The Role of Microbiota in the Pathogenesis of Bullous Pemphigoid and Pemphigus Vulgaris: Evidence, Controversies, and Perspectives
Abstract
1. Introduction
2. The Gut Microbiota: General Features
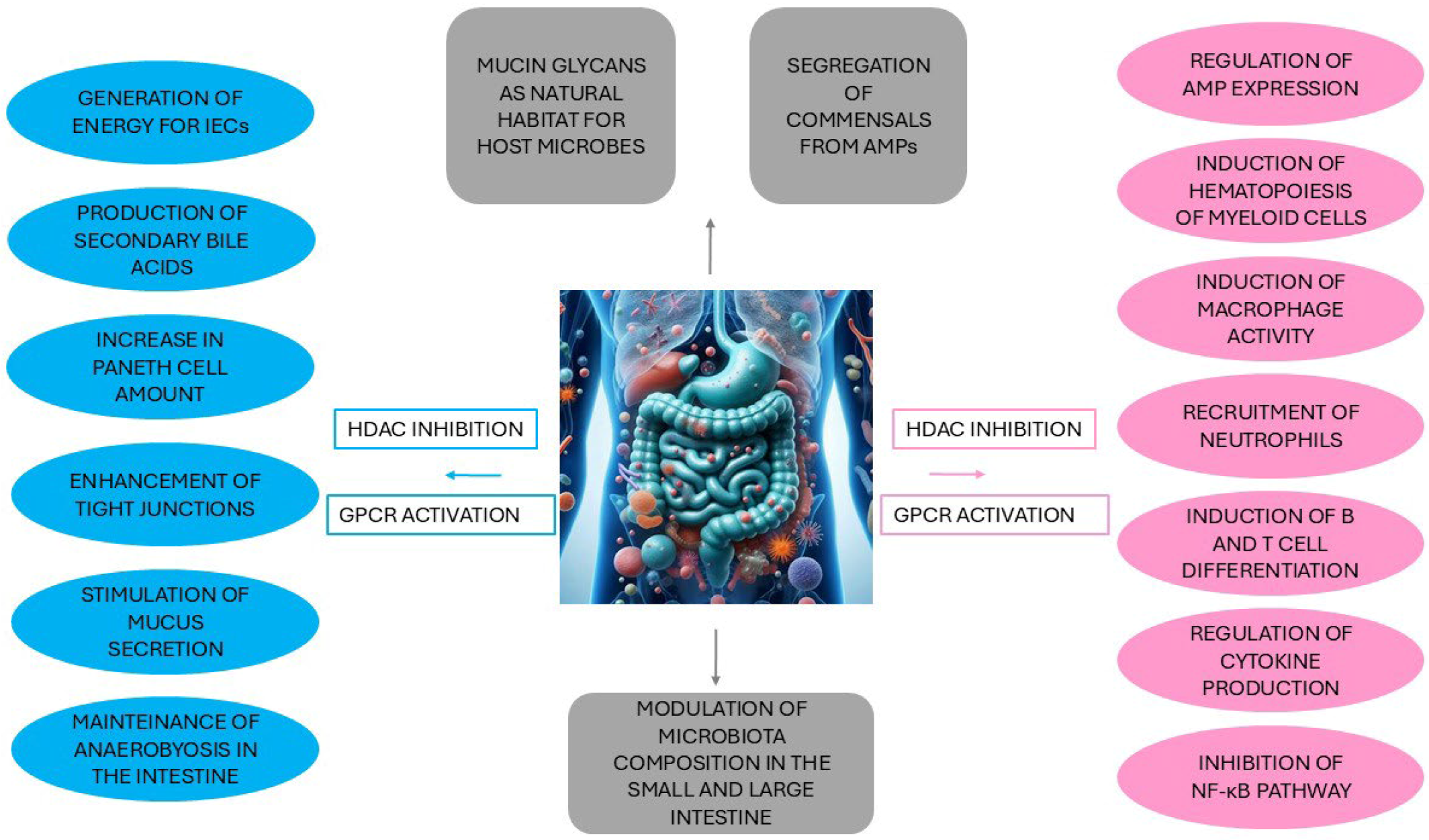
2.1. The Crosstalk Between the Gut Microbiota and Intestinal Epithelial Cells
2.2. Interaction Between the Gut Microbiota and Immunity
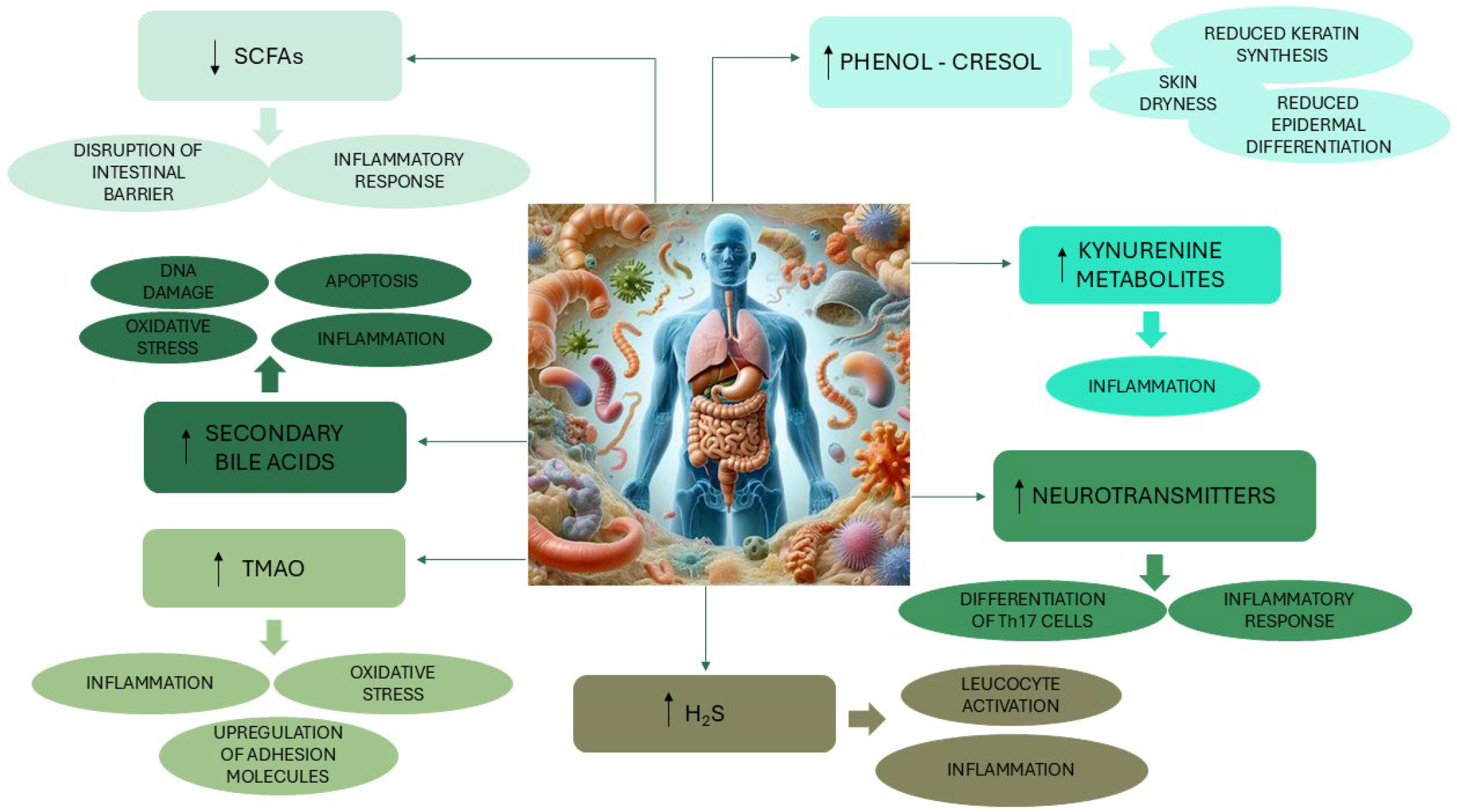
3. The Gut–Skin Axis
4. The Gut Microbiota and the Relationship with Autoimmune Bullous Diseases
4.1. The Association of the Gut Microbiota with Bullous Pemphigoid
4.2. The Association of the Gut Microbiota with Pemphigus Vulgaris
5. The Oral Microbiota and the Relationship with the Health-Disease Status
The Association of the Oral Microbiota with Pemphigus Vulgaris
6. The Skin Microbiota and the Relationship with the Health–Disease Status
The Association of the Skin Microbiota with Bullous Pemphigoid and Pemphigus Vulgaris
7. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial peptide |
| BA | Bile acid |
| BP | Bullous pemphigoid |
| Dsg | Desmoglein |
| F/B | Firmicutes/Bacteroidetes ratio |
| FMT | Fecal microbiota transplantation |
| GABA | γ-aminobutyric acid |
| GAD | Glutamate decarboxylase |
| GALT | Gut-associated lymphoid tissues |
| GPCR | G protein-coupled receptor |
| H2S | Hydrogen sulfide |
| IEC | Intestinal epithelium cells |
| IFN-γ | Interferon gamma |
| Ig | Immunoglobulin |
| IL | Interleukin |
| LCA | Lithocholic acid |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOD | Nucleotide-binding oligomerization domain |
| PAMP | Pathogen-associated molecular pattern |
| rRNA | ribosomal RNA |
| SCFAs | Short-chain fatty acids |
| Th | T helper |
| TLR | Toll-like receptor |
| TMAO | Trimethylamine-N-oxide |
| TNF-α | Tumor necrosis factor-alpha |
| Treg | Regulatory T cells |
References
- Kridin, K. Pemphigus group: Overview, epidemiology, mortality, and comorbidities. Immunol. Res. 2018, 66, 255–270. [Google Scholar] [CrossRef]
- Di Lernia, V.; Casanova, D.M.; Goldust, M.; Ricci, C. Pemphigus Vulgaris and Bullous Pemphigoid: Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020050. [Google Scholar] [CrossRef] [PubMed]
- Egami, S.; Yamagami, J.; Amagai, M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J. Allergy Clin. Immunol. 2020, 145, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Lo Schiavo, A.; Ruocco, E.; Brancaccio, G.; Caccavale, S.; Ruocco, V.; Wolf, R. Bullous pemphigoid: Etiology, pathogenesis, and inducing factors: Facts and controversies. Clin. Dermatol. 2013, 31, 391–399. [Google Scholar] [CrossRef]
- Miodovnik, M.; Künstner, A.; Langan, E.A.; Zillikens, D.; Gläser, R.; Sprecher, E.; Baines, J.F.; Schmidt, E.; Ibrahim, S.M. A distinct cutaneous microbiota profile in autoimmune bullous disease patients. Exp. Dermatol. 2017, 26, 1221–1227. [Google Scholar] [CrossRef]
- Lin, L.; Hwang, B.J.; Culton, D.A.; Li, N.; Burette, S.; Koller, B.H.; Messingham, K.A.; Fairley, J.A.; Lee, J.J.; Hall, R.P.; et al. Eosinophils Mediate Tissue Injury in the Autoimmune Skin Disease Bullous Pemphigoid. J. Investig. Dermatol. 2018, 138, 1032–1043. [Google Scholar] [CrossRef]
- Nätynki, A.; Tuusa, J.; Hervonen, K.; Kaukinen, K.; Lindgren, O.; Huilaja, L.; Kokkonen, N.; Salmi, T.; Tasanen, K. Autoantibodies Against the Immunodominant Bullous Pemphigoid Epitopes Are Rare in Patients With Dermatitis Herpetiformis and Coeliac Disease. Front. Immunol. 2020, 11, 575805. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.N.; Khan, Z.A.; Ali, M.H.; Almas, T.; Khedro, T.; Raj Nagarajan, V. A blistering new era for bullous pemphigoid: A scoping review of current therapies, ongoing clinical trials, and future directions. Ann. Med. Surg. 2021, 70, 102799. [Google Scholar] [CrossRef]
- Persson, M.S.M.; Begum, N.; Grainge, M.J.; Harman, K.E.; Grindlay, D.; Gran, S. The global incidence of bullous pemphigoid: A systematic review and meta-analysis. Br. J. Dermatol. 2022, 186, 414–425. [Google Scholar] [CrossRef]
- Liu, X.; van Beek, N.; Cepic, A.; Andreani, N.A.; Chung, C.J.; Hermes, B.M.; Yilmaz, K.; Benoit, S.; Drenovska, K.; Gerdes, S.; et al. The gut microbiome in bullous pemphigoid: Implications of the gut-skin axis for disease susceptibility. Front. Immunol. 2023, 14, 1212551. [Google Scholar] [CrossRef]
- Cozzani, E.; Marzano, A.V.; Caproni, M.; Feliciani, C.; Calzavara-Pinton, P.; Cutaneous Immunology Group of SIDeMaST. Bullous pemphigoid: Italian guidelines adapted from the EDF/EADV guidelines. Ital. Dermatol. Venereol. 2018, 153, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.S.M.; Harman, K.E.; Vinogradova, Y.; Langan, S.M.; Hippisley-Cox, J.; Thomas, K.S.; Gran, S. Incidence, prevalence and mortality of bullous pemphigoid in England 1998–2017: A population-based cohort study. Br. J. Dermatol. 2021, 184, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Borradori, L.; Van Beek, N.; Feliciani, C.; Tedbirt, B.; Antiga, E.; Bergman, R.; Böckle, B.C.; Caproni, M.; Caux, F.; Chandran, N.S.; et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1689–1704. [Google Scholar] [CrossRef]
- Porro, A.M.; Seque, C.A.; Ferreira, M.C.C.; Enokihara, M.M.S.E.S. Pemphigus vulgaris. An. Bras. Dermatol. 2019, 94, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Schmidt, E. Epidemiology of Pemphigus. JID Innov. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Quintarelli, L.; Coi, A.; Maglie, R.; Corrà, A.; Mariotti, E.B.; Aimo, C.; Ruffo di Calabria, V.; Verdelli, A.; Bianchi, B.; Del Bianco, E.; et al. Clinical Patterns, Survival, Comorbidities, and Treatment Regimens in 149 Patients With Pemphigus in Tuscany (Italy): A 12-Year Hospital-Based Study. Front. Immunol. 2022, 13, 895490. [Google Scholar] [CrossRef]
- Li, S.Z.; Wu, Q.Y.; Fan, Y.; Guo, F.; Hu, X.M.; Zuo, Y.G. Gut Microbiome Dysbiosis in Patients with Pemphigus and Correlation with Pathogenic Autoantibodies. Biomolecules 2024, 14, 880. [Google Scholar] [CrossRef]
- Langan, S.M.; Smeeth, L.; Hubbard, R.; Fleming, K.M.; Smith, C.J.; West, J. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: Population based cohort study. BMJ 2008, 337, a180. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Wang, M. The Global Incidence Rate of Pemphigus Vulgaris: A Systematic Review and Meta-Analysis. Dermatology 2023, 239, 514–522. [Google Scholar] [CrossRef]
- Didona, D.; Maglie, R.; Eming, R.; Hertl, M. Pemphigus: Current and Future Therapeutic Strategies. Front. Immunol. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Šuler Baglama, Š.; Trčko, K. Skin and gut microbiota dysbiosis in autoimmune and inflammatory skin diseases. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, 105–109. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef] [PubMed]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Senchukova, M.A. Microbiota of the gastrointestinal tract: Friend or foe? World J. Gastroenterol. 2023, 29, 19–42. [Google Scholar] [CrossRef]
- Chai, J.; Deng, F.; Li, Y.; Wei, X.; Zhao, J. Editorial: The gut-skin axis: Interaction of gut microbiome and skin diseases. Front. Microbiol. 2024, 15, 1427770. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Gorini, F.; Tonacci, A. Vitamin D: An Essential Nutrient in the Dual Relationship between Autoimmune Thyroid Diseases and Celiac Disease-A Comprehensive Review. Nutrients 2024, 16, 1762. [Google Scholar] [CrossRef]
- Mohammad, S.; Karim, M.R.; Iqbal, S.; Lee, J.H.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.U.; Yang, D.C. Atopic dermatitis: Pathophysiology, microbiota, and metabolome—A comprehensive review. Microbiol. Res. 2024, 281, 127595. [Google Scholar] [CrossRef] [PubMed]
- Peterle, L.; Sanfilippo, S.; Tonacci, A.; Li Pomi, F.; Borgia, F.; Gangemi, S. Common pathogenetic traits of atopic dermatitis and autism spectrum disorders, potential connections and treatments: Trivial Th2 inflammation or much more? Front. Immunol. 2023, 14, 1201989. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Scaglione, G.L.; Fania, L.; De Paolis, E.; De Bonis, M.; Mazzanti, C.; Di Zenzo, G.; Lechiancole, S.; Messinese, S.; Capoluongo, E. Evaluation of cutaneous, oral and intestinal microbiota in patients affected by pemphigus and bullous pemphigoid: A pilot study. Exp. Mol. Pathol. 2020, 112, 104331. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Q.; Fan, Y.; Guo, F.; Li, S.; Zhang, S.; Zuo, Y.G. Identification of gut microbiota dysbiosis in bullous pemphigoid under different disease activity. Exp. Dermatol. 2023, 32, 2149–2159. [Google Scholar] [CrossRef]
- Syromyatnikov, M.; Nesterova, E.; Gladkikh, M.; Smirnova, Y.; Gryaznova, M.; Popov, V. Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms 2022, 10, 1866. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best. Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Ames, N.J.; Ranucci, A.; Moriyama, B.; Wallen, G.R. The Human Microbiome and Understanding the 16S rRNA Gene in Translational Nursing Science. Nurs. Res. 2017, 66, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, P.; Li, R.; Tan, P.; Xie, J.; Zhang, R.; Li, J. Multicenter assessment of microbial community profiling using 16S rRNA gene sequencing and shotgun metagenomic sequencing. J. Adv. Res. 2020, 26, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA gene amplicon sequencing using Japanese gut microbiome data from the V1-V2 and V3-V4 primer sets. BMC Genomics. 2021, 22, 527. [Google Scholar] [CrossRef]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Shi, H.; Li, J.; Zhang, M.; Zhao, J.; Su, X. Comprehensive Assessment of 16S rRNA Gene Amplicon Sequencing for Microbiome Profiling across Multiple Habitats. Microbiol. Spectr. 2023, 11, e0056323. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef]
- Schoenborn, A.A.; von Furstenberg, R.J.; Valsaraj, S.; Hussain, F.S.; Stein, M.; Shanahan, M.T.; Henning, S.J.; Gulati, A.S. The enteric microbiota regulates jejunal Paneth cell number and function without impacting intestinal stem cells. Gut Microbes. 2019, 10, 45–58. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Donato, J., Jr. The role of leptin in health and disease. Temperature (Austin) 2017, 4, 258–291. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Su, C.; Zhao, Q.; Shi, A.; Zhao, F.; Tang, L.; Xu, D.; Xiang, Z.; Wang, Y.; Wang, Y.; et al. Gut Microbiota-Mediated Elevated Production of Secondary Bile Acids in Chronic Unpredictable Mild Stress. Front. Pharmacol. 2022, 13, 837543. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, E.Y.; Shi, H. Tryptophan and Its Metabolite Serotonin Impact Metabolic and Mental Disorders via the Brain–Gut–Microbiome Axis: A Focus on Sex Differences. Cells 2025, 14, 384. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Gubatan, J.; Holman, D.R.; Puntasecca, C.J.; Polevoi, D.; Rubin, S.J.; Rogalla, S. Antimicrobial peptides and the gut microbiome in inflammatory bowel disease. World J. Gastroenterol. 2021, 27, 7402–7422. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, S.; Deng, B.; Tan, C.; Deng, J.; Zhu, G.; Yin, Y.; Ren, W. Implication of G Protein-Coupled Receptor 43 in Intestinal Inflammation: A Mini-Review. Front. Immunol. 2018, 9, 1434. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, Y.; Ye, Z.H.; Chen, L.P.; Xu, J.X.; Han, J.; Xie, L.; Xing, S.; Tian, D.A.; Seidler, U.; et al. Suppression of MyD88 disturbs gut microbiota and activates the NLR pathway and hence fails to ameliorate DSS-induced colitis. Precis. Clin. Med. 2024, 7, pbae013. [Google Scholar] [CrossRef]
- Price, A.E.; Shamardani, K.; Lugo, K.A.; Deguine, J.; Roberts, A.W.; Lee, B.L.; Barton, G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560–575.e6. [Google Scholar] [CrossRef]
- Wang, S.; Charbonnier, L.M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity 2015, 43, 289–303. [Google Scholar] [CrossRef]
- Manshouri, S.; Seif, F.; Kamali, M.; Bahar, M.A.; Mashayekh, A.; Molatefi, R. The interaction of inflammasomes and gut microbiota: Novel therapeutic insights. Cell Commun. Signal. 2024, 22, 209. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef]
- Thye, A.Y.; Bah, Y.R.; Law, J.W.; Tan, L.T.; He, Y.W.; Wong, S.H.; Thurairajasingam, S.; Chan, K.G.; Lee, L.H.; Letchumanan, V. Gut-Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Yan, D.; Issa, N.; Afifi, L.; Jeon, C.; Chang, H.W.; Liao, W. The Role of the Skin and Gut Microbiome in Psoriatic Disease. Curr. Dermatol. Rep. 2017, 6, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Sanchez-Lopez, M.F.; Barrero-Caicedo, P.A.; Olmos-Carval, H.M.; Torres-Medina, A.F.; Alzate-Granados, J.P. Relationship between skin and gut microbiota dysbiosis and inflammatory skin diseases in adult patients: A systematic review. Microbe 2025, 7, 100342. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, M.; Peng, Q.; Sha, K.; Liu, T.; Xia, J.; Xie, H.; Li, J.; Xu, S.; Deng, Z. Lithocholic acid promotes rosacea-like skin inflammation via G protein-coupled bile acid receptor 1. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166563. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.; Roumain, M.; Picalausa, C.; Thibaut, M.M.; Clerbaux, L.A.; Tailleux, A.; Staels, B.; Muccioli, G.G.; Bindels, L.B.; Leclercq, I.A. A gut microbiota-independent mechanism shapes the bile acid pool in mice with MASH. JHEP Rep. 2024, 6, 101148. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; He, J.; He, X. The role of bile acid receptor TGR5 in regulating inflammatory signalling. Scand. J. Immunol. 2024, 99, e13361. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C.; et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef]
- Al-Rubaye, H.; Perfetti, G.; Kaski, J.C. The Role of Microbiota in Cardiovascular Risk: Focus on Trimethylamine Oxide. Curr. Probl. Cardiol. 2019, 44, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; de Alteriis, G.; Maisto, M.; Donnarumma, M.; Tenore, G.C.; Colao, A.; Fabbrocini, G.; Savastano, S. Association of Trimethylamine N-Oxide (TMAO) with the Clinical Severity of Hidradenitis Suppurativa (Acne Inversa). Nutrients 2021, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Stepaniuk, A.; Baran, A.; Flisiak, I. Kynurenine Pathway in Psoriasis-a Promising Link? Dermatol. Ther. 2023, 13, 1617–1627. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Talamantes, A.K.; Gómez-González, B.A.; Uriarte-Mayorga, D.F.; Martínez-Guzman, M.A.; Wheber-Hidalgo, K.A.; Alvarado-Navarro, A. Neurotransmitters, neuropeptides and their receptors interact with immune response in healthy and psoriatic skin. Neuropeptides 2020, 79, 102004. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A.; Nedoszytko, B.; Żmijewski, M.A.; Cubała, W.J.; Slominski, A.T. The Brain-Skin Axis in Psoriasis-Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int. J. Mol. Sci. 2022, 23, 669. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masuoka, N.; Kano, M.; Iizuka, R. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes. 2014, 5, 121–128. [Google Scholar] [CrossRef]
- Buret, A.G.; Allain, T.; Motta, J.P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2022, 36, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.; Mathai, P.P.; Lopez, S.; Matson, M.; Elkin, B.; Kozysa, D.; Kabage, A.J.; Hamilton, M.; Vaughn, B.P.; Sadowsky, M.J.; et al. Differential hydrogen sulfide production by a human cohort in response to animal- and plant-based diet interventions. Clin. Nutr. 2022, 41, 1153–1162. [Google Scholar] [CrossRef]
- Gorini, F.; Del Turco, S.; Sabatino, L.; Gaggini, M.; Vassalle, C. H2S as a Bridge Linking Inflammation, Oxidative Stress and Endothelial Biology: A Possible Defense in the Fight against SARS-CoV-2 Infection? Biomedicines 2021, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiong, L.; Tang, J.; Li, L.; Li, L. Hydrogen Sulfide in Skin Diseases: A Novel Mediator and Therapeutic Target. Oxid. Med. Cell Longev. 2021, 2021, 6652086. [Google Scholar] [CrossRef] [PubMed]
- Coavoy-Sánchez, S.A.; Costa, S.K.P.; Muscará, M.N. Hydrogen sulfide and dermatological diseases. Br. J. Pharmacol. 2020, 177, 857–865. [Google Scholar] [CrossRef]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2022, 12, 746998. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, X.; Zhou, X.; Zhan, T.; Dai, Q.; Zhang, Y.; Zhang, W.; Shu, Y.; Li, W.; Xu, H. Association of gut microbiome and metabolites with onset and treatment response of patients with pemphigus vulgaris. Front. Immunol. 2023, 14, 1114586. [Google Scholar] [CrossRef]
- Stirnadel-Farrant, H.A.; Xu, X.; Kwiatek, J.; Jain, P.; Meyers, J.; Candrilli, S.; Mines, D.; Datto, C.J. Characteristics, treatment patterns, health care resource utilization and costs in patients with bullous pemphigoid: A retrospective analysis of US health insurance claims data. JAAD Int. 2023, 13, 117–125. [Google Scholar] [CrossRef]
- Hsu, D.; Brieva, J.; Silverberg, J.I. Costs of Care for Hospitalization for Pemphigus in the United States. JAMA Dermatol. 2016, 152, 645–654. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Genovese, G.; Di Zenzo, G.; Cozzani, E.; Berti, E.; Cugno, M.; Marzano, A.V. New Insights Into the Pathogenesis of Bullous Pemphigoid: 2019 Update. Front. Immunol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Hesari, R.; Thibaut, D.; Schur, N.; Thoutireddy, S.; Witcher, R.; Julian, E. Bullous Pemphigoid and Human Leukocyte Antigen (HLA)-DQA1: A Systematic Review. Cureus 2023, 15, e39923. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, L.; Jiang, F. Study of cytokine-induced immunity in bullous pemphigoid: Recent developments. Ann. Med. 2023, 55, 2280991. [Google Scholar] [CrossRef]
- Gupta, A.; Dhakan, D.B.; Maji, A.; Saxena, R.; PK, V.P.; Mahajan, S.; Pulikkan, J.; Kurian, J.; Gomez, A.M.; Scaria, J.; et al. Association of Flavonifractor plautii, a Flavonoid-Degrading Bacterium, with the Gut Microbiome of Colorectal Cancer Patients in India. mSystems 2019, 4, e00438–e19. [Google Scholar] [CrossRef]
- Ogita, T.; Yamamoto, Y.; Mikami, A.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Strongly Suppresses Th2 Immune Responses in Mice. Front. Immunol. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii, a Bacteria Increased With Green Tea Consumption, Promotes Recovery From Acute Colitis in Mice via Suppression of IL-17. Front. Nutr. 2021, 7, 610946. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, M.; Yang, X.; Hong, N.; Yu, C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J. Crohns Colitis. 2013, 7, e558–e568. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best. Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef]
- El Atty, K.A.; Nouh, H.; Abdelsalam, S.; Ellakany, A.; Abdaalah, H.; Header, D. Study of Fecalibacteria prausntzii in Egyptian patients with inflammatory bowel disease. Prz. Gastroenterol. 2024, 19, 151–158. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Tran, P.; Prindle, A. Potential Roles for Gamma-Aminobutyric Acid Signaling in Bacterial Communities. Bioelectricity. 2021, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Bhatia, S.K.; Gurav, R.; Choi, Y.K.; Jeon, J.M.; Yoon, J.J.; Choi, K.Y.; Ahn, J.; Kim, H.T.; Yang, Y.H. Gamma aminobutyric acid (GABA) production in Escherichia coli with pyridoxal kinase (pdxY) based regeneration system. Enzyme Microb. Technol. 2022, 155, 109994. [Google Scholar] [CrossRef]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A.; Golani, O.; Dori-Bachash, M.; Moresi, C.; Shapiro, H.; Del Mare-Roumani, S.; et al. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Bui, A.; Lee, K.; Truong, A.; Milush, J.M.; Somsouk, M.; Liao, W. Multiomic Analysis of the Gut Microbiome in Psoriasis Reveals Distinct Host—Microbe Associations. JID Innov. 2022, 2, 100115. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Thirion, F.; Guilly, S.; Fromentin, S.; Plaza Oñate, F.; Alvarez, A.S.; Le Chatelier, E.; Pons, N.; Levenez, F.; Quinquis, B.; Ehrlich, S.; et al. Changes in Gut Microbiota of Patients with Atopic Dermatitis During Balneotherapy. Clin. Cosmet. Investig. Dermatol. 2022, 15, 163–176. [Google Scholar] [CrossRef]
- Andermann, T.; Antonelli, A.; Barrett, R.L.; Silvestro, D. Estimating Alpha, Beta, and Gamma Diversity Through Deep Learning. Front. Plant Sci. 2022, 13, 839407. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- West, C.E.; Rydén, P.; Lundin, D.; Engstrand, L.; Tulic, M.K.; Prescott, S.L. Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin. Exp. Allergy. 2015, 45, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Birzele, L.T.; Depner, M.; Ege, M.J.; Engel, M.; Kublik, S.; Bernau, C.; Loss, G.J.; Genuneit, J.; Horak, E.; Schloter, M.; et al. Environmental and mucosal microbiota and their role in childhood asthma. Allergy 2017, 72, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Manasson, J.; Shen, N.; Garcia Ferrer, H.R.; Ubeda, C.; Iraheta, I.; Heguy, A.; Von Feldt, J.M.; Espinoza, L.R.; Garcia Kutzbach, A.; Segal, L.N.; et al. Gut Microbiota Perturbations in Reactive Arthritis and Postinfectious Spondyloarthritis. Arthritis Rheumatol. 2018, 70, 242–254. [Google Scholar] [CrossRef]
- Ćesić, D.; Lugović Mihić, L.; Ozretić, P.; Lojkić, I.; Buljan, M.; Šitum, M.; Zovak, M.; Vidović, D.; Mijić, A.; Galić, N.; et al. Association of Gut Lachnospiraceae and Chronic Spontaneous Urticaria. Life 2023, 13, 1280. [Google Scholar] [CrossRef]
- Zaplana, T.; Miele, S.; Tolonen, A.C. Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics. Front. Bioeng. Biotechnol. 2024, 11, 1324396. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, P.; Hu, R.; Qi, S.; Zhao, Y.; Miao, Y.; Han, Y.; Zhou, L.; Yang, Q. Gut microbiota characterization in Chinese patients with alopecia areata. J. Dermatol. Sci. 2021, 102, 109–115. [Google Scholar] [CrossRef]
- Lam, S.Y.; Radjabzadeh, D.; Eppinga, H.; Nossent, Y.R.A.; van der Zee, H.H.; Kraaij, R.; Konstantinov, S.R.; Fuhler, G.M.; Prens, E.P.; Thio, H.B.; et al. A microbiome study to explore the gut-skin axis in hidradenitis suppurativa. J. Dermatol. Sci. 2021, 101, 218–220. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High Molecular Weight Barley β-Glucan Alters Gut Microbiota Toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella Induces the Production of Th17 Cells in the Colon of Mice. J. Immunol. Res. 2020, 2020, 9607328. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Dong, Z.; Hecht, D.K.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014, 7, 983–994. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zheng, Z.; Shao, T.; Liu, L.; Xie, Z.; Le Chatelier, E.; He, Z.; Zhong, W.; Fan, Y.; Zhang, L.; et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 2017, 18, 142. [Google Scholar]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Tett, A.; Tarallo, S.; Naccarati, A.; De Angelis, M.; Neviani, E.; Cocolin, L.; Gobbetti, M.; Segata, N.; et al. Distinct Genetic and Functional Traits of Human Intestinal Prevotella copri Strains Are Associated with Different Habitual Diets. Cell Host Microbe 2019, 25, 444–453.e3. [Google Scholar] [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host Microbe 2019, 26, 666–679.e7. [Google Scholar] [CrossRef]
- Zhang, S.M.; Huang, S.L. The Commensal Anaerobe Veillonella dispar Reprograms Its Lactate Metabolism and Short-Chain Fatty Acid Production during the Stationary Phase. Microbiol. Spectr. 2023, 11, e0355822. [Google Scholar] [CrossRef] [PubMed]
- Fultz, R.; Ticer, T.; Ihekweazu, F.D.; Horvath, T.D.; Haidacher, S.J.; Hoch, K.M.; Bajaj, M.; Spinler, J.K.; Haag, A.M.; Buffington, S.A.; et al. Unraveling the Metabolic Requirements of the Gut Commensal Bacteroides ovatus. Front. Microbiol. 2021, 12, 745469. [Google Scholar] [CrossRef]
- Han, Z.; Fan, Y.; Wu, Q.; Guo, F.; Li, S.; Hu, X.; Zuo, Y.G. Comparison of gut microbiota dysbiosis between pemphigus vulgaris and bullous pemphigoid. Int. Immunopharmacol. 2024, 128, 111470. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- You, S.; Ouyang, J.; Wu, Q.; Zhang, Y.; Gao, J.; Luo, X.; Wang, Y.; Wu, Y.; Jiang, F. Comparison of serum cytokines and chemokines levels and clinical significance in patients with pemphigus vulgaris-A retrospective study. Exp. Dermatol. 2024, 33, e15173. [Google Scholar] [CrossRef] [PubMed]
- Timoteo, R.P.; da Silva, M.V.; Miguel, C.B.; Silva, D.A.; Catarino, J.D.; Rodrigues Junior, V.; Sales-Campos, H.; Freire Oliveira, C.J. Th1/Th17-Related Cytokines and Chemokines and Their Implications in the Pathogenesis of Pemphigus Vulgaris. Mediators Inflamm. 2017, 2017, 7151285. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.X.; Liu, F.L.; Zhou, N.; Jiang, C.Y.; Wang, C.Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. Imeta 2022, 1, e58. [Google Scholar] [CrossRef]
- Gu, B.H.; Choi, J.P.; Park, T.; Kim, A.S.; Jung, H.Y.; Choi, D.Y.; Lee, S.J.; Chang, Y.S.; Kim, M.; Park, H.K. Adult asthma with symptomatic eosinophilic inflammation is accompanied by alteration in gut microbiome. Allergy 2023, 78, 1909–1921. [Google Scholar] [CrossRef]
- Notting, F.; Pirovano, W.; Sybesma, W.; Kort, R. The butyrate-producing and spore-forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut Microbiome 2023, 4, e16. [Google Scholar] [CrossRef]
- Coello, K.; Hansen, T.H.; Sørensen, N.; Ottesen, N.M.; Miskowiak, K.W.; Pedersen, O.; Kessing, L.V.; Vinberg, M. Affective disorders impact prevalence of Flavonifractor and abundance of Christensenellaceae in gut microbiota. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110300. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yiu, N.; Hu, Z.; Zhou, W.; Long, X.; Yang, M.; Liao, J.; Zhang, G.; Lu, Q.; Zhao, M. Alterations of fecal microbiome and metabolome in pemphigus patients. J. Autoimmun. 2023, 141, 103108. [Google Scholar] [CrossRef]
- Daniel, B.S.; Hertl, M.; Werth, V.P.; Eming, R.; Murrell, D.F. Severity score indexes for blistering diseases. Clin. Dermatol. 2012, 30, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar]
- Anand, P.K. Lipids, inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 297, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Xiao, X.; Kumar, M.; Bachman, M.; Borregaard, N.; Joe, B.; Vijay-Kumar, M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat. Commun. 2015, 6, 7113. [Google Scholar] [CrossRef]
- Houot, L.; Chang, S.; Pickering, B.S.; Absalon, C.; Watnick, P.I. The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J. Bacteriol. 2010, 192, 3055–3067. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Linnemann, R.W.; Mansbach, J.M.; Ajami, N.J.; Espinola, J.A.; Petrosino, J.F.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Thompson, A.D.; et al. The Fecal Microbiota Profile and Bronchiolitis in Infants. Pediatrics 2016, 138, e20160218. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Sharma, G.; Garg, N.; Hasan, S.; Shirodkar, S. Prevotella: An insight into its characteristics and associated virulence factors. Microb. Pathog. 2022, 169, 105673. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Kohara, C.; Watanabe, E.; Takahashi, S.; Ozawa, G.; Inomata, K.; Suzuki, K.; Mizukami, M.; Nagai, E.; Okabe, M.; et al. Impact of Intestinal Microbiota on Reconstitution of Circulating Monocyte, Dendritic Cell, and Natural Killer Cell Subsets in Adults Undergoing Single-Unit Cord Blood Transplantation. Biol. Blood Marrow Transplant. 2020, 26, e292–e297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shi, Y.; Ni, Y.; Ni, M.; Yang, Y.; Zhang, X. Gestational diabetes-combined excess weight gain exacerbates gut microbiota dysbiosis in newborns, associated with reduced abundance of Clostridium, Coriobacteriaceae, and Collinsella. Front. Cell Infect. Microbiol. 2024, 14, 1496447. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Chang, S.H.; Choi, Y. Gut dysbiosis in autoimmune diseases: Association with mortality. Front. Cell Infect. Microbiol. 2023, 13, 1157918. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Ou, R.; Tang, N.; Su, W.; Yang, R.; Yu, X.; Zhang, G.; Jiao, J.; Zhou, X. Alternation of the gut microbiota in irritable bowel syndrome: An integrated analysis based on multicenter amplicon sequencing data. J. Transl. Med. 2023, 21, 117. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2022, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Ma, Y.; Liu, J.; Cui, Y.; Yuan, Y.; Xiang, C.; Ma, D.; Liu, H. The microbiome types of colorectal tissue are potentially associated with the prognosis of patients with colorectal cancer. Front. Microbiol. 2023, 14, 1100873. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci Hum Well. 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Fan, W.; Lei, N.; Zheng, Y.; Liu, J.; Cao, X.; Su, T.; Su, Z.; Lu, Y. Oral microbiota diversity in moderate to severe plaque psoriasis, nail psoriasis and psoriatic arthritis. Sci. Rep. 2024, 14, 18402. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Mahootchi, P.; Safari, S.; Rahimi, H.; Aghili, S.S. Interactions between systemic diseases and oral microbiota shifts in the aging community: A narrative review. J. Basic. Microbiol. 2023, 63, 831–854. [Google Scholar] [CrossRef]
- Macklis, P.; Adams, K.; Kaffenberger, J.; Kumar, P.; Krispinsky, A.; Kaffenberger, B. The Association Between Oral Health and Skin Disease. J. Clin. Aesthet. Dermatol. 2020, 13, 48–53. [Google Scholar] [PubMed]
- Qiao, P.; Shi, Q.; Zhang, R.; E, L.; Wang, P.; Wang, J.; Liu, H. Psoriasis Patients Suffer From Worse Periodontal Status-A Meta-Analysis. Front. Med. 2019, 6, 212. [Google Scholar] [CrossRef]
- Murata, T.; Yamaga, T.; Iida, T.; Miyazaki, H.; Yaegaki, K. Classification and examination of halitosis. Int Dent J. 2002, 52, 181–186. [Google Scholar] [CrossRef]
- Zorba, M.; Melidou, A.; Patsatsi, A.; Poulopoulos, A.; Gioula, G.; Kolokotronis, A.; Minti, F. The role of oral microbiome in pemphigus vulgaris. Arch. Microbiol. 2021, 203, 2237–2247. [Google Scholar] [CrossRef]
- Subadra, K.; S, S.; Warrier, S.A. Oral Pemphigus Vulgaris. Cureus 2021, 13, e18005. [Google Scholar] [CrossRef]
- Murphy, E.C.; Frick, I.M. Gram-positive anaerobic cocci–commensals and opportunistic pathogens. FEMS Microbiol. Rev. 2013, 37, 520–553. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Higashi, D.L.; Krieger, M.C.; Qin, H.; Zou, Z.; Palmer, E.A.; Kreth, J.; Merritt, J. Who is in the driver’s seat? Parvimonas micra: An understudied pathobiont at the crossroads of dysbiotic disease and cancer. Environ. Microbiol. Rep. 2023, 15, 254–264. [Google Scholar]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar]
- Komiya, Y.; Shimomura, Y.; Higurashi, T.; Sugi, Y.; Arimoto, J.; Umezawa, S.; Uchiyama, S.; Matsumoto, M.; Nakajima, A. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 2019, 68, 1335–1337. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Chiscuzzu, F.; Crescio, C.; Varrucciu, S.; Rizzo, D.; Sali, M.; Delogu, G.; Bussu, F. Current Evidence on the Relation Between Microbiota and Oral Cancer-The Role of Fusobacterium nucleatum-A Narrative Review. Cancers 2025, 17, 171. [Google Scholar] [CrossRef]
- Begić, G.; Badovinac, I.J.; Karleuša, L.; Kralik, K.; Cvijanovic Peloza, O.; Kuiš, D.; Gobin, I. Streptococcus salivarius as an Important Factor in Dental Biofilm Homeostasis: Influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in Mixed Biofilm. Int. J. Mol. Sci. 2023, 24, 7249. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Fact. 2022, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Amagai, M. Dissecting skin microbiota and microenvironment for the development of therapeutic strategies. Curr. Opin. Microbiol. 2023, 74, 102311. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Nakamura, Y.; Núñez, G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol. Int. 2017, 66, 539–544. [Google Scholar] [CrossRef]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Celoria, V.; Rosset, F.; Pala, V.; Dapavo, P.; Ribero, S.; Quaglino, P.; Mastorino, L. The Skin Microbiome and Its Role in Psoriasis: A Review. Psoriasis 2023, 13, 71–78. [Google Scholar] [CrossRef]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Rainer, B.M.; Thompson, K.G.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Bui, J.; Fischer, A.H.; Pasieka, H.B.; Garza, L.A.; Kang, S.; et al. Characterization and Analysis of the Skin Microbiota in Rosacea: A Case-Control Study. Am. J. Clin. Dermatol. 2020, 21, 139–147. [Google Scholar] [CrossRef]
- Tutka, K.; Żychowska, M.; Reich, A. Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea-A Systematic Review of the Literature. Microorganisms 2020, 8, 1756. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Lee, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Characterization and Analysis of the Skin Microbiota in Rosacea: Impact of Systemic Antibiotics. J. Clin. Med. 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Ferček, I.; Lugović-Mihić, L.; Tambić-Andrašević, A.; Ćesić, D.; Grginić, A.G.; Bešlić, I.; Mravak-Stipetić, M.; Mihatov-Štefanović, I.; Buntić, A.M.; Čivljak, R. Features of the Skin Microbiota in Common Inflammatory Skin Diseases. Life 2021, 11, 962. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Hong, J.; Buddenkotte, J.; Berger, T.G.; Steinhoff, M. Management of itch in atopic dermatitis. Semin. Cutan. Med. Surg. 2011, 30, 71–86. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Ruta, L.; Pioggia, G.; Gangemi, S. Association Between Atopic Dermatitis and Autism Spectrum Disorders: A Systematic Review. Am. J. Clin. Dermatol. 2015, 16, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ohzono, A.; Teye, K.; Numata, S.; Hiroyasu, S.; Tsuruta, D.; Hachiya, T.; Kuroda, K.; Hashiguchi, M.; Kawakami, T.; et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br. J. Dermatol. 2017, 177, 141–151. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Messingham, K.N.; Cahill, M.P.; Kilgore, S.H.; Munjal, A.; Schlievert, P.M.; Fairley, J.A. TSST-1+ Staphylococcus aureus in Bullous Pemphigoid. J. Investig. Dermatol. 2022, 142, 1032—1039.e6. [Google Scholar] [CrossRef]
- Conti, F.; Ceccarelli, F.; Iaiani, G.; Perricone, C.; Giordano, A.; Amori, L.; Miranda, F.; Massaro, L.; Pacucci, V.A.; Truglia, S.; et al. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Gimza, B.D.; Cassat, J.E. Mechanisms of Antibiotic Failure During Staphylococcus aureus Osteomyelitis. Front. Immunol. 2021, 12, 638085. [Google Scholar] [CrossRef]
- Gobao, V.C.; Alfishawy, M.; Smith, C.; Byers, K.E.; Yassin, M.; Urish, K.L.; Shah, N.B. Risk Factors, Screening, and Treatment Challenges in Staphylococcus aureus Native Septic Arthritis. Open Forum Infect. Dis. 2020, 8, ofaa593. [Google Scholar] [CrossRef]
- Powers, M.E.; Bubeck Wardenburg, J. Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog. 2014, 10, e1003871. [Google Scholar] [CrossRef]
- van der Kooi-Pol, M.M.; Duipmans, J.C.; Jonkman, M.F.; van Dijl, J.M. Host-pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Belheouane, M.; Hermes, B.M.; Van Beek, N.; Benoit, S.; Bernard, P.; Drenovska, K.; Gerdes, S.; Gläser, R.; Goebeler, M.; Günther, C.; et al. Characterization of the skin microbiota in bullous pemphigoid patients and controls reveals novel microbial indicators of disease. J. Adv. Res. 2023, 44, 71–79. [Google Scholar] [CrossRef]
- Srinivas, G.; Möller, S.; Wang, J.; Künzel, S.; Zillikens, D.; Baines, J.F.; Ibrahim, S.M. Genome-wide mapping of gene-microbiota interactions in susceptibility to autoimmune skin blistering. Nat. Commun. 2013, 4, 2462. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, M.; Elalouf, O.; Anderson, M.; Chandran, V. The skin microbiome in psoriatic disease: A systematic review and critical appraisal. J. Transl. Autoimmun. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Rauer, L.; Reiger, M.; Bhattacharyya, M.; Brunner, P.M.; Krueger, J.G.; Guttman-Yassky, E.; Traidl-Hoffmann, C.; Neumann, A.U. Skin microbiome and its association with host cofactors in determining atopic dermatitis severity. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 772–782. [Google Scholar] [CrossRef]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef]
- Nakamura, K.; O’Neill, A.M.; Williams, M.R.; Cau, L.; Nakatsuji, T.; Horswill, A.R.; Gallo, R.L. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep. 2020, 10, 21237. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef] [PubMed]
| Study Design | Country— Study Period | Population | Main Findings | Limitations | References |
|---|---|---|---|---|---|
| Cross-sectional | Italy— January 2018–June 2018 | 8 BP patients: 5 females, mean age 70 ± 18 years | Firmicutes relative abundance: p50 (%, min–max): 47.7 (38.8–65.3). Bacteroidetes relative abundance: p50 (%, min–max): 43.8 (33.0–50.9). Proteobacteria relative abundance: p50 (%, min–max): 7.8 (5.2–12.7). | Small sample size. | [38] |
| Cross-sectional | Germany (N = 14; Finland (N = 3); Bulgaria (N = 3)— June 2014–July 2020 | 18 BP patients: 46 females, mean age 40.26 (63–98) years 66 healthy controls: 46 females, mean age 80.64 (62–100) years. Two subgroups: 54 pairs, first diagnosis (BPF and CLF), and 11 pairs, relapse (BPR and CLR) | Significant decrease and a lower trend in the Chao and Shannon indexes, respectively, in BP patients. Significant decrease in Chao index in BPR compared with the CLR group. Significant difference in Bray–Curtis dissimilarity between BP patients and their healthy controls and between the first diagnosis cases and their controls but not between relapsed cases and their matched controls. Microbial composition affected by study center, disease status, and age. At the genus level, Flavonifractor significantly and primarily enriched in BPR cases, while Faecalibacterium reduced in both subgroups of patients. At the species level, Ruthenibacterium lactatiformans, Anaerotruncus colihominis, and Eubacterium callanderi significantly increased, and Prevotella copri, Faecalibacterium prausnitzii, and Faecalibacterium sp. I417 significantly decreased in BP patients if compared with their controls. Ruthenibacterium lactatiformans, Anaerotruncus colihominis, Bacteroides eggerthii, and Bifidobacterium dentium more enriched, and Sutterella wadsworthensis reduced in the BPF group compared with the CLF group. Significant increase in six bacterial species, including Flavonifractor plautii, and significant decline of three species, including Alistipes shahii, in BPR cases compared with their matched controls. Twelve gut microbial pathways significantly affected in BP patients. 30 species and 49 pathways significantly associated with BPDAI score. | Lack of longitudinal and metabolome data. Small sample size. Controls included subjects with basal cell carcinoma or squamous cell carcinoma. | [10] |
| Cross-sectional | China | 24 BP-O patients: 7 females, mean age 69.75 ± 10.28 years. 24 BP-R patients: 11 females, mean age 69.92 ± 13.50 years 26 healthy controls: 14 females, mean age 63.58 ± 9.15 tears | No significant differences in ACE index between the three groups, except for a higher score in the BP-O group compared with healthy controls and a lower score in BP-R patients than the two other groups. No significant differences in Bray–Curtis dissimilarity between the three groups. At the family level, the BP-O group showed an increased proportion of Bacteroidaceae, Ruminococcaceae, and Enterobacteriaceae and a lower proportion of Lachnospiraceae, Prevotellaceae, and Veillonellaceae compared with the healthy controls. In the BP-R group, the proportion of Lachnospiraceae and Veillonellaceae increased and that of Ruminococcaceae, Bacteroidaceae, Prevotellaceae and Enterobacteriaceae decreased compared with the control group. At the ASV level, similarity between BP-R and control groups and significant differences in enriched and depleted ASVs between BP-O and the other two groups. Significantly higher relative abundance of Prevotella copri and significant depletion of Vellonella dispar and Bacteroides ovatus in the BP-O group compared with other groups. Barnesiella intestinihominis and Veillonella dispar inversely correlated with anti- BP180, while Ruminococcus albus, Vescimonas coprocola, Sporobacter termitidis, Alistipes shahii, and Bifidobacterium adolescentis positively correlated with anti-BP180. Prevotella copri, Roseburia intestinalis, and Sutterella wadsworthensis inversely correlated with EOS%, while Blautia hominis positively correlated with EOS%. In BP-O patients, enrichment of Ruminococcaceae spp., Clostridium XIVb, Coprococcus, Oscillibacter, and Escherichia Shigella. In BP-R patients, enrichment of Lachnospiracea incertae sediswere and Sutterella. | Lack of longitudinal data. Small sample size. Single-center study. No standardized criteria for patients’ complications. The effect of the treatment on the gut microbiota not evaluated. Lack of assessment of the relationship between gut microbiota composition and anti-BP230. | [39] |
| Prospective | China— October 2016–March 2022 | 38 BP patients: 12 females, mean age 67.8 ± 11.4 years 38 healthy controls: 22 females, mean age 65.5 ± 10.3 years | No significant difference in the Shannon index between BP patients and controls. Bray–Curtis dissimilarity significantly different between cases and controls. At the phylum level, enrichment of Proteobacteria and Actinobacteria in BP patients. At the genus level, BP patients show an increase in Bacteroides and Prevotella and decrease in Escherichia-Shigella and Faecalibacterium. Significant differences between cases and controls in the microbial composition of Bacteroidetes and Firmicutes phyla. Faecalibacterium negatively correlated with anti-BP180. Alterations in 14 functional pathways significantly increased in BP patients. | Small sample size. Single-center study. Lack of metagenomic analysis. | [164] |
| Study Design | Country— Study Period | Population | Main Findings | Limitations | References |
|---|---|---|---|---|---|
| Cross-sectional | Italy— January 2018–June 2018 | 12 PV patients: 6 females, mean age 55 ± 14 years | Firmicutes relative abundance: p50 (%, min–max): 43.3 (31.8–75.0). Bacteroidetes relative abundance: p50 (%, min–max): 50.9 (20.5–66.6). Proteobacteria relative abundance: p50 (%, min–max): 10.3 (5.7–21.4). | Small sample size. | [38] |
| Cross-sectional | China— January 2017–May 2020 | 18 PV patients: 9 females, mean age 45.78 ± 13.45 years 14 healthy controls: 5 females, mean age 44.57 ± 14.72 years | No significant differences in the Shannon and Simpson diversity indexes between cases and controls. Ten taxa significantly different between the two groups. At the family level, higher abundance of Carnobacteriaceae, Enterobacteriaceae, and Burkholderiales and reduced levels of Enterobacteriales in PV. At the genus level, PV patients have decreased levels of Lachnospiracea_incertae_sedis and Coprococcus and increased levels of Granulicatella and Flavonifractor. Significantly higher concentration of six cytokines (IL-1β, IL-2R, IL-7, IL-8, C5a, YKL-40) out of 21 overall assessed in PV group than in the control group. Plasma IL-5, IL-6, IL-17A, and IL-21 show an increasing trend in PV patients. Significant positive correlation between Flavonifractor and plasma levels of C5a, IL-1β, IL-6, IL-7, IL-8, and IL-21. Significant inverse correlation of Lachnospiracea_incertae_sedis and Coprococcus with plasma IL-17A concentration. | Lack of longitudinal data. Small sample size. Single-center study. Half of PV patients under treatment with systemic corticosteroids. Lack of possibility to infer a causal relationship due to the study design. | [153] |
| Cross-sectional | China | 43 PV patients: 15 females, mean age 51.89 ± 15.61 years 26 healthy controls: 11 females, mean age 52.92 ± 15.21 years | No significant differences in the Richness, Chao, and Shannon indexes between cases and controls. Simpson index significantly higher in healthy controls than in PV patients. High level of dissimilarity between the two groups based on Bray–Curtis analysis. Decrease in the relative abundance of Firmicutes and increase in that of Proteobacteria and Verrucomicrobia in PV patients. At the genus level, higher proportions of Bacteroides, Escherichia, Akkermansia, and Klebsiella and lower proportions of Faecalibacterium and Roseburia in the case group. Opportunistic pathogens (unclassified Klebsiella, Bacteroides fragilis, and Bacteroides thetaiotaomicron) positively correlated with PDAI and anti-Dsg1 and anti-Dsg3 antibody levels. 215 significant associations between enriched bacterial species and metabolites. | Lack of longitudinal data. Single-center study. Small disease cohort. Results data-driven. Lack of possibility to infer a causal relationship due to the study design. | [173] |
| Cross-sectional | China— November 2017–April 2019 | 60 PV patients: 33 females, mean age 47.38 ± 12.82 years 19 matched healthy family members: 9 females, mean age 41.00 ± 14.81 years 100 fecal samples (60 treatment-naïve, 21 matched post-treatment, and 19 controls) | No significant differences in alpha diversity. Significantly high degree of dissimilarity between the two groups (beta-diversity). Three enterotypes—E1, E2, E3—identified in the two groups: E2 (Escherichia predominant) and E3 (Bacteroides predominant) significantly enriched in PV and healthy controls, respectively. At the phylum level, Actinobacteria predominant in PV patients, while Bacteroidetes predominant in healthy controls. At the species level, PV patients have a significant decrease in Bacteroidesovatus, Bacteroides uniformis, Eubacterium rectale, Eubacterium ventriosum, Roseburia intestinalis, and Roseburia inulinivorans and significant enrichment in Escherichia coli. Lachnospiraceae bacterium 5.1.57FAA abundance significantly and positively correlated with anti-Dsg3 antibodies and PDAI scores. Eubacterium ventriosum strongly and positively correlated with the ΔPDAI (an index that reflects the response to glucocorticoid treatment). Higher abundance of Escherichia coli in responders than in non-responders to therapy. No significant variations in alpha and beta diversity after glucocorticoid treatment; however, after one month of therapy, there was a decrease in the relative abundance of Escherichia coli and an increase in the probiotic abundance. PTS pathway, the most represented in PV patients, showing the strongest correlation with Escherichia coli. Fatty acid biosynthesis enriched in healthy controls and had the highest correlation with Bacteroides ovatus. | Lack of longitudinal data. Single-center study. Small sample size. Lack of a control group for other autoimmune diseases. The mechanisms by which Escherichia coli participates in the development of PV not investigated. Lack of possibility to infer a causal relationship due to the study design. | [117] |
| Cross-sectional | China— November 2016–May 2022 | 20 patients with AP (15 of whom with PV): 11 females, mean age 52.80 ± 16.79 years 11 patients with PR (7 of whom with PV): 6 females, mean age 60.36 ± 12.31 years 47 healthy controls (most of them spouses of the patients): 29 females, mean age 62.62 ± 11.45 years | No significant differences in the indexes of alpha diversity, but a progressive decrease in ACE and Chao indexes from healthy controls to the PR group and then to the AP group and a slight decrease of Shannon and Simpson indexes in the healthy control group compared with the other groups. No significant differences in beta diversity. Firmicutes and Bacteroidetes dominant phyla in all three groups, with a decreasing, albeit not significant, trend of the F/B ratio in AP patients. At the family level, the highest relative abundance of Lachnospiraceae in PR and the lowest relative abundance of Veillonellaceae in AP. Prevotalleceae abundance progressively increased in the AP, healthy control, and PR groups without significant differences. At the genus level, Blautia abundance is significantly higher in the AP than in the PR group, while that of Prevotella shows a not significant increase across AP, healthy control, and PR groups. | Lack of longitudinal data. Small sample size. Single-center study. Lack of adjustment for dietary habits between cases and controls. Some patients under systemic corticosteroid treatment. Significant age differences between the three groups. Lack of possibility to infer a causal relationship due to the study design. | [17] |
| Cross-sectional | China— October 2016–March 2022 | 19 PV patients: 11 females, mean age 59.9 ± 15.0 years 38 healthy controls: 22 females, mean age 65.5 ± 10.3 years | No significant difference in the Shannon index and Bray–Curtis dissimilarity between PV patients and controls. At the phylum level, enrichment of Proteobacteria and Actinobacteria in PV patients. At the genus level, increase in Bacteroides and Faecalibacterium and a decrease in Escherichia-Shigella and Prevotella among cases. At the species level, enrichment in Intestinibacter bartletti in PV patients and Blautia wexlerae and Bifidobacterium catenulatum in controls. Significant differences between cases and controls in the microbial composition of Bacteroidetes and Proteobacteria phyla. Enterobacter positively correlated with anti-Dsg3. | Lack of longitudinal data. Small sample size. Single-center study. Lack of metagenomic analysis. | [164] |
| Study Design | Country— Study Period | Population | Main Findings | Limitations | References |
|---|---|---|---|---|---|
| Cross-sectional | Italy— January 2018–June 2018 | 12 PV patients: 6 females, mean age 55 ± 14 years | Firmicutes relative abundance: p50 (%, min–max): 45.5 (27.1–72.6) in PV patients vs. 39.6 (32.3–73.4) in healthy controls. Fusobacteria relative abundance: p50 (%, min–max): 28.0 (10.4–41.6) in PV patients vs. 8.5 (1.9–13.2) in healthy controls. Bacteroidetes relative abundance: p50 (%, min–max): 7.2 (5.7–12.6) in PV patients vs. 8.5 (1.9–13.2) in healthy controls. Proteobacteria relative abundance: p50 (%, min–max): 15.2 (5.1–23.9) in PV patients vs. 13.3 (10.5–42.6) in healthy controls. Actinobacteria relative abundance: p50 (%, min–max): 5.5 (2.8–27.0) in PV patients vs. 2.4 (1.4–5.3) in healthy controls. | Small sample size. | [38] |
| Cross-sectional | Greece— January 2016–December 2018 | 15 PV patients: 9 females 15 healthy controls | At the phylum level, significant differences in the relative abundance of Firmicutes (61.27% in patients vs. 55.88% in controls), Proteobacteria (12.33% in patients vs. 9.17% in controls), Fusobacteria (4.09% vs. 3.39%). At the family level, significant differences in the relative abundance of Bacillales incertae sedis (5.98% in patients vs. 1.41% in controls) and Fusobacteriaceae (3.91% in patients vs. 2.56% in controls). At the genus level, significant differences in the relative abundance of Streptococcus (34.37% in patients vs. 33.30% in controls), Fusobacterium (4.51% in patients vs. 4.13% in controls), and Gemella (7.13% in patients vs. 5.80% in controls). Alpha diversity: no significant differences in Shannon, Simpson, and Fischer indexes. Eleven taxa significantly increased in abundance in controls and 30 taxa in patients. Patients showing the highest mean of the phylum Firmicutes, families Clostridiales Family XI Incertae Sedis and Carnobacteriaceae, genera Actibacillus, Aggregatibacter, Selenomonas, Prevotella with species P. maculosa, nigrescens, oris, and other sp., and Streptococcus with species S. intermedius, mitis, sanguinis, and thermophilus. Bray–Curtis dissimilarity significantly different between patients and controls, with Firmicutes and Fusobacteria significantly enriched in cases compared with healthy subjects. Smoker patients with a high abundance of Rothia mucilaginosa, Streptococcus salivarius, Haemophilus parainfluenzae, Granulicatella adiacens, and Streptococcus pseudopneumoniae. 11 species only detected in smokers. Eleven significantly discriminative taxa between smokers and non-smoker patients, with Firmicutes having the highest proportion in smokers and Proteobacteria in non-smokers. | Lack of longitudinal data. Small sample size. Single-center study. | [200] |
| Study Design | Country— Study Period | Population | Main Findings | Limitations | References |
|---|---|---|---|---|---|
| Cross-sectional | Germany— August 2014–January 2015 | 12 BP patients: 9 females, mean age 79.8 ± 9.9 years 12 healthy controls: 7 females, mean age 81.7 ± 7.5 years | Shannon, Simpson, and Chao1 indexes not significantly different between perilesional and non-lesional sites in BP patients. Bray–Curtis dissimilarity index significantly different by sample location, blistering disease status, group affiliation, and between perilesional and non-lesional sites within patients. Significant decrease in Actinobacteria abundance in back, elbow, and perilesional samples from patients and in Proteobacteria in perilesional sites. Significant enrichment of Firmicutes and the genus Staphylococcus in patient perilesional sites compared with control sites matched controls. At the species level, relative abundance of Staphylococcus epidermis significantly different between patients and controls on perilesional sites. Actinobacteria abundance significantly different between perilesional and non-lesional sites in patients. Proteobacteria as the most abundant phylum in control and patient non-lesional sites; Actinobacteria and Firmicutes as the second most abundant phylum in control and patient non-lesional sites, respectively. Higher relative abundance of Firmicutes in perilesional sites in patients. Four-fold increase in the Firmicutes/Proteobacteria ratio in BP compared with control subjects. | Lack of longitudinal data. Small sample size. Single-center study. | [5] |
| Cross-sectional | Italy— January 2018–June 2018 | 12 PV patients: 6 females, mean age 55 ± 14 years 8 BP patients: 5 females, mean age 70 ± 18 years | Firmicutes relative abundance: p50 (%, min–max): 82.4 (82.1–83.2) in PV patients vs. 99.3 (55.7–99.9) in BP patients. Actinobacteria relative abundance: p50 (%, min–max): 17.4 (15.8–17.5) in PV patients vs. 30.7 in BP patients (only one subject). Proteobacteria relative abundance: p50 (%, min–max): 13.4 in BP patients (only one subject). Staphylococcus and Corynebacterium species enriched in BP patients. Overall increased diversity of bacterial species in PV patients Shannon index significantly different between the two groups. | Small sample size. Single-center study. | [38] |
| Cross-sectional | Germany; France; Bulgaria; Greece; Finland— October 2015–September 2019 | 228 BP patients: 113 females, mean age 80 ± 8.95 years 190 healthy controls: 86 females, mean age 80 ± 8.51 years | Shannon and Chao1 indexes not significantly different at sites rarely affected by BP. Control corresponding sites showing higher bacterial diversity than patient contralateral sites. Contralateral sites showing higher bacterial diversity than perilesional sites in patients. Shannon index significantly correlated with study center, disease status, and sex. Chao1 index significantly correlated with the study center and disease status. Disease status associated with a decrease in Shannon index in perilesional and contralateral lesions in patients and with a decrease in Chao1 index, even after adjustment for covariates. BPDAI not significantly associated with alpha diversity indexes at perilesional and contralateral skin sites. Bray–Curtis dissimilarity index significantly correlated with disease status. Disease status, blistering status, and study center accounting for a portion of the variance in the beta-diversity. Cutibacterium acnes abundance significantly correlated with study center, blistering status, and sex and higher relative abundance at control corresponding sites than at perilesional sites in patients. Staphylococcus hominis abundance significantly correlated with disease status and body site and significantly inversely correlated with BPDAI at patient contralateral sites but not perilesional sites. Staphylococcus aureus abundance significantly correlated with disease status at rarely affected sites. Decrease in Staphylococcus aureus abundance in control corresponding sites compared with an increase in patient perilesional sites. Staphylococcus aureus positively correlated with BPDAI at perilesional and contralateral sites but not correlated with age at any patient sites. Staphylococcus hominis and Staphylococcus aureus significantly negatively correlated with patient perilesional and contralateral sites but not with any matched control sites. Staphylococcus aureus significantly negatively correlated with Cutibacterium acnes at all patient sites but not in matched controls. | Lack of longitudinal data. | [236] |
| Autoimmune Skin Disease | Body District | Changes in the Microbiota | Level of Evidence |
|---|---|---|---|
| Bullous pemphigoid | Gut | Alpha diversity—no significant variations; beta-diversity significantly different in cases vs. controls | Moderate |
| Oral cavity | No studies | - | |
| Skin | Alpha diversity significantly correlated with the disease status in one study; beta-diversity significantly different in cases vs. controls in one study | Low | |
| Pemphigus vulgaris | Gut | Alpha diversity generally not significantly different between cases and controls; high degree of changes in the beta diversity in some studies | Low |
| Oral cavity | Alpha diversity—no significant variations; beta-diversity significantly different in cases vs. in one study | Low | |
| Skin | Significant variations in the alpha diversity in one study | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Coi, A.; Santoro, M.; Tonacci, A.; Sansone, F.; Mariotti, E.B.; Donati, M.; Verdelli, A.; Nasca, M.R.; Amerio, P.; et al. The Role of Microbiota in the Pathogenesis of Bullous Pemphigoid and Pemphigus Vulgaris: Evidence, Controversies, and Perspectives. Int. J. Mol. Sci. 2025, 26, 6076. https://doi.org/10.3390/ijms26136076
Gorini F, Coi A, Santoro M, Tonacci A, Sansone F, Mariotti EB, Donati M, Verdelli A, Nasca MR, Amerio P, et al. The Role of Microbiota in the Pathogenesis of Bullous Pemphigoid and Pemphigus Vulgaris: Evidence, Controversies, and Perspectives. International Journal of Molecular Sciences. 2025; 26(13):6076. https://doi.org/10.3390/ijms26136076
Chicago/Turabian StyleGorini, Francesca, Alessio Coi, Michele Santoro, Alessandro Tonacci, Francesco Sansone, Elena Biancamaria Mariotti, Marta Donati, Alice Verdelli, Maria Rita Nasca, Paolo Amerio, and et al. 2025. "The Role of Microbiota in the Pathogenesis of Bullous Pemphigoid and Pemphigus Vulgaris: Evidence, Controversies, and Perspectives" International Journal of Molecular Sciences 26, no. 13: 6076. https://doi.org/10.3390/ijms26136076
APA StyleGorini, F., Coi, A., Santoro, M., Tonacci, A., Sansone, F., Mariotti, E. B., Donati, M., Verdelli, A., Nasca, M. R., Amerio, P., Antiga, E., Barletta, E., & Caproni, M. (2025). The Role of Microbiota in the Pathogenesis of Bullous Pemphigoid and Pemphigus Vulgaris: Evidence, Controversies, and Perspectives. International Journal of Molecular Sciences, 26(13), 6076. https://doi.org/10.3390/ijms26136076








