Alkaline Phosphatase as a Potential Biomarker of Muscle Function: A Pilot Study in Patients with Hypophosphatasia
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population According to HPP
2.2. Correlations Between ALP and Muscle Strength and Mass in General
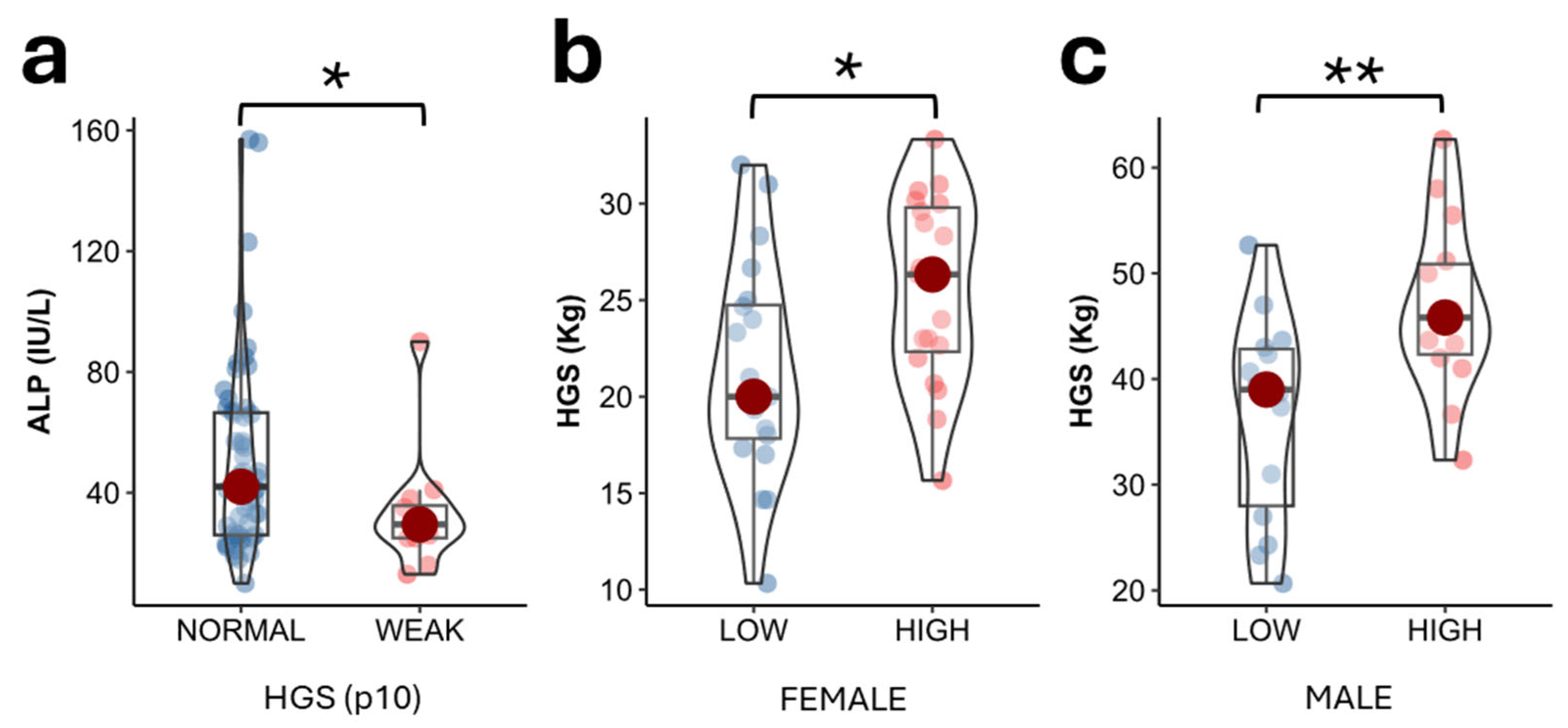
2.3. ALP Values According to Muscle Strength in the Study Population
2.4. Parameters Determining Muscle Strength in Study Population
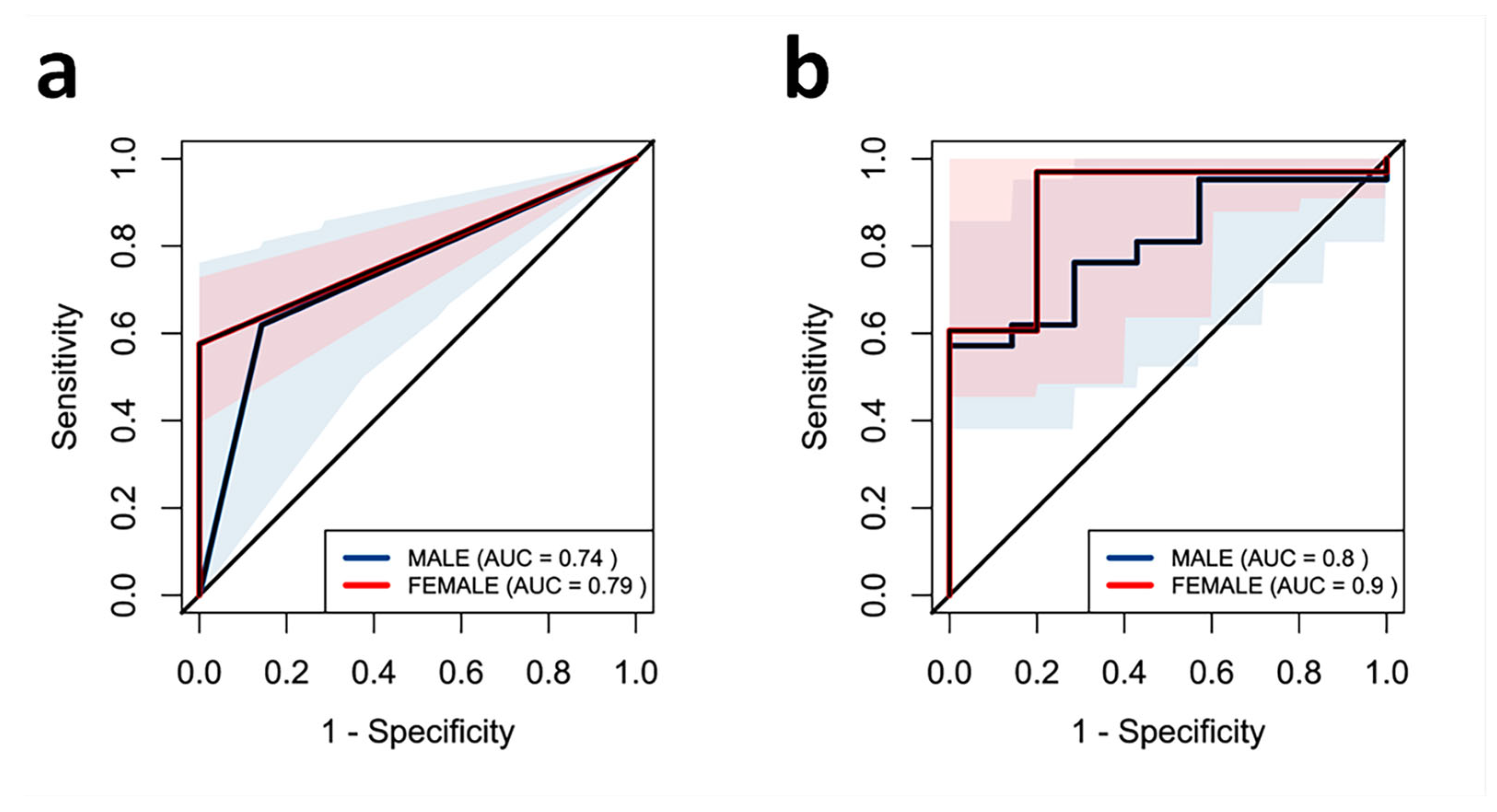
2.5. Effect of ALP on Muscle Strength in the Study Population
3. Discussion
4. Materials and Methods
4.1. Study Design and Recruitment of Participants
4.2. Assessment of Muscle Functionality: Muscle Strength
4.3. Body Composition Measures
4.3.1. Anthropometric Measures
4.3.2. Body Composition by Dual Energy X-Ray Absorptiometry
4.3.3. Muscle Ultrasonography of the Quadriceps Rectus Femoris
4.4. Biochemical Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| TNSALP | tissue-nonspecific alkaline phosphatase |
| PPi | inorganic pyrophosphate |
| HPP | hypophosphatasia |
| ATP | adenosine triphosphate |
| ROS | reactive oxygen species |
| BMD | bone mineral density |
| BMI | body mass index |
| FFM | fat-free mass |
| FFMI | fat-free mass index |
| FM | fat mass |
| DXA | dual-energy X-ray absorptiometry |
| CMR | muscular circumference rectus |
| MARA | muscular area rectus anterior |
| HGS | handgrip strength |
| CMB | bone mineral content |
| aBMD | areal bone mineral density |
| TH | total hip |
| FN | femoral neck |
| LS | lumbar spine |
References
- Harris, H. The Human Alkaline Phosphatases: What We Know and What We Don’t Know. Clin. Chim. Acta 1990, 186, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L. Hypophosphatasia: An Overview of the Disease and Its Treatment. Osteoporos. Int. 2015, 26, 2743–2757. [Google Scholar] [CrossRef]
- Hofmann, C.; Girschick, H.J.; Mentrup, B.; Graser, S.; Seefried, L.; Liese, J.; Jakob, F. Clinical Aspects of Hypophosphatasia: An Update. Clin. Rev. Bone Min. Miner. Metab. 2013, 11, 60–70. [Google Scholar] [CrossRef]
- Whyte, M.P. Hypophosphatasia—Aetiology, Nosology, Pathogenesis, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2016, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Mornet, E. Hypophosphatasia. Best Pract. Res. Clin. Rheumatol. 2008, 22, 113–127. [Google Scholar] [CrossRef]
- Pendleton, E.G.; Nichenko, A.S.; Mcfaline-Figueroa, J.; Raymond-Pope, C.J.; Schifino, A.G.; Pigg, T.M.; Barrow, R.P.; Greising, S.M.; Call, J.A.; Mortensen, L.J. Compromised Muscle Properties in a Severe Hypophosphatasia Murine Model. Int. J. Mol. Sci. 2023, 24, 15905. [Google Scholar] [CrossRef]
- Hepp, N.; Frederiksen, A.L.; Duno, M.; Jørgensen, N.R.; Jensen, J.-E.B. Biochemical and Clinical Manifestations in Adults with Hypophosphatasia: A National Cross-Sectional Study. Osteoporos. Int. 2022, 33, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Yasuda, S.; Kakoi, S.; Ohata, Y.; Shimizu, Y.; Hasegawa, C.; Hayakawa, A.; Akiyama, T.; Yagi, T.; Aotani, D.; et al. Effect of Asfotase Alfa on Muscle Weakness in a Japanese Adult Patient of Hypophosphatasia with Low ALP Levels. Intern. Med. 2020, 59, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Sato, Y.; Ishikawa, M.; Arakawa, Y.; Iijima, M.; Akiyama, T.; Takano, K.; Watanabe, A.; Kosho, T. Marked Motor Function Improvement in a 32-Year-Old Woman with Childhood-Onset Hypophosphatasia by Asfotase Alfa Therapy: Evaluation Based on Standardized Testing Batteries Used in Duchenne Muscular Dystrophy Clinical Trials. Mol. Genet. Metab. Rep. 2020, 25, 100643. [Google Scholar] [CrossRef]
- Kitoh, H.; Izawa, M.; Kaneko, H.; Kitamura, A.; Matsuyama, S.; Kato, K.; Ogi, T. Two Children with Hypophosphatasia with a Heterozygous c.1559delT Variant in the ALPL Gene, the Most Common Variant in Japanese Populations. Bone Rep. 2022, 17, 101626. [Google Scholar] [CrossRef]
- Zhang, Z.; Nam, H.K.; Crouch, S.; Hatch, N.E. Tissue Nonspecific Alkaline Phosphatase Function in Bone and Muscle Progenitor Cells: Control of Mitochondrial Respiration and ATP Production. Int. J. Mol. Sci. 2021, 22, 1140. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, A.-R.; Lee, Y.-J. Relationship between Serum Alkaline Phosphatase and Low Muscle Mass Index Among Korean Adults: A Nationwide Population-Based Study. Biomolecules 2021, 11, 842. [Google Scholar] [CrossRef]
- Xiao, L.; Dai, M.; Zhao, F.; Shen, Y.; Kwan, R.Y.C.; Salvador, J.T.; Zhang, L.; Luo, Y.; Liu, Q.; Yang, P. Assessing the Risk Factors Associated with Sarcopenia in Patients with Liver Cirrhosis: A Case–Control Study. Sci. Rep. 2023, 13, 21845. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Do, J.Y.; Kim, J.C. Association Between Alkaline Phosphatase and Muscle Mass, Strength, or Physical Performance in Patients on Maintenance Hemodialysis. Front. Med. 2021, 8, 657957. [Google Scholar] [CrossRef]
- Pettengill, M.; Matute, J.D.; Tresenriter, M.; Hibbert, J.; Burgner, D.; Richmond, P.; Luis Millán, J.; Ozonoff, A.; Strunk, T.; Currie, A.; et al. Human Alkaline Phosphatase Dephosphorylates Microbial Products and Is Elevated in Preterm Neonates with a History of Late-Onset Sepsis. PLoS ONE 2017, 12, e0175936. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.; Geraci, S.; Heemskerk, S.; Wilmer, M.J.; Bilos, A.; Kraenzlin, B.; Gretz, N.; Pickkers, P.; Masereeuw, R. Alkaline Phosphatase Protects against Renal Inflammation through Dephosphorylation of Lipopolysaccharide and Adenosine Triphosphate. Br. J. Pharmacol. 2015, 172, 4932–4945. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Thompson, L.F.; Karhausen, J.; Cotta, R.J.; Ibla, J.C.; Robson, S.C.; Colgan, S.P. Endogenous Adenosine Produced during Hypoxia Attenuates Neutrophil Accumulation: Coordination by Extracellular Nucleotide Metabolism. Blood 2004, 104, 3986–3992. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Rosenstein, E.D.; Kramer, S.B.; Weissmann, G.; Hirschhorn, R. Adenosine; a Physiologic Modulator of Superoxide Anion Generation by Human Neutrophils. Adenosine Acts via an A2 Receptor on Human Neutrophils. J. Immunol. 1985, 135, 1366–1371. [Google Scholar] [CrossRef]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo Marín, M.; Rojo Martínez, G.; Olveira, G. Normative Reference Values for Hand Grip Dynamometry in Spain. Association with Lean Mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef]
- Durrough, C.; Colazo, J.M.; Simmons, J.; Hu, J.-R.; Hudson, M.; Black, M.; de Riesthal, M.; Dahir, K. Characterization of Physical, Functional, and Cognitive Performance in 15 Adults with Hypophosphatasia. Bone 2021, 142, 115695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, X.; Jiang, Y.; Su, N.; Li, J.; Kang, L.; Zhang, Y.; Yang, M. Evaluation of Appendicular Muscle Mass in Sarcopenia in Older Adults Using Ultrasonography: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 1174–1198. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Sung, J.H.; Park, J.-W.; Son, M.H.; Lee, J.H.; Kim, B.-J. Usefulness of Muscle Ultrasound in Appendicular Skeletal Muscle Mass Estimation for Sarcopenia Assessment. PLoS ONE 2023, 18, e0280202. [Google Scholar] [CrossRef] [PubMed]
- Naruse, M.; Trappe, S.; Trappe, T.A. Human Skeletal Muscle Size with Ultrasound Imaging: A Comprehensive Review. J. Appl. Physiol. 2022, 132, 1267–1279. [Google Scholar] [CrossRef]
- Sun, Y.; Rahbani, J.F.; Jedrychowski, M.P.; Riley, C.L.; Vidoni, S.; Bogoslavski, D.; Hu, B.; Dumesic, P.A.; Zeng, X.; Wang, A.B.; et al. Mitochondrial TNAP Controls Thermogenesis by Hydrolysis of Phosphocreatine. Nature 2021, 593, 580–585. [Google Scholar] [CrossRef]
- Krötzsch, E.; Salgado, R.m.; Caba, D.; Lichtinger, A.; Padilla, L.; Di Silvio, M. 162 Alkaline Phosphatase Activity Is Related to Acute Inflammation and Collagen Turnover During Acute and Chronic Wound Healing. Wound Repair. Regen. 2005, 13, A28–A48. [Google Scholar] [CrossRef]
- Kang, K.Y.; Hong, Y.S.; Park, S.-H.; Ju, J.H. Increased Serum Alkaline Phosphatase Levels Correlate with High Disease Activity and Low Bone Mineral Density in Patients with Axial Spondyloarthritis. Semin. Arthritis Rheum. 2015, 45, 202–207. [Google Scholar] [CrossRef]
- Antuña, E.; Potes, Y.; Baena-Huerta, F.J.; Cachán-Vega, C.; Menéndez-Coto, N.; Álvarez Darriba, E.; Fernández-Fernández, M.; Burgos Bencosme, N.; Bermúdez, M.; López Álvarez, E.M.; et al. NLRP3 Contributes to Sarcopenia Associated to Dependency Recapitulating Inflammatory-Associated Muscle Degeneration. Int. J. Mol. Sci. 2024, 25, 1439. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Ma, A.; Chen, H.; Wang, B.; Zhao, G. The Correlation between Serum Alkaline Phosphatase and Grip Strength in Middle-Aged and Elderly People: NHANES 2011–2014. BMC Musculoskelet. Disord. 2025, 26, 191. [Google Scholar] [CrossRef]
- González-Cejudo, T.; Villa-Suárez, J.M.; Ferrer-Millán, M.; Andújar-Vera, F.; Contreras-Bolívar, V.; Andreo-López, M.C.; Gómez-Vida, J.M.; Martínez-Heredia, L.; González-Salvatierra, S.; De Haro Muñoz, T.; et al. Mild Hypophosphatasia May Be Twice as Prevalent as Previously Estimated: An Effective Clinical Algorithm to Detect Undiagnosed Cases. Clin. Chem. Lab. Med. 2024, 62, 128–137. [Google Scholar] [CrossRef]
- Curiel, M.D.; Carrasco De La Peña, J.L.; Perez, J.H.; Cano, R.P.; Rapado, A.; Martinez, I.R. Study of Bone Mineral Density in Lumbar Spine and Femoral Neck in a Spanish Population. Osteoporos. Int. 1997, 7, 59–64. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Kanis, J.A. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-Calibration and Least Significant Change, Spinal Cord Injury, Peri-Prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional Ultrasound®: Conceptualisation, Technical Considerations and Standardisation. Endocrinol. Diabetes Y Nutr. (Engl. ed.) 2023, 70, 74–84. [Google Scholar] [CrossRef]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 6. Reference procedure for the measure-ment of catalytic concentration of gam-ma-glutamyltransferase. Clin. Chem. Lab. Med. 2002, 40, 643–648. [Google Scholar]

| Controls (n = 34) | HPP (n = 34) | p-Value | |
|---|---|---|---|
| Clinical variables | |||
| Sex (% females) | 56 | 56 | 0.761 |
| Age (years) | 49 (18–76) | 50 (18–79) | 0.968 |
| ALP (IU/L) | 66.5 (37–157) | 26 (10–47) | <0.001 * |
| Muscle strength | |||
| HGS (Kg) | 30 (15–62) | 25 (10–52) | 0.039 * |
| Low muscle strength or dynapenia (%) | 6 | 30 | 0.009 * |
| Body composition parameters | |||
| BMI (Kg/m2) | 25.8 (16.9–36.8) | 25.6 (17.6–37.9) | 0.662 |
| FFM (DXA) (Kg) | 44.28 (30–66.9) | 43.57 (29–78.6) | 0.882 |
| Leg circumference (cm) | 39.0 (33.0–44.5) | 36 (24.3–55) | 0.096 |
| Tricipital skinfold (mm) | 17.5 (9.0–34.0) | 20.0 (5.0–47.0) | 0.344 |
| FFMI, females (Kg/m2) | 15.5 (11.8–19.6) | 14.84 (11.2–21.7) | 0.551 |
| FFMI, males (Kg/m2) | 19.96 (16.1–21.4) | 20.23 (17.2–25) | 0.262 |
| Low FFM (%) | 11 (33) | 11 (30) | 0.634 |
| FM (DXA) (%) | 34.5 (15.9–49.3) | 33.7 (14.5–48.3) | 0.611 |
| MARA (cm2) | 3.76 (1.8–17.1) | 4.15 (1.27–10.4) | 0.551 |
| CMR (cm) | 8.25(6.64–16.3) | 9.14(1.59–13.6) | 0.262 |
| X-axis (cm) | 3.41 (1.02–5.71) | 3.69(1.37–5.14) | 0.634 |
| Y-axis (cm) | 1.41(0.82–3.12) | 1.34 (0.8–3.74) | 0.89 |
| Bone parameters | |||
| BMD (g/cm2) | |||
| TH | 0.95 (0.58–1.33) | 0.98 (0.61–1.32) | 0.469 |
| FN | 0.89 (0.54–1.31) | 0.85 (0.50–1.25) | 0.034 * |
| LS | 1.12 (0.73–1.47) | 1.06 (0.66–1.42) | 0.16 |
| Non-Standardized Coefficients | |||||
|---|---|---|---|---|---|
| B | Error Typ. | Beta | 95% CI | p-Value | |
| HGS | |||||
| FFMI | 1.368 | 0.364 | 0.367 | 0.640–2.097 | <0.001 |
| % FM | −0.585 | 0.137 | −0.42 | −0.858–(−0.312) | <0.001 |
| ALP | 0.094 | 0.034 | 0.24 | 0.025–0.163 | 0.008 |
| Model 1 | |||
| Variables | Exp (B) | CI 95% | p-Value |
| ALP cutoff, male | 6.88 | 1.15–75 | 0.034 * |
| ALP cutoff, female | 13.13 | 1317–1775.01 | 0.018 * |
| Model 2 | |||
| Variables | Exp (B) | CI 95% | p-Value |
| ALP cutoff, male | 9.25 | 1.31–120.47 | 0.024 * |
| FFMI | 1.18 | 0.79–1.87 | 0.428 |
| ALP cutoff, female | 14.84 | 1.49–2007.6 | 0.018 * |
| FFMI | 1.23 | 0.82–2.2 | 0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreo-López, M.C.; Contreras-Bolívar, V.; Martínez-Heredia, L.; Andújar-Vera, F.; Becerra-García, D.; González-Cejudo, T.; González-Salvatierra, S.; García-Fontana, C.; García-Fontana, B.; Muñoz-Torres, M. Alkaline Phosphatase as a Potential Biomarker of Muscle Function: A Pilot Study in Patients with Hypophosphatasia. Int. J. Mol. Sci. 2025, 26, 6153. https://doi.org/10.3390/ijms26136153
Andreo-López MC, Contreras-Bolívar V, Martínez-Heredia L, Andújar-Vera F, Becerra-García D, González-Cejudo T, González-Salvatierra S, García-Fontana C, García-Fontana B, Muñoz-Torres M. Alkaline Phosphatase as a Potential Biomarker of Muscle Function: A Pilot Study in Patients with Hypophosphatasia. International Journal of Molecular Sciences. 2025; 26(13):6153. https://doi.org/10.3390/ijms26136153
Chicago/Turabian StyleAndreo-López, María Carmen, Victoria Contreras-Bolívar, Luis Martínez-Heredia, Francisco Andújar-Vera, Diego Becerra-García, Trinidad González-Cejudo, Sheila González-Salvatierra, Cristina García-Fontana, Beatriz García-Fontana, and Manuel Muñoz-Torres. 2025. "Alkaline Phosphatase as a Potential Biomarker of Muscle Function: A Pilot Study in Patients with Hypophosphatasia" International Journal of Molecular Sciences 26, no. 13: 6153. https://doi.org/10.3390/ijms26136153
APA StyleAndreo-López, M. C., Contreras-Bolívar, V., Martínez-Heredia, L., Andújar-Vera, F., Becerra-García, D., González-Cejudo, T., González-Salvatierra, S., García-Fontana, C., García-Fontana, B., & Muñoz-Torres, M. (2025). Alkaline Phosphatase as a Potential Biomarker of Muscle Function: A Pilot Study in Patients with Hypophosphatasia. International Journal of Molecular Sciences, 26(13), 6153. https://doi.org/10.3390/ijms26136153







