 113, 1094–1104. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, N.; Li, H.; Liu, S.; Chen, X.; Yu, S.; Wu, N.; Bian, X.W.; Shen, H.Y.; Li, C.; et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget 2017, 8, 37511–37524. [Google Scholar] [CrossRef]
- Kast, R.E.; Skuli, N.; Karpel-Massler, G.; Frosina, G.; Ryken, T.; Halatsch, M.E. Blocking epithelial-to-mesenchymal transition in glioblastoma with a sextet of repurposed drugs: The EIS regimen. Oncotarget 2017, 8, 60727–60749. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, T.; Wan, Q.; Wang, Q.; Chen, Z.; Gao, Y.; Ye, Y.; Lin, J.; Zhao, B.; Wang, H.; et al. TRAF4 Maintains Deubiquitination of Caveolin-1 to Drive Glioblastoma Stemness and Temozolomide Resistance. Cancer Res. 2022, 82, 3573–3587. [Google Scholar] [CrossRef]
- Somuncu, O.S.; Karaman, I.; Saracoglu, H.P.; Yilmaz, E.; Akin, D. Modeling Schizophrenia with Glioblastoma Cells: In Vitro Analysis of Risperidone Treatment on Glial Spheroids. Psychiatry Clin. Psychopharmacol. 2021, 31, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Faraz, S.; Pannullo, S.; Rosenblum, M.; Smith, A.; Wernicke, A.G. Long-term survival in a patient with glioblastoma on antipsychotic therapy for schizophrenia: A case report and literature review. Ther. Adv. Med. Oncol. 2016, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Stringer, B.W.; De Silva, M.I.; Greenberg, Z.; Puerta, A.N.; Adams, R.; Milky, B.; Zabolocki, M.; Hurk, M.v.D.; Ebert, L.M.; Bishop, C.F.; et al. Human cerebrospinal fluid affects chemoradiotherapy sensitivities in tumor cells from patients with glioblastoma. Sci. Adv. 2023, 9, eadf1332. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Ekpo, O.E.; Semenya, D.M.; Jardine, A.; Prince, S. Exploitation of a novel phenothiazine derivative for its anti-cancer activities in malignant glioblastoma. Apoptosis 2020, 25, 261–274. [Google Scholar] [CrossRef]
- Johannessen, T.; Hasan-Olive, M.; Zhu, H.; Denisova, O.; Grudic, A.; Latif, A.; Saed, H.; Varughese, J.K.; Røsland, G.V.; Yang, N.; et al. Thioridazine inhibits autophagy and sensitizes glioblastoma cells to temozolomide. Int. J. Cancer 2019, 144, 1735–1745. [Google Scholar] [CrossRef]
- Chu, C.-W.; Ko, H.-J.; Chou, C.-H.; Cheng, T.-S.; Cheng, H.-W.; Liang, Y.-H.; Lai, Y.-L.; Lin, C.-Y.; Wang, C.; Loh, J.-K.; et al. Thioridazine Enhances P62-Mediated Autophagy and Apoptosis Through Wnt/β-Catenin Signaling Pathway in Glioma Cells. Int. J. Mol. Sci. 2019, 20, 473. [Google Scholar] [CrossRef]
- Cheng, H.W.; Liang, Y.H.; Kuo, Y.L.; Chuu, C.P.; Lin, C.Y.; Lee, M.H.; Wu, A.T.; Yeh, C.T.; Chen, E.I.; Whang-Peng, J.; et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015, 6, e1753. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, J.Y.; Kang, S.S. COX-2 Acts as a Key Mediator of Trifluoperazine-induced Cell Death in U87MG Glioma Cells. Anticancer. Res. 2022, 42, 5773–5781. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Sears, T.; Cortopassi, G.; Woolard, K.; Angelastro, J.M. Repurposing FDA approved drugs inhibiting mitochondrial function for targeting glioma-stem like cells. Biomed. Pharmacother. 2021, 133, 111058. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, J.M.; Jeon, B.; Elkamhawy, A.; Paik, S.; Hong, J.; Oh, S.J.; Paek, S.H.; Lee, C.J.; Hassan, A.H.E.; et al. Repositioning of the antipsychotic trifluoperazine: Synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents. Eur. J. Med. Chem. 2018, 151, 186–198. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, R.; Zhang, C.; Xu, Y.; Han, M.; Huang, B.; Chen, A.; Qiu, C.; Thorsen, F.; Prestegarden, L.; et al. Trifluoperazine, a novel autophagy inhibitor, increases radiosensitivity in glioblastoma by impairing homologous recombination. J. Exp. Clin. Cancer Res. 2017, 36, 118. [Google Scholar] [CrossRef]
- Ramis, G.; Villalonga-Planells, R.; Serra-Sitjar, M.; Brell, M.; de Mattos, S.F.; Villalonga, P. The tumor suppressor FOXO3a mediates the response to EGFR inhibition in glioblastoma cells. Cell Oncol. 2019, 42, 521–536. [Google Scholar] [CrossRef]
- Bhat, K.; Saki, M.; Vlashi, E.; Cheng, F.; Duhachek-Muggy, S.; Alli, C.; Yu, G.; Medina, P.; He, L.; Damoiseaux, R.; et al. The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 11085–11096. [Google Scholar] [CrossRef]
- Misganaw, D. Heteromerization of dopaminergic receptors in the brain: Pharmacological implications. Pharmacol. Res. 2021, 170, 105600. [Google Scholar] [CrossRef]
- Perreault, M.L.; Hasbi, A.; O’Dowd, B.F.; George, S.R. Heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef]
- Khan, S.S.; Lee, F.J. Delineation of domains within the cannabinoid CB1 and dopamine D2 receptors that mediate the formation of the heterodimer complex. J. Mol. Neurosci. 2014, 53, 10–21. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska-Sendo, A.; Łabędź-Masłowska, A.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 receptor and dopamine D2 receptor cooperatively contribute to the regulation of neutrophil adhesion to endothelial cells. Acta Biochim. Pol. 2018, 65, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.V.; Madhukar, N.S.; Gilvary, C.; Kline, C.L.B.; Oster, S.; El-Deiry, W.S.; Elemento, O.; Doherty, F.; VanEngelenburg, A.; Durrant, J.; et al. Dopamine Receptor D5 is a Modulator of Tumor Response to Dopamine Receptor D2 Antagonism. Clin. Cancer Res. 2019, 25, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M. How antipsychotics work in schizophrenia: A primer on mechanisms. CNS Spectr. 2024, 30, e6. [Google Scholar] [CrossRef]
- Broyd, A.; Balzan, R.P.; Woodward, T.S.; Allen, P. Dopamine, cognitive biases and assessment of certainty: A neurocognitive model of delusions. Clin. Psychol. Rev. 2017, 54, 96–106. [Google Scholar] [CrossRef]
- Yakubov, R.; Kaloti, R.; Persaud, P.; McCracken, A.; Zadeh, G.; Bunda, S. It’s all downstream from here: RTK/Raf/MEK/ERK pathway resistance mechanisms in glioblastoma. J. Neurooncol. 2025, 172, 327–345. [Google Scholar] [CrossRef]
- Hasan, S.; Mahmud, Z.; Hossain, M.; Islam, S. Harnessing the role of aberrant cell signaling pathways in glioblastoma multiforme: A prospect towards the targeted therapy. Mol. Biol. Rep. 2024, 51, 1069. [Google Scholar] [CrossRef]
- Kast, R.E. Adding perphenazine to increase effectiveness of standard glioblastoma chemoirradiation. J. Balk. Union Oncol. 2020, 25, 1676–1686. [Google Scholar]
- Kast, R.E.; Ellingson, B.M.; Marosi, C.; Halatsch, M.E. Glioblastoma treatment using perphenazine to block the subventricular zone’s tumor trophic functions. J. Neurooncol. 2014, 116, 207–212. [Google Scholar] [CrossRef][Green Version]
- Wesner, K.; Hiemke, C.; Bergemann, N.; Egberts, K.M.; Fekete, S.; Gerlach, M.; Havemann-Reinecke, U.; Lense, X.M.; Riemer, T.G.; Schoretsanitis, G.; et al. Therapeutic Reference Range for Olanzapine in Schizophrenia: Systematic Review on Blood Concentrations, Clinical Effects, and Dopamine Receptor Occupancy. J. Clin. Psychiatry 2023, 84, 47964. [Google Scholar] [CrossRef]
- Akam, E.; Strange, P.G. Inverse agonist properties of atypical antipsychotic drugs. Biochem. Pharmacol. 2004, 67, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.; Perry, K.W.; Nelson, D.L.; Wong, D.T.; Rasmussen, K.; Moore, N.A.; Calligaro, D.O. Olanzapine: A basic science update. Br. J. Psychiatry 1999, 174 (Suppl. S37), 36–40. [Google Scholar] [CrossRef]
- Kapur, S.; Zipursky, R.B.; Remington, G.; Jones, C.; DaSilva, J.; Wilson, A.A.; Houle, S. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: A PET investigation. Am. J. Psychiatry. 1998, 155, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Aravagiri, M.; Teper, Y.; Marder, S.R. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm. Drug Dispos. 1999, 20, 369–377. [Google Scholar] [CrossRef]
- Nedahl, M.; Johansen, S.; Linnet, K. Reference Brain and Blood Concentrations of Olanzapine in Postmortem Cases. J. Anal. Toxicol. 2018, 42, 650–654. [Google Scholar] [CrossRef]
- Kast, R.E.; Foley, K.F. Cancer chemotherapy and cachexia: Mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur. J. Cancer Care 2007, 16, 351–354. [Google Scholar] [CrossRef]
- Vig, S.; Seibert, L.; Green, M.R. Olanzapine is effective for refractory chemotherapy induced nausea and vomiting irrespective of chemotherapy emetogenicity. J. Cancer Res. Clin. Oncol. 2014, 140, 77–82. [Google Scholar] [CrossRef]
- Navari, R.M.; Qin, R.; Ruddy, K.J.; Liu, H.; Powell, S.F.; Bajaj, M.; Dietrich, L.; Biggs, D.; Lafky, J.M.; Loprinzi, C.L. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 375, 134–142. [Google Scholar] [CrossRef]
- Trifilio, S.; Welles, C.; Seeger, K.; Mehta, S.; Fishman, M.; McGowan, K.; Strejcek, K.; Eiten, E.; Pirotte, C.; Lucier, E.; et al. Olanzapine Reduces Chemotherapy induced Nausea and Vomiting Compared with Aprepitant in Myeloma Patients Receiving High-dose Melphalan Before Stem Cell Transplantation: A Retrospective Study. Clin. Lymphoma Myeloma Leuk. 2017, 17, 584–589. [Google Scholar] [CrossRef]
- Murakami, M.; Miyata, Y.; Nakashima, K.; Abe, M.; Nishimura, J.; Wada, M.; Iino, K.; Akechi, T.; Iihara, H.; Imamura, C.K.; et al. Clinical benefits of adding olanzapine to 5-HT3 receptor antagonist, NK1 receptor antagonist, and dexamethasone for the prevention of nausea and vomiting in highly emetogenic chemotherapy: A systematic review and meta-analysis of the Clinical Practice Guidelines for Antiemesis 2023 from the Japan Society of Clinical Oncology. Int. J. Clin. Oncol. 2025, 30, 27–39. [Google Scholar] [CrossRef]
- Slimano, F.; Netzer, F.; Borget, I.; Lemare, F.; Besse, B. Olanzapine as antiemetic drug in oncology: A retrospective study in non-responders to standard antiemetic therapy. Int. J. Clin. Pharm. 2018, 40, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Wu, H.; Wang, D.; Burschinski, A.; Nomura, N.; Takeuchi, H.; Schneider-Thoma, J.; Davis, J.M.; Leucht, S. Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: A systematic review and dose-response meta-analysis. Mol. Psychiatry 2023, 28, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Saudemont, G.; Prod’Homme, C.; Da Silva, A.; Villet, S.; Reich, M.; Penel, N.; Gamblin, V. The use of olanzapine as an antiemetic in palliative medicine: A systematic review of the literature. BMC Palliat. Care 2020, 19, 56. [Google Scholar] [CrossRef]
- Šoukalová, Z. Olanzapine in oncology palliative care. Klin. Onkol. 2022, 35, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.O.; Ajewole, V.; Braun, U.K. A Review of Olanzapine in the Treatment of Cancer Anorexia Cachexia Syndrome. Pharmacy 2024, 12, 34. [Google Scholar] [CrossRef]
- Sharpley, A.L.; Attenburrow, M.E.; Hafizi, S.; Cowen, P.J. Olanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patients. J. Clin. Psychiatry 2005, 66, 450–454. [Google Scholar] [CrossRef]
- Emamzadeh, N.; Abbasi, F.; Delfan, N.; Etemadi, M.H.; Iranmehr, A. Prevalence, risk factors, and impacts of sleep disturbances in patients with primary brain tumors: A systematic review. Neurosurg. Rev. 2025, 48, 375. [Google Scholar] [CrossRef]
- Robertson, M.E.; McSherry, F.; Herndon, J.E.; Peters, K.B. Insomnia and its associations in patients with recurrent glial neoplasms. Springerplus 2016, 5, 823. [Google Scholar] [CrossRef][Green Version]
- Armstrong, T.S.; Shade, M.Y.; Breton, G.; Gilbert, M.R.; Mahajan, A.; Scheurer, M.E.; Vera, E.; Berger, A.M. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017, 19, 323–335. [Google Scholar] [CrossRef]
- Tsamakis, K.; Mueller, C.; Hortis, I.; Kallergi, M.; Tolos, I.; Alevyzakis, E.; Siafakas, N.; Ouranidis, A.; Tsiptsios, D.; Kympouropoulos, S.; et al. Association of antipsychotic use with raised eosinophil count. Exp. Ther. Med. 2021, 21, 513. [Google Scholar] [CrossRef]
- Vaios, E.J.; Winter, S.F.; Muzikansky, A.; Nahed, B.V.; Dietrich, J. Eosinophil and lymphocyte counts predict bevacizumab response and survival in recurrent glioblastoma. Neurooncol. Adv. 2020, 2, vdaa031. [Google Scholar] [CrossRef] [PubMed]
- Spina, C.; Hsieh, K.; Wolthuis, B.; Elliston, C.; Baez, F.; Smith, D.; Wang, T. Treatment-induced increase in eosinophil count is associated with improved overall survival in glioblastoma patients. Int. J. Radiat. Oncol. 2018, 102, e315. [Google Scholar] [CrossRef]
- Vieira, B.M.; de São José, V.S.; Niemeyer Filho, P.S.; Moura-Neto, V. Eosinophils induces glioblastoma cell suppression and apoptosis—Roles of GM-CSF and cysteinyl leukotrienes. Int. Immunopharmacol. 2023, 123, 110729. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, L.; Hou, Z.; Zhang, P.; Li, G.; Xie, J. Eosinophils and other peripheral blood biomarkers in glioma grading: A preliminary study. BMC Neurol. 2019, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Madhugiri, V.S.; Venkatesan, S.; Dutt, A.; Moiyadi, A.V.; Shetty, P.; Gupta, T.; Epari, S.; Jalali, R.; Sasidharan, G.M.; Kumar, V.R.; et al. An Analysis of Eosinophil- and Basophil-Based Indices in Patients with Glioblastoma and their Correlation with Survival. World Neurosurg. 2023, 170, e292–e300. [Google Scholar] [CrossRef]
- Vas, C.; Jain, A.; Trivedi, M.; Jha, M.K.; Mathew, S.J. Pharmacotherapy for Treatment Resistant Depression: Antidepressants and Atypical Antipsychotics. Psychiatr. Clin. N. Am. 2023, 46, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Mathew, S.J. Pharmacotherapies for Treatment-Resistant Depression: How Antipsychotics Fit in the Rapidly Evolving Therapeutic Landscape. Am. J. Psychiatry 2023, 180, 190–199. [Google Scholar] [CrossRef]
- van der Meer, P.B.; Dirven, L.; Hertler, C.; Boele, F.W.; Batalla, A.; Walbert, T.; Rooney, A.G.; Koekkoek, J.A.F. Depression and anxiety in glioma patients. Neurooncol. Pract. 2023, 10, 335–343. [Google Scholar] [CrossRef]
- Dong, J.; Chen, Q.; Weng, S.; Liu, L.; Wang, J.; Fang, S.; Fan, X.; Jiang, T. The effect of depression and anxiety on survival in patients with glioma: A systematic review and meta-analysis. J. Neurooncol. 2024, 170, 265–275. [Google Scholar] [CrossRef]
- Dufner, V.; Kessler, A.F.; Just, L.; Hau, P.; Bumes, E.; Pels, H.J.; Grauer, O.M.; Wiese, B.; Löhr, M.; Jordan, K.; et al. The Emesis Trial: Depressive Glioma Patients Are More Affected by Chemotherapy-Induced Nausea and Vomiting. Front. Neurol. 2022, 13, 773265. [Google Scholar] [CrossRef]
- Vecht, C.J.; Kerkhof, M.; Duran-Pena, A. Seizure prognosis in brain tumors: New insights and evidence-based management. Oncologist 2014, 19, 751–759. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, M.E.; van der Meer, P.B.; Dirven, L.; Taphoorn, M.J.B.; Koekkoek, J.A.F. Efficacy of antiepileptic drugs in glioma patients with epilepsy: A systematic review. Neurooncol. Pract. 2021, 8, 501–517. [Google Scholar] [CrossRef]
- Esparza, R.; Azad, T.D.; Feroze, A.H.; Mitra, S.S.; Cheshier, S.H. Glioblastoma stem cells and stem cell-targeting immunotherapies. J. Neurooncol. 2015, 123, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Schrøder, H.D.; Kristensen, B.W. CD133+ niches and single cells in glioblastoma have different phenotypes. J. Neurooncol. 2011, 104, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Lombardi, G.; Villani, V.; Benincasa, D.; Abbruzzese, C.; Cestonaro, I.; Corrà, M.; Padovan, M.; Cerretti, G.; Caccese, M.; et al. Efficacy and safety of chlorpromazine as an adjuvant therapy for glioblastoma in patients with unmethylated MGMT gene promoter: RACTAC, a phase II multicenter trial. Front. Oncol. 2023, 13, 1320710. [Google Scholar] [CrossRef]
- McCarthy, C.I.; Mustafá, E.R.; Cornejo, M.P.; Yaneff, A.; Rodríguez, S.S.; Perello, M.; Raingo, J. Chlorpromazine, an Inverse Agonist of D1R-Like, Differentially Targets Voltage-Gated Calcium Channel (CaV) Subtypes in mPFC Neurons. Mol. Neurobiol. 2023, 60, 2644–2660. [Google Scholar] [CrossRef]
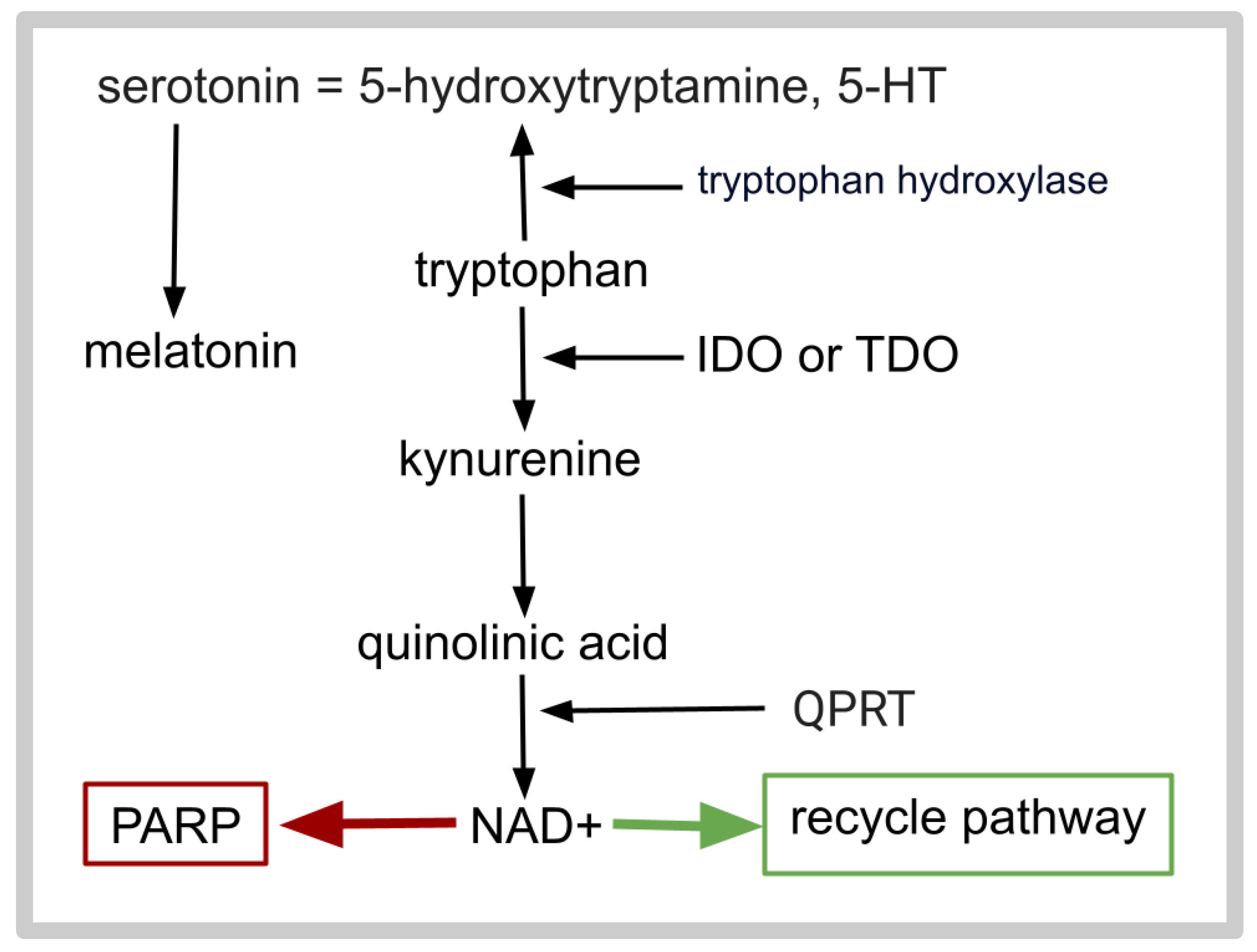
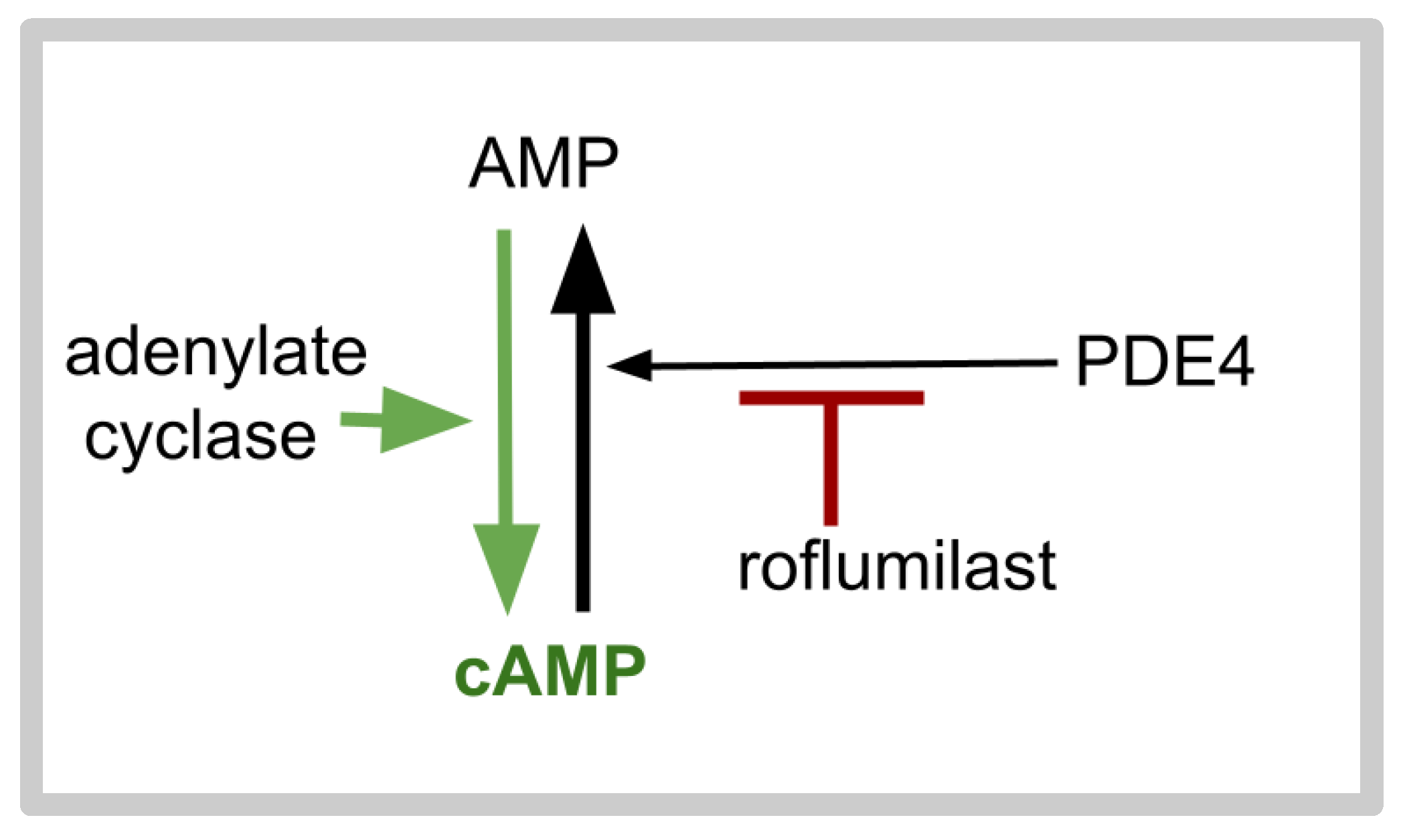
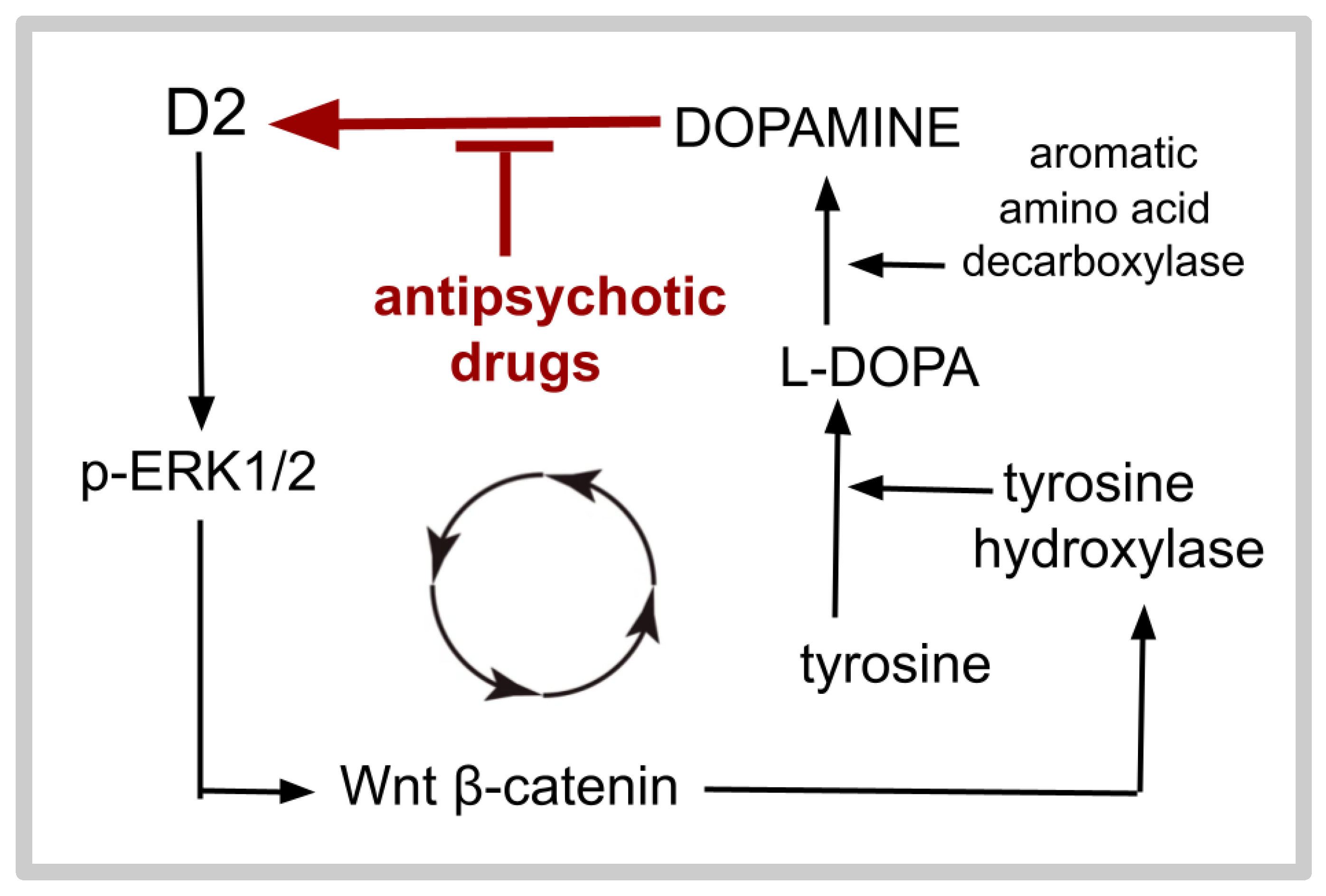
| Drug | Dose | T1/2 | CYP Inhibition | CYP Metabolism |
|---|---|---|---|---|
| aprepitant | 80 mg/d | ~12 h | 3A4 | 3A4 |
| vortioxetine | 20 mg/d | 2–3 days | 2D6 | 3A4, 2C19, 2D6 |
| olanzapine | 5 mg h/s. | ~1 to 2 d | 2D6 | 1A2 |
| roflumilast | 250–500 μg/d | ~1 d | ---- | 3A4, 1A2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kast, R.E.; Marques Vieira, B.; Barros da Silva, E., Jr. A New Adjuvant Treatment for Glioblastoma Using Aprepitant, Vortioxetine, Roflumilast and Olanzapine: The AVRO Regimen. Int. J. Mol. Sci. 2025, 26, 6158. https://doi.org/10.3390/ijms26136158
Kast RE, Marques Vieira B, Barros da Silva E Jr. A New Adjuvant Treatment for Glioblastoma Using Aprepitant, Vortioxetine, Roflumilast and Olanzapine: The AVRO Regimen. International Journal of Molecular Sciences. 2025; 26(13):6158. https://doi.org/10.3390/ijms26136158
Chicago/Turabian StyleKast, Richard E., Bruno Marques Vieira, and Erasmo Barros da Silva, Jr. 2025. "A New Adjuvant Treatment for Glioblastoma Using Aprepitant, Vortioxetine, Roflumilast and Olanzapine: The AVRO Regimen" International Journal of Molecular Sciences 26, no. 13: 6158. https://doi.org/10.3390/ijms26136158
APA StyleKast, R. E., Marques Vieira, B., & Barros da Silva, E., Jr. (2025). A New Adjuvant Treatment for Glioblastoma Using Aprepitant, Vortioxetine, Roflumilast and Olanzapine: The AVRO Regimen. International Journal of Molecular Sciences, 26(13), 6158. https://doi.org/10.3390/ijms26136158






