Balancing Immunity: GSK-3’s Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses
Abstract
1. Introduction
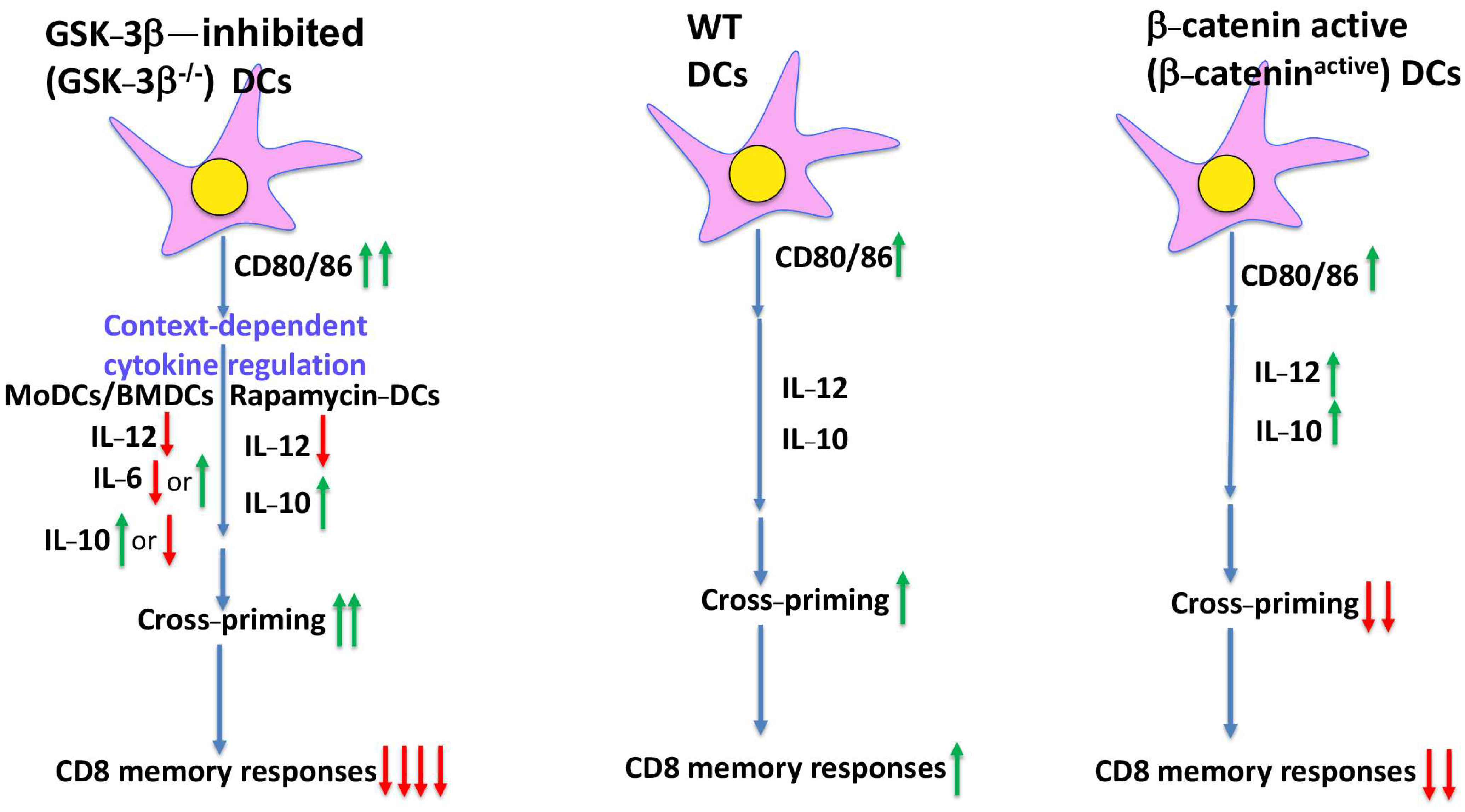
2. GSK-3 in DCs, a β-Catenin-Related Affair
3. Unexpected Dual Roles of GSK-3 in DC Vaccine-Induced CD8 T-Cell Responses Independent of β-Catenin
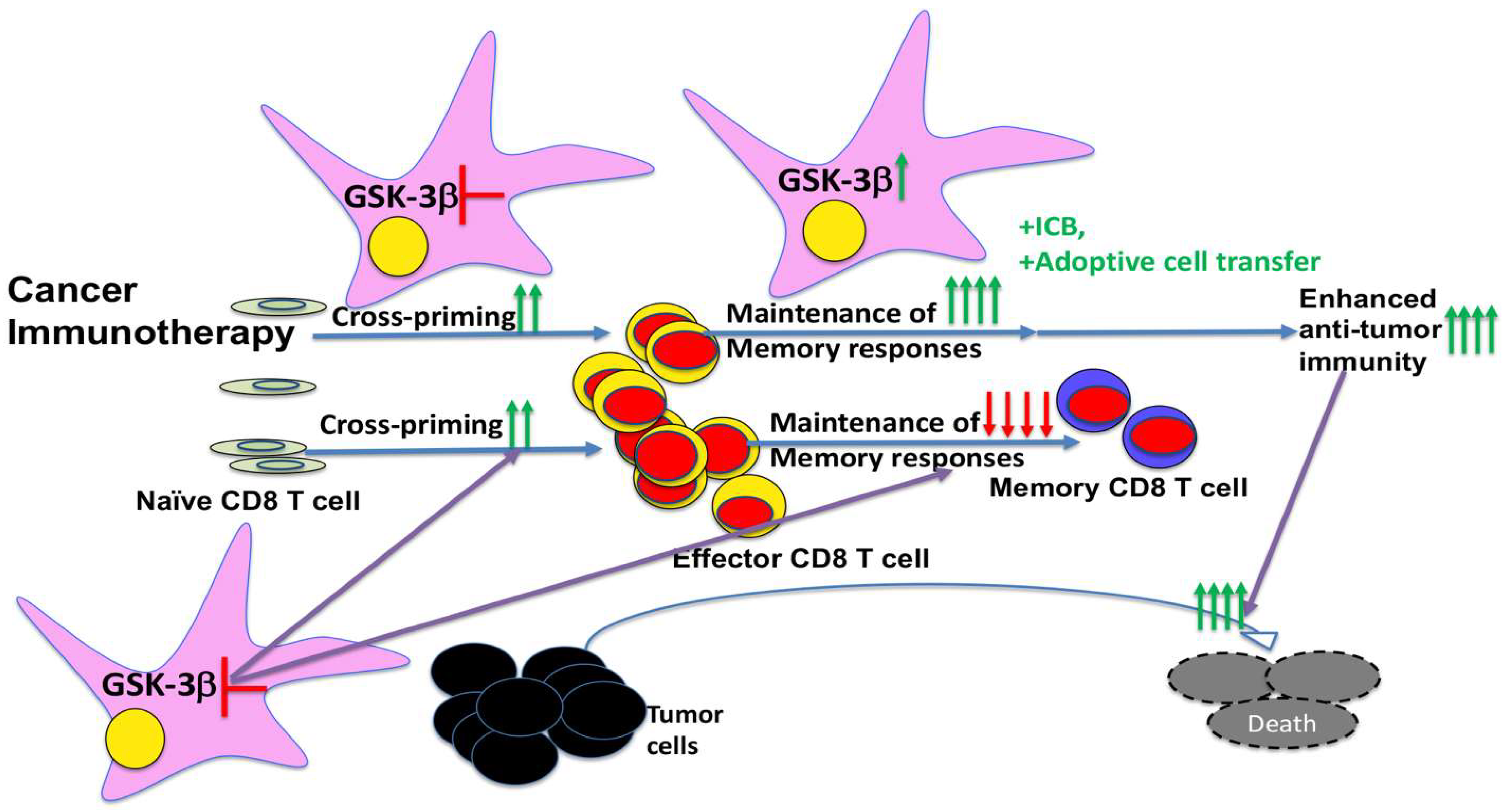
4. Implications for Therapeutic Targeting of GSK-3 in Cancer Immunotherapies
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCs | dendritic cells |
| APCs | antigen-presenting cells |
| ICB | immune checkpoint blockade |
| MoDCs | monocyte-derived DCs |
| BMDCs | bone marrow-derived DCs |
| GSK-3 | glycogen synthase kinase-3 |
| IDO | indoleamine 2,3-dioxygenase |
| LPS | lipopolysaccharide |
References
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288. [Google Scholar] [CrossRef]
- Bevan, M.J. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 1976, 117, 2233–2238. [Google Scholar] [CrossRef]
- Kurts, C.; Robinson, B.W.; Knolle, P.A. Cross-priming in health and disease. Nat. Rev. Immunol. 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.M.; Ohlfest, J.R. Increasing the efficacy of tumor cell vaccines by enhancing cross priming. Cancer Lett. 2012, 325, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fabian, K.L.; Taylor, J.L.; Storkus, W.J. Therapeutic Use of Dendritic Cells to Promote the Extranodal Priming of Anti-Tumor Immunity. Front. Immunol. 2013, 4, 388. [Google Scholar] [CrossRef]
- Fuertes, M.B.; Woo, S.R.; Burnett, B.; Fu, Y.X.; Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013, 34, 67–73. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Dendritic cells in cancer immunology. Cell Mol. Immunol. 2022, 19, 3–13. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, A.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Ma, T.; Zhou, L.; Mi, Q.S.; Jiang, A. Dendritic Cell-Based Vaccines Against Cancer: Challenges, Advances and Future Opportunities. Immunol. Investig. 2022, 51, 2133–2158. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, P.; Brocker, T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J. Immunol. 2003, 170, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Yewdall, A.W.; Drutman, S.B.; Jinwala, F.; Bahjat, K.S.; Bhardwaj, N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE 2010, 5, e11144. [Google Scholar] [CrossRef]
- Ferris, S.T.; Ohara, R.A.; Ou, F.; Wu, R.; Huang, X.; Kim, S.; Chen, J.; Liu, T.T.; Schreiber, R.D.; Murphy, T.L.; et al. cDC1 Vaccines Drive Tumor Rejection by Direct Presentation Independently of Host cDC1. Cancer Immunol. Res. 2022, 10, 920–931. [Google Scholar] [CrossRef]
- Veglia, F.; Gabrilovich, D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017, 45, 43–51. [Google Scholar] [CrossRef]
- Chrisikos, T.T.; Zhou, Y.; Slone, N.; Babcock, R.; Watowich, S.S.; Li, H.S. Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol. Immunol. 2019, 110, 24–39. [Google Scholar] [CrossRef]
- Sanchez-Paulete, A.R.; Teijeira, A.; Cueto, F.J.; Garasa, S.; Perez-Gracia, J.L.; Sanchez-Arraez, A.; Sancho, D.; Melero, I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol. 2017, 28, xii44–xii55. [Google Scholar] [CrossRef]
- Bandola-Simon, J.; Roche, P.A. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol. 2018, 113, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Liang, X.; Fu, C.; Cui, W.; Ober-Blobaum, J.L.; Zahner, S.P.; Shrikant, P.A.; Clausen, B.E.; Flavell, R.A.; Mellman, I.; Jiang, A. beta-catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8(+) T cells. J. Leukoc. Biol. 2014, 95, 179–190. [Google Scholar] [CrossRef]
- Hong, Y.; Manoharan, I.; Suryawanshi, A.; Majumdar, T.; Angus-Hill, M.L.; Koni, P.A.; Manicassamy, B.; Mellor, A.L.; Munn, D.H.; Manicassamy, S. beta-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 2015, 75, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Holtzhausen, A.; Zhao, F.; Evans, K.S.; Tsutsui, M.; Orabona, C.; Tyler, D.S.; Hanks, B.A. Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol. Res. 2015, 3, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xiao, C.; Evans, K.S.; Theivanthiran, T.; DeVito, N.; Holtzhausen, A.; Liu, J.; Liu, X.; Boczkowski, D.; Nair, S.; et al. Paracrine Wnt5a-beta-Catenin Signaling Triggers a Metabolic Program that Drives Dendritic Cell Tolerization. Immunity 2018, 48, 147–160.e147. [Google Scholar] [CrossRef]
- Jiang, A.; Bloom, O.; Ono, S.; Cui, W.; Unternaehrer, J.; Jiang, S.; Whitney, J.A.; Connolly, J.; Banchereau, J.; Mellman, I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 2007, 27, 610–624. [Google Scholar] [CrossRef]
- Manicassamy, S.; Reizis, B.; Ravindran, R.; Nakaya, H.; Salazar-Gonzalez, R.M.; Wang, Y.C.; Pulendran, B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010, 329, 849–853. [Google Scholar] [CrossRef]
- Fu, C.; Liang, X.; Cui, W.; Ober-Blobaum, J.L.; Vazzana, J.; Shrikant, P.A.; Lee, K.P.; Clausen, B.E.; Mellman, I.; Jiang, A. beta-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc. Natl. Acad. Sci. USA 2015, 112, 2823–2828. [Google Scholar] [CrossRef]
- Patel, P.; Woodgett, J.R. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr. Top. Dev. Biol. 2017, 123, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Augello, G.; Emma, M.R.; Cusimano, A.; Azzolina, A.; Montalto, G.; McCubrey, J.A.; Cervello, M. The Role of GSK-3 in Cancer Immunotherapy: GSK-3 Inhibitors as a New Frontier in Cancer Treatment. Cells 2020, 9, 1427. [Google Scholar] [CrossRef]
- Crespo, A.R.; Luna, S.G.; Moes, B.; Rodriguez, A.; Rudd, C.E. The many faceted role of glycogen synthase kinase-3 (GSK-3) in T cells and cancer immunotherapy. J. Cancer Biol. 2024, 5, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, E.; Conzelmann, M.; Maraskovsky, E.; Hess, M.; Kirsch, M.; Giese, T.; Ho, A.D.; Zoller, M.; Dreger, P.; Luft, T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood 2007, 109, 1584–1592. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Gu, Z.; Garcia, C.A.; Liang, R.; Alard, P.; Beurel, E.; Jope, R.S.; Greenway, T.; Martin, M. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-beta-signaling pathways regulates the innate inflammatory response. J. Immunol. 2011, 186, 5217–5226. [Google Scholar] [CrossRef]
- Alessandrini, A.; De Haseth, S.; Fray, M.; Miyajima, M.; Colvin, R.B.; Williams, W.W.; Benedict Cosimi, A.; Benichou, G. Dendritic cell maturation occurs through the inhibition of GSK-3beta. Cell Immunol. 2011, 270, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Yanagawa, Y.; Iwabuchi, K.; Nonomura, K.; Onoe, K. Glycogen synthase kinase 3 activity during development of bone marrow-derived dendritic cells (DCs) essential for the DC function to induce T helper 2 polarization. Immunology 2007, 122, 189–198. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Cardinal, J.; Macedo, C.; Rosborough, B.R.; Sumpter, T.L.; Geller, D.A.; Metes, D.; Thomson, A.W. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 2010, 115, 4758–4769. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Garcia, C.A.; Tang, Y.; Benakanakere, M.R.; Greenway, T.; Alard, P.; Kinane, D.F.; Martin, M. The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J. Immunol. 2011, 186, 675–684. [Google Scholar] [CrossRef]
- Noh, K.T.; Son, K.H.; Jung, I.D.; Kang, T.H.; Choi, C.H.; Park, Y.M. Glycogen Synthase Kinase-3beta (GSK-3beta) Inhibition Enhances Dendritic Cell-based Cancer Vaccine Potency via Suppression of Interferon-gamma-induced Indoleamine 2,3-Dioxygenase Expression. J. Biol. Chem. 2015, 290, 12394–12402. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Ma, T.; Yin, C.; Zhou, L.; Clausen, B.E.; Mi, Q.S.; Jiang, A. GSK-3beta in Dendritic Cells Exerts Opposite Functions in Regulating Cross-Priming and Memory CD8 T Cell Responses Independent of beta-Catenin. Vaccines 2024, 12, 1037. [Google Scholar] [CrossRef]
- Liang, J.; Yu, M.; Li, Y.; Zhao, L.; Wei, Q. Glycogen synthase kinase-3: A potential immunotherapeutic target in tumor microenvironment. Biomed. Pharmacother. 2024, 173, 116377. [Google Scholar] [CrossRef]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bienz, M. Inhibition of GSK3 by Wnt signalling–two contrasting models. J. Cell Sci. 2011, 124, 3537–3544. [Google Scholar] [CrossRef]
- Escribano, C.; Delgado-Martin, C.; Rodriguez-Fernandez, J.L. CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J. Immunol. 2009, 183, 6282–6295. [Google Scholar] [CrossRef]
- Larabee, J.L.; Maldonado-Arocho, F.J.; Pacheco, S.; France, B.; DeGiusti, K.; Shakir, S.M.; Bradley, K.A.; Ballard, J.D. Glycogen synthase kinase 3 activation is important for anthrax edema toxin-induced dendritic cell maturation and anthrax toxin receptor 2 expression in macrophages. Infect. Immun. 2011, 79, 3302–3308. [Google Scholar] [CrossRef]
- Spinnler, K.; Mezger, M.; Steffens, M.; Sennefelder, H.; Kurzai, O.; Einsele, H.; Loeffler, J. Role of glycogen synthase kinase 3 (GSK-3) in innate immune response of human immature dendritic cells to Aspergillus fumigatus. Med. Mycol. 2010, 48, 589–597. [Google Scholar] [CrossRef]
- Qian, C.; Qian, L.; Yu, Y.; An, H.; Guo, Z.; Han, Y.; Chen, Y.; Bai, Y.; Wang, Q.; Cao, X. Fas signal promotes the immunosuppressive function of regulatory dendritic cells via ERK/beta-catenin pathway. J. Biol. Chem. 2013, 288, 27825–27835. [Google Scholar] [CrossRef]
- Lopez Gonzalez, M.; Oosterhoff, D.; Lindenberg, J.J.; Milenova, I.; Lougheed, S.M.; Martianez, T.; Dekker, H.; Quixabeira, D.C.A.; Hangalapura, B.; Joore, J.; et al. Constitutively active GSK3beta as a means to bolster dendritic cell functionality in the face of tumour-mediated immune suppression. Oncoimmunology 2019, 8, e1631119. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Ma, T.; Yin, C.; Zhou, L.; Clausen, B.E.; Mi, Q.S.; Jiang, A. beta-Catenin in Dendritic Cells Negatively Regulates CD8 T Cell Immune Responses through the Immune Checkpoint Molecule Tim-3. Vaccines 2024, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Nagai, S.; Kondo, S.; Mizuno, S.; Nakamura, K.; Tanabe, M.; Takeuchi, T.; Matsuda, S.; Koyasu, S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 2008, 112, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Lee, Y.L.; Yang, Y.Y.; Shih, N.Y.; Ho, C.C.; Wu, Y.C.; Huang, T.S.; Huang, M.C.; Liu, H.C.; Shen, W.W.; et al. Modulation of the development of human monocyte-derived dendritic cells by lithium chloride. J. Cell Physiol. 2011, 226, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.D.; Wang, J.; Yuan, T.L.; Li, Y.H.; Yang, H.; Liu, Y.; Zhao, Y.; Herrmann, M. Interactions between canonical Wnt signaling pathway and MAPK pathway regulate differentiation, maturation and function of dendritic cells. Cell Immunol. 2016, 310, 170–177. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. Differential regulation of STAT family members by glycogen synthase kinase-3. J. Biol. Chem. 2008, 283, 21934–21944. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Tsai, C.C.; Kai, J.I.; Huang, W.C.; Wang, C.Y.; Wang, Y.; Chen, C.L.; Fang, Y.T.; Lin, Y.S.; Anderson, R.; Chen, S.H.; et al. Glycogen synthase kinase-3beta facilitates IFN-gamma-induced STAT1 activation by regulating Src homology-2 domain-containing phosphatase 2. J. Immunol. 2009, 183, 856–864. [Google Scholar] [CrossRef]
- Rudd, C.E.; Chanthong, K.; Taylor, A. Small Molecule Inhibition of GSK-3 Specifically Inhibits the Transcription of Inhibitory Co-receptor LAG-3 for Enhanced Anti-tumor Immunity. Cell Rep. 2020, 30, 2075–2082.e2074. [Google Scholar] [CrossRef]
- Taylor, A.; Rudd, C.E. Small Molecule Inhibition of Glycogen Synthase Kinase-3 in Cancer Immunotherapy. Adv. Exp. Med. Biol. 2019, 1164, 225–233. [Google Scholar] [CrossRef]
- Issa, M.E.; Rudd, C.E. Glycogen Synthase Kinase-3 (GSK-3) Regulation of Inhibitory Coreceptor Expression in T-cell Immunity. J. Cell Immunol. 2021, 3, 336–342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Ma, T.; Zhou, L.; Mi, Q.-S.; Jiang, A. Balancing Immunity: GSK-3’s Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses. Int. J. Mol. Sci. 2025, 26, 6078. https://doi.org/10.3390/ijms26136078
Fu C, Ma T, Zhou L, Mi Q-S, Jiang A. Balancing Immunity: GSK-3’s Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses. International Journal of Molecular Sciences. 2025; 26(13):6078. https://doi.org/10.3390/ijms26136078
Chicago/Turabian StyleFu, Chunmei, Tianle Ma, Li Zhou, Qing-Sheng Mi, and Aimin Jiang. 2025. "Balancing Immunity: GSK-3’s Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses" International Journal of Molecular Sciences 26, no. 13: 6078. https://doi.org/10.3390/ijms26136078
APA StyleFu, C., Ma, T., Zhou, L., Mi, Q.-S., & Jiang, A. (2025). Balancing Immunity: GSK-3’s Divergent Roles in Dendritic Cell-Mediated T-Cell Priming and Memory Responses. International Journal of Molecular Sciences, 26(13), 6078. https://doi.org/10.3390/ijms26136078





