Exogenous Administration of Delta-9-Tetrahydrocannabinol Affects Adult Hippocampal Neurotransmission in Female Wistar Rats
Abstract
1. Introduction
1.1. Overview of Delta-9-Tetrahydrocannabinol and Cannabis
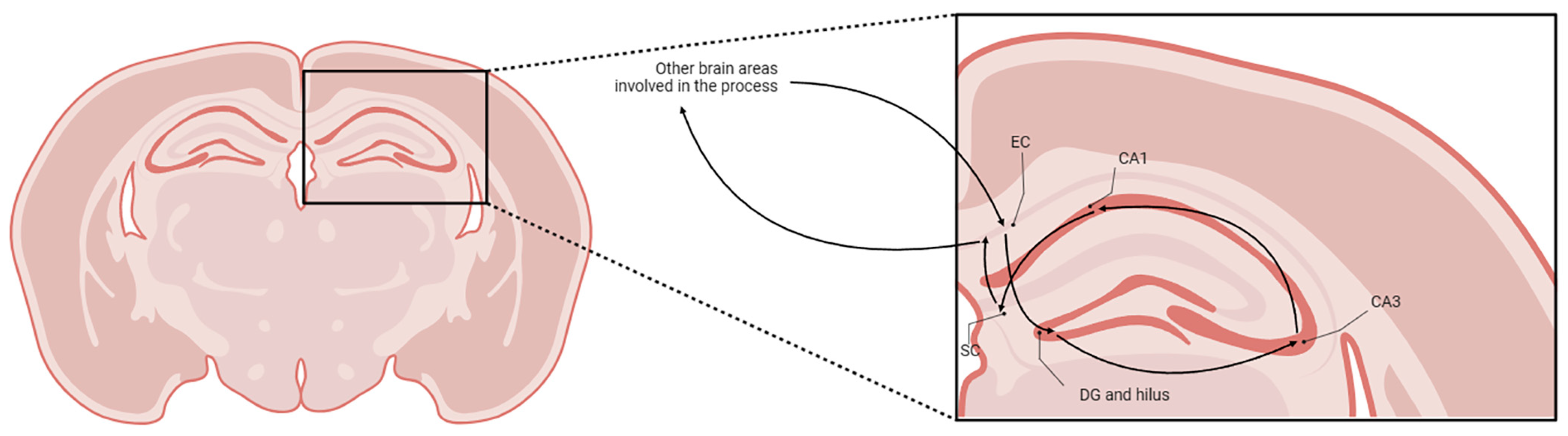
1.2. Hippocampal Formation and Cannabis
1.3. Sexual Dimorphism in Cannabis’ Effects
1.4. Aim of the Study
2. Results
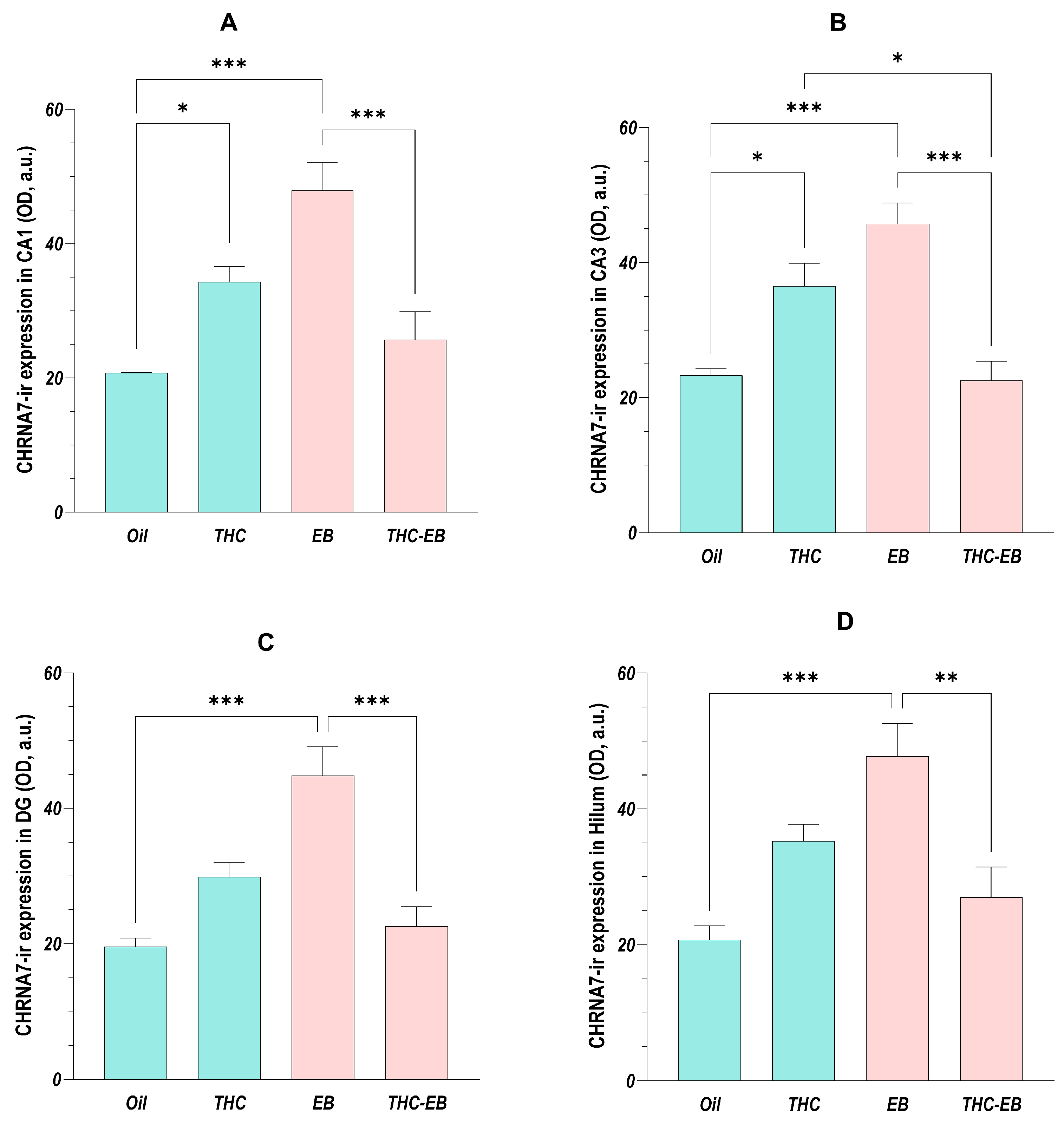
2.1. Effects of THC Administration on the Expression of CHRNA7 in HF Areas
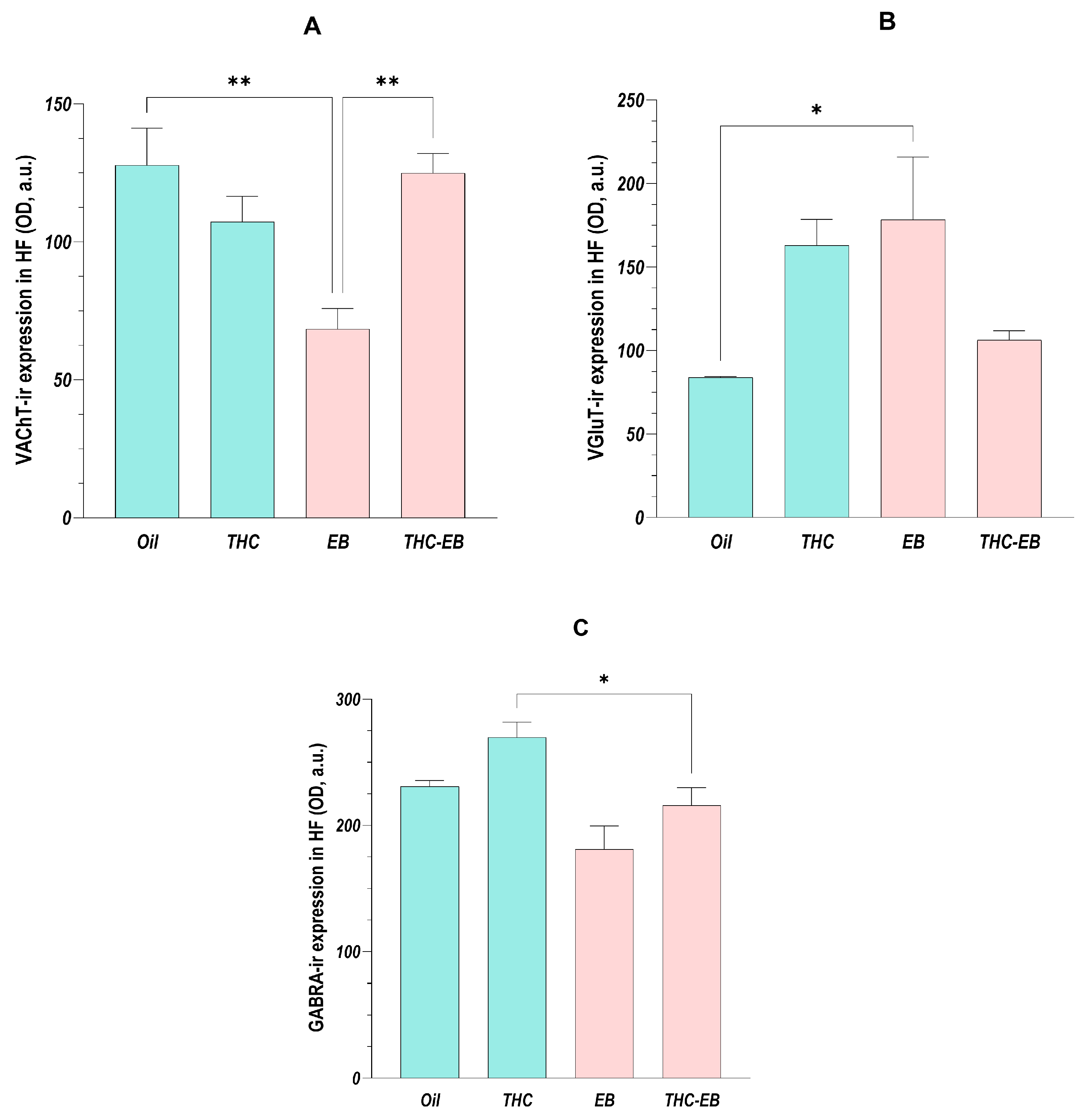
2.2. Effects of THC Administration on the Expression of VAChT in the HF
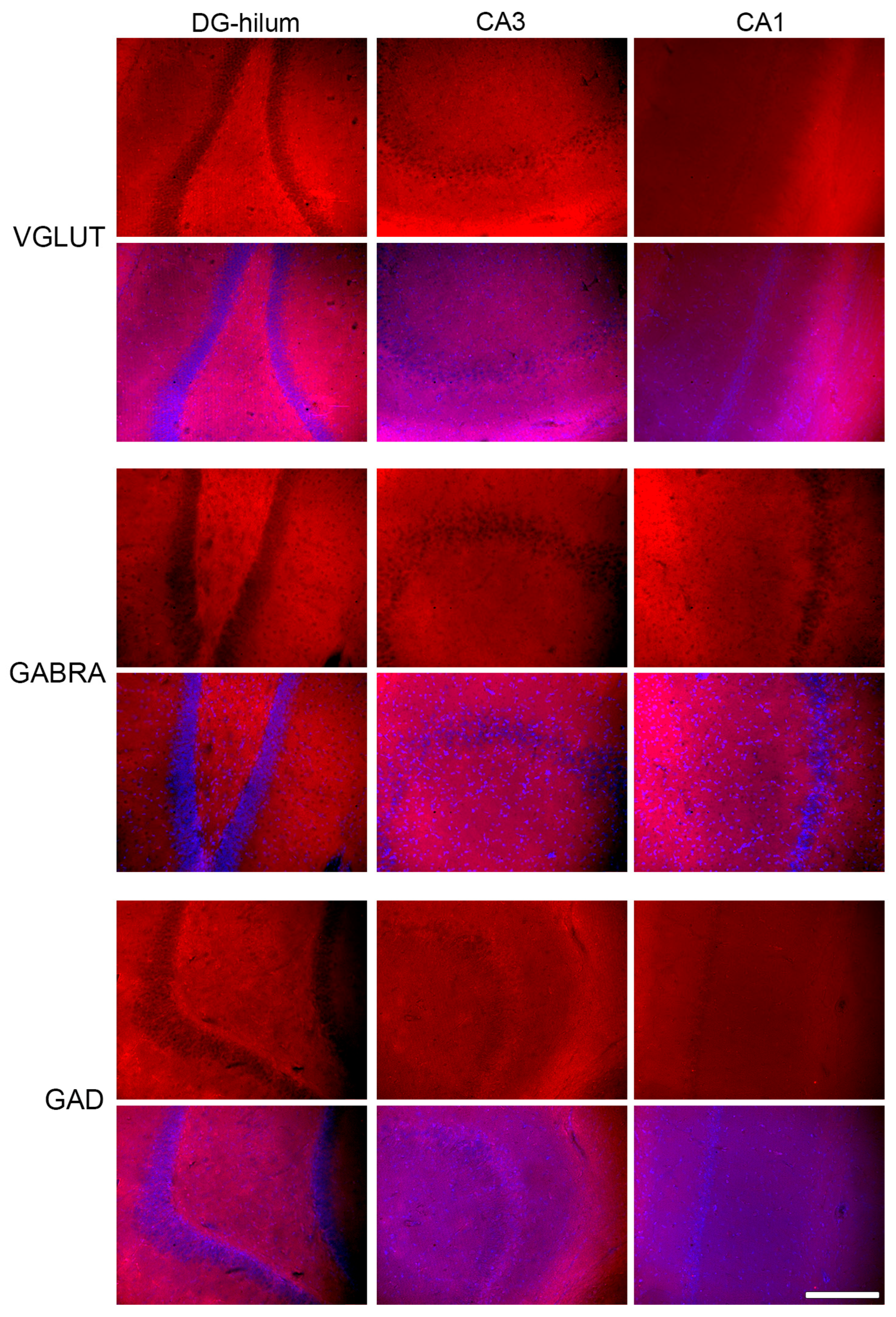
2.3. Effects of THC Administration on the Expression of VGLUT in the HF
2.4. Effects of THC Administration on the Expression of GABRA in the HF
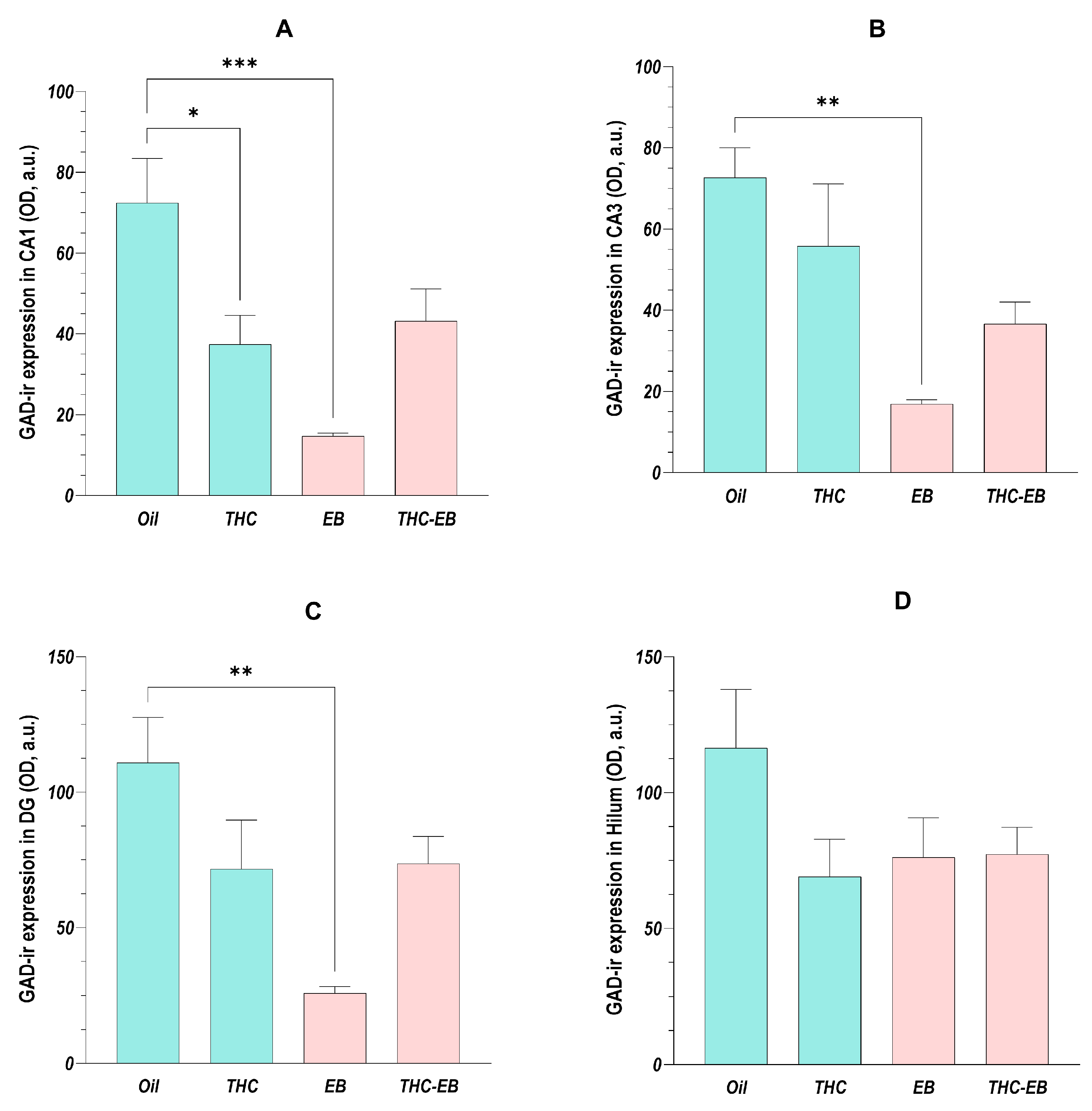
2.5. Effects of THC Administration on the Expression of GAD in HF Areas
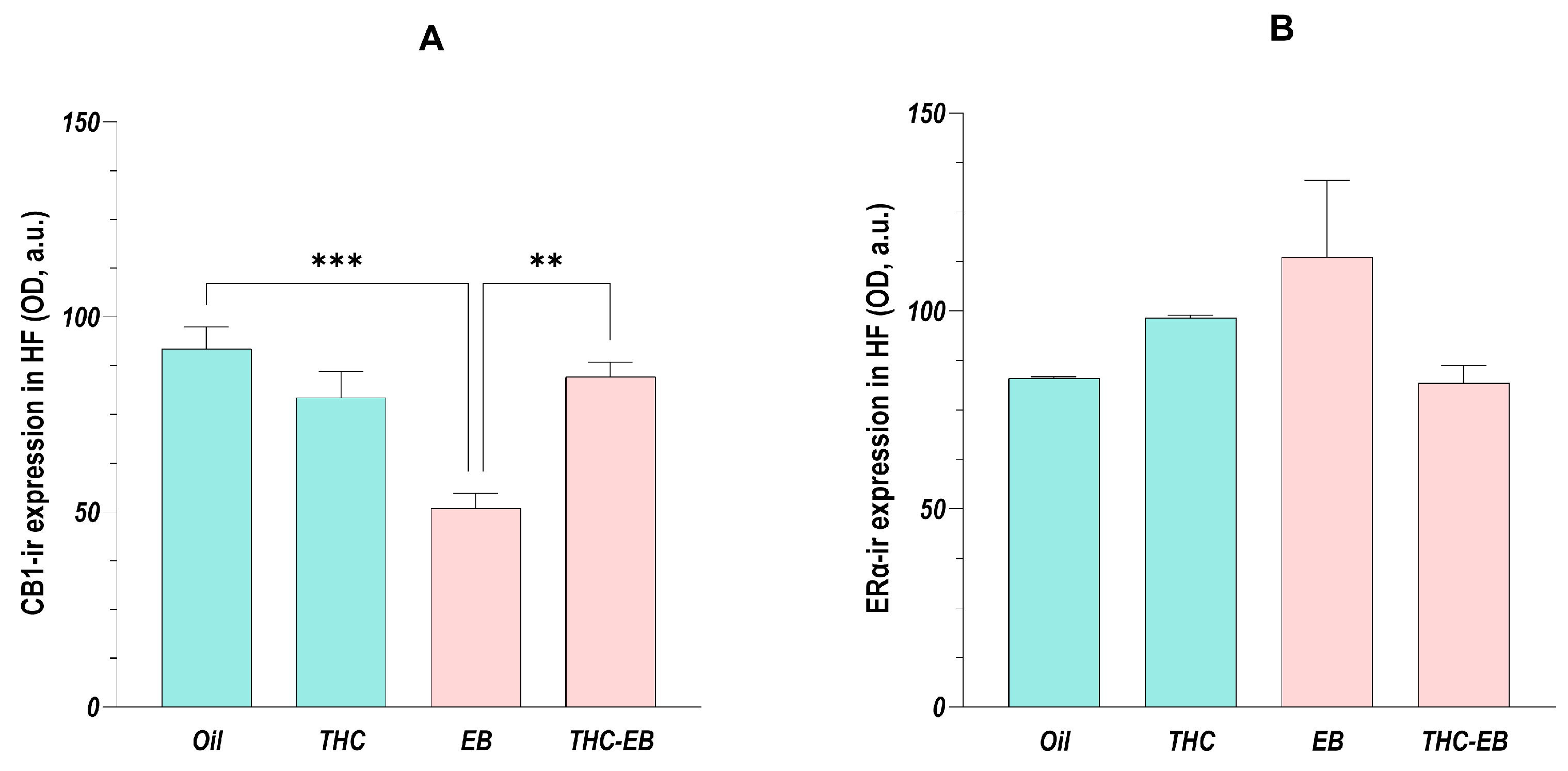
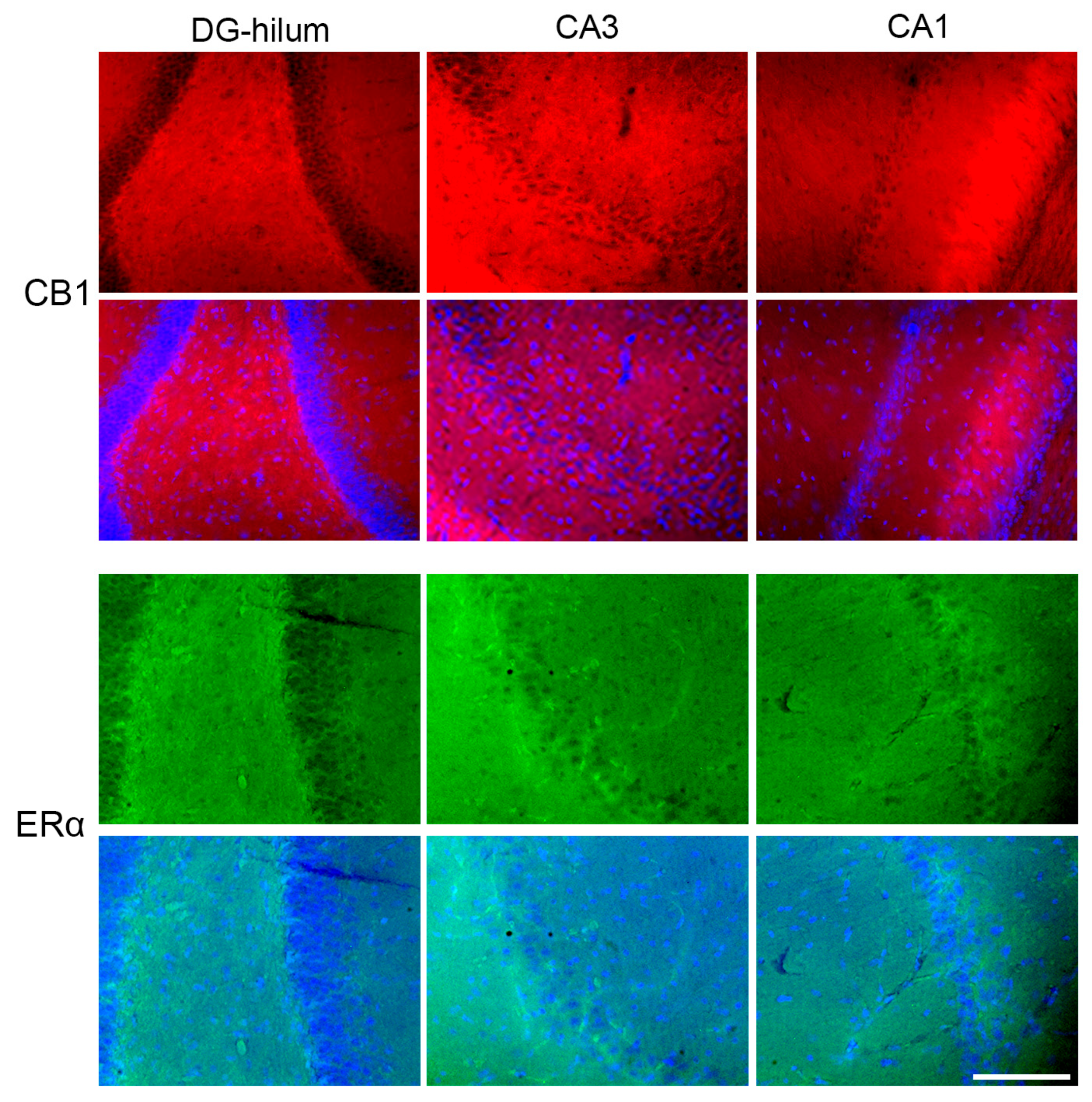
2.6. Effects of THC Administration on the Expression of CB1 in the HF
2.7. Effects of THC Administration on the Expression of ERα in the HF
3. Discussion
3.1. The Cholinergic System
3.2. The Glutamatergic and GABAergic Systems
3.3. CB1 and ERα Receptors
3.4. Limitations of the Study
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Immunohistochemistry
4.3. Fluorescence Quantification
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Cornu ammonis |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| CHRNA7 | Cholinergic receptor alpha 7 subunit |
| CNS | Central nervous system |
| DG | Dentate gyrus |
| EB | Estradiol benzoate |
| EBα | Estradiol receptor alpha |
| EC | Entorhinal cortex |
| GABA | Gamma-aminobutyric acid |
| GABRA | Gamma-aminobutyric acid type A receptor |
| GAD | Glutamic acid decarboxylase |
| HF | Hippocampal formation |
| OD | Optic density |
| PBS | Phosphate-buffered saline |
| SC | Subicular complex |
| SEM | Standard error of the mean |
| THC | Delta-9-tetrahydrocannabinol |
| VAChT | Vesicular acetylcholine transporter |
| VGLUT | Vesicular glutamate transporter |
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D.; Craft, R.M. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 2018, 43, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef] [PubMed]
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Wise, L.E.; Thorpe, A.J.; Lichtman, A.H. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology 2009, 34, 2072–2080. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Fan, N.; Teng, Z.Q.; Wu, Y.; Yang, H.; Tang, Y.P.; Sun, H.; Song, Y.; Chen, C. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 2013, 155, 1154–1165. [Google Scholar] [CrossRef]
- Volkow, N.D.; Swanson, J.M.; Evins, A.E.; DeLisi, L.E.; Meier, M.H.; Gonzalez, R.; Bloomfield, M.A.P.; Curran, H.V.; Baler, R. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 2016, 73, 292–297. [Google Scholar] [CrossRef]
- Schultz, C.; Engelhardt, M. Anatomy of the hippocampal formation. Front. Neurol. Neurosci. 2014, 34, 6–17. [Google Scholar]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian. Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [PubMed]
- Tamminga, C.A.; Southcott, S.; Sacco, C.; Wagner, A.D.; Ghose, S. Glutamate dysfunction in hippocampus: Relevance of dentate gyrus and CA3 signaling. Schizophr. Bull. 2012, 38, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142 (Suppl. S2), 111–121. [Google Scholar] [CrossRef]
- Dolfen, N.; Veldman, M.P.; Gann, M.A.; von Leupoldt, A.; Puts, N.A.J.; Edden, R.A.E.; Mikkelsen, M.; Swinnen, S.; Schwabe, L.; Albouy, G.; et al. A role for GABA in the modulation of striatal and hippocampal systems under stress. Commun. Biol. 2021, 4, 1033. [Google Scholar] [CrossRef]
- Colizzi, M.; McGuire, P.; Pertwee, R.G.; Bhattacharyya, S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci. Biobehav. Rev. 2016, 64, 359–381. [Google Scholar] [CrossRef]
- Goonawardena, A.V.; Robinson, L.; Hampson, R.E.; Riedel, G. Cannabinoid and cholinergic systems interact during performance of a short-term memory task in the rat. Learn. Mem. 2010, 17, 502–511. [Google Scholar] [CrossRef]
- Coimbra, N.C.; Mendes-Gomes, J.; Da Silva, J.A.; Dos Anjos-Garcia, T.; Ullah, F.; Almada, R.C. Chapter e14—New Ethological and Morphological Perspectives for the Investigation of Panicolytic-Like Effects of Cannabidiol. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. e140–e149. [Google Scholar]
- Cuttler, C.; Mischley, L.K.; Sexton, M. Sex Differences in Cannabis Use and Effects: A Cross-Sectional Survey of Cannabis Users. Cannabis Cannabinoid Res. 2016, 1, 166–175. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and gonadal hormones: Bidirectional interactions in physiology and behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef]
- Habayeb, O.M.; Bell, S.C.; Konje, J.C. Endogenous cannabinoids: Metabolism and their role in reproduction. Life Sci. 2002, 70, 1963–1977. [Google Scholar] [CrossRef]
- Ryan, K.S.; Bash, J.C.; Hanna, C.B.; Hedges, J.C.; Lo, J.O. Effects of marijuana on reproductive health: Preconception and gestational effects. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 558–565. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Shi, P.; Yuan, J.; Jia, Q.; Pi, C.; Chen, T.; Xiong, L.; Chen, J.; Tang, J.; et al. α7 Nicotinic acetylcholine receptor: A key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect. J. Neuroinflamm. 2023, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Zhang, W.J.; Su, D.F.; Zhang, G.Q.; Miao, C.Y. Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: A narrative review. Ann. Transl. Med. 2021, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- de Castro, B.M.; De Jaeger, X.; Martins-Silva, C.; Lima, R.D.F.; Amaral, E.; Menezes, C.; Lima, P.; Neves, C.M.L.; Pires, R.G.; Gould, T.W.; et al. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell. Biol. 2009, 29, 5238–5250. [Google Scholar] [CrossRef]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic acetylcholine receptors: From structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 1–46. [Google Scholar]
- Muratori, B.G.; da Veiga, I.E.T.; Medeiros, G.N.; Silva, S.M.; Soliani, A.G.; Prado, C.M.; Cerutti, S.M. Standardized extract of Ginkgo biloba induced memory consolidation in female mice with hypofunction of vesicular acetylcholine transporter. Behav. Brain Res. 2025, 482, 115455. [Google Scholar] [CrossRef]
- Newhouse, P.; Dumas, J. Estrogen-cholinergic interactions: Implications for cognitive aging. Horm. Behav. 2015, 74, 173–185. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, Y.; Park, D. Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. [Google Scholar] [CrossRef]
- Benfante, R.; Di Lascio, S.; Cardani, S.; Fornasari, D. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: A new therapeutic perspective in aging-related disorders. Aging Clin. Exp. Res. 2021, 33, 823–834. [Google Scholar] [CrossRef]
- Piovesana, R.; Salazar Intriago, M.S.; Dini, L.; Tata, A.M. Cholinergic Modulation of Neuroinflammation: Focus on α7 Nicotinic Receptor. Int. J. Mol. Sci. 2021, 22, 4912. [Google Scholar] [CrossRef]
- Yakel, J.L. Nicotinic ACh receptors in the hippocampal circuit; functional expression and role in synaptic plasticity. J. Physiol. 2014, 592, 4147–4153. [Google Scholar] [CrossRef]
- Niewiadomska, G.; Baksalerska-Pazera, M.; Riedel, G. The septo-hippocampal system, learning and recovery of function. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Martyn, A.C.; De Jaeger, X.; Magalhães, A.C.; Kesarwani, R.; Gonçalves, D.F.; Raulic, S.; Guzman, M.S.; Jackson, M.F.; Izquierdo, I.; MacDonald, J.F.; et al. Elimination of the vesicular acetylcholine transporter in the forebrain causes hyperactivity and deficits in spatial memory and long-term potentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 17651–17656. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Fonseca, B.M.; Sá, S.I. The effects of delta-9-tetrahydrocannabinol administration in the regulation of female rat sociosexual behaviour. Eur. J. Neurosci. 2023, 57, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Ågmo, A.; Laan, E. Sexual incentive motivation, sexual behavior, and general arousal: Do rats and humans tell the same story? Neurosci. Biobehav. Rev. 2022, 135, 104595. [Google Scholar] [CrossRef]
- Ayoub, S.M.; Vemuri, S.; Hoang, E.B.; Jha, N.A.; Minassian, A.; Young, J.W. Beneficial and adverse effects of THC on cognition in the HIV-1 transgenic rat model: Importance of exploring task- and sex-dependent outcomes. Brain Behav. Immun. 2025, 128, 571–588. [Google Scholar] [CrossRef]
- Rombo, D.M.; Ribeiro, J.A.; Sebastião, A.M. Hippocampal GABAergic transmission: A new target for adenosine control of excitability. J. Neurochem. 2016, 139, 1056–1070. [Google Scholar] [CrossRef]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Egerton, A.; Allison, C.; Brett, R.R.; Pratt, J.A. Cannabinoids and prefrontal cortical function: Insights from preclinical studies. Neurosci. Biobehav. Rev. 2006, 30, 680–695. [Google Scholar] [CrossRef]
- Coutts, A.A.; Anavi-Goffer, S.; Ross, R.A.; MacEwan, D.J.; Mackie, K.; Pertwee, R.G.; Irving, A.J. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J. Neurosci. 2001, 21, 2425–2433. [Google Scholar] [CrossRef]
- Hsieh, C.; Brown, S.; Derleth, C.; Mackie, K. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 1999, 73, 493–501. [Google Scholar] [CrossRef]
- Dobovišek, L.; Hojnik, M.; Ferk, P. Overlapping molecular pathways between cannabinoid receptors type 1 and 2 and estrogens/androgens on the periphery and their involvement in the pathogenesis of common diseases (Review). Int. J. Mol. Med. 2016, 38, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.D.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Rebouças, E.C.; Leal, S.; Sá, S.I. Regulation of NPY and α-MSH expression by estradiol in the arcuate nucleus of Wistar female rats: A stereological study. Neurol. Res. 2016, 38, 740–747. [Google Scholar] [CrossRef]
- Morgan, M.M.; Stickney, J.D.; Wilson-Poe, A.R. Low-Dose Δ9-THC Produces Antinociception in Female, But Not Male Rats. Cannabis Cannabinoid Res. 2023, 8, 603–607. [Google Scholar] [CrossRef]
- Leite, C.; Madeira, M.D.; Sá, S.I. Effects of sex steroids and estrogen receptor agonists on the expression of estrogen receptor alpha in the principal division of the bed nucleus of the stria terminalis of female rats. Brain Res. 2014, 1582, 99–106. [Google Scholar] [CrossRef]
- Martins, S.I.; Madeira, M.D.; Sá, S.I. Effects of gonadal steroids and of estrogen receptor agonists on the expression of estrogen receptor alpha in the medial preoptic nucleus of female rats. Neuroscience 2015, 310, 63–72. [Google Scholar] [CrossRef]


| Antibody | Antibody Registry ID | Dilution | Supplier |
|---|---|---|---|
| Primary antibodies | |||
| Rabbit anti-CHRNA7 Polyclonal Antibody (PA5-116644) | AB_2901275 | 1:500 | Thermo Fisher Scientific (Biogen Científica, Madrid, Spain) |
| Goat Anti-VAChT Polyclonal Antibody (AG260) | AB_10000324 | 1:500 | Merck Millipore (Algés, Portugal) |
| Mouse anti-VGLUT2 Monoclonal Antibody (MAB5504) | AB_2187552 | 1:1000 | Merck Millipore (Algés, Portugal) |
| Mouse anti-GABRA5 Monoclonal Antibody (MA5-27700) | AB_2735193 | 1:500 | Thermo Fisher Scientific (Biogen Científica, Madrid, Spain) |
| Mouse anti-GAD67 Monoclonal Antibody (MAB5406) | AB_2278725 | 1:2000 | Merck Millipore (Algés, Portugal) |
| Rabbit anti-Cannabinoid Receptor 1 Polyclonal Antibody (ab23703) | AB_447623 | 1:500 | Merck Millipore (Algés, Portugal) |
| Rabbit anti-ERα Polyclonal Antibody (sc-542) | AB_631470 | 1:1000 | Merck Millipore (Algés, Portugal) |
| Secondary antibodies | |||
| DyLight 488 Goat anti-rabbit (DI-1488) | AB_2336402 | 1:1000 | Vector Laboratories (Oxford, UK) |
| Alexa Fluor™ 488 Donkey anti-Goat IgG (A-11055) | AB_2534102 | 1:1000 | Thermo Fisher Scientific (Biogen Científica, Madrid, Spain) |
| Texas Red Horse anti-mouse (TI-2000) | AB_2336178 | 1:1000 | Vector Laboratories (Oxford, UK) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, A.M.; Leal, S.; Fonseca, B.M.; Sá, S.I. Exogenous Administration of Delta-9-Tetrahydrocannabinol Affects Adult Hippocampal Neurotransmission in Female Wistar Rats. Int. J. Mol. Sci. 2025, 26, 6144. https://doi.org/10.3390/ijms26136144
Neves AM, Leal S, Fonseca BM, Sá SI. Exogenous Administration of Delta-9-Tetrahydrocannabinol Affects Adult Hippocampal Neurotransmission in Female Wistar Rats. International Journal of Molecular Sciences. 2025; 26(13):6144. https://doi.org/10.3390/ijms26136144
Chicago/Turabian StyleNeves, Ana M., Sandra Leal, Bruno M. Fonseca, and Susana I. Sá. 2025. "Exogenous Administration of Delta-9-Tetrahydrocannabinol Affects Adult Hippocampal Neurotransmission in Female Wistar Rats" International Journal of Molecular Sciences 26, no. 13: 6144. https://doi.org/10.3390/ijms26136144
APA StyleNeves, A. M., Leal, S., Fonseca, B. M., & Sá, S. I. (2025). Exogenous Administration of Delta-9-Tetrahydrocannabinol Affects Adult Hippocampal Neurotransmission in Female Wistar Rats. International Journal of Molecular Sciences, 26(13), 6144. https://doi.org/10.3390/ijms26136144






