Artificial Intelligence in the Management of Hereditary and Acquired Hemophilia: From Genomics to Treatment Optimization
Abstract
1. Introduction
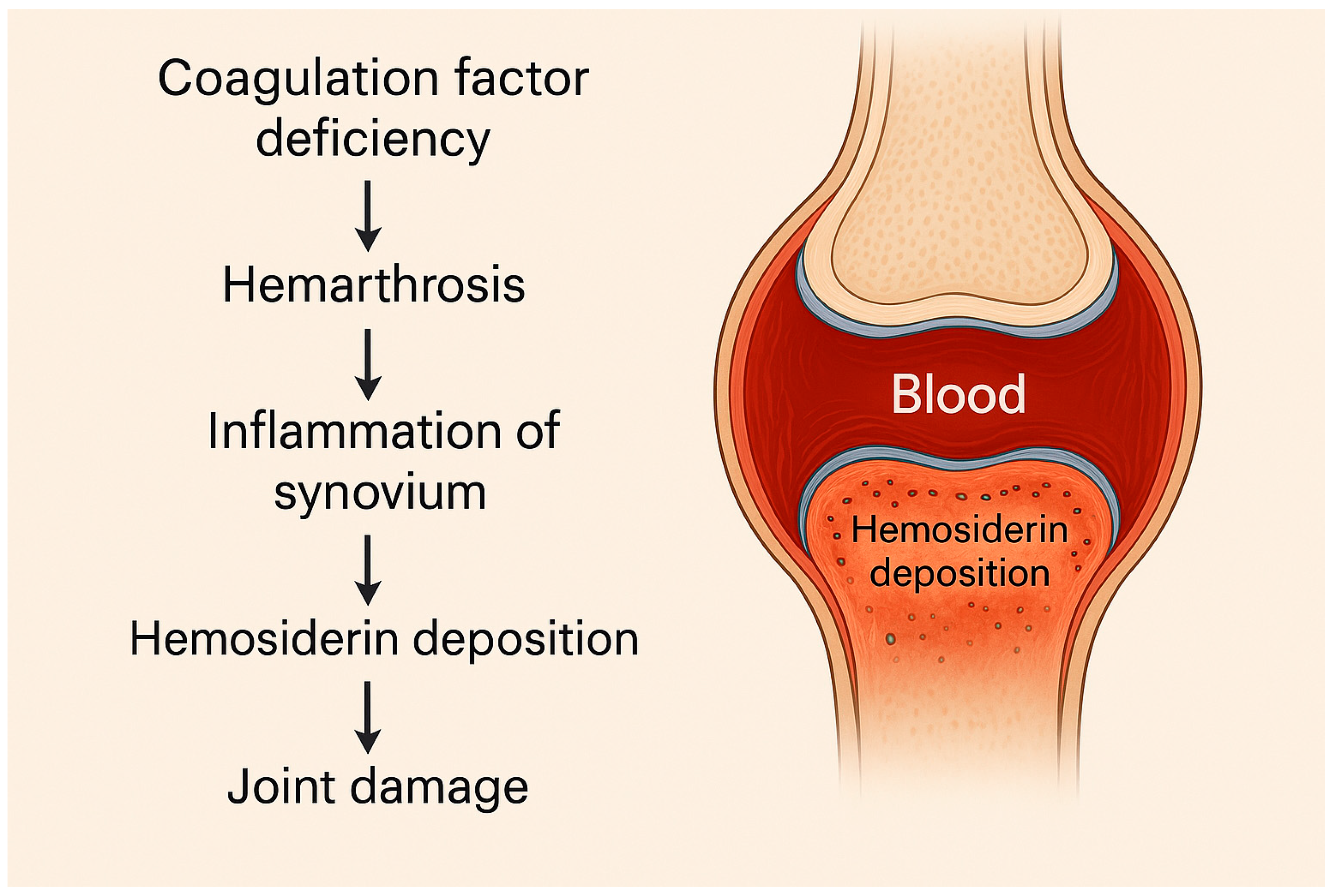
1.1. General Considerations on Clinical Background of Hemophilia
1.2. Hemophilia A
1.3. Hemophilia B
1.4. Diagnosis of Hereditary Hemophilia
1.5. Acquired Hemophilia
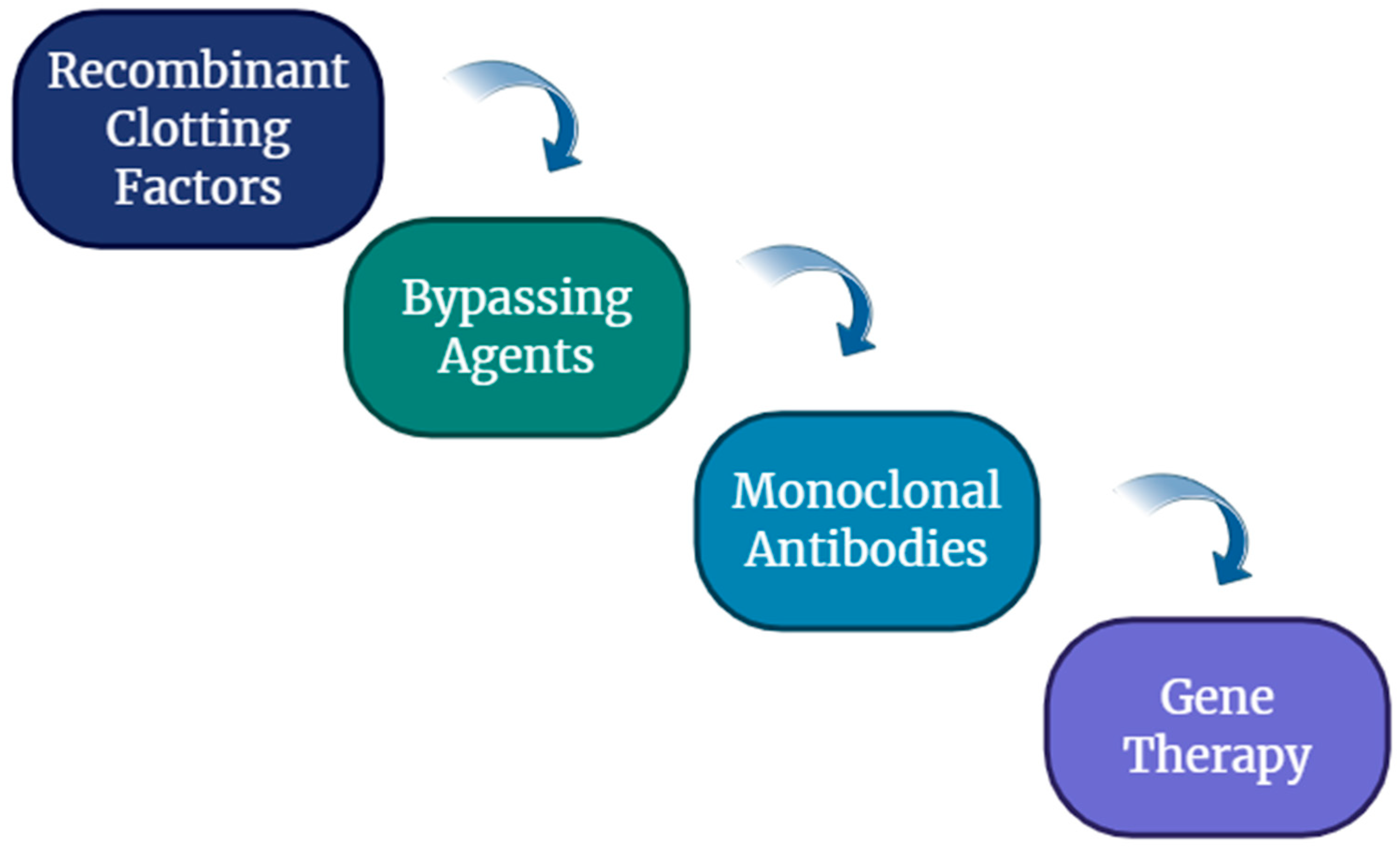
1.6. Management of Hemophilia
2. Focus on Artificial Intelligence
3. AI in Hematology
4. AI in Hemophilia
4.1. Diagnostic Applications of AI in Hemophilia
4.2. Prognostic Modeling and Risk Stratification
4.3. Treatment Optimization and Clinical Decision Support
4.4. Monitoring, Rehabilitation, and Long-Term Care
5. Ethical and Regulatory Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewandowska, M.; Nasr, S.; Shapiro, A.D. Emerging Therapies in Hemophilia: Improving Equitable Access to Care. J. Blood Med. 2025, 16, 95–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanchette, V.S.; Key, N.S.; Ljung, L.R.; Manco-Johnson, M.J.; van den Berg, H.M.; Srivastava, A. Definitions in hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- van Galen, K.P.M.; d’Oiron, R.; James, P.; Abdul-Kadir, R.; Kouides, P.A.; Kulkarni, R.; Mahlangu, J.N.; Othman, M.; Peyvandi, F.; Rotellini, D.; et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2021, 19, 1883–1887. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. S6), 1–158. [Google Scholar] [CrossRef]
- Tiede, A.; Collins, P.; Knoebl, P.; Teitel, J.; Kessler, C.; Shima, M.; Di Minno, G.; d’Oiron, R.; Salaj, P.; Jiménez-Yuste, V.; et al. International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica 2020, 105, 1791–1801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, J.; Miesbach, W.; Prüller, F.; Siegemund, T.; Scholz, U.; Sachs, U.J.; Standing Commission Labor (STAEKOLA) of the Society of Thrombosis and Haemostasis Research (GTH). An Update on Laboratory Diagnostics in Haemophilia A and B. Hamostaseologie 2022, 42, 248–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, C.E.; Batt, K.M. Hemophilia clinical gene therapy: Brief review. Transl. Res. 2013, 161, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Rath, G.; Goyal, A.K. Advancement in the treatment of haemophilia. Int. J. Biol. Macromol. 2018, 118 Pt A, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Okaygoun, D.; Oliveira, D.D.; Soman, S.; Williams, R. Advances in the management of haemophilia: Emerging treatments and their mechanisms. J. Biomed. Sci. 2021, 28, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crossnohere, N.L.; Elsaid, M.; Paskett, J.; Bose-Brill, S.; Bridges, J.F.P. Guidelines for Artificial Intelligence in Medicine: Literature Review and Content Analysis of Frameworks. J. Med. Internet Res. 2022, 24, e36823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senbekov, M.; Saliev, T.; Bukeyeva, Z.; Almabayeva, A.; Zhanaliyeva, M.; Aitenova, N.; Toishibekov, Y.; Fakhradiyev, I. The recent progress and applications of digital technologies in healthcare: A review. Int. J. Telemed. Appl. 2020, 2020, 8830200. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, A.I.; Kitsios, F.; Talias, M.A. Digital transformation in healthcare: Technology acceptance and its applications. Int. J. Environ. Res. Public Health 2023, 20, 3407. [Google Scholar] [CrossRef]
- Nitiéma, P. Artificial Intelligence in Medicine: Text Mining of Health Care Workers’ Opinions. J. Med. Internet Res. 2023, 25, e41138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schramm, W. The history of haemophilia—A short review. Thromb. Res. 2014, 134 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Primers 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Hosack, L.; Backstein, D.; Spangehl, M. Recurrent Hemarthrosis After Total Knee Arthroplasty: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 2019, 27, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, H.A.; FernAndez-Palazzi, F.; Gilbert, M.S. Haemophilic pseudotumours of the limbs and their percutaneous treatment. Haemophilia 2002, 8, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.Q.; Gonzalez-Fernandez, E.; Bhatia, K.; Divani, A.A.; Di Napoli, M.; Hinduja, A.; Datta, Y.H. Neurological Complications Associated with Hereditary Bleeding Disorders. Curr. Neurol. Neurosci. Rep. 2023, 23, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Bick, L.; Lauritzen, U.M.; Andersen, S.; Galili, J.; Blichfeldt-Eckhardt, L. Haematoma in the airways as the first symptom of acquired haemophilia A. Ugeskr. Laeger 2022, 184, V05220323. [Google Scholar] [PubMed]
- Shapiro, S.; Benson, G.; Evans, G.; Harrison, C.; Mangles, S.; Makris, M. Cardiovascular disease in hereditary haemophilia: The challenges of longevity. Br. J. Haematol. 2022, 197, 397–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolton-Maggs, P.H.; Pasi, K.J. Haemophilias A and B. Lancet 2003, 361, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Garagiola, I.; Young, G. The past and future of haemophilia: Diagnosis, treatments, and its complications. Lancet 2016, 388, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rocino, A.; Coppola, A.; Franchini, M.; Castaman, G.; Santoro, C.; Zanon, E.; Santagostino, E.; Morfini, M.; Italian Association of Haemophilia Centres (AICE) Working Party. Principles of treatment and update of recommendations for the management of haemophilia and congenital bleeding disorders in Italy. Blood Transfus. 2014, 12, 575–598, Erratum in Blood Transfus. 2015, 13, 167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruse-Jarres, R.; Kempton, C.L.; Baudo, F.; Collins, P.W.; Knoebl, P.; Leissinger, C.A.; Tiede, A.; Kessler, C.M. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am. J. Hematol. 2017, 92, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Dolan, G.; Benson, G.; Bowyer, A.; Eichler, H.; Hermans, C.; Jiménez-Yuste, V.; Ljung, R.; Pollard, D.; Santagostino, E.; Šalek, S.Z. Principles of care for acquired hemophilia. Eur. J. Haematol. 2021, 106, 762–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pai, M. Acquired Hemophilia A. Hematol. Oncol. Clin. N. Am. 2021, 35, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Platton, S.; Sivapalaratnam, S.; Raheja, P. Diagnosis and laboratory monitoring of acquired hemophilia A. Hematol. Am. Soc. Hematol. Educ. Program 2023, 2023, 11–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehoczki, A.; Fekete, M.; Mikala, G.; Bodó, I. Acquired hemophilia A as a disease of the elderly: A comprehensive review of epidemiology, pathogenesis, and novel therapy. Geroscience 2025, 47, 503–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zdziarska, J.; Musiał, J. Acquired hemophilia A: An underdiagnosed, severe bleeding disorder. Pol. Arch. Med. Wewn. 2014, 124, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Lechner, K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb. Haemost. 1981, 45, 200–203. [Google Scholar] [CrossRef]
- Delgado, J.; Jimenez-Yuste, V.; Hernandez-Navarro, F.; Villar, A. Acquired haemophilia: Review and meta-analysis focused on therapy and prognostic factors. Br. J. Haematol. 2003, 121, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.W.; Hirsch, S.; Baglin, T.P.; Dolan, G.; Hanley, J.; Makris, M.; Keeling, D.M.; Liesner, R.I.; Brown, S.A.; Hay, C.R.; et al. Acquired hemophilia A in the United Kingdom: A 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007, 109, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.; Duncan, E.; Singhal, D.; Al-Qunfoidi, R.; Coghlan, D.; Jaksic, W.; Szabo, F.; McRae, S.; Lloyd, J. Twelve years of experience of acquired hemophilia A: Trials and tribulations in South Australia. Semin. Thromb. Hemost. 2009, 35, 769–777. [Google Scholar] [CrossRef]
- Knoebl, P.; Marco, P.; Baudo, F.; Collins, P.; Huth-Kühne, A.; Nemes, L.; Pellegrini, F.; Tengborn, L.; Lévesque, H.; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). J. Thromb. Haemost. 2012, 10, 622–631. [Google Scholar] [CrossRef]
- Franchini, M.; Zaffanello, M.; Lippi, G. Acquired hemophilia in pediatrics: A systematic review. Pediatr. Blood Cancer 2010, 55, 606–611. [Google Scholar] [CrossRef]
- Oldenburg, J.; Zeitler, H.; Pavlova, A. Genetic markers in acquired haemophilia. Haemophilia 2010, 16 (Suppl. S3), 41–45. [Google Scholar] [CrossRef]
- Ma, A.D.; Carrizosa, D. Acquired factor VIII inhibitors: Pathophysiology and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2006, 2006, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.; Zeitler, H.; Scharrer, I.; Brackmann, H.H.; Oldenburg, J. HLA genotype in patients with acquired haemophilia A. Haemophilia 2010, 16, 107–112. [Google Scholar] [CrossRef]
- Baudo, F.; Collins, P.; Huth-Kuhne, A.; Levesque, H.; Marco, P.; Nemes, L.; Pellegrini, F.; Tengborn, L.; Knoebl, P. Management of bleeding in acquired hemophilia A: Results from the European Acquired Haemophilia (EACH2) registry. Blood 2012, 120, 39–46. [Google Scholar] [CrossRef]
- Collins, P.; Baudo, F.; Knoebl, P.; Lévesque, H.; Nemes, L.; Pellegrini, F.; Marco, P.; Tengborn, L.; Huth-Kühne, A. Immunosuppression for acquired hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). Blood 2012, 120, 47–55. [Google Scholar] [CrossRef]
- Bitting, R.L.; Bent, S.; Li, Y.; Kohlwes, J. The prognosis and treatment of acquired hemophilia: A systematic review and meta-analysis. Blood Coagul. Fibrinol. 2009, 20, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Werwitzke, S.; Geisen, U.; Nowak-Gottl, U.; Eichler, H.; Stephan, B.; Scholz, U.; Holstein, K.; Klamroth, R.; Knöbl, P.; Huth-Kühne, A.; et al. Diagnostic and prognostic value of factor VIII binding antibodies in acquired hemophilia A: Data from the GTH-AH 01/2010 study. J. Thromb. Haemost. 2016, 14, 940–947. [Google Scholar] [CrossRef]
- Tiede, A.; Werwitzke, S.; Scharf, R.E. Laboratory diagnosis of acquired hemophilia A: Limitations, consequences, and challenges. Semin. Thromb. Hemost. 2014, 40, 803–811. [Google Scholar]
- Collins, P.W.; Chalmers, E.; Hart, D.P.; Liesner, R.; Rangarajan, S.; Talks, K.; Williams, M.; Hay, C.R. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia. Br. J. Haematol. 2013, 160, 153–170. [Google Scholar] [CrossRef]
- Huth-Kuhne, A.; Baudo, F.; Collins, P.; Ingerslev, J.; Kessler, C.M.; Lévesque, H.; Castellano, M.E.M.; Shima, M.; St-Louis, J. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica 2009, 94, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Miesbach, W.; Baghaei, F.; Boban, A.; Chowdary, P.; Coppens, M.; Hart, D.P.; Jimenez-Yuste, V.; Klamroth, R.; Makris, M.; Noone, D.; et al. Gene therapy of hemophilia: Hub centres should be haemophilia centres: A joint publication of EAHAD and EHC. Haemophilia 2022, 28, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- Puetz, J. Hemophilia Gene Therapy: Another Blessing or Another Curse? Mo. Med. 2024, 121, 231–234. [Google Scholar] [PubMed] [PubMed Central]
- Collins, P.W. Therapeutic challenges in acquired factor VIII deficiency. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 369–374. [Google Scholar] [CrossRef]
- Kruse-Jarres, R.; St-Louis, J.; Greist, A.; Shapiro, A.; Smith, H.; Chowdary, P.; Drebes, A.; Gomperts, E.; Bourgeois, C.; Mo, M.; et al. Efficacy and safety of OBI-1, an antihaemophilic factor VIII (recombinant), porcine sequence, in subjects with acquired haemophilia A. Haemophilia 2015, 21, 162–170. [Google Scholar] [CrossRef]
- Hvas, A.M.; Sorensen, H.T.; Norengaard, L.; Christiansen, K.; Ingerslev, J.; Sorensen, B. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J. Thromb. Haemost. 2007, 5, 2408–2414. [Google Scholar] [CrossRef]
- Varadi, K.; Negrier, C.; Berntorp, E.; Astermark, J.; Bordet, J.C.; Morfini, M.; Linari, S.; Schwarz, H.P.; Turecek, P.L. Monitoring the bioavailability of FEIBA with a thrombin generation assay. J. Thromb. Haemost. 2003, 1, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Hueber, A.J.; Leipe, J.; Manger, B.; Schett, G.; Kallert, S. A case report of plasma exchange, steroids, mycophenolate mofetil and cyclophosphamide in acquired factor VIII inhibitors. Ther. Apher. Dial. 2008, 12, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Dedeken, L.; St-Louis, J.; Demers, C.; Meilleur, C.; Rivard, G.E. Postpartum acquired haemophilia: A single centre experience with rituximab. Haemophilia 2009, 15, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, M.; Wang, S.; Li, X.; Sun, Y. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun. 2020, 40, 154–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majumder, A.; Sen, D. Artificial intelligence in cancer diagnostics and therapy: Current perspectives. Indian J. Cancer 2021, 58, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Stanfill, M.H.; Marc, D.T. Health Information Management: Implications of Artificial Intelligence on Healthcare Data and Information Management. Yearb. Med. Inform. 2019, 28, 56–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moritz, S.; Romeike, B.; Stosch, C.; Tolks, D. Generative AI (gAI) in medical education: Chat-GPT and co. GMS J. Med. Educ. 2023, 40, Doc54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allegra, A.; Tonacci, A.; Sciaccotta, R.; Genovese, S.; Musolino, C.; Pioggia, G.; Gangemi, S. Machine Learning and Deep Learning Applications in Multiple Myeloma Diagnosis, Prognosis, and Treatment Selection. Cancers 2022, 14, 606. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Tonacci, A.; Genovese, S.; Pioggia, G.; Gangemi, S. Machine Learning Approaches in Diagnosis, Prognosis and Treatment Selection of Cardiac Amyloidosis. Int. J. Mol. Sci. 2023, 24, 5680. [Google Scholar] [CrossRef]
- Stagno, F.; Russo, S.; Murdaca, G.; Mirabile, G.; Alvaro, M.E.; Nasso, M.E.; Zemzem, M.; Gangemi, S.; Allegra, A. Utilization of Machine Learning in the Prediction, Diagnosis, Prognosis, and Management of Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2025, 26, 2535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stagno, F.; Mirabile, G.; Rizzotti, P.; Bottaro, A.; Pagana, A.; Gangemi, S.; Allegra, A. Using Artificial Intelligence to Enhance Myelodysplastic Syndrome Diagnosis, Prognosis, and Treatment. Biomedicines 2025, 13, 835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rösler, W.; Altenbuchinger, M.; Baeßler, B.; Beissbarth, T.; Beutel, G.; Bock, R.; von Bubnoff, N.; Eckardt, J.N.; Foersch, S.; Loeffler, C.M.L.; et al. An overview and a roadmap for artificial intelligence in hematology and oncology. J. Cancer Res. Clin. Oncol. 2023, 149, 7997–8006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Amico, S.; Dall’Olio, D.; Sala, C.; Dall’Olio, L.; Sauta, E.; Zampini, M.; Asti, G.; Lanino, L.; Maggioni, G.; Campagna, A.; et al. Synthetic Data Generation by Artificial Intelligence to Accelerate Research and Precision Medicine in Hematology. JCO Clin. Cancer Inform. 2023, 7, e2300021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alim, M.S.; Bappon, S.D.; Sabuj, S.M.; Islam, M.J.; Tarek, M.M.; Azam, M.S.; Islam, M.M. Integrating convolutional neural networks for microscopic image analysis in acute lymphoblastic leukemia classification: A deep learning approach for enhanced diagnostic precision. Syst. Soft Comput. 2024, 6, 200121. [Google Scholar] [CrossRef]
- Eckhardt, C.M.; Madjarova, S.J.; Williams, R.J.; Ollivier, M.; Karlsson, J.; Pareek, A.; Nwachukwu, B.U. Unsupervised machine learning methods and emerging applications in healthcare. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 376–381. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Savani, B.; Mohty, M.; Nagler, A. Machine learning and artificial intelligence in haematology. Br. J. Haematol. 2021, 192, 239–250. [Google Scholar] [CrossRef]
- Walter, W.; Pohlkamp, C.; Meggendorfer, M.; Nadarajah, N.; Kern, W.; Haferlach, C.; Haferlach, T. Artificial intelligence in hematological diagnostics, Game changer or gadget? Blood Rev. 2023, 58, 101019. [Google Scholar] [CrossRef] [PubMed]
- El Alaoui, Y.; Elomri, A.; Qaraqe, M.; Padmanabhan, R.; Yasin Taha, R.; El Omri, H.; El Omri, A.; Aboumarzouk, O. A Review of Artificial Intelligence Applications in Hematology Management, Current Practices and Future Prospects. J. Med. Int. Res. 2022, 24, e36490. [Google Scholar] [CrossRef]
- Radakovich, N.; Nagy, M.; Nazha, A. Artificial Intelligence in Hematology: Current Challenges and Opportunities. Curr. Hematol. Malig. Rep. 2020, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Kim, G.Y.E.; Suarez, C.J.; Chen, J.H. Applications of machine learning in routine laboratory medicine, Current state and future directions. Clin. Biochem. 2022, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.R.M.; Xiang, H.; Scott, M.; Collins, P.W.; Liesner, R.; Dolan, G.; Hollingsworth, R. The haemtrack home therapy reporting system: Design, implementation, strengths and weaknesses: A report from UK Haemophilia Centre Doctors Organisation. Haemophilia 2017, 23, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.; Desai, V.; Xu, Y.; Ridgeway, G.; Finkle, W.; Solari, P.; Sullivan, S.; Lanes, S. Development and validation of an algorithm for identifying patients with hemophilia A in an administrative claims database. Value Health 2018, 21, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Chelle, P.; Yeung, C.H.T.; Bonanad, S.; Morales Muñoz, J.C.; Ozelo, M.C.; Megías Vericat, J.E.; Iorio, A.; Spears, J.; Mir, R.; Edginton, A. Routine clinical care data for population pharmacokinetic modeling: The case for Fanhdi/Alphanate in hemophilia A patients. J. Pharmacokinet. Pharmacodyn. 2019, 46, 427–438. [Google Scholar] [CrossRef]
- Singh, V.K.; Maurya, N.S.; Mani, A.; Yadav, R.S. Machine learning method using position-specific mutation based classification outperforms one hot coding for disease severity prediction in haemophilia ‘A’. Genomics 2020, 112, 5122–5128. [Google Scholar] [CrossRef]
- Ai, D.; Cui, C.; Tang, Y.; Wang, Y.; Zhang, N.; Zhang, C.; Zhen, Y.; Li, G.; Huang, K.; Liu, G.; et al. Machine learning model for predicting physical activity related bleeding risk in Chinese boys with haemophilia A. Thromb. Res. 2023, 232, 43–53. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J.; Li, Z.; Chen, Z.; Liu, G.; Yao, W.; Li, G.; Zhen, Y.; Cheng, X.; Ai, D.; et al. Low-dose immune tolerance induction therapy in severe hemophilia a children in China: Starting earlier resulted in better inhibitor eradication outcomes. Thromb. Res. 2023, 225, 33–38. [Google Scholar] [CrossRef]
- van Velzen, A.S.; Eckhardt, C.L.; Peters, M.; Oldenburg, J.; Cnossen, M.; Liesner, R.; Morfini, M.; Castaman, G.; McRae, S.; INSIGHT Consortium; et al. Product type and the risk of inhibitor development in nonsevere haemophilia A patients: A case-control study. Br. J. Haematol. 2020, 189, 1182–1191. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Skelton, S.; Sansom, C.E.; Gomez, K.; Moss, D.S.; Hart, D.P. A large-scale computational study of inhibitor risk in non-severe haemophilia A. Br. J. Haematol. 2015, 168, 413–420. [Google Scholar] [CrossRef]
- Janssen, A.; Leebeek, F.W.G.; Cnossen, M.H.; Mathôt RAAOPTI-CLOT Study Group and SYMPHONY Consortium. Deep compartment models: A deep learning approach for the reliable prediction of time-series data in pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 934–945. [Google Scholar] [CrossRef]
- Janssen, A.; Hoogendoorn, M.; Cnossen, M.H.; Mathôt RAAOPTI-CLOT Study Group and SYMPHONY Consortium. Application of SHAP values for inferring the optimal functional form of covariates in pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, A.S.; Eckhardt, C.L.; Peters, M.; Leebeek, F.W.G.; Escuriola-Ettingshausen, C.; Hermans, C.; Fijnvandraat, K. Intensity of factor VIII treatment and the development of inhibitors in non-severe hemophilia A patients: Results of the INSIGHT case-control study. J. Thromb. Haemost. 2017, 15, 1422–1429. [Google Scholar] [CrossRef]
- Chowdary, P.; Hampton, K.; Jiménez-Yuste, V.; Young, G.; El Fegoun, S.B.; Cooper, A.; Scalfaro, E.; Tiede, A. Predictive modeling identifies total bleeds at 12-weeks Postswitch to N8-GP prophylaxis as a predictor of treatment response. Thromb. Haemost. 2022, 122, 913–925. [Google Scholar] [CrossRef]
- Cuesta-Barriuso, R.; López-Pina, J.A.; Nieto-Munuera, J.; Sagarra-Valls, G.; Panisello-Royo, J.M.; Torres-Ortuño, A. Effectiveness of the Medtep Hemophilia online platform for adherence to prophylactic treatment in haemophilia patients: Results from a 1-year observational study. Haemophilia 2018, 24, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Banchev, A.; Goldmann, G.; Marquardt, N.; Klein, C.; Horneff, S.; Langenkamp, R. Impact of telemedicine tools on record keeping and compliance in haemophilia care. Hamostaseologie 2019, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mondorf, W.; Eichler, H.; Fischer, R.; Holstein, K.; Klamroth, R.; Nimtz-Talaska, A. Smart Medication™, an electronic diary for surveillance of haemophilia home care and optimization of resource distribution. Hamostaseologie 2019, 39, 339–346. [Google Scholar] [CrossRef]
- Russell, S.; Whitehart, S.; Mason, J.; Window, P. Does the method of telehealth delivery affect the physiotherapy management of adults with bleeding disorders? A comparison of audioconferencing and videoconferencing. Haemophilia 2023, 29, 1589–1596. [Google Scholar] [CrossRef]
- Tiede, A.; Bonanad, S.; Santamaria, A.; Goldmann, G.; Canaro, M.; Palomero, A.; Frade, L.J.G.; Megias-Vericat, J.E.; Martinez, F.; Candel, F.G.; et al. Quality of electronic treatment records and adherence to prophylaxis in haemophilia and von Willebrand disease: Systematic assessments from an electronic diary. Haemophilia 2020, 26, 999–1008. [Google Scholar] [CrossRef]
- Jacobson, K.; Hooke, M.C. Telehealth videoconferencing for children with hemophilia and their families: A clinical project. J. Pediatr. Oncol. Nurs. 2016, 33, 282–288. [Google Scholar] [CrossRef]
- Germini, F.; Borg Debono, V.; Page, D.; Zuk, V.; Kucher, A.; Cotoi, C.; Hobson, N.; Sevestre, M.; Skinner, M.W.; PROBE Investigators. User-centered development and testing of the online patientreported outcomes, burdens, and experiences (PROBE) survey and the myPROBE app and integration with the Canadian bleeding disorder registry: Mixed methods study. JMIR Hum. Factors 2022, 9, e30797. [Google Scholar] [CrossRef]
- Babington-Ashaye, A.; de Moerloose, P.; Diop, S.; Geissbuhler, A. Design, development and usability of an educational AI chatbot for people with haemophilia in Senegal. Haemophilia 2023, 29, 1063–1073. [Google Scholar] [CrossRef]
- Cruz-Montecinos, C.; Maas, H.; Cerda, M.; Pérez-Alenda, S. Altered neural control of gait and its association with pain and joint impairment in adults with haemophilic arthropathy: Clinical and methodological implications. Haemophilia 2022, 28, 497–504. [Google Scholar] [CrossRef]
- Kahl, F.; Kapsecker, M.; Nissen, L.; Bresser, L.; Heinemann, M.; Reimer, L.M.; Jonas, S.M. Digital Technologies in Hereditary Coagulation Disorders: A Systematic Review. Hamostaseologie 2024, 44, 446–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rawal, A.; Kidchob, C.; Ou, J.; Sauna, Z.E. Application of machine learning approaches for predicting hemophilia A severity. J. Thromb. Haemost. 2024, 22, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Soldà, G.; Asselta, R. Applying artificial intelligence to uncover the genetic landscape of coagulation factors. J. Thromb. Haemost. 2025, 23, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.J.S.; Rios, R.; Nogueira, T.; Mello, R.F. Protein residue network analysis reveals fundamental properties of the human coagulation factor VIII. Sci. Rep. 2021, 11, 12625. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.J.S.; Rios, R.; Nogueira, T.; Mello, R.F. Prediction of hemophilia A severity using a small-input machine-learning framework. NPJ Syst. Biol. Appl. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, M.V.; Nogueira, T.; Rios, R.A.; Lopes, T.J.S. A graph-based machine learning framework identifies critical properties of FVIII that lead to hemophilia A. Front. Bioinform. 2023, 3, 1152039. [Google Scholar] [CrossRef]
- Rawal, A.; Kidchob, C.; Ou, J.; Yogurtcu, O.N.; Yang, H.; Sauna, Z.E. A machine learning approach for identifying variables associated with risk of developing neutralizing antidrug antibodies to factor VIII. Heliyon 2023, 9, e16331. [Google Scholar] [CrossRef]
- Lopes, T.J.S.; Nogueira, T.; Rios, R. A machine learning framework predicts the clinical severity of hemophilia B caused by point-mutations. Front. Bioinform. 2022, 2, 912112. [Google Scholar] [CrossRef]
- Ferreira-Martins, A.J.; Castaldoni, R.; Alencar, B.M.; Ferreira, M.V.; Nogueira, T.; Rios, R.A.; Lopes, T.J.S. Full-scale network analysis reveals properties of the FV protein structure organization. Sci. Rep. 2023, 13, 9546. [Google Scholar] [CrossRef]
- Nagao, A.; Inagaki, Y.; Nogami, K.; Yamasaki, N.; Iwasaki, F.; Liu, Y.; Murakami, Y.; Ito, T.; Takedani, H. Artificial intelligence-assisted ultrasound imaging in hemophilia: Research, development, and evaluation of hemarthrosis and synovitis detection. Res. Pract. Thromb. Haemost. 2024, 8, 102439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. The current role of artificial intelligence in hemophilia. Expert Rev. Hematol. 2022, 15, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, C.; Rogers, B.; Chandler, M.; Santos, J. Application of Artificial Intelligence and Machine Learning Was Not Able to Reliably Predict Poor Outcomes in People with Hemophilia. Cureus 2024, 16, e66810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; See, K.C.; Ngiam, K.Y.; Celi, L.A.; Sun, X.; Feng, M. Reinforcement Learning for Clinical Decision Support in Critical Care: Comprehensive Review. J. Med. Internet Res. 2020, 22, e18477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcedo Andrade, P.E.; Mannucci, P.M.; Kessler, C.M. Emicizumab: The hemophilia A game-changer. Haematologica 2024, 109, 1334–1347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knoebl, P.; Thaler, J.; Jilma, P.; Quehenberger, P.; Gleixner, K.; Sperr, W.R. Emicizumab for the treatment of acquired hemophilia A. Blood 2021, 137, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Zanon, E.; Mannucci, P.M.; Peyvandi, F. Emicizumab in acquired hemophilia A: Pros and cons of a new approach to the prevention and treatment of bleeding. Blood Transfus. 2023, 21, 549–556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N. Engl. J. Med. 2018, 379, 811–822. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Monahan, P.E.; Eyster, M.E.; Sullivan, S.K.; Ragni, M.V.; Croteau, S.E.; Rasko, J.E.J.; Recht, M.; Samelson-Jones, B.J.; MacDougall, A.; et al. Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A. N. Engl. J. Med. 2021, 385, 1961–1973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tandon, A.; Cobb, B.; Centra, J.; Izmailova, E.; Manyakov, N.V.; McClenahan, S.; Patel, S.; Sezgin, E.; Vairavan, S.; Vrijens, B.; et al. Human Factors, Human-Centered Design, and Usability of Sensor-Based Digital Health Technologies: Scoping Review. J. Med. Internet Res. 2024, 26, e57628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz-Montecinos, C.; Rivera-Lillo, G.; Núñez-Cortés, R.; Contreras-Sepulveda, F.; Tapia, C. How can artificial intelligence help rehabilitation programs for people with hemophilia in the coming decades? Eur. J. Haematol. 2023, 111, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Canclini, M.; Saviolo-Negrin, N.; Zanon, E.; Bertoletti, R.; Girolami, A.; Pagnan, A. Psychological aspects and coping in haemophilic patients: A case-control study. Haemophilia 2003, 9, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Iannone, M.; Pennick, L.; Tom, A.; Cui, H.; Gilbert, M.; Weihs, K.; Stopeck, A.T. Prevalence of depression in adults with haemophilia. Haemophilia 2012, 18, 868–874. [Google Scholar] [CrossRef]
- Tran, D.Q.; Barry, V.; Antun, A.; Ribeiro, M.; Stein, S.; Kempton, C.L. Physician trust and depression influence adherence to factor replacement: A single-centre cross-sectional study. Haemophilia 2017, 23, 98–104. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Powers, B.; Vogeli, C.; Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019, 366, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Vlahou, A.; Hallinan, D.; Apweiler, R.; Argiles, A.; Beige, J.; Benigni, A.; Bischoff, R.; Black, P.C.; Boehm, F.; Céraline, J.; et al. Data Sharing Under the General Data Protection Regulation: Time to Harmonize Law and Research Ethics? Hypertension 2021, 77, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez Llamas, J.; Vranckaert, K.; Preuveneers, D.; Joosen, W. Balancing Security and Privacy: Web Bot Detection, Privacy Challenges, and Regulatory Compliance under the GDPR and AI Act. Open Res. Eur. 2025, 5, 76. [Google Scholar] [PubMed] [PubMed Central]
- Sung, J. Artificial intelligence in medicine: Ethical, social and legal perspectives. Ann. Acad. Med. Singap. 2023, 52, 695–699. [Google Scholar] [CrossRef] [PubMed]
| Application | AI Technology Used | Clinical Impact | Status of Implementation | |
|---|---|---|---|---|
| Early Diagnosis | Supervised machine learning (e.g., classification algorithms) for genotype–phenotype correlation and severity prediction | Timely identification of hemophilia A and B severity, including in underdiagnosed female carriers | Still in research | [94,97] |
| Risk Prediction | Predictive analytics, machine learning models | Identification of bleeding risks and inhibitor development | Still in research | [76] |
| Imaging Diagnostics | Convolutional neural networks (CNNs) for AI-assisted ultrasound | Enhanced detection of joint damage and synovitis | Still in research | [102] |
| Prognostic Modeling | Supervised learning, Reinforcement learning | Prediction of disease progression and joint outcomes | Still in research | [66,67,68,69,70,106] |
| Treatment Optimization | Pharmacokinetics modeling, data analysis | Precision in therapy adjustments and dosage planning | Still in research | [93,112] |
| Monitoring and Long-Term Care | Wearable sensors, digital health tools | Improved patient monitoring and rehabilitation | Still in research | [114,115,116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, L.; Pagana, A.G.; Minciullo, P.L.; Fazio, M.; Stagno, F.; Gangemi, S.; Genovese, S.; Allegra, A. Artificial Intelligence in the Management of Hereditary and Acquired Hemophilia: From Genomics to Treatment Optimization. Int. J. Mol. Sci. 2025, 26, 6100. https://doi.org/10.3390/ijms26136100
Giordano L, Pagana AG, Minciullo PL, Fazio M, Stagno F, Gangemi S, Genovese S, Allegra A. Artificial Intelligence in the Management of Hereditary and Acquired Hemophilia: From Genomics to Treatment Optimization. International Journal of Molecular Sciences. 2025; 26(13):6100. https://doi.org/10.3390/ijms26136100
Chicago/Turabian StyleGiordano, Laura, Antonio Gaetano Pagana, Paola Lucia Minciullo, Manlio Fazio, Fabio Stagno, Sebastiano Gangemi, Sara Genovese, and Alessandro Allegra. 2025. "Artificial Intelligence in the Management of Hereditary and Acquired Hemophilia: From Genomics to Treatment Optimization" International Journal of Molecular Sciences 26, no. 13: 6100. https://doi.org/10.3390/ijms26136100
APA StyleGiordano, L., Pagana, A. G., Minciullo, P. L., Fazio, M., Stagno, F., Gangemi, S., Genovese, S., & Allegra, A. (2025). Artificial Intelligence in the Management of Hereditary and Acquired Hemophilia: From Genomics to Treatment Optimization. International Journal of Molecular Sciences, 26(13), 6100. https://doi.org/10.3390/ijms26136100






