Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection
Abstract
1. Introduction
2. Results and Discussion
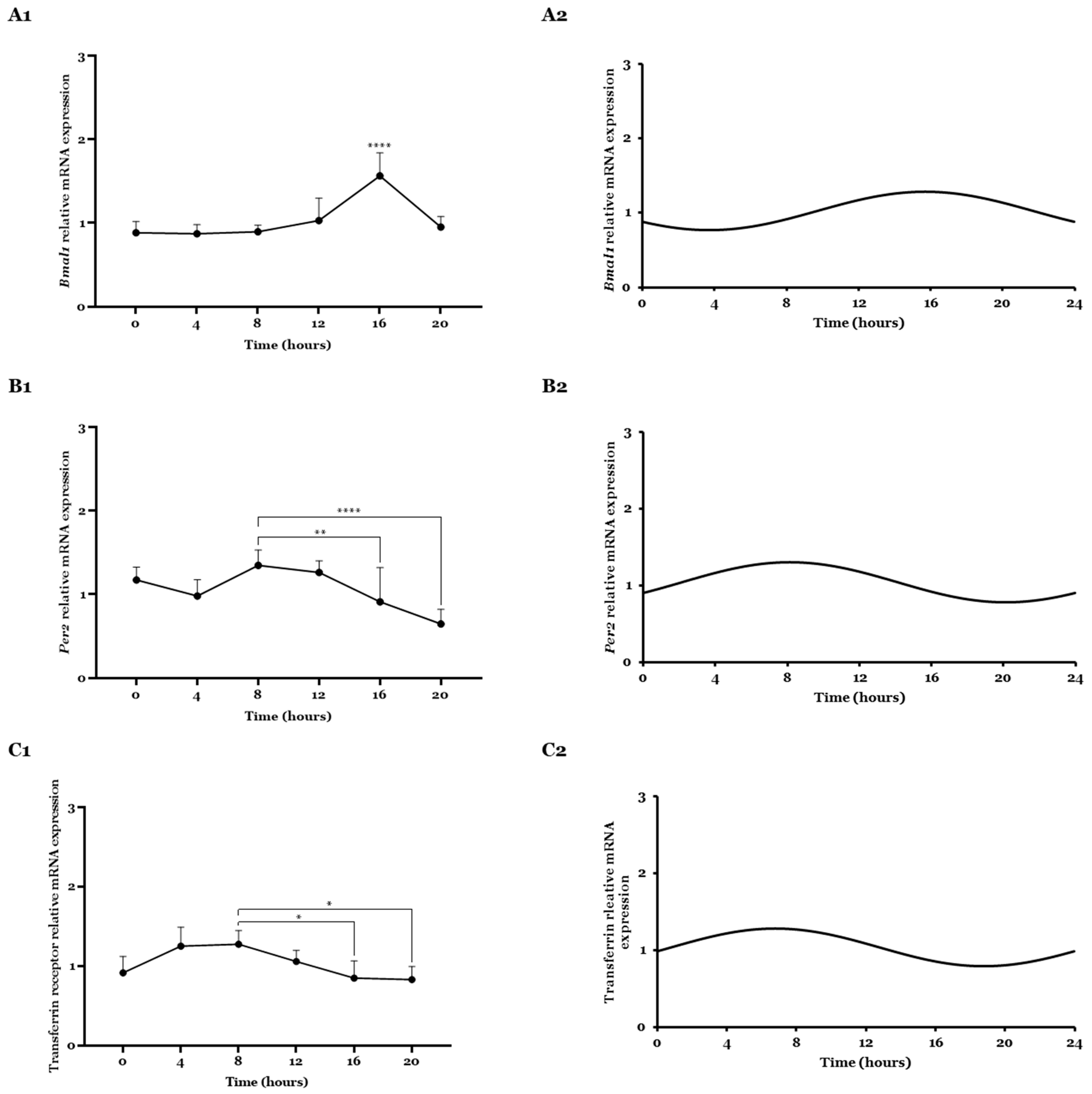
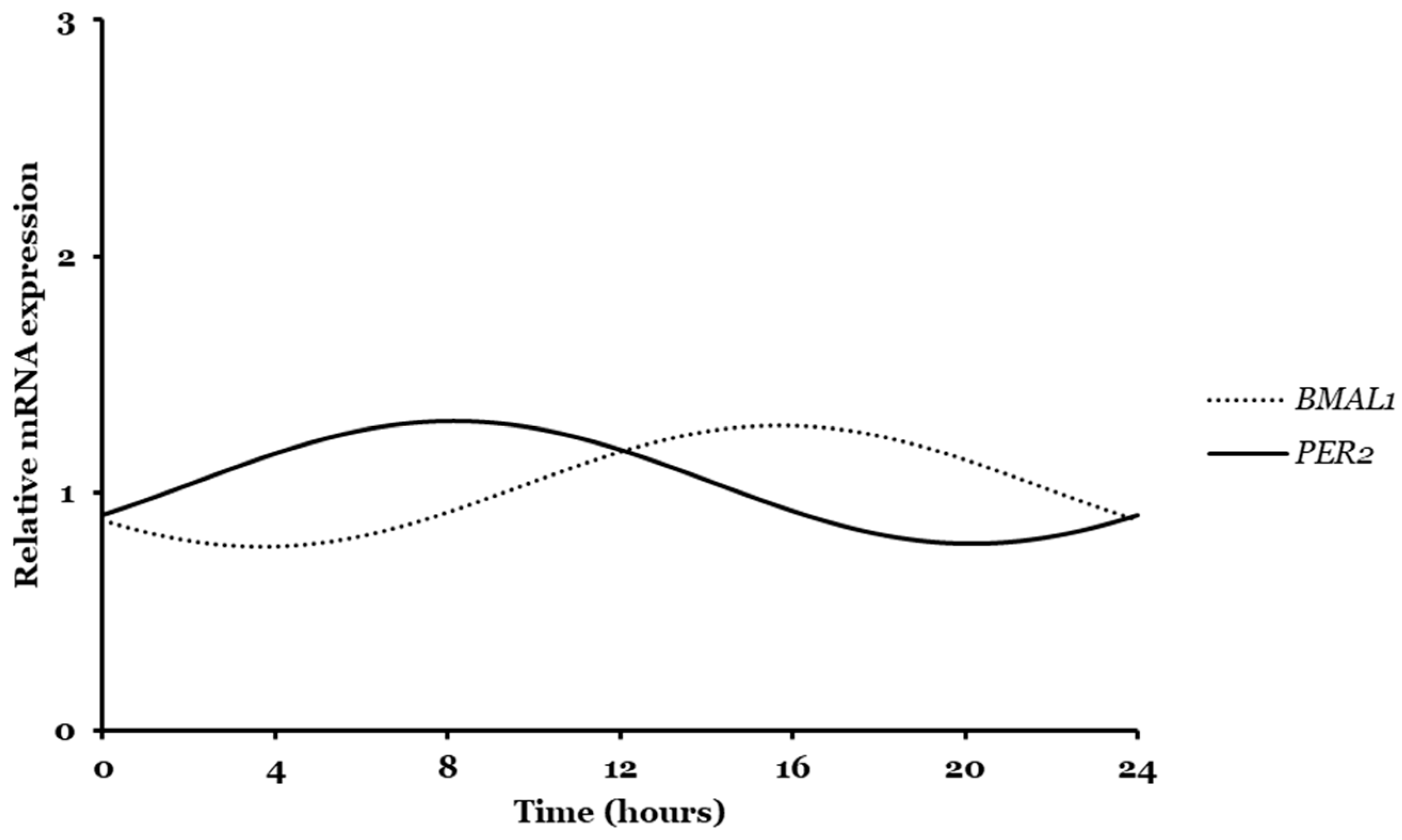
2.1. Bmal1, Per2, and Transferrin Gene Expression Analyzed by CircWave
2.2. Bmal1, Per2, and Transferrin Gene Expressions Analyzed by CosinorPy
2.3. Transferrin Receptor Expression
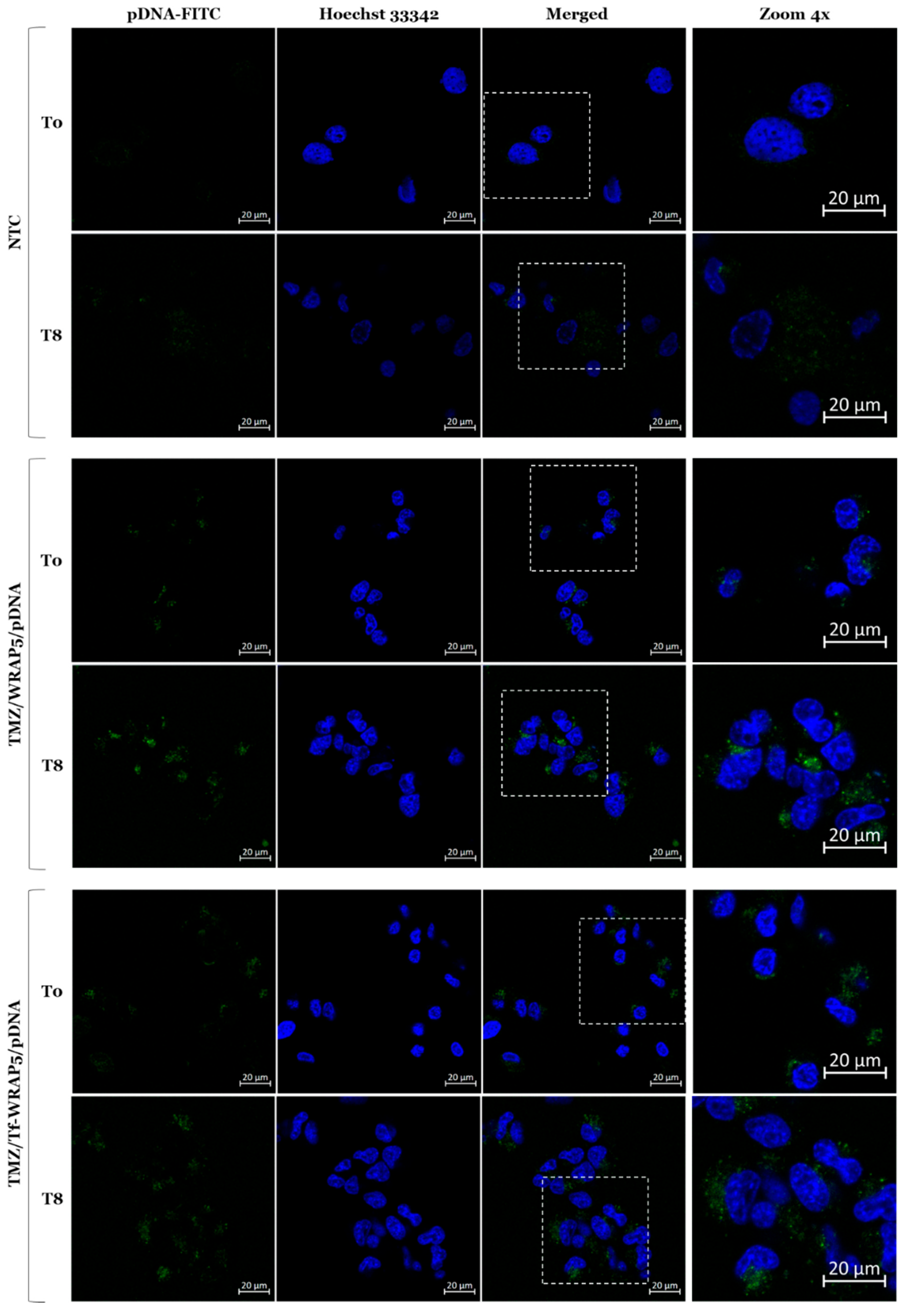
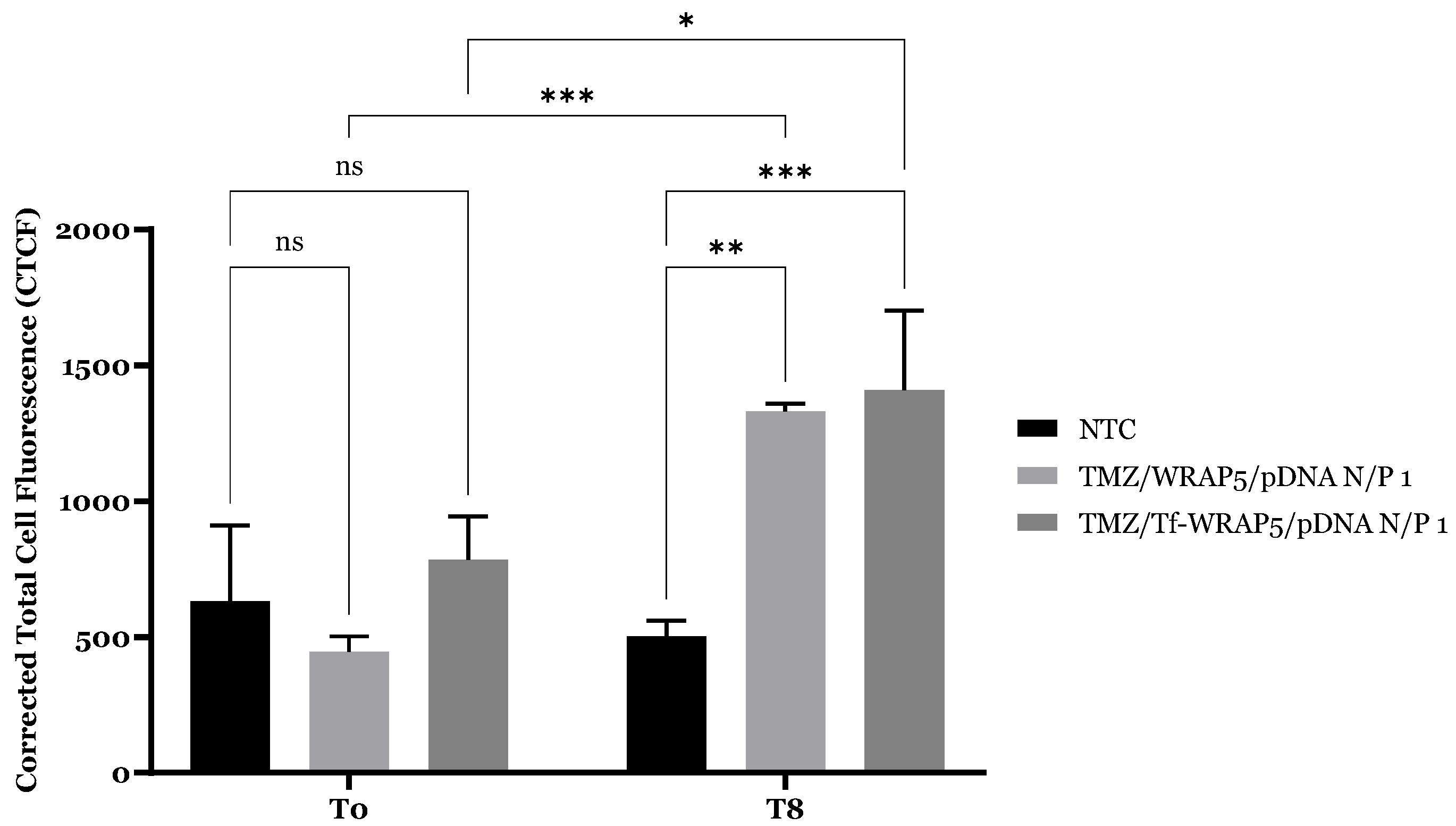
2.4. Cellular Internalization and Complexes’ Co-Localization
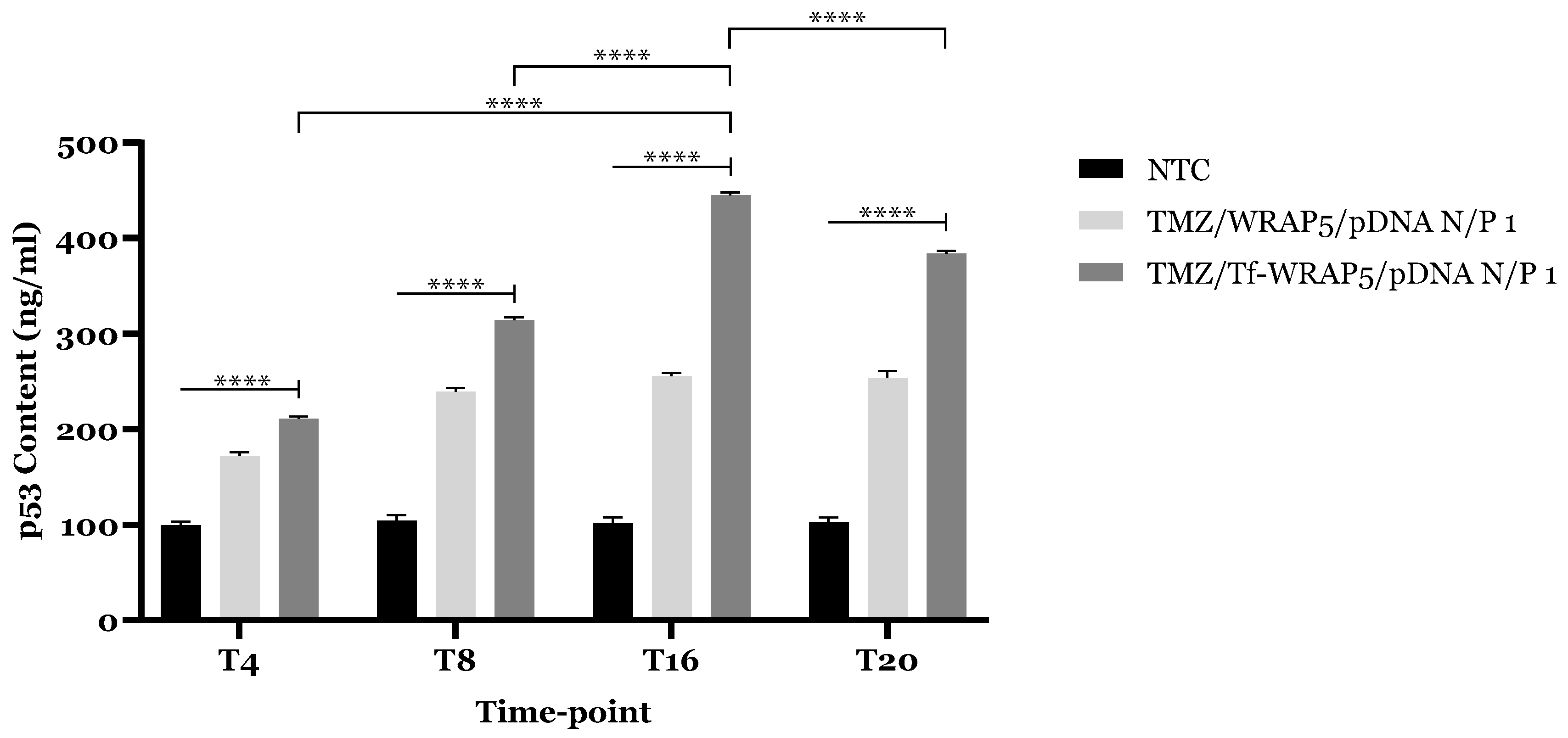
2.5. TP53 and p53 Expression
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Cell Culture
3.2.2. Cell Synchronization
3.2.3. Rhythmic Oscillations of Bmal1, Per2, and Transferrin Receptor Gene Analyzed by Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
3.2.4. Transferrin Receptor Quantification
3.2.5. Formulation and Characterization of Complexes
3.2.6. Live Cell Imaging Assay
FITC Plasmid Labelling
Cellular Uptake
3.2.7. TP53 Expression Evaluation
3.2.8. p53 Expression Evaluation
3.2.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petković, M.; Henis, M.; Heese, O.; Relógio, A. Chronotherapy in Glioblastoma: State of the art and future perspectives. eBioMedicine 2023, 89, 104470. [Google Scholar] [CrossRef]
- Albuquerque, T.; Neves, A.R.; Quintela, T.; Costa, D. Exploring the link between chronobiology and drug delivery: Effects on cancer therapy. J. Mol. Med. 2021, 99, 1349–1371. [Google Scholar] [CrossRef]
- Catozzi, S.; Assaad, S.; Delrieu, L.; Favier, B.; Dumas, E.; Hamy, A.-S.; Latouche, A.; Crochet, H.; Blay, J.-Y.; Mullaert, J.; et al. Early morning immune checkpoint blockade and overall survival of patients with metastatic cancer: An In-depth chronotherapeutic study. Eur. J. Cancer 2024, 199, 113571. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Quintela, T.; Costa, D. Circadian rhythm and disease: Relationship, new insights, and future perspectives. J. Cell. Physiol. 2022, 237, 3239–3256. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Brancaccio, M.; Gonzalez-Aponte, M.F.; Herzog, E.D. Circadian Rhythms and Astrocytes: The Good, the Bad, and the Ugly. Annu. Rev. Neurosci. 2023, 46, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Irfan, M.; Ullah, S.; Fathi-karkan, S.; Kharaba, Z.; Rahdar, A.; Aliahmad, M.; Aboudzadeh, M.A. Nanotechnology in glioblastoma therapy: Advances in drug delivery systems and diagnostic approaches. J. Drug Deliv. Sci. Technol. 2024, 102, 106322. [Google Scholar] [CrossRef]
- Rusak, A.; Wiatrak, B.; Krawczyńska, K.; Górnicki, T.; Zagórski, K.; Zadka, Ł.; Fortuna, W. Starting points for the development of new targeted therapies for glioblastoma multiforme. Transl. Oncol. 2025, 51, 102187. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 13302. [Google Scholar] [CrossRef]
- Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. Association of IDH1 Mutation and MGMT Promoter Methylation with Clinicopathological Parameters in an Ethnically Diverse Population of Adults with Gliomas in England. Biomedicines 2024, 12, 2732. [Google Scholar] [CrossRef]
- Orasanu, C.I.; Aschie, M.; Deacu, M.; Bosoteanu, M.; Vamesu, S.; Enciu, M.; Bălţătescu, G.I.; Cozaru, G.C.; Mitroi, A.F.; Voda, R.I. Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type. Clin. Pract. 2022, 12, 1054–1068. [Google Scholar] [CrossRef]
- Chai, R.; Li, G.; Liu, Y.; Zhang, K.; Zhao, Z.; Wu, F.; Chang, Y.; Pang, B.; Li, J.; Li, Y.; et al. Predictive value of MGMT promoter methylation on the survival of TMZ treated IDH-mutant glioblastoma. Cancer Biol. Med. 2021, 18, 272–282. [Google Scholar] [CrossRef]
- Chien, C.-H.; Hsueh, W.-T.; Chuang, J.-Y.; Chang, K.-Y. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J. Biomed. Sci. 2021, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Slat, E.A.; Sponagel, J.; Marpegan, L.; Simon, T.; Kfoury, N.; Kim, A.; Binz, A.; Herzog, E.D.; Rubin, J.B. Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma. J. Biol. Rhythm. 2017, 32, 121–129. [Google Scholar] [CrossRef]
- Gonzalez-Aponte, M.F.; Damato, A.R.; Trebucq, L.L.; Simon, T.; Cárdenas-García, S.P.; Cho, K.; Patti, G.J.; Golombek, D.A.; Chiesa, J.J.; Rubin, J.B.; et al. Circadian regulation of MGMT expression and promoter methylation underlies daily rhythms in TMZ sensitivity in glioblastoma. J. Neuro-Oncol. 2024, 166, 419–430. [Google Scholar] [CrossRef]
- Damato, A.R.; Luo, J.; Katumba, R.G.N.; Talcott, G.R.; Rubin, J.B.; Herzog, E.D.; Campian, J.L. Temozolomide chronotherapy in patients with glioblastoma: A retrospective single-institute study. Neuro-Oncol. Adv. 2021, 3, vdab041. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, G.; Qu, M.; Gimple, R.C.; Wu, Q.; Qiu, Z.; Prager, B.C.; Wang, X.; Kim, L.J.Y.; Morton, A.R.; et al. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov. 2019, 9, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.M.; Fornasier, S.J.; Guido, M.E. Pharmacological Modulation of the Cytosolic Oscillator Affects Glioblastoma Cell Biology. Cell. Mol. Neurobiol. 2024, 44, 51. [Google Scholar] [CrossRef]
- Deguchi, S.; Ohka, F.; Shiba, Y.; Yamaguchi, J.; Sato, A.; Shinjo, K.; Arakawa, Y.; Narita, Y.; Kondo, Y.; Saito, R. Investigator-initiated phase I trial of an oligonucleotide therapeutic targeting long noncoding RNA TUG 1 for recurrent glioblastoma. BMC Cancer 2025, 25, 251. [Google Scholar] [CrossRef]
- Giordano, F.A.; Layer, J.P.; Leonardelli, S.; Friker, L.L.; Turiello, R.; Corvino, D.; Zeyen, T.; Schaub, C.; Müller, W.; Sperk, E.; et al. L-RNA aptamer-based CXCL12 inhibition combined with radiotherapy in newly-diagnosed glioblastoma: Dose escalation of the phase I/II GLORIA trial. Nat. Commun. 2024, 15, 4210. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference–based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Meng, W.; Huang, L.; Guo, J.; Xin, Q.; Liu, J.; Hu, Y. Innovative Nanomedicine Delivery: Targeting Tumor Microenvironment to Defeat Drug Resistance. Pharmaceutics 2024, 16, 1549. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fang, Z.; Wang, J.; Liu, K. A nano-targeted co-delivery system based on gene regulation and molecular blocking strategy for synergistic enhancement of platinum chemotherapy sensitivity in ovarian cancer. Int. J. Pharm. 2023, 640, 123022. [Google Scholar] [CrossRef]
- Guo, Q.; Kong, F.; Pan, X.; Cao, S. Co-delivery of docetaxel and p53 gene from cationic nanoparticles based on poly (l-lactide) and low-molecular-weight polyethyleneimine (PEA). Pharm. Dev. Technol. 2022, 27, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.S.; Rekha, M.R. Synergistic effect of p53 gene/DOX intracellular delivery and P-gp inhibition by pullulan thiomers on cancer cells: In vitro and in vivo evaluations. J. Mater. Chem. B 2023, 11, 1365–1377. [Google Scholar] [CrossRef]
- Wu, H.; Gao, W.; Chen, P.; Wei, Y.; Zhao, H.; Wang, F. Research progress of drug resistance mechanism of temozolomide in the treatment of glioblastoma. Heliyon 2024, 10, e39984. [Google Scholar] [CrossRef]
- Hani, U.; Choudhary, V.T.; Ghazwani, M.; Alghazwani, Y.; Osmani, R.A.M.; Kulkarni, G.S.; Shivakumar, H.G.; Wani, S.U.D.; Paranthaman, S. Nanocarriers for Delivery of Anticancer Drugs: Current Developments, Challenges, and Perspectives. Pharmaceutics 2024, 16, 1527. [Google Scholar] [CrossRef]
- Aldrian, G.; Vaissière, A.; Konate, K.; Seisel, Q.; Vivès, E.; Fernandez, F.; Viguier, V.; Genevois, C.; Couillaud, F.; Démèné, H.; et al. PEGylation rate influences peptide-based nanoparticles mediated siRNA delivery in vitro and in vivo. J. Control. Release 2017, 256, 79–91. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Faria, R.; Gonçalves, A.M.; Santos, C.; Vivès, E.; Boisguérin, P.; Passarinha, L.A.; Sousa, Â.; Costa, D. Development of WRAP5 Peptide Complexes for Targeted Drug/Gene Co-Delivery toward Glioblastoma Therapy. Pharmaceutics 2022, 14, 2213. [Google Scholar] [CrossRef]
- Koo, J.; Shin, Y.; Jeon, H.; Cheong, J.; Cho, S.; Park, C.; Song, E.C.; Ramsey, J.D.; Lim, C.; Oh, K.T. Enhancing glioblastoma therapy via intranasal administration of highly potent cell-penetrating peptide decorated nanoparticles. J. Control. Release 2025, 378, 997–1012. [Google Scholar] [CrossRef]
- Jana, A.; Narula, P.; Chugh, A.; Kulshreshtha, R. Efficient delivery of anti-miR-210 using Tachyplesin, a cell penetrating peptide, for glioblastoma treatment. Int. J. Pharm. 2019, 572, 118789. [Google Scholar] [CrossRef]
- Neves, A.; Albuquerque, T.; Faria, R.; Santos, C.R.A.; Vivès, E.; Boisguérin, P.; Carneiro, D.; Bruno, D.F.; Pavlaki, M.D.; Loureiro, S.; et al. Evidence That a Peptide-Drug/p53 Gene Complex Promotes Cognate Gene Expression and Inhibits the Viability of Glioblastoma Cells. Pharmaceutics 2024, 16, 781. [Google Scholar] [CrossRef]
- Lin, H.-H.; Qraitem, M.; Lian, Y.; Taylor, S.R.; Farkas, M.E. Analyses of BMAL1 and PER2 Oscillations in a Model of Breast Cancer Progression Reveal Changes With Malignancy. Integr. Cancer Ther. 2019, 18, 1534735419836494. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Torres, I.D.; Loureiro, J.A.; Lima, J.; Pereira, M.C. Transferrin-Conjugated PLGA Nanoparticles for Co-Delivery of Temozolomide and Bortezomib to Glioblastoma Cells. ACS Appl. Nano Mater. 2023, 6, 14191–14203. [Google Scholar] [CrossRef] [PubMed]
- Kusmierz, C.D.; Callmann, C.E.; Kudruk, S.; Distler, M.E.; Mirkin, C.A. Transferrin Aptamers Increase the In Vivo Blood–Brain Barrier Targeting of Protein Spherical Nucleic Acids. Bioconjugate Chem. 2022, 33, 1803–1810. [Google Scholar] [CrossRef]
- Ni, X.-R.; Zhao, Y.-Y.; Cai, H.-P.; Yu, Z.-H.; Wang, J.; Chen, F.-R.; Yu, Y.-J.; Feng, G.-K.; Chen, Z.-P. Transferrin receptor 1 targeted optical imaging for identifying glioma margin in mouse models. J. Neuro-Oncol. 2020, 148, 245–258. [Google Scholar] [CrossRef]
- Rosager, A.M.; Sørensen, M.D.; Dahlrot, R.H.; Hansen, S.; Schonberg, D.L.; Rich, J.N.; Lathia, J.D.; Kristensen, B.W. Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value. PLoS ONE 2017, 12, e0182954. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Lück, S.; Westermark, P.O. Circadian mRNA expression: Insights from modeling and transcriptomics. Cell. Mol. Life Sci. CMLS 2016, 73, 497–521. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef] [PubMed]
- Van Drunen, R.; Dai, Y.; Wei, H.; Fekry, B.; Noori, S.; Shivshankar, S.; Bravo, R.; Zhao, Z.; Yoo, S.-h.; Justice, N.; et al. Cell-specific regulation of the circadian clock by BMAL1 in the paraventricular nucleus: Implications for regulation of systemic biological rhythms. Cell Rep. 2024, 43, 114380. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Zhou, M.; Han, L.; Wu, S.; Wu, L.; Yang, J. Cryptochrome 2 Suppresses Epithelial-Mesenchymal Transition by Promoting Trophoblastic Ferroptosis in Unexplained Recurrent Spontaneous Abortion. Am. J. Pathol. 2024, 194, 1197–1217. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ren, Q.; Wang, X.; Bai, H.; Tian, D.; Gao, G.; Wang, F.; Yu, P.; Chang, Y.-Z. Cellular iron depletion enhances behavioral rhythm by limiting brain Per1 expression in mice. CNS Neurosci. Ther. 2024, 30, e14592. [Google Scholar] [CrossRef]
- Okazaki, F.; Matsunaga, N.; Okazaki, H.; Azuma, H.; Hamamura, K.; Tsuruta, A.; Tsurudome, Y.; Ogino, T.; Hara, Y.; Suzuki, T.; et al. Circadian Clock in a Mouse Colon Tumor Regulates Intracellular Iron Levels to Promote Tumor Progression. J. Biol. Chem. 2016, 291, 7017–7028. [Google Scholar] [CrossRef]
- Okazaki, F.; Matsunaga, N.; Okazaki, H.; Utoguchi, N.; Suzuki, R.; Maruyama, K.; Koyanagi, S.; Ohdo, S. Circadian Rhythm of Transferrin Receptor 1 Gene Expression Controlled by c-Myc in Colon Cancer–Bearing Mice. Cancer Res. 2010, 70, 6238–6246. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Pettikiriarachchi, A.; McLean, A.R.D.; Harding, R.; Blewitt, M.E.; Seillet, C.; Pasricha, S.-R. Serum iron variation is circadian-regulated and linked to the harmonic circadian oscillations of erythropoiesis and hepatic Tfrc expression in mice. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Pettikiriarachchi, A.; McLean, A.R.D.; Harding, R.; Blewitt, M.E.; Seillet, C.; Pasricha, S.-R. Serum iron and transferrin saturation variation are circadian regulated and linked to the harmonic circadian oscillations of erythropoiesis and hepatic expression in mice. Am. J. Hematol. 2024, 99, 2075–2083. [Google Scholar] [CrossRef]
- van der Spek, R.; Fliers, E.; la Fleur, S.E.; Kalsbeek, A. Daily Gene Expression Rhythms in Rat White Adipose Tissue Do Not Differ Between Subcutaneous and Intra-Abdominal Depots. Front. Endocrinol. 2018, 9, 206. [Google Scholar] [CrossRef]
- Moškon, M. CosinorPy: A python package for cosinor-based rhythmometry. BMC Bioinform. 2020, 21, 485. [Google Scholar] [CrossRef]
- Moškon, M. Identification and characterisation of variable rhythms using CosinorPy. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ryabinina, A.Y.; Bryk, A.A.; Blagonravov, M.L.; Goryachev, V.A.; Mozhaev, A.A.; Ovechkina, V.S. Circadian Rhythms of Body Temperature and Locomotor Activity in Spontaneously Hypertensive Rats under Frequent Changes in Light Conditions. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2024, 31, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Spick, M.; Isherwood, C.M.; Gethings, L.A.; Hughes, C.J.; Daly, M.E.; Hassanin, H.; van der Veen, D.R.; Skene, D.J.; Johnston, J.D. Challenges and opportunities for statistical power and biomarker identification arising from rhythmic variation in proteomics. npj Biol. Timing Sleep 2025, 2, 3. [Google Scholar] [CrossRef]
- Shiba, A.; de Goede, P.; Tandari, R.; Foppen, E.; Korpel, N.L.; Coopmans, T.V.; Hellings, T.P.; Jansen, M.W.; Ruitenberg, A.; Ritsema, W.I.G.R.; et al. Synergy between time-restricted feeding and time-restricted running is necessary to shift the muscle clock in male wistar rats. Neurobiol. Sleep Circadian Rhythm. 2024, 17, 100106. [Google Scholar] [CrossRef]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef]
- Carvalho-Moreira, J.P.; de Oliveira Guarnieri, L.; Passos, M.C.; Emrich, F.; Bargi-Souza, P.; Peliciari-Garcia, R.A.; Moraes, M.F.D. CircadiPy: An open-source toolkit for analyzing chronobiology time series. J. Neurosci. Methods 2024, 411, 110245. [Google Scholar] [CrossRef]
- Parsons, R.; Jayasinghe, O.; White, N.; Chunduri, P.; Rawashdeh, O. GLMMcosinor: Flexible cosinor modeling with a generalized linear mixed modeling framework to characterize rhythmic time series. bioRxiv 2024. [Google Scholar] [CrossRef]
- van Rheede, J.J.; Feldmann, L.K.; Busch, J.L.; Fleming, J.E.; Mathiopoulou, V.; Denison, T.; Sharott, A.; Kühn, A.A. Diurnal modulation of subthalamic beta oscillatory power in Parkinson’s disease patients during deep brain stimulation. npj Park. Dis. 2022, 8, 88. [Google Scholar] [CrossRef]
- Motiei, M.; Abu-Dawud, R.; Relógio, A.; Assaf, C. Circadian rhythms in haematological malignancies: Therapeutic potential and personalised interventions. eBioMedicine 2024, 110, 105451. [Google Scholar] [CrossRef]
- Zhu, X.; Maier, G.; Panda, S. Learning from circadian rhythm to transform cancer prevention, prognosis, and survivorship care. Trends Cancer 2024, 10, 196–207. [Google Scholar] [CrossRef]
- Wang, J.; Shao, F.; Yu, Q.-X.; Ye, L.; Wusiman, D.; Wu, R.; Tuo, Z.; Wang, Z.; Li, D.; Cho, W.C.; et al. The Common Hallmarks of Aging, Circadian Rhythms and Cancer: Implications for Therapeutic Strategies. Research 2025, 8, 0612. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, Z.; Wu, M.; Chen, F.; Chen, L. Circadian rhythm regulates the function of immune cells and participates in the development of tumors. Cell Death Discov. 2024, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Relógio, A. Molecular mechanisms of tumour development in glioblastoma: An emerging role for the circadian clock. npj Precis. Oncol. 2024, 8, 40. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Meng, Y.; Fang, J.; Xu, D.; Lu, Z. The interplay of the circadian clock and metabolic tumorigenesis. Trends Cell Biol. 2024, 34, 742–755. [Google Scholar] [CrossRef]
- Tavsanli, N.; Erözden, A.A.; Çalışkan, M. Evaluation of small-molecule modulators of the circadian clock: Promising therapeutic approach to cancer. Mol. Biol. Rep. 2024, 51, 848. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Cheng, Z.; Teo, G.; Krueger, S.; Rock, T.M.; Koh, H.W.; Choi, H.; Vogel, C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol. Syst. Biol. 2016, 12, 855. [Google Scholar] [CrossRef]
- Hanson, R.L.; Porter, J.R.; Batchelor, E. Protein stability of p53 targets determines their temporal expression dynamics in response to p53 pulsing. J. Cell Biol. 2019, 218, 1282–1297. [Google Scholar] [CrossRef]
- Faria, R.; Vivés, E.; Boisguerin, P.; Sousa, A.; Costa, D. Development of Peptide-Based Nanoparticles for Mitochondrial Plasmid DNA Delivery. Polymers 2021, 13, 1836. [Google Scholar] [CrossRef]
- Albuquerque, T.; Neves, A.R.; Paul, M.; Biswas, S.; Vuelta, E.; García-Tuñón, I.; Sánchez-Martin, M.; Quintela, T.; Costa, D. A Potential Effect of Circadian Rhythm in the Delivery/Therapeutic Performance of Paclitaxel-Dendrimer Nanosystems. J. Funct. Biomater. 2023, 14, 362. [Google Scholar] [CrossRef]
- Furtado, A.; Duarte, A.C.; Costa, A.R.; Gonçalves, I.; Santos, C.R.A.; Gallardo, E.; Quintela, T. Circadian ABCG2 Expression Influences the Brain Uptake of Donepezil across the Blood–Cerebrospinal Fluid Barrier. Int. J. Mol. Sci. 2024, 25, 5014. [Google Scholar] [CrossRef] [PubMed]
- Moškon, M. GitHub, CosinorPy. Available online: https://github.com/mmoskon/CosinorPy?tab=readme-ov-file (accessed on 8 May 2025).




| Gene | p-Value | Centre of Gravity (COG) (h) |
|---|---|---|
| Bmal1 | 0.028 | 15.71 |
| Per2 | 0.022 | 8.14 |
| Transferrin | 0.005 | 6.78 |
| Gene | p-Value | Period (τ) | MESOR (M) | Amplitude (A) | Acrophase (Ø) (Radians) | Centre of Gravity (COG) (h) |
|---|---|---|---|---|---|---|
| 24 h | ||||||
| Bmal1 | 0.05 | 4.94 | 1.30 | 0.60 | −1.50 | 18.82 |
| Per2 | 0.03 | 15.36 | 1.00 | 0.50 | 3.01 | 7.36 |
| Transferrin | 0.09 | 22.31 | 1.05 | 0.45 | −1.13 | 20.00 |
| 48 h | ||||||
| Bmal1 | 0.002 | 21.86 | 1.25 | 0.65 | 2.22 | 7.72 |
| Per2 | 0.001 | 29.42 | 1.35 | 0.85 | −2.26 | 33.42 |
| Software | p-Value | Period (τ) | MESOR (M) | Amplitude (A) | Acrophase (Radians) | Centre of Gravity (COG) (h) |
|---|---|---|---|---|---|---|
| CircWave | 0.000 | 24.00 | - | - | - | 9.59 |
| CosinorPy | 0.083 | 25.11 | 199.95 | 120.71 | −2.00 | 16.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, A.R.; Vivès, E.; Boisguérin, P.; Quintela, T.; Costa, D. Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection. Int. J. Mol. Sci. 2025, 26, 6130. https://doi.org/10.3390/ijms26136130
Neves AR, Vivès E, Boisguérin P, Quintela T, Costa D. Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection. International Journal of Molecular Sciences. 2025; 26(13):6130. https://doi.org/10.3390/ijms26136130
Chicago/Turabian StyleNeves, Ana R., Eric Vivès, Prisca Boisguérin, Telma Quintela, and Diana Costa. 2025. "Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection" International Journal of Molecular Sciences 26, no. 13: 6130. https://doi.org/10.3390/ijms26136130
APA StyleNeves, A. R., Vivès, E., Boisguérin, P., Quintela, T., & Costa, D. (2025). Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection. International Journal of Molecular Sciences, 26(13), 6130. https://doi.org/10.3390/ijms26136130










