The Effect of Rosavin, a Characteristic Compound of Rhodiola rosea, on BMP-2 Induction and Osteoblast Proliferation In Vitro
Abstract
1. Introduction
2. Results
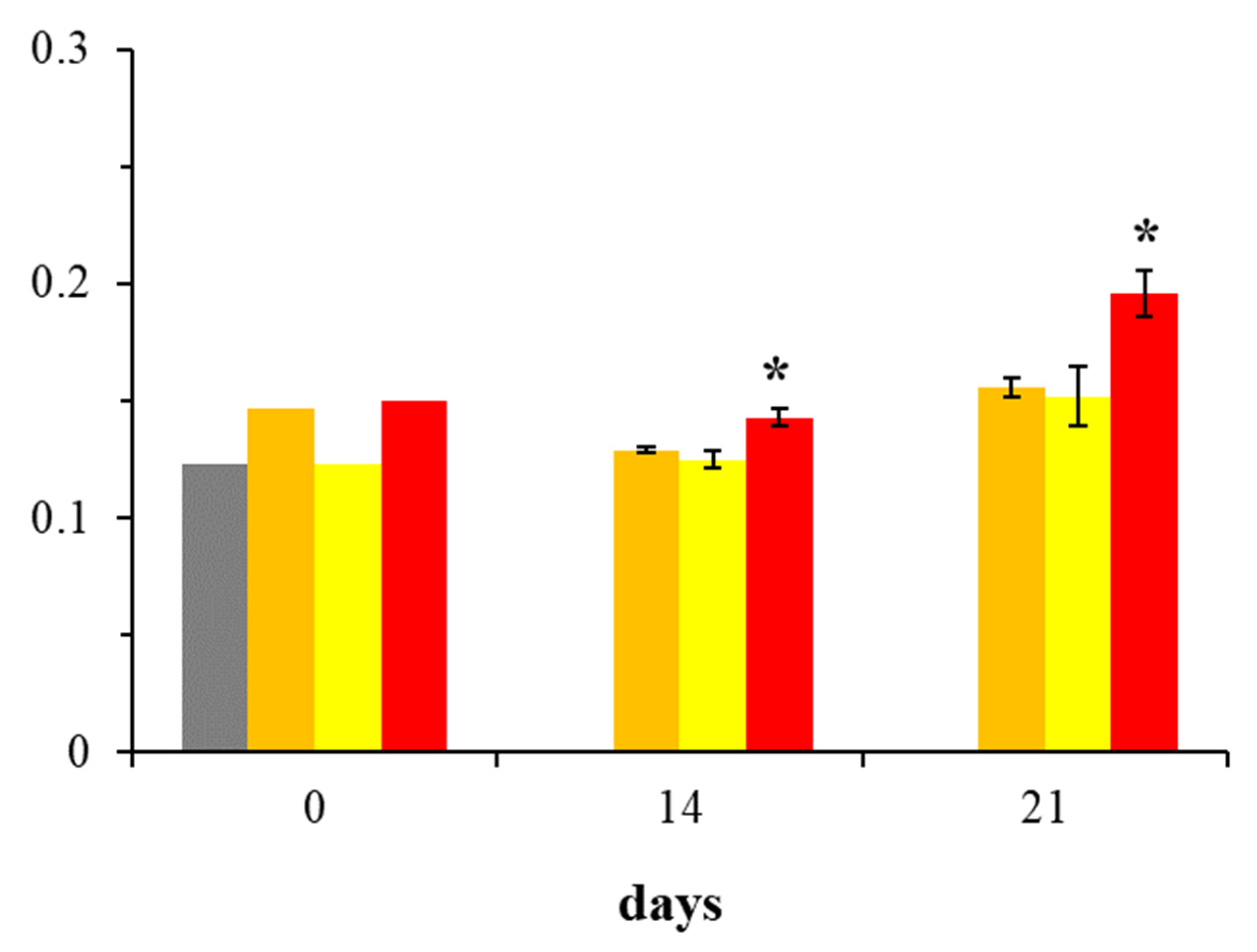
2.1. Assessment of BMP-2 Expression in Osteoblast Cultures
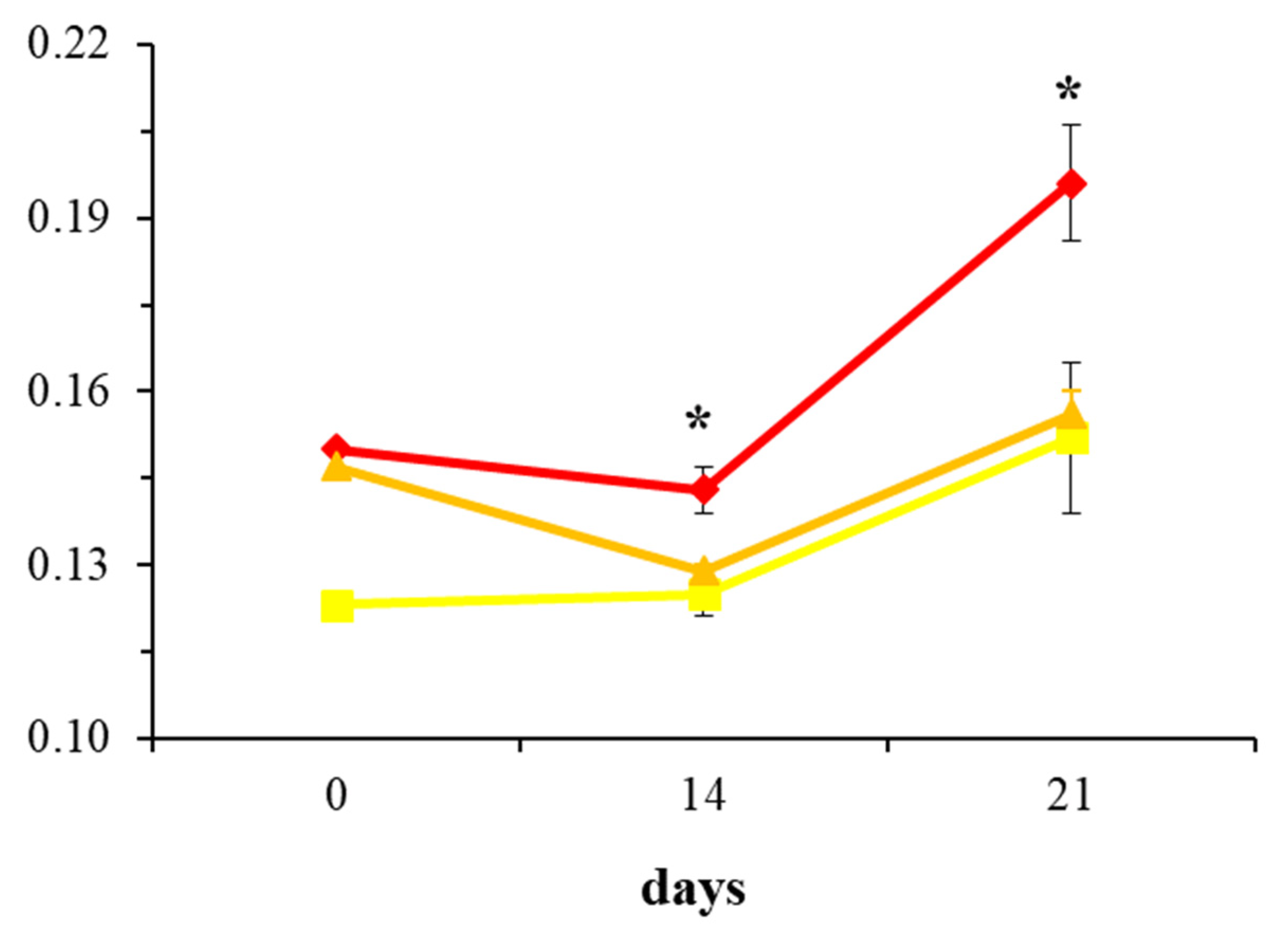
2.2. Evaluation of Collagen Production in Osteoblast Cultures
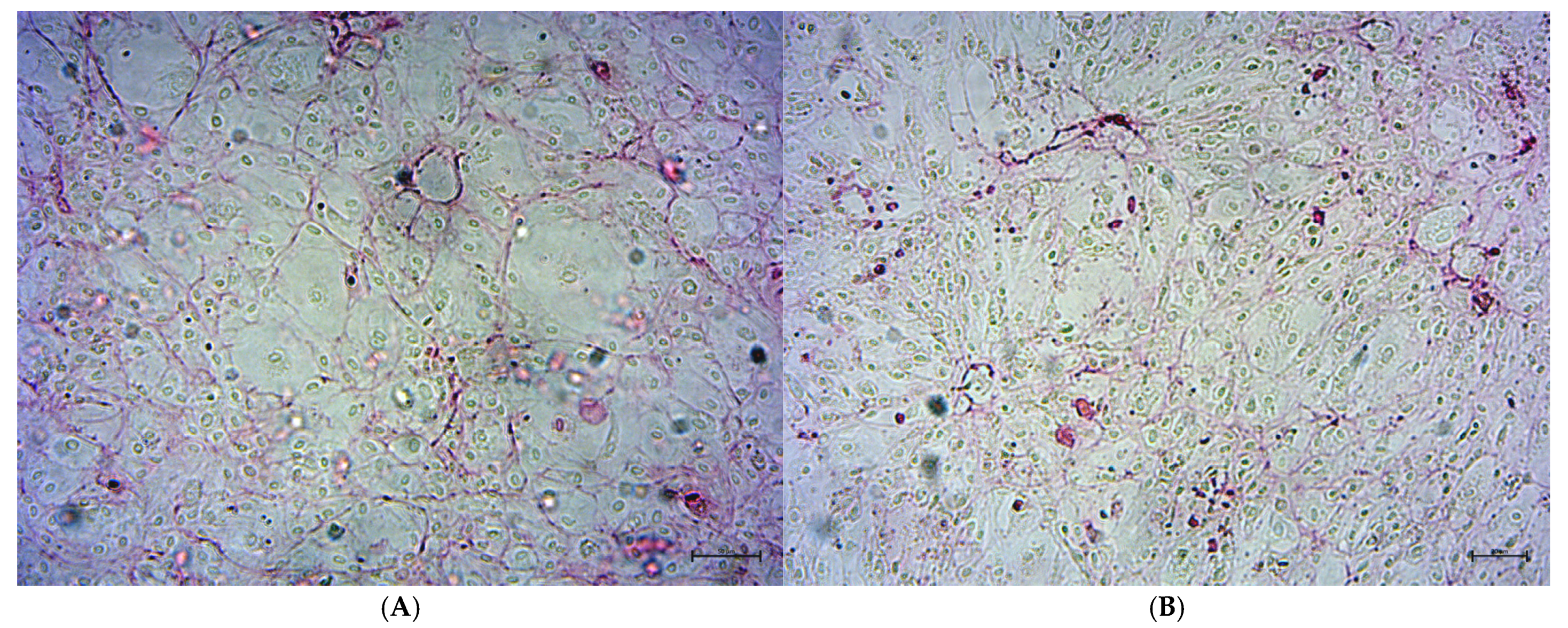
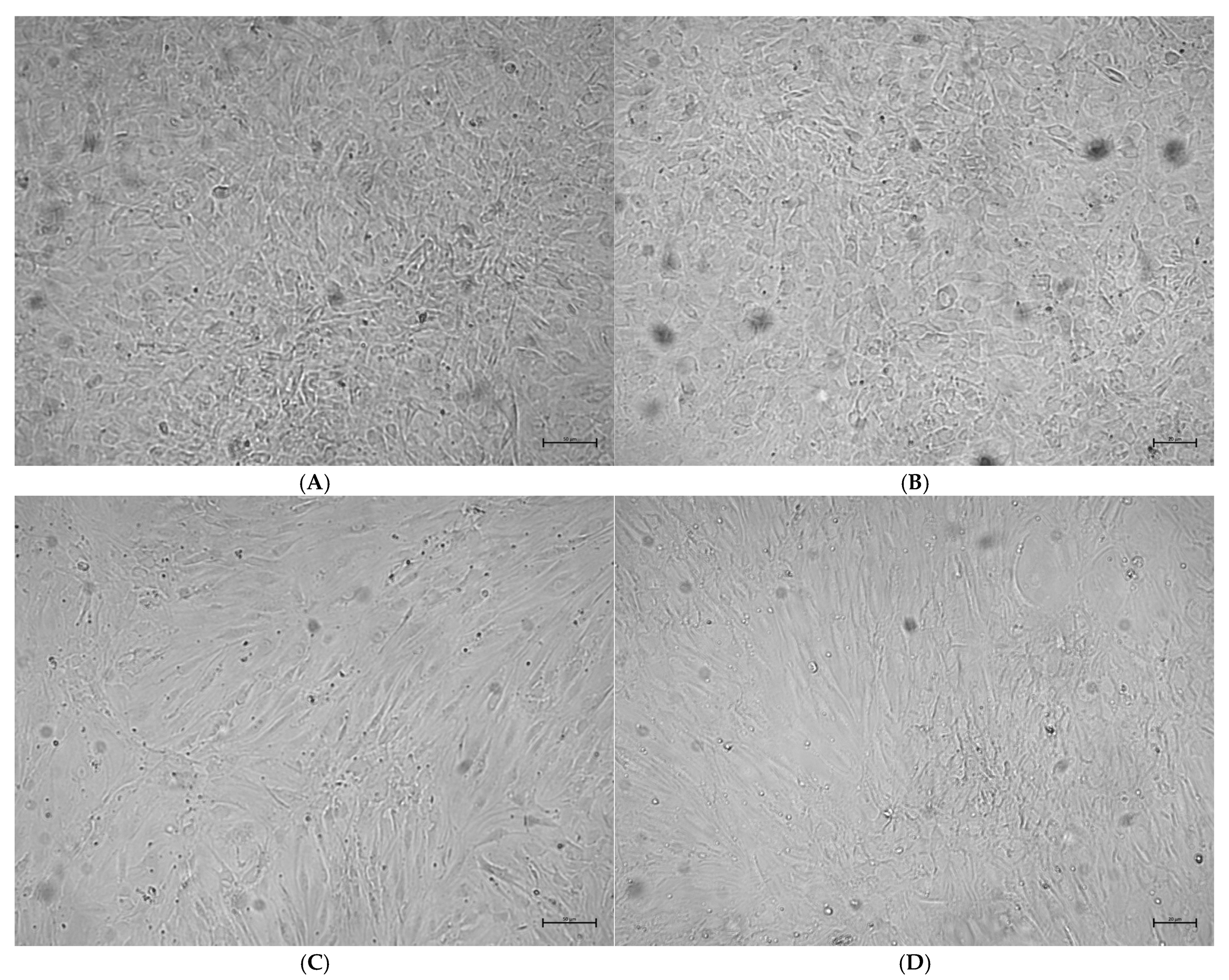
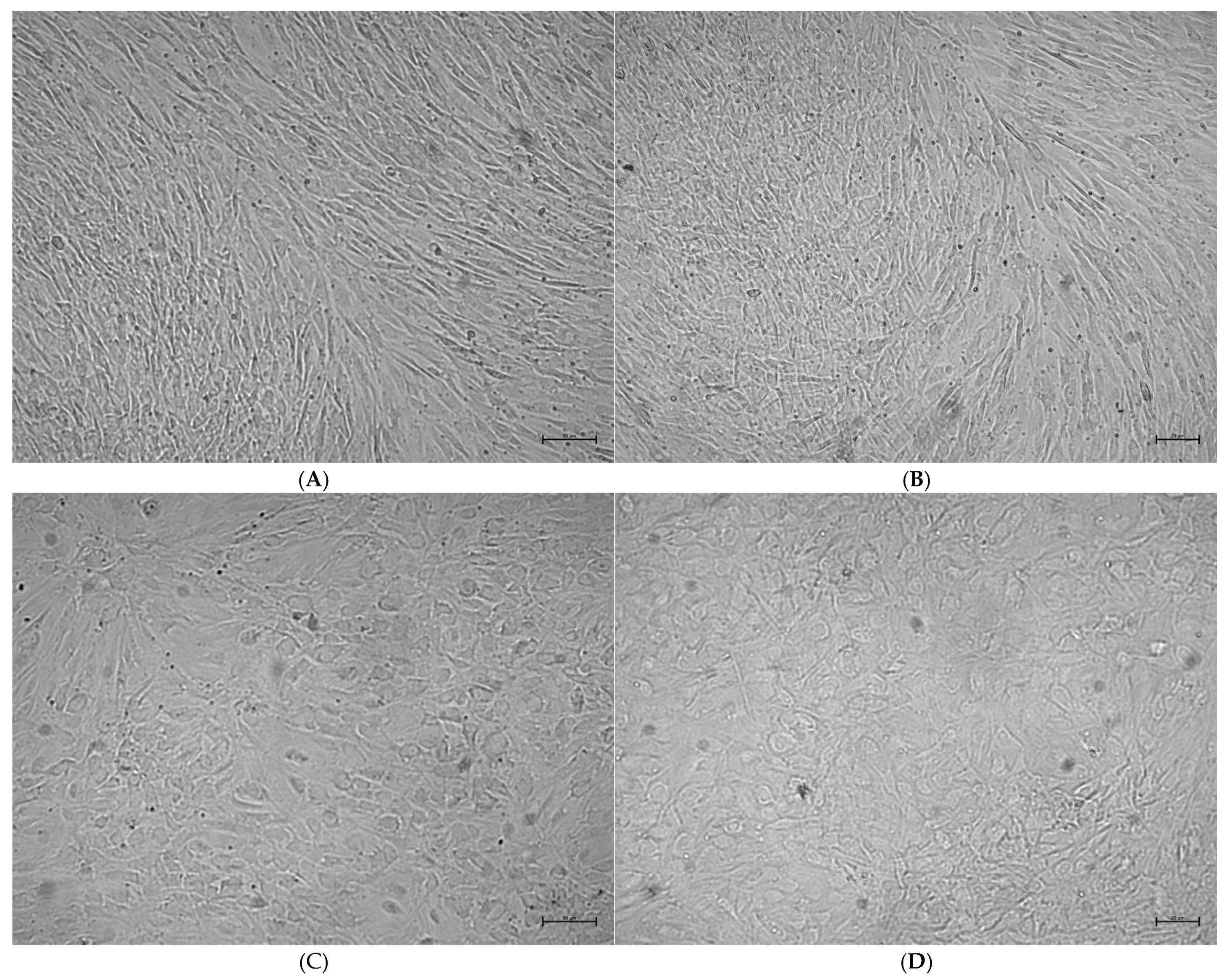
2.3. Analysis of Osteoblast Confluence in Culture
3. Discussion
4. Materials and Methods
4.1. Culture of Human Osteoblasts Line
- HOBs containing the osteoblast growth medium, including Basal Medium and SupplementMix (DMEM g).
- HOBs containing the osteoblast mineralization medium (PromoCell, C-27020) and SupplementMix (C-39616) (DMEM m).
- HOBs containing the osteoblast mineralization medium, SupplementMix, and 50 µM rosavin in final dilution (R50).
- HOBs containing the osteoblast mineralization medium, SupplementMix, and 100 µM rosavin in final dilution (R100).
4.2. Preparation of Rosavin Solution
4.3. Enzyme-Linked Immunosorbent Assay
4.4. Determination of Collagen Production and Cell Confluence in Cell Cultures
4.5. Bioethical Issues
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions-Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Perfumi, M.; Mattioli, L. Adaptogenic and central nervous system effects of Rhodiola rosea extract. Phytomedicine 2007, 14, 106–115. [Google Scholar]
- Marcos-Frutos, D.; Leban, Ž.; Li, Z.; Zhang, X.; Lara, P.M.; Alix-Fages, C.; Jiménez-Martínez, P.; Zebboudji, N.; Caillet, A.; Redondo, B.; et al. The Impact of Rhodiola Rosea Extract on Strength Performance in Alternative Bench-Press and Bench-Pull Exercises Under Resting and Mental Fatigue Conditions: A Randomized, Triple-Blinded, Placebo-Controlled, Crossover Trial. Nutrients 2025, 17, 940. [Google Scholar] [CrossRef]
- Linde, K.; Berner, M.M.; Kriston, L. The effectiveness of Rhodiola rosea in pediatric use in Sweden. BMC Complement. Altern. Med. 2009, 9, 26. [Google Scholar]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an Adaptogen to Enhance Exercise Performance: A Review of the Literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, Y.; Tong, Y.; Yuan, H.; Pang, H. Mechanism of low-intensity pulse ultrasound combined with Rhodiola to promote bone formation in spinal fusion. Cell. Mol. Biol. 2024, 70, 136–141. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Cominacini, M.; Trabetti, E.; Bombieri, C.; Pessoa, J.; Romanelli, M.G.; Valenti, M.T. The bone microenvironment: New insights into the role of stem cells and cell communication in bone regeneration. Stem Cell Res. Ther. 2025, 16, 169. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Kalal, A.A.; Mohapatra, S. A Comprehensive Review Exploring the Role of Bone Morphogenetic Proteins [BMP]: Biological Mechanisms. Curr. Issues Mol. Biol. 2025, 47, 156. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Shi, L.; Wang, J.; Zhou, J.; Rui, C.; Yin, Y.; Du, W.; Chang, S.; Rui, Y. Global, regional, and national burdens of hip fractures in elderly individuals from 1990 to 2021 and predictions up to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Arch. Gerontol. Geriatr. 2025, 133, 105832. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis. Endocr. Pract. 2010, 16 (Suppl. 3), 1–37. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.S.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef]
- Surisaeng, T.; Wisitrasameewong, W.; Champaiboon, C.; Sa-Ard-Iam, N.; Chanamuangkon, T.; Thongnuek, P.; Tam, Y.K.; Muramatsu, H.; Weissman, D.; Pardi, N.; et al. BMP-2 mRNA-transfected BMSCs promote superior calvarial bone regeneration. Sci. Rep. 2025, 15, 15022. [Google Scholar] [CrossRef]
- López De Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef]
- Koblenzer, M.; Weiler, M.; Fragoulis, A.; Rütten, S.; Pufe, T.; Jahr, H. Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells 2022, 11, 2702. [Google Scholar] [CrossRef]
- Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Őrfi, L.; Szabó, A.J.; Vannay, Á.; Veres-Székely, A. Optimization of Sirius Red-Based Microplate Assay to Investigate Collagen Production In Vitro. Int. J. Mol. Sci. 2023, 24, 17435. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone Health and Osteoporosis Prevention and Treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Haider, M.F. A comprehensive review on glucocorticoids induced osteoporosis: A medication caused disease. Steroids 2024, 207, 109440. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Y.; Zheng, L.; He, P.; Tan, J.; Cai, J.; Wu, M.; Ye, X. Rosavin: Research Advances in Extraction and Synthesis, Pharmacological Activities and Therapeutic Effects on Diseases of the Characteristic Active Ingredients of Rhodiola rosea L. Molecules 2023, 28, 7412. [Google Scholar] [CrossRef]
- Bernatonienė, J.; Jakštas, V.; Kopustinskienė, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Hung, S.K.; Perry, R.; Ernst, E. The effectiveness and efficacy of Rhodiola rosea L.: A systematic review of randomized clinical trials. Phytomedicine 2011, 18, 235–244. [Google Scholar] [CrossRef]
- Evstatieva, L.; Todorova, M.; Antonova, D.; Staneva, J. Chemical composition of the essential oils of Rhodiola rosea L. of three different origins. Pharmacogn. Mag. 2010, 6, 256–258. [Google Scholar]
- Mao, Y.; Li, Y.; Yao, N. Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 45, 510–515. [Google Scholar] [CrossRef]
- Liang, K.; Ma, S.; Luo, K.; Wang, R.; Xiao, C.; Zhang, X.; Gao, Y.; Li, M. Salidroside: An Overview of Its Promising Potential and Diverse Applications. Pharmaceuticals 2024, 17, 1703. [Google Scholar] [CrossRef]
- Aktar, A.; Bhuia, M.S.; Chowdhury, R.; Hasan, R.; Rakib, A.I.; Al Hasan, S.; Sonia, F.A.; Islam, M.T. Therapeutic promises of bioactive rosavin: A comprehensive review with mechanistic insight. Chem. Biodivers. 2024, 21, e202400286. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Turczyn, P.; Lach-Gruba, A.; Poniatowski, Ł.A.; Purrahman, D.; Mahmoudian-Sani, M.-R.; Szukiewicz, D. The Role of Rosavin in the Pathophysiology of Bone Metabolism. Int. J. Mol. Sci. 2024, 25, 2117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Huo, L.; Chai, Y.; Liu, Z.; Ren, Z.; Yu, C. Rosavin suppresses osteoclastogenesis in vivo and in vitro by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Ann. Transl. Med. 2021, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, L.; Wang, F.; Chen, M.; Li, H. Rosavin regulates bone homeostasis through HDAC1-induced epigenetic regulation of EEF2. Chem.-Biol. Interact. 2023, 384, 110696. [Google Scholar] [CrossRef] [PubMed]
- Haÿ, E.; Lemonnier, J.; Fromigué, O.; Marie, P.J. Bone Morphogenetic Protein-2 Promotes Osteoblast Apoptosis through a Smad-Independent, Protein Kinase C-Dependent Signaling Pathway. J. Biol. Chem. 2001, 276, 29028–29036. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef]
- DeConde, A.S.; Sidell, D.; Lee, M.; Bezouglaia, O.; Low, K.; Elashoff, D.; Grogan, T.; Tetradis, S.; Aghaloo, T.; St John, M. Bone Morphogenetic Protein-2-Impregnated Biomimetic Scaffolds Successfully Induce Bone Healing in a Marginal Mandibular Defect. Laryngoscope 2013, 123, 1149–1155. [Google Scholar] [CrossRef]
- Matsubara, H.; Hogan, D.E.; Morgan, E.F.; Mortlock, D.P.; Einhorn, T.A.; Gerstenfeld, L.C. Vascular Tissues Are a Primary Source of BMP2 Expression during Bone Formation Induced by Distraction Osteogenesis. Bone 2012, 51, 168–180. [Google Scholar] [CrossRef]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.; Sone, E.D. In vitro models of collagen biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2020, 32, e2004418. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The effect of curcumin on breast cancer cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Song, Y.A.; Park, Y.L.; Myung, E.; Chung, C.-Y.; Park, K.-J.; Cho, S.-B.; Lee, W.-S.; Kim, H.-S.; Rew, J.-S.; et al. Epigallocatechin-3-gallate Inhibits LPS-Induced NF-κB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M. An Overview of Bone Cells and their Regulating Factors of Differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar] [PubMed Central]
- Naoki, T.; Sakamoto, T.; Hoshi, K.; Hikita, A. Spatiotemporal analysis of osteoblast morphology and Wnt signal induced osteoblast reactivation during bone modeling in vitro. JBMR Plus 2022, 6, e10689. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, R.; Shi, X.; Wei, T.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Substance P Stimulates Bone Marrow Stromal Cell Osteogenic Activity, Osteoclast Differentiation, and Resorption Activity In Vitro. Bone 2009, 45, 309–320. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 595–611. [Google Scholar] [CrossRef]
- Park, H.; Bergeron, E.; Senta, H.; Guillemette, K.; Beauvais, S.; Blouin, R.; Sirois, J.; Faucheux, N. Sanguinarine induces apoptosis of human osteosarcoma cells through the extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2010, 399, 446–451. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in cancer treatment and cancer prevention—Review on epidemiological data and clinical trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Olsson, E.M.; von Schéele, B.; Panossian, A.G. A randomized, double-blind, placebo-controlled, parallel-group study of the standardized extract SHR-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009, 75, 105–112. [Google Scholar] [CrossRef]
- Spasov, A.A.; Wikman, G.K.; Mandrikov, V.B.; Mironova, I.A.; Neumoin, V.V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 2000, 7, 85–89. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, 16–19 October 2013. [Google Scholar]
- International Society for Stem Cell Research (ISSCR). Guidelines for Stem Cell Research and Clinical Translation. In Proceedings of the ISSCR, Virtual, 21–26 June 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdasiewicz, P.; Wróbel, E.; Stolarczyk, K.; Stolarczyk, E.U.; Mikulska, A.; Szukiewicz, D. The Effect of Rosavin, a Characteristic Compound of Rhodiola rosea, on BMP-2 Induction and Osteoblast Proliferation In Vitro. Int. J. Mol. Sci. 2025, 26, 6075. https://doi.org/10.3390/ijms26136075
Wojdasiewicz P, Wróbel E, Stolarczyk K, Stolarczyk EU, Mikulska A, Szukiewicz D. The Effect of Rosavin, a Characteristic Compound of Rhodiola rosea, on BMP-2 Induction and Osteoblast Proliferation In Vitro. International Journal of Molecular Sciences. 2025; 26(13):6075. https://doi.org/10.3390/ijms26136075
Chicago/Turabian StyleWojdasiewicz, Piotr, Edyta Wróbel, Krzysztof Stolarczyk, Elżbieta U. Stolarczyk, Agnieszka Mikulska, and Dariusz Szukiewicz. 2025. "The Effect of Rosavin, a Characteristic Compound of Rhodiola rosea, on BMP-2 Induction and Osteoblast Proliferation In Vitro" International Journal of Molecular Sciences 26, no. 13: 6075. https://doi.org/10.3390/ijms26136075
APA StyleWojdasiewicz, P., Wróbel, E., Stolarczyk, K., Stolarczyk, E. U., Mikulska, A., & Szukiewicz, D. (2025). The Effect of Rosavin, a Characteristic Compound of Rhodiola rosea, on BMP-2 Induction and Osteoblast Proliferation In Vitro. International Journal of Molecular Sciences, 26(13), 6075. https://doi.org/10.3390/ijms26136075







