Effects of High Glucose on Simulated Ischemia/Reperfusion Injury in Isolated Cardiomyocytes
Abstract
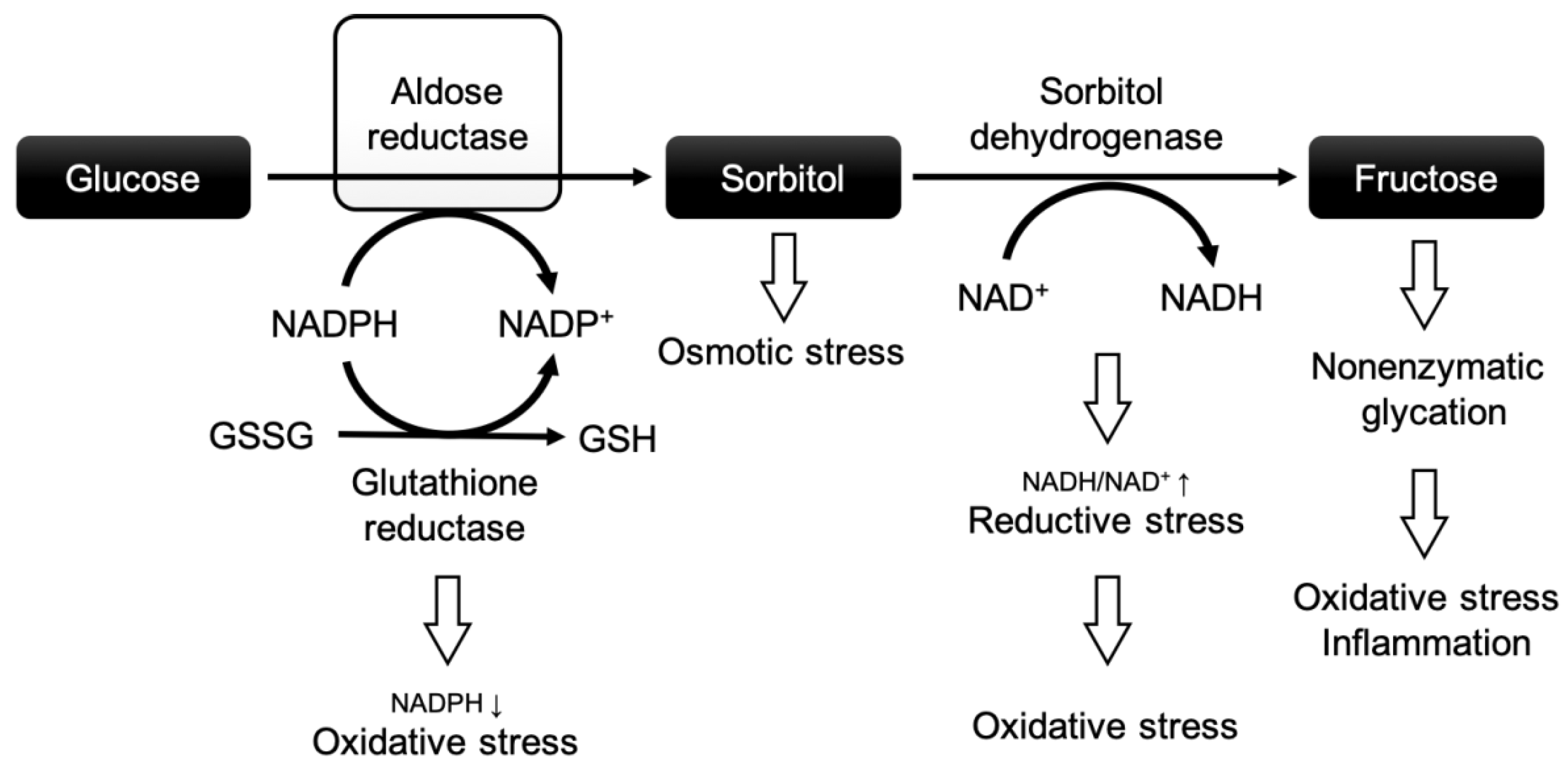
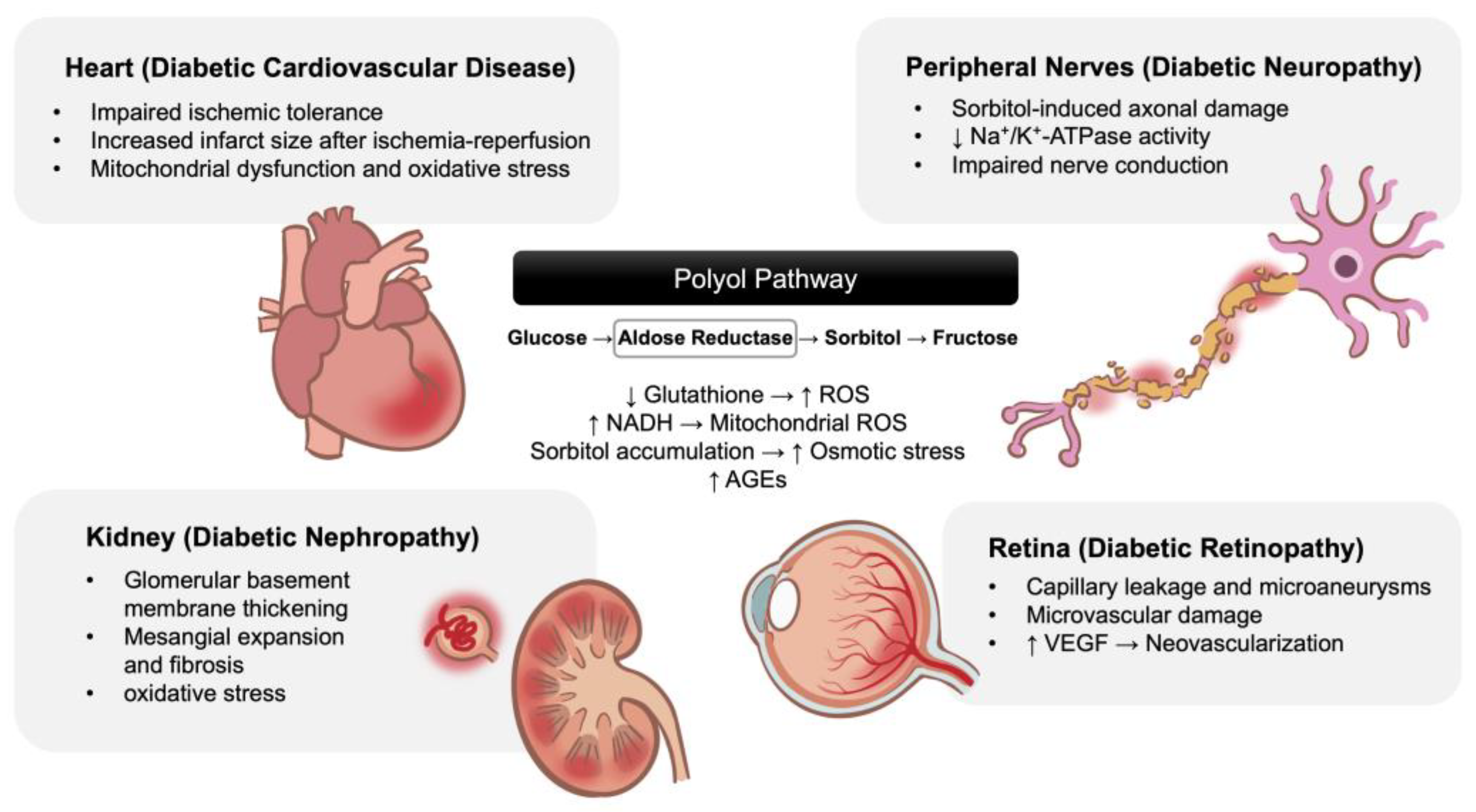
1. Introduction
- Investigate the injury process in cardiomyocytes after exposure to high glucose concentrations followed by simulated IR injury.
- Assess the potential of the AR inhibitor Epalrestat to attenuate simulated IR injury after high glucose exposure.
2. Results
2.1. Effects of High Glucose Exposure Followed by Simulated Ischemia/Reperfusion Injury in Cardiomyocytes
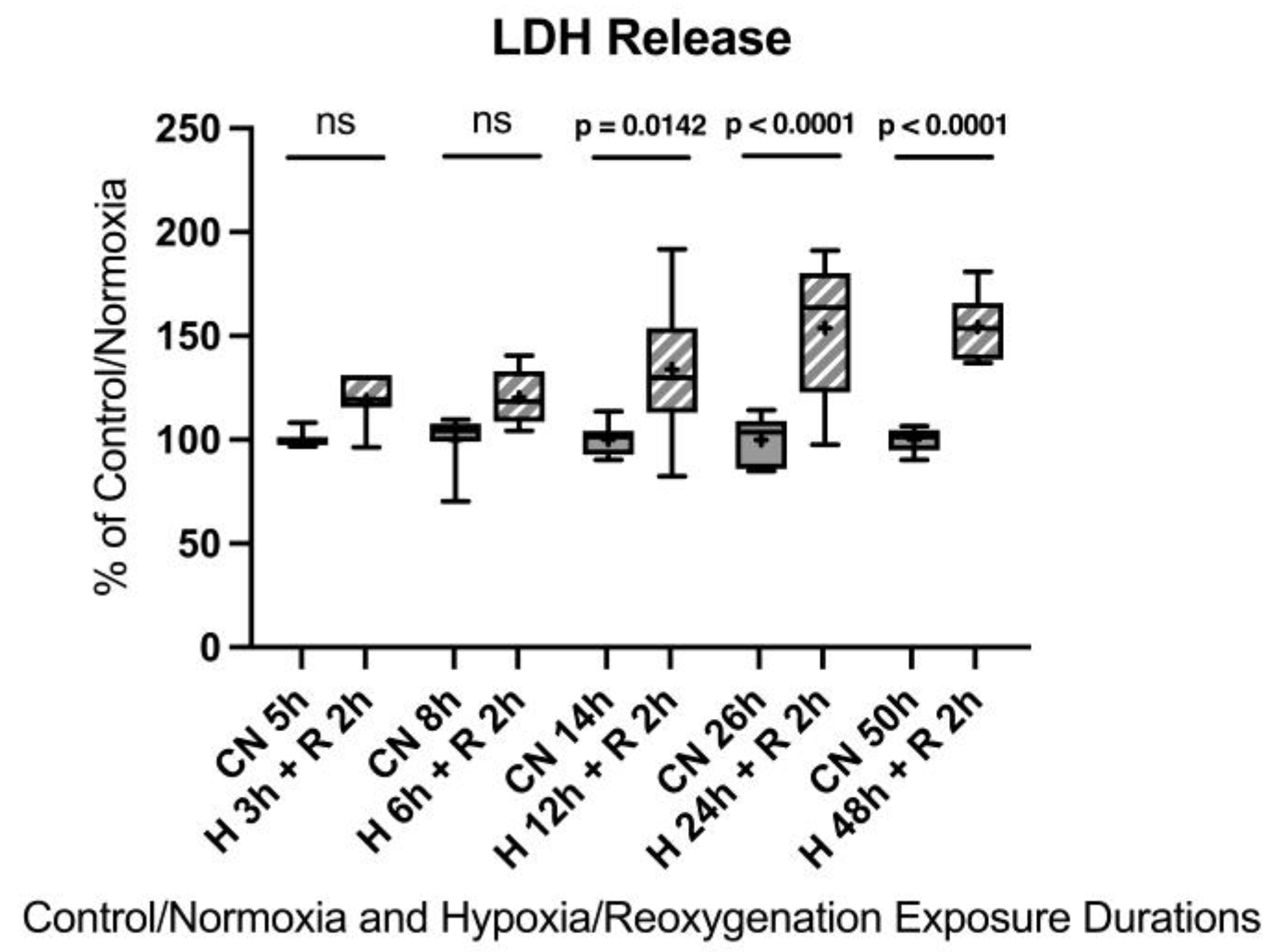
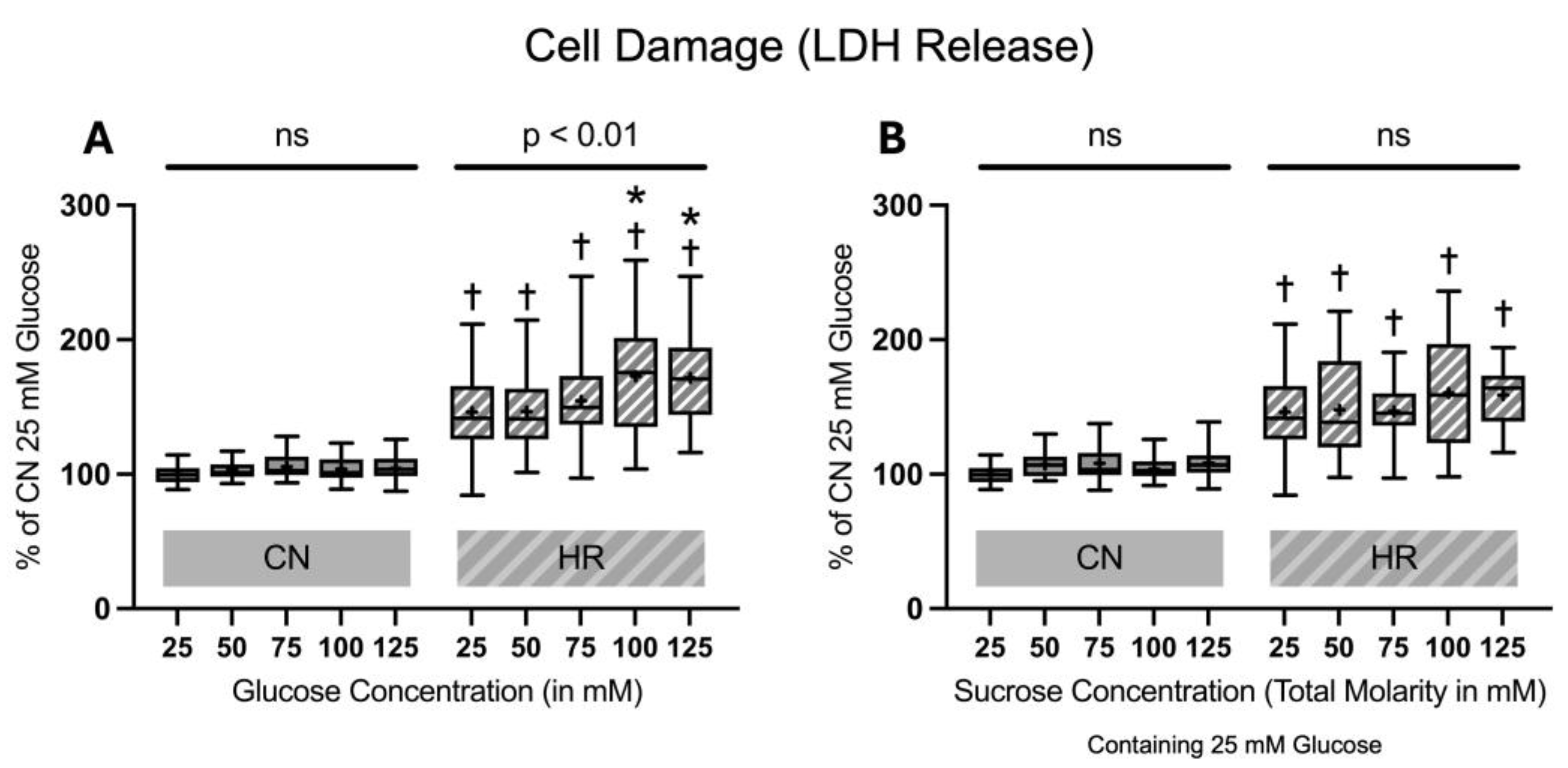
2.1.1. Cell Damage Assessed by Lactate Dehydrogenase Release
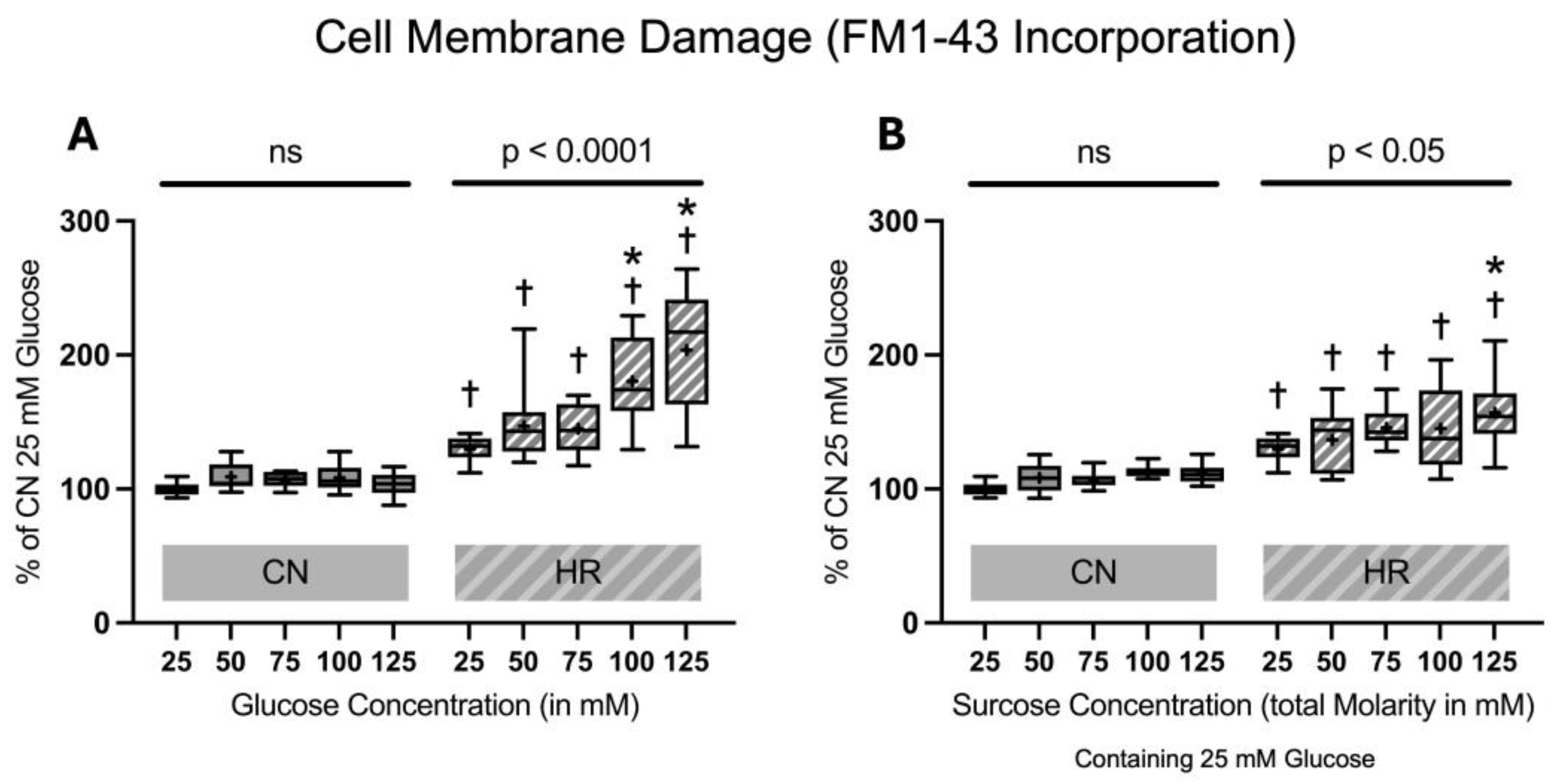
2.1.2. Cell Membrane Damage Assessed by FM1-43 Incorporation into the Cell Membrane
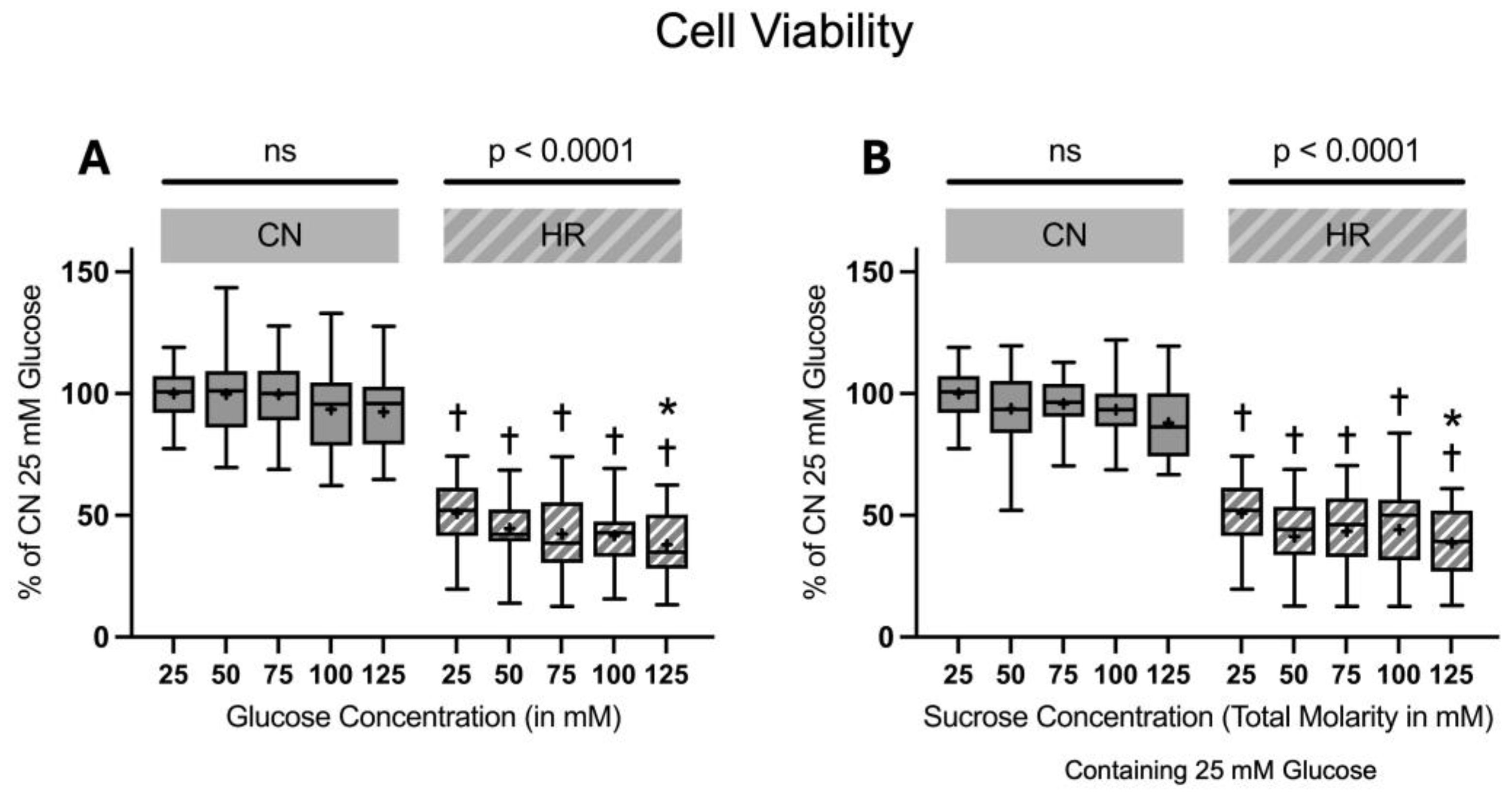
2.1.3. Cell Viability and Toxicity
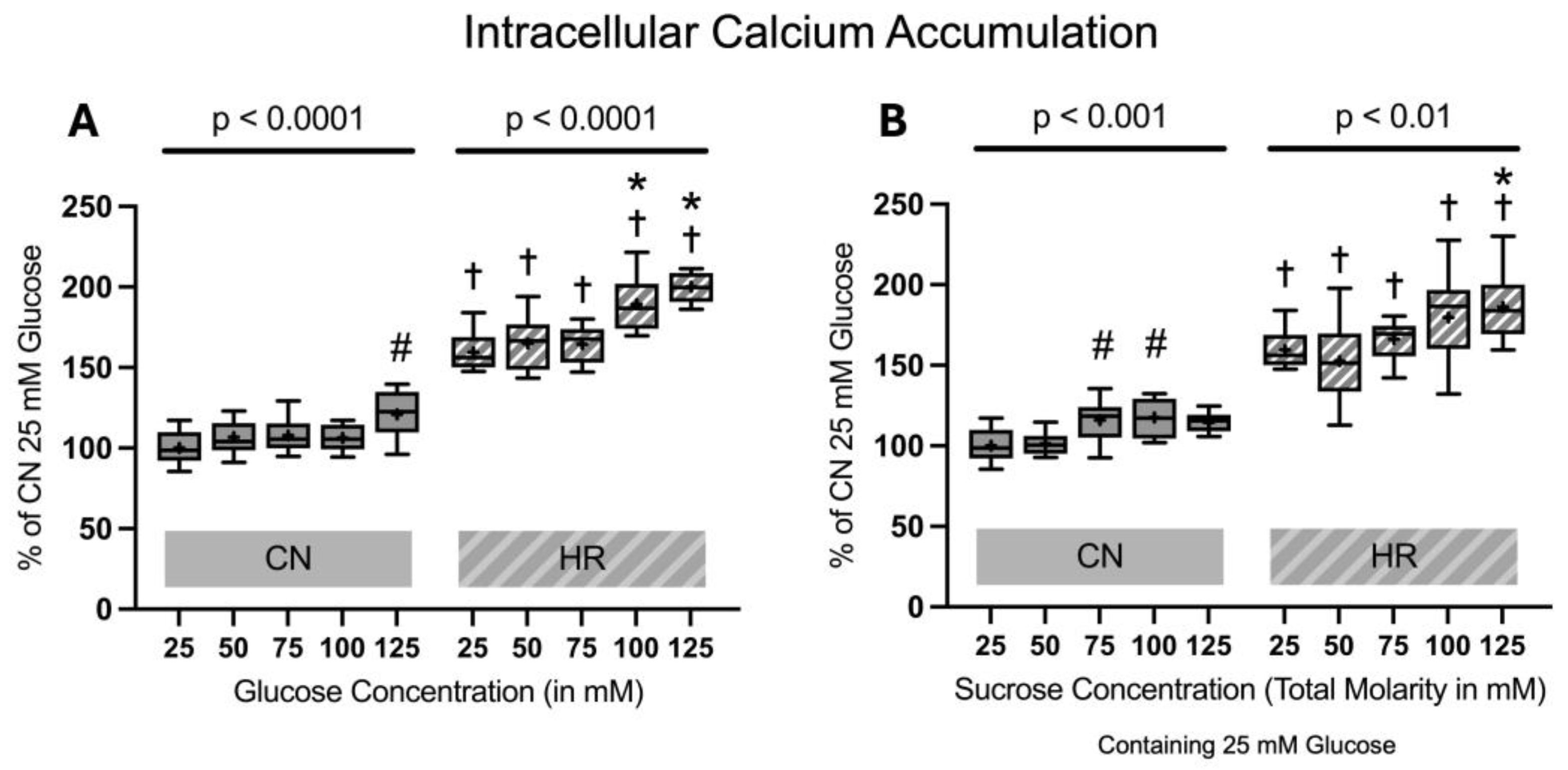
2.1.4. Intracellular Calcium Accumulation
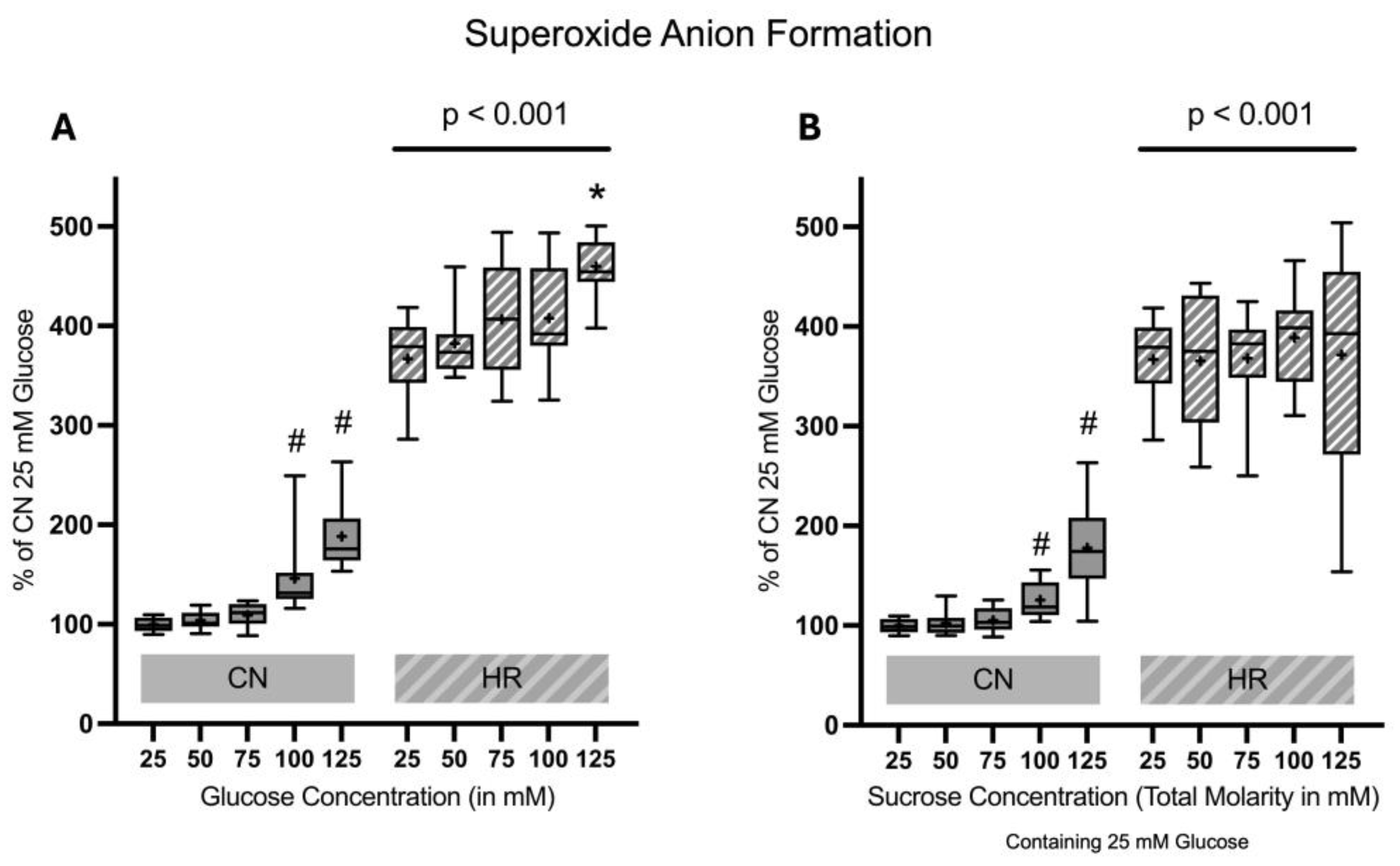
2.1.5. Superoxide Anion Formation
2.2. Effects of Aldose Reductase Inhibitor on Lactate Dehydrogenase Release of Cardiomyocytes Exposed to High Glucose or Sucrose Concentrations Followed by Hypoxia/Reoxygenation
3. Discussion
3.1. Effects of High Glucose and Ischemia/Reperfusion Injury in Cardiomyocytes
3.2. Aldose Reductase Inhibitors and Their Cardioprotective Effects
3.3. Study Limitations
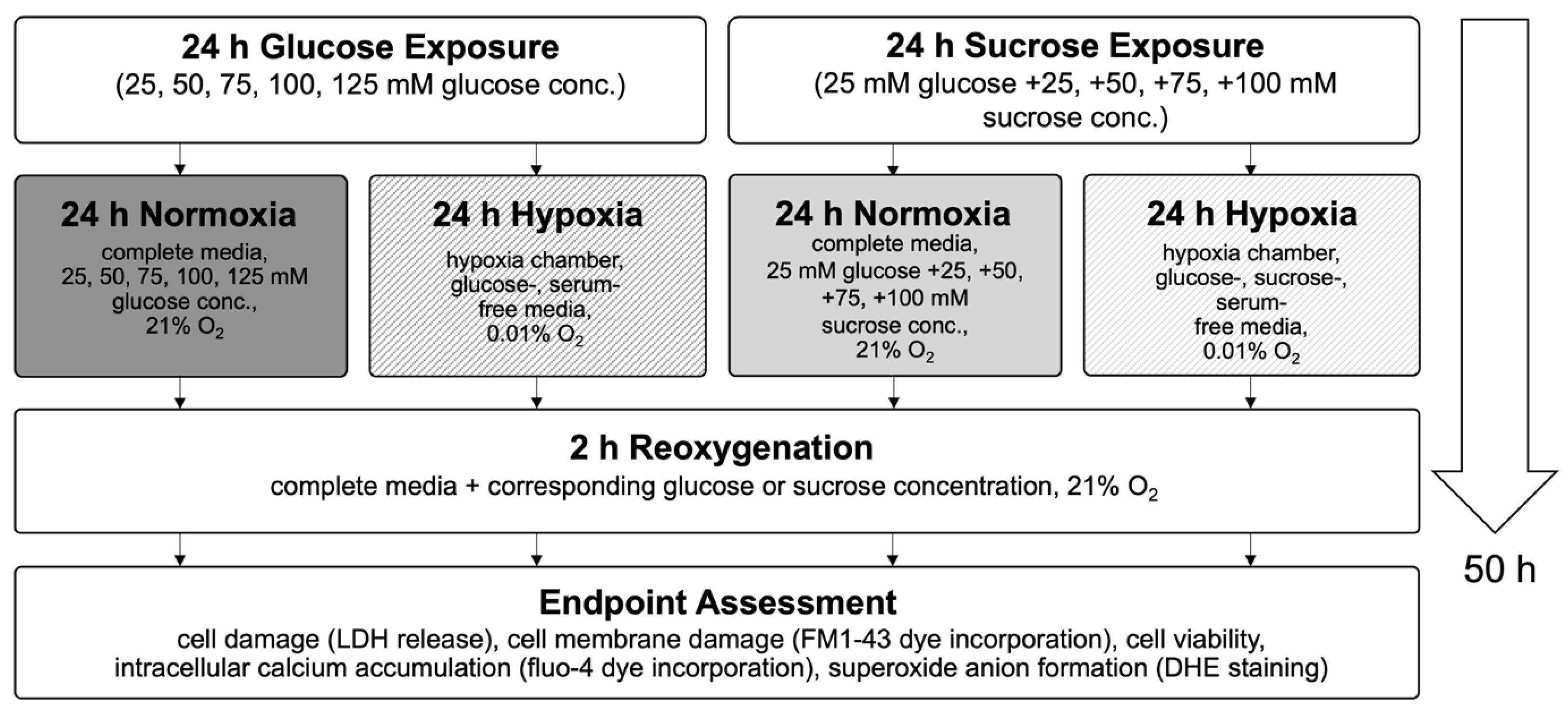
4. Materials and Methods
4.1. Cell Culture
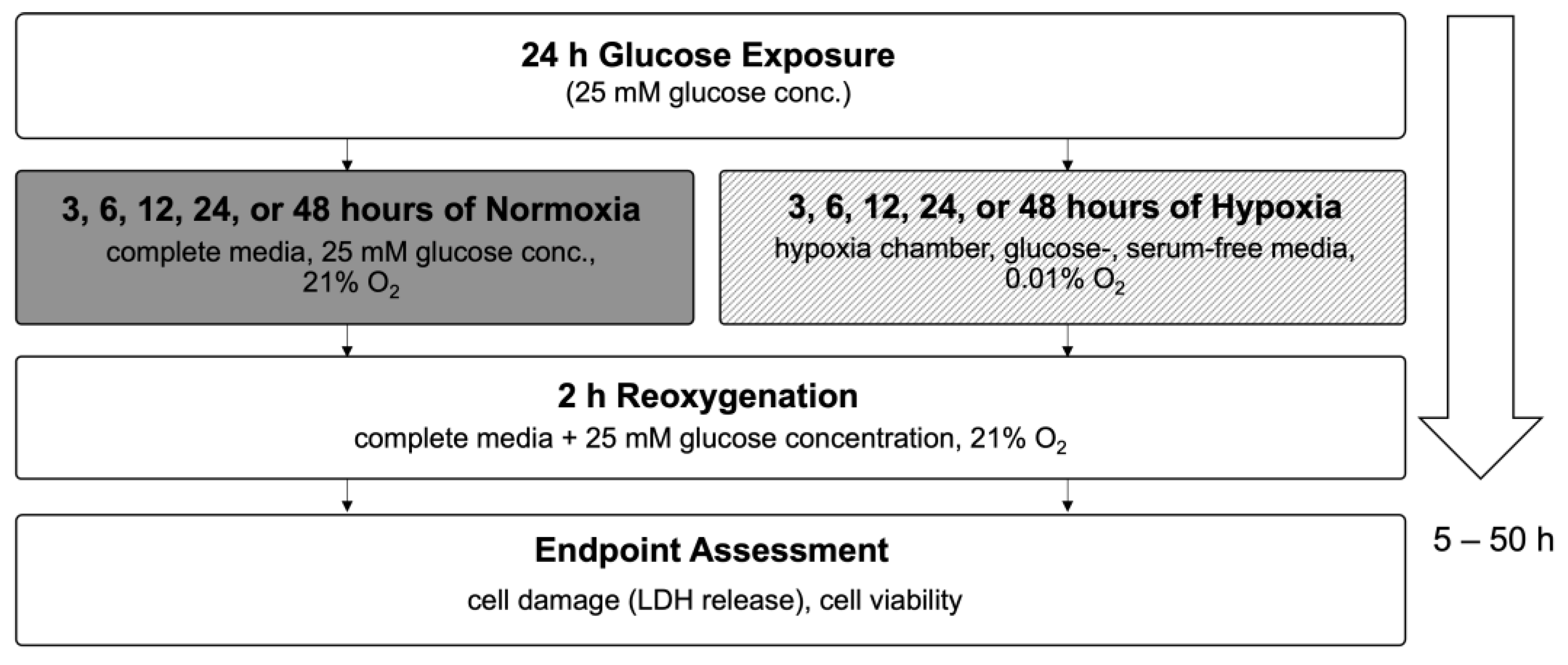
4.2. Optimization of Hypoxia/Reoxygenation Conditions for Confluent H9c2 Cardiomyocytes
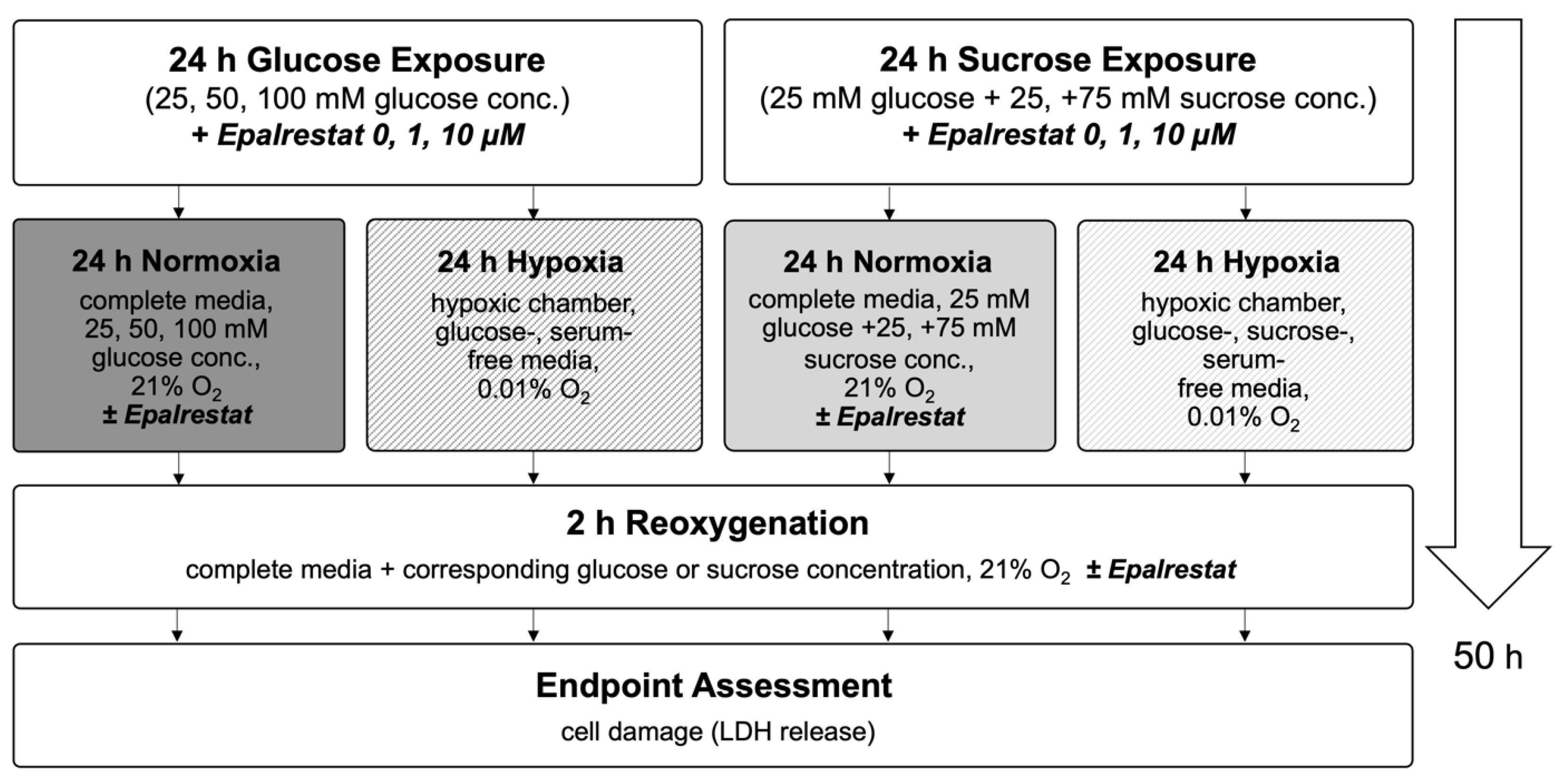
4.3. High Glucose Exposure and Hypoxia/Reoxygenation Injury Model
4.4. Aldose Reductase Inhibitor—Epalrestat
4.5. Endpoint Assessments
4.5.1. Lactate Dehydrogenase Release
4.5.2. FM1-43 Incorporation into the Cellular Membrane
4.5.3. Cell Viability and Cell Toxicity
4.5.4. Intracellular Calcium Accumulation
4.5.5. Reactive Oxygen Species Formation
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Aldose reductase |
| CN | Control/Normoxia |
| CVDs | Cardiovascular diseases |
| DHE | Dihydroethidium |
| Em | Emission |
| Ex | Excitation |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| HR | Hypoxia/reoxygenation |
| IR | Ischemia/reperfusion |
| LDH | Lactate dehydrogenase |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide + Hydrogen |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate + Hydrogen |
| PBS | Phosphate-buffered saline |
| ROS | Reactive oxygen species |
| T2DM | Type 2 diabetes mellitus |
Appendix A
| Name | Company | Catalog No. |
|---|---|---|
| −20 °C Freezer | Frigidaire | FFU17FHW4 |
| Synergy H1 Hybrid Multi-Mode Reader | BioTek Instruments, Inc. | H1MF |
| Bio-Safety Cabinet | NuAire | NU425400 |
| Hypoxia Incubator Chamber | Stemcell Technologies | 27310 |
| Centrifuge | Anstel Enterprises Inc. | 4235 |
| Cell Counter | Invitrogen | C10281 |
| Cell Culture Incubator | NuAire | Nu-5500 |
| Refrigerator (4 °C, Cell Culture Room) | Kelvinator | MRT18NREW1 |
| UltraRocker™ Rocking Platform | Bio-Rad Laboratories, Inc. | 1660719 |
| Liquid Nitrogen Tank (Cell Storage) | Thermo Fisher Scientific | CY509109 |
| Various Volume Pipets | Mettler-Toledo Rainin, LLC | 17008708 |
| Inverted Light Microscope | Nikon | TMS214343 |
| 4 °C Refrigerator | Frigidaire | FFU17G4JW25 |
| Inverted Laboratory Microscope | Leica Microsystems CMS GmbH | DM IL LED |
| CCD Microscope Camera | Leica Microsystems CMS GmbH | DFC365 FX |
| Software platform for life science LAS X Life Science | Leica Microsystems CMS GmbH | Version 2.0 |
| Name | Company | Catalog No. |
|---|---|---|
| PrestoBlue® Cell Reagent | Invitrogen by Thermo Fisher Scientific | A13262 |
| CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit | Promega | G1780 |
| Fluo-4 Direct™ Calcium Assay Kit | Invitrogen by Thermo Fisher Scientific | F10471 |
| Dihydroethidium (Hydroethidine) | Invitrogen by Thermo Fisher Scientific | D11347 |
| Membrane Impermeant Styryl Dye FM™1-43 | Molecular Probes | T3163 |
| Epalrestat | Merck by Sigma-Aldrich | SML0527 |
| D-(+)-Glucose | Sigma-Aldrich | G8270 |
| Sucrose | Sigma-Aldrich | S0389 |
| Ethanol, 200 Proof | Thermo Fisher Scientific | BP2818 |
| Sterile Water | Thermo Fisher Scientific | I9030 |
| AquaGuard Water Bath Cleaner | Thermo Fisher Scientific | 19161E |
| CO2 Cylinder for Cell Incubator | A-L Compressed Gases | UN1013 |
| 0.01% O2 Cylinder (Hypoxic Mixture) | A-L Compressed Gases | UN1956 |
| Liquid Nitrogen Tank | A-L Compressed Gases | UN4052 |
| Name | Company | Catalog No. |
|---|---|---|
| 96-well Clear Flat Bottom Polystyrene TC-treated Microplate, with Low Evaporation Lid, Sterile | Corning | 1016742 |
| Sterile Syringe Filter 0.22 µm | Fisherbrand | 09720004 |
| Cell Counting Chambers w/Trypan Blue Dye | Fisher | C10228 |
| 10 mL Pipette | CELLSTAR Serological Pipettes; Greiner Bio-One | 607 180 |
| 50 mL Pipette | CELLSTAR Serological Pipettes; Greiner Bio-One | 768 180 |
| 15 mL and 50 mL Conical Centrifuge Tubes | Thermo Fisher Scientific | 22-171-716 and 14-432-22 |
| Microcentrifuge Tubes (1.5 mL) | Research Products International | 145515A |
| Reagent Reservoirs 25 mL, Sterile | Thermo Fisher Scientific | 8096-11 |
| Pipet Tips, Various Sizes to Fit Rainin Pipets | VWR | 83009-688 |
| Plastic Weigh Boats, Small | VWR | 611-9171 |
| Paper Towels | Thermo Fisher Scientific | 06-666-114 |
| Bench Protectors (Blue Pads) | VWR | 82020-845 |
| Delicate Task Wipes | KimTech | 34155 |
| Nitrile Gloves | B. Braun | 9209817 |
| Name | Company | Catalog No. |
|---|---|---|
| H9c2(2-1) clonal cell line derived from embryonic BD1X rat heart tissue | ATCC | CRL-1446 |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | ATCC | 30-2002 |
| Fetal Bovine Serum (FBS) | ATCC | 30-2020 |
| Penicillin-Streptomycin 10.000 U/mL | Gibco by Thermo Fisher Scientific | 15140122 |
| Dulbecco’s Modified Eagle’s Medium (DMEM), Glucose- and Serum Free, Glutamine Free, without Phenol Red | Gibco by Thermo Fisher Scientific | A1443001 |
| Trypsin-EDTA (0.25%) Phenol Red (100 mL) | Gibco by Thermo Fisher Scientific | 25200056 |
| 1X Phosphate-Buffered Saline (500 mL) | Thermo Fisher Scientific | J61196.AP |
| H9c2(2-1) clonal cell line derived from embryonic BD1X rat heart tissue | ATCC | CRL-1446 |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | ATCC | 30-2002 |
| Fetal Bovine Serum (FBS) | ATCC | 30-2020 |
| Penicillin-Streptomycin 10.000 U/mL | Gibco by Thermo Fisher Scientific | 15140122 |
| Dulbecco’s Modified Eagle’s Medium (DMEM), Glucose- and Serum Free, Glutamine Free, without Phenol Red | Gibco by Thermo Fisher Scientific | A1443001 |
| Trypsin-EDTA (0.25%) Phenol Red (100 mL) | Gibco by Thermo Fisher Scientific | 25200056 |
| 1X Phosphate-Buffered Saline (500 mL) | Thermo Fisher Scientific | J61196.AP |


Appendix B
Appendix B.1. Optimization of Hypoxia Exposure Time
Appendix B.2. Cell Damage Assessed by Lactate Dehydrogenase Release
Appendix B.3. Cell Viability and Toxicity

References
- World Heart Federation World Heart Report. Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 10 September 2023).
- World Health Organization Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 10 September 2023).
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Chapter Six—Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 298, pp. 229–317. [Google Scholar]
- Braunwald, E.; Kloner, R.A. Myocardial reperfusion: A double-edged sword? J. Clin. Investig. 1985, 76, 1713–1719. [Google Scholar] [CrossRef]
- Piper, H.M.; Garcña-Dorado, D.; Ovize, M. A fresh look at reperfusion injury. Cardiovasc. Res. 1998, 38, 291–300. [Google Scholar] [CrossRef]
- Gottlieb, R.A. Cell Death Pathways in Acute Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 233–238. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Pencina, M.J.; Wilson, P.W.F.; Paynter, N.P.; Vasan, R.S.; D’Agostino, R.B., Sr. Lifetime Risk of Cardiovascular Disease Among Individuals With and Without Diabetes Stratified by Obesity Status in the Framingham Heart Study. Diabetes Care 2008, 31, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Fang, F.; John, R.; Van, J.A.D.; Barr, M.; Thaveau, F.; Chakfe, N.; Geny, B.; Scholey, J.W. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell. Cardiol. 2016, 91, 11–22. [Google Scholar] [CrossRef]
- Candido, R.; Srivastava, P.; Cooper, M.E.; Burrell, L.M. Diabetes mellitus: A cardiovascular disease. Curr. Opin. Investig. Drugs Lond. Engl. 2000 2003, 4, 1088–1094. [Google Scholar]
- Alegria, J.R.; Miller, T.D.; Gibbons, R.J.; Yi, Q.-L.; Yusuf, S. Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am. Heart J. 2007, 154, 743–750. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Balzer, C.; Cleveland, W.J.; Li, Z.; Riess, M.L. Buffer glucose adjustment affects myocardial function after ischemia-reperfusion in long-term diabetic rat isolated hearts. Physiol. Rep. 2022, 10, e15387. [Google Scholar] [CrossRef]
- Rossetti, L.; Giaccari, A.; DeFronzo, R.A. Glucose Toxicity. Diabetes Care 1990, 13, 610–630. [Google Scholar] [CrossRef]
- Heilig, C.W.; Brosius, F.C.; Henry, D.N. Glucose transporters of the glomerulus and the implications for diabetic nephropathy. Kidney Int. Suppl. 1997, 60, S91–S99. [Google Scholar] [PubMed]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Rev. Med. Chem. 2016, 16, 120–162. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 145–153. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Rolo, A.P.; Palmeira, C.M. The NAD ratio redox paradox: Why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods 2013, 23, 297–302. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, L.; Yu, N.; Ren, Z.; Wang, T.; Zhang, Y.; Zhao, X.; Yu, T. Effects of advanced glycation end products (AGEs) on the differentiation potential of primary stem cells: A systematic review. Stem Cell Res. Ther. 2023, 14, 74. [Google Scholar] [CrossRef]
- Ido, Y.; Kilo, C.; Williamson, J.R. Interactions between the sorbitol pathway, non-enzymatic glycation, and diabetic vascular dysfunction. Nephrol. Dial. Transplant. 1996, 11 (Suppl. 5), 72–75. [Google Scholar] [CrossRef]
- Dia, M.; Paccalet, A.; Pillot, B.; Leon, C.; Ovize, M.; Crola Da Silva, C.; Bochaton, T.; Paillard, M. Myocardial Ischemia-Reperfusion and Diabetes: Lessons Learned From Bedside to Bench. Front. Cardiovasc. Med. 2021, 8, 660698. [Google Scholar] [CrossRef]
- Russo, I.; Penna, C.; Musso, T.; Popara, J.; Alloatti, G.; Cavalot, F.; Pagliaro, P. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc. Diabetol. 2017, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, D. Research progress on the effects of novel hypoglycemic drugs in diabetes combined with myocardial ischemia/reperfusion injury. Ageing Res. Rev. 2023, 86, 101884. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E. Neuropathy: Metabolically-Induced. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 767–775. ISBN 978-0-08-045046-9. [Google Scholar]
- Son, N.-H.; Ananthakrishnan, R.; Yu, S.; Khan, R.S.; Jiang, H.; Ji, R.; Akashi, H.; Li, Q.; O’Shea, K.; Homma, S.; et al. Cardiomyocyte Aldose Reductase Causes Heart Failure and Impairs Recovery from Ischemia. PLoS ONE 2012, 7, e46549. [Google Scholar] [CrossRef]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. J. Diabetes Res. 2007, 2007, 061038. [Google Scholar] [CrossRef] [PubMed]
- Larkins, R.G.; Dunlop, M.E. The link between hyperglycaemia and diabetic nephropathy. Diabetologia 1992, 35, 499–504. [Google Scholar] [CrossRef]
- Song, L.; Ye, J.; Cheng, Q. Therapeutic effects of mecobalamin combined with epalrestat on diabetic peripheral neuropathy: Reduction of inflammatory factors and improvement in electromyogram indices. Am. J. Transl. Res. 2025, 17, 2898–2906. [Google Scholar] [CrossRef]
- Ohi, T.; Saita, K.; Furukawa, S.; Ohta, M.; Hayashi, K.; Matsukura, S. Therapeutic effects of aldose reductase inhibitor on experimental diabetic neuropathy through synthesis/secretion of nerve growth factor. Exp. Neurol. 1998, 151, 215–220. [Google Scholar] [CrossRef]
- He, J.; Gao, H.; Yang, N.; Zhu, X.; Sun, R.; Xie, Y.; Zeng, C.; Zhang, J.; Wang, J.; Ding, F.; et al. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol. Sin. 2019, 40, 86–97. [Google Scholar] [CrossRef]
- Tsugawa, T.; Shinohara, R.; Nagasaka, A.; Nakano, I.; Takeda, F.; Nagata, M.; Oda, N.; Sawai, Y.; Hayakawa, N.; Suzuki, A.; et al. Alteration of urinary sorbitol excretion in WBN-kob diabetic rats—Treatment with an aldose reductase inhibitor. J. Endocrinol. 2004, 181, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, I.; Shimizu, K.; Kamanaka, Y.; Ebata, K.; Kikkawa, R.; Haneda, M.; Shigeta, Y. The effect of an aldose reductase inhibitor (Epalrestat) on diabetic nephropathy in rats. Diabetes Res. Clin. Pract. 1994, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Reihanifar, T.; Şahin, M.; Stefek, M.; Ceylan, A.F.; Karasu, Ç. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group Cemtirestat, an aldose reductase inhibitor and antioxidant compound, induces ocular defense against oxidative and inflammatory stress in rat models for glycotoxicity. Cell Biochem. Funct. 2023, 41, 622–632. [Google Scholar] [CrossRef]
- Dong, F.; Ren, J. Fidarestat improves cardiomyocyte contractile function in db/db diabetic obese mice through a histone deacetylase Sir2-dependent mechanism. J. Hypertens. 2007, 25, 2138–2147. [Google Scholar] [CrossRef]
- Robison, W.G.; Laver, N.M.; Jacot, J.L.; Glover, J.P. Sorbinil prevention of diabetic-like retinopathy in the galactose-fed rat model. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2368–2380. [Google Scholar]
- Verma, S.; Maitland, A.; Weisel, R.D.; Li, S.-H.; Fedak, P.W.M.; Pomroy, N.C.; Mickle, D.A.G.; Li, R.-K.; Ko, L.; Rao, V. Hyperglycemia exaggerates ischemia-reperfusion-induced cardiomyocyte injury: Reversal with endothelin antagonism. J. Thorac. Cardiovasc. Surg. 2002, 123, 1120–1124. [Google Scholar] [CrossRef]
- Jun, J.H.; Jun, N.-H.; Shim, J.-K.; Shin, E.J.; Kwak, Y.-L. Erythropoietin protects myocardium against ischemia-reperfusion injury under moderate hyperglycemia. Eur. J. Pharmacol. 2014, 745, 1–9. [Google Scholar] [CrossRef]
- Han, R.-H.; Huang, H.-M.; Han, H.; Chen, H.; Zeng, F.; Xie, X.; Liu, D.-Y.; Cai, Y.; Zhang, L.-Q.; Liu, X.; et al. Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia. Mil. Med. Res. 2021, 8, 58. [Google Scholar] [CrossRef]
- Yu, F.; Liu, F.; Luo, J.-Y.; Zhao, Q.; Wang, H.-L.; Fang, B.-B.; Li, X.-M.; Yang, Y.-N. Targeted activation of ERK1/2 reduces ischemia and reperfusion injury in hyperglycemic myocardium by improving mitochondrial function. Ann. Transl. Med. 2022, 10, 1238. [Google Scholar] [CrossRef]
- Pálóczi, J.; Paál, Á.; Pigler, J.; Kiss, B.; Rhoden, A.; Varga, Z.V.; Ferdinandy, P.; Eschenhagen, T.; Görbe, A. Organ-specific model of simulated ischemia/reperfusion and hyperglycemia based on engineered heart tissue. Vascul. Pharmacol. 2023, 152, 107208. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, Z.; Jiang, L.; Wang, L.; Yang, Y.; Wang, Q.; Qian, X.; Zeng, W.; Yang, W.; Liang, R.; et al. Oxytocin ameliorates high glucose- and ischemia/reperfusion-induced myocardial injury by suppressing pyroptosis via AMPK signaling pathway. Biomed. Pharmacother. 2022, 153, 113498. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Physiological and Pathological Roles of Aldose Reductase. Metabolites 2021, 11, 655. [Google Scholar] [CrossRef]
- Galvez, A.; Morales, M.P.; Eltit, J.M.; Ocaranza, P.; Carrasco, L.; Campos, X.; Sapag-Hagar, M.; Díaz-Araya, G.; Lavandero, S. A rapid and strong apoptotic process is triggered by hyperosmotic stress in cultured rat cardiac myocytes. Cell Tissue Res. 2001, 304, 279–285. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, W.; Guo, J.; Wang, X. Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na+/H+ exchanger. Sci. Signal. 2022, 15, eabn5881. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Peana, D.; Veteto, A.B.; Lambert, M.D.; Nourian, Z.; Karasseva, N.G.; Hill, M.A.; Lindman, B.R.; Baines, C.P.; Krenz, M.; et al. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc. Res. 2019, 115, 46–56. [Google Scholar] [CrossRef]
- Eisner, V.; Criollo, A.; Quiroga, C.; Olea-Azar, C.; Santibañez, J.F.; Troncoso, R.; Chiong, M.; Díaz-Araya, G.; Foncea, R.; Lavandero, S. Hyperosmotic stress-dependent NFkappaB activation is regulated by reactive oxygen species and IGF-1 in cultured cardiomyocytes. FEBS Lett. 2006, 580, 4495–4500. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Raskin, P.; Pietri, A.O.; Unger, R.; Shannon, W.A. The effect of diabetic control on the width of skeletal-muscle capillary basement membrane in patients with Type I diabetes mellitus. N. Engl. J. Med. 1983, 309, 1546–1550. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Štambuk, T.; Gornik, O. Protein Glycosylation in Diabetes. Adv. Exp. Med. Biol. 2021, 1325, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. WJG 2009, 15, 4137–4142. [Google Scholar] [CrossRef]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Krinsley, J.S. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin. Proc. 2003, 78, 1471–1478. [Google Scholar] [CrossRef]
- Ansley, D.M.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013, 229, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Itoh, T.; Sunaga, D.; Miura, T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc. Diabetol. 2012, 11, 67. [Google Scholar] [CrossRef]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef]
- Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Complicat. 2010, 24, 354–360. [Google Scholar] [CrossRef]
- Ramirez, M.A.; Borja, N.L. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 2008, 28, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Hotta, N.; Kawamori, R.; Fukuda, M.; Shigeta, Y. Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: Multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet. Med. J. Br. Diabet. Assoc. 2012, 29, 1529–1533. [Google Scholar] [CrossRef]
- Hotta, N.; Kawamori, R.; Atsumi, Y.; Baba, M.; Kishikawa, H.; Nakamura, J.; Oikawa, S.; Yamada, N.; Yasuda, H.; Shigeta, Y.; et al. Stratified analyses for selecting appropriate target patients with diabetic peripheral neuropathy for long-term treatment with an aldose reductase inhibitor, epalrestat. Diabet. Med. J. Br. Diabet. Assoc. 2008, 25, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Hotta, N. Is there a place for inhibition of transforming growth factor-β and the polyol pathway in therapy for diabetic retinopathy? J. Diabetes Investig. 2010, 1, 134–136. [Google Scholar] [CrossRef]
- Ramasamy, R.; Oates, P.J.; Schaefer, S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 1997, 46, 292–300. [Google Scholar] [CrossRef]
- Trueblood, N.; Ramasamy, R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, H75–H83. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Kaneko, M.; Bakr, S.; Liao, H.; Lu, Y.; Lewis, E.R.; Yan, S.; Ii, S.; Itakura, M.; Rui, L.; et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004, 18, 1192–1199. [Google Scholar] [CrossRef]
- Watarai, A.; Nakashima, E.; Hamada, Y.; Watanabe, G.; Naruse, K.; Miwa, K.; Kobayashi, Y.; Kamiya, H.; Nakae, M.; Hamajima, N.; et al. Aldose reductase gene is associated with diabetic macroangiopathy in Japanese Type 2 diabetic patients. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 894–899. [Google Scholar] [CrossRef]
- Johnson, B.F.; Nesto, R.W.; Pfeifer, M.A.; Slater, W.R.; Vinik, A.I.; Chyun, D.A.; Law, G.; Wackers, F.J.T.; Young, L.H. Cardiac abnormalities in diabetic patients with neuropathy: Effects of aldose reductase inhibitor administration. Diabetes Care 2004, 27, 448–454. [Google Scholar] [CrossRef]
- Gaztanaga, J.; Ramasamy, R.; Schmidt, A.M.; Fishman, G.; Schendelman, S.; Thangavelu, K.; Perfetti, R.; Katz, S.D. A pilot open-label study of aldose reductase inhibition with AT-001 (caficrestat) in patients hospitalized for COVID-19 infection: Results from a registry-based matched-control analysis. Diabetes Metab. Syndr. 2021, 15, 102328. [Google Scholar] [CrossRef]
- Kato, A.; Kobayashi, K.; Narukawa, K.; Minoshima, Y.; Adachi, I.; Hirono, S.; Nash, R.J. 6,7-Dihydroxy-4-phenylcoumarin as inhibitor of aldose reductase 2. Bioorg. Med. Chem. Lett. 2010, 20, 5630–5633. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Sickinger, S.; Frotschnig, S.; Grimm, M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 276–284. [Google Scholar] [CrossRef]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. Vitro Cell. Dev. Biol. Anim. 2011, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ascuitto, R.J.; Ross-Ascuitto, N.T. Substrate metabolism in the developing heart. Semin. Perinatol. 1996, 20, 542–563. [Google Scholar] [CrossRef]
- Bartelds, B.; Knoester, H.; Smid, G.B.; Takens, J.; Visser, G.H.; Penninga, L.; van der Leij, F.R.; Beaufort-Krol, G.C.; Zijlstra, W.G.; Heymans, H.S.; et al. Perinatal changes in myocardial metabolism in lambs. Circulation 2000, 102, 926–931. [Google Scholar] [CrossRef]
- Piquereau, J.; Ventura-Clapier, R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front. Physiol. 2018, 9, 959. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gérard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease Modeling and Phenotypic Drug Screening for Diabetic Cardiomyopathy using Human Induced Pluripotent Stem Cells. Cell Rep. 2014, 9, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Salzman, M.M.; Bartos, J.A.; Yannopoulos, D.; Riess, M.L. Poloxamer 188 Protects Isolated Adult Mouse Cardiomyocytes from Reoxygenation Injury. Pharmacol. Res. Perspect. 2020, 8, e00639. [Google Scholar] [CrossRef]
- Ramasamy, R.; Goldberg, I.J. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ. Res. 2010, 106, 1449–1458. [Google Scholar] [CrossRef]
- Walter, M.J.; Shiota, M.; Barajas, M.B.; Oyama, T.; Riess, M.L. Glucose Toxicity in Isolated Cardiomyocytes Undergoing Hypoxia/Reoxygenation. Anästhesiol. Intensivmed. 2023, 64, S220. [Google Scholar]
- Karasu, Ç. Time course of changes in endothelium-dependent and -independent relaxation of chronically diabetic aorta: Role of reactive oxygen species. Eur. J. Pharmacol. 2000, 392, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shiota, M.; Li, Z.; Barajas, M.B.; Oyama, T.; Riess, M.L. Abstract 176: The Pathological Role of Aldose Reductase in Isolated Cardiomyocytes Undergoing Hypoxia/Reoxygenation After Prior Exposure to High Glucose Concentrations. Circulation 2023, 148, A176. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef] [PubMed]
- Cochilla, A.J.; Angleson, J.K.; Betz, W.J. Monitoring secretory membrane with FM1-43 fluorescence. Annu. Rev. Neurosci. 1999, 22, 1–10. [Google Scholar] [CrossRef]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, M.J.K.; Shiota, M.; Li, Z.; Barajas, M.B.; Oyama, T.; Riess, M.L. Effects of High Glucose on Simulated Ischemia/Reperfusion Injury in Isolated Cardiomyocytes. Int. J. Mol. Sci. 2025, 26, 6050. https://doi.org/10.3390/ijms26136050
Walter MJK, Shiota M, Li Z, Barajas MB, Oyama T, Riess ML. Effects of High Glucose on Simulated Ischemia/Reperfusion Injury in Isolated Cardiomyocytes. International Journal of Molecular Sciences. 2025; 26(13):6050. https://doi.org/10.3390/ijms26136050
Chicago/Turabian StyleWalter, Miriam J. K., Masakazu Shiota, Zhu Li, Matthew B. Barajas, Takuro Oyama, and Matthias L. Riess. 2025. "Effects of High Glucose on Simulated Ischemia/Reperfusion Injury in Isolated Cardiomyocytes" International Journal of Molecular Sciences 26, no. 13: 6050. https://doi.org/10.3390/ijms26136050
APA StyleWalter, M. J. K., Shiota, M., Li, Z., Barajas, M. B., Oyama, T., & Riess, M. L. (2025). Effects of High Glucose on Simulated Ischemia/Reperfusion Injury in Isolated Cardiomyocytes. International Journal of Molecular Sciences, 26(13), 6050. https://doi.org/10.3390/ijms26136050







