Untargeted Metabolomics to Harness Ideal Protein Concept and Mitigate Environmental Impact in Rabbit Models
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Statement
4.2. Experimental Diets
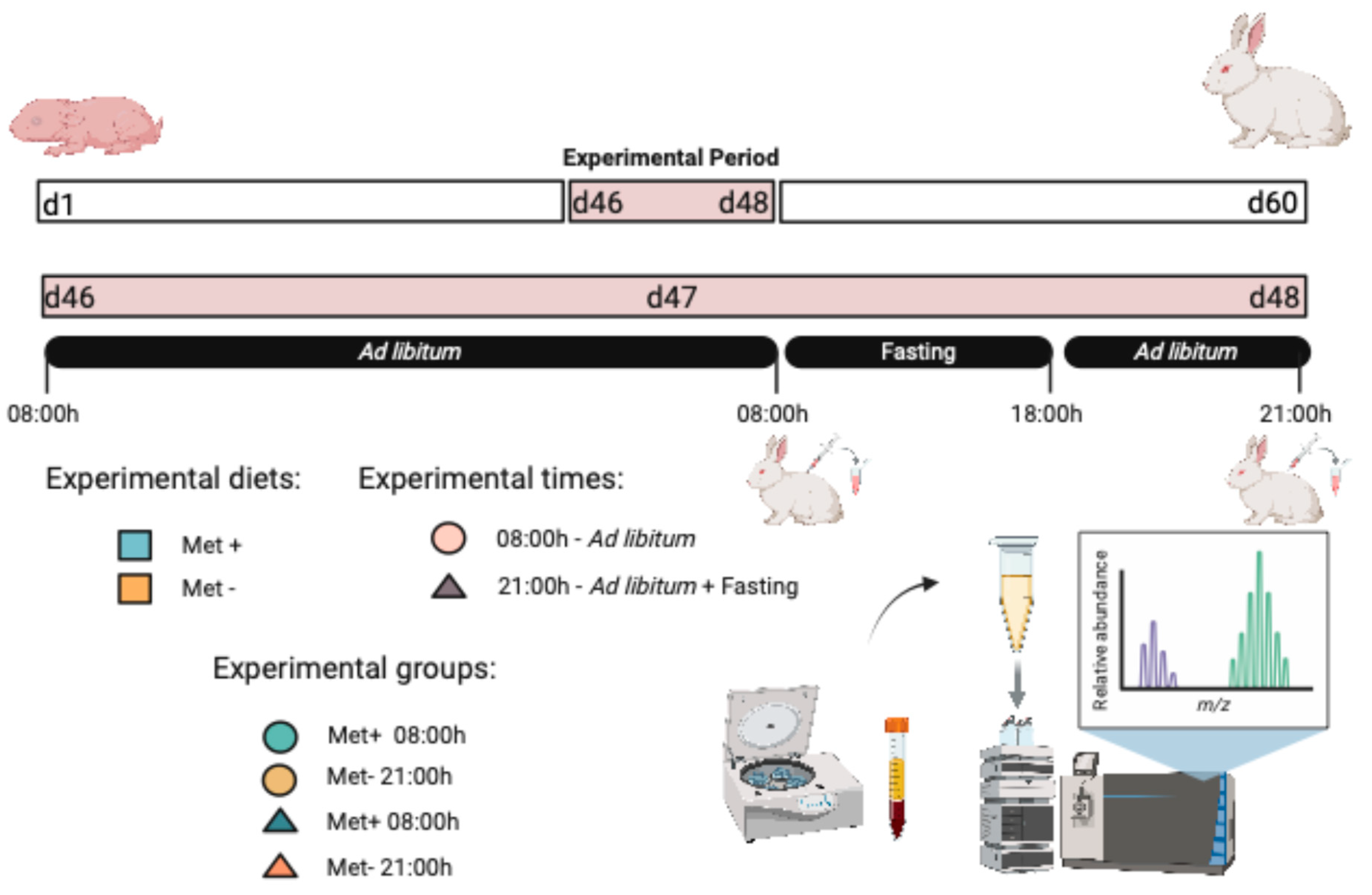
4.3. Experimental Design
4.4. Chemical Analysis
4.5. LC-MS Metabolomics Analysis of Plasma
4.5.1. Chemical Solvents and Standards for Metabolomics Analysis
4.5.2. Sample Preparation and LC-MS Analysis
4.5.3. Sample Quality Control and Metabolomics Data Pre-Processing
4.5.4. Metabolite Identification
4.5.5. Metabolites Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| LC−MS | liquid chromatography−mass spectrometry |
| PCA | principal component analysis |
| PLS-DA | partial least-squares discriminant analysis |
| ACN | acetonitrile |
| IS | internal standards |
| HPLC | high-performance liquid chromatography |
| m/z | mass to charge ratio |
| QC | quality control |
| LPCs | lysophosphatidylcholines |
References
- Velthof, G.L.; Hou, Y.; Oenema, O. Nitrogen excretion factors of livestock in the European Union: A review: Nitrogen excretion factors of livestock in EU. J. Sci. Food Agric. 2015, 95, 3004–3014. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Webb, J.; Sørensen, P.; Velthof, G.; Amon, B.; Pinto, M.; Rodhe, L.; Salomon, E.; Hutchings, N.; Burczyk, P.; Reid, J. An Assessment of the Variation of Manure Nitrogen Efficiency throughout Europe and an Appraisal of Means to Increase Manure-N Efficiency. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2013; Volume 119, pp. 371–442. [Google Scholar]
- Langner, J.; Bergström, R.; Foltescu, V. Impact of climate change on surface ozone and deposition of sulphur and nitrogen in Europe. Atmos. Environ. 2005, 39, 1129–1141. [Google Scholar] [CrossRef]
- Van Milgen, J.; Dourmad, J.-Y. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Hou, Y.; Bai, Z.; Lesschen, J.P.; Staritsky, I.G.; Sikirica, N.; Ma, L.; Velthof, G.L.; Oenema, O. Feed use and nitrogen excretion of livestock in EU-27. Agric. Ecosyst. Environ. 2016, 218, 232–244. [Google Scholar] [CrossRef]
- Matsumoto, S.; Häberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders—Update. J. Hum. Genet. 2019, 64, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; Ródenas, L.; Martínez-Paredes, E.; Cambra-López, M.; Blas, E.; Pascual, J. A moderate protein diet does not cover the requirements of growing rabbits with high growth rate. Anim. Feed. Sci. Technol. 2020, 264, 114495. [Google Scholar] [CrossRef]
- Emmert, J.L.; Baker, D.H. Use of the Ideal Protein Concept for Precision Formulation of Amino Acid Levels in Broiler Diets. J. Appl. Poult. Res. 1997, 6, 462–470. [Google Scholar] [CrossRef]
- Puntmann, V.O. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Esko, T.; Hirschhorn, J.N.; AFeldman, H.; Hsu, Y.-H.H.; ADeik, A.; Clish, C.B.; Ebbeling, C.B.; Ludwig, D.S. Metabolomic profiles as reliable biomarkers of dietary composition. Am. J. Clin. Nutr. 2017, 105, 547–554. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; López-Luján, M.C.; Ródenas, L.; Martínez-Paredes, E.M.; Blas, E.; Pascual, J.J. Plasmatic Urea Nitrogen in Growing Rabbits with Different Combinations of Dietary Levels of Lysine, Sulphur Amino Acids and Threonine. Animals 2020, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; López-Luján, M.d.C.; Ródenas, L.; Martínez-Paredes, E.M.; Blas, E.; Pascual, J.J. Plasma urea nitrogen as an indicator of amino acid imbalance in rabbit diets. World Rabbit. Sci. 2020, 28, 63. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L.; López-Lujan, M.C.; Cambra-López, M.; Blas, E.; Pascual, J.J. Urea Nitrogen Metabolite Can Contribute to Implementing the Ideal Protein Concept in Monogastric Animals. Animals 2022, 12, 2344. [Google Scholar] [CrossRef]
- He, Q.; Kong, X.; Wu, G.; Ren, P.; Tang, H.; Hao, F.; Huang, R.; Li, T.; Tan, B.; Li, P.; et al. Metabolomic analysis of the response of growing pigs to dietary l-arginine supplementation. Amino Acids 2009, 37, 199–208. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L.; Cambra-López, M.; Blas, E.; Larsen, T.; Pascual, J.J.; Hedemann, M.S. Biomarkers for ideal protein: Rabbit diet metabolomics varying key amino acids. Commun. Biol. 2024, 7, 712. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Piles, M.; Sánchez, J.P.; Pascual, M.; Llobat, L.; Pascual, J.J.; Hedemann, M.S. Untargeted urine metabolomics suggests that ascorbic acid may serve as a promising biomarker for reduced feed intake in rabbits. Sci. Rep. 2024, 14, 29180. [Google Scholar] [CrossRef]
- Taboada, E.; Mendez, J.; De Blas, J. The response of highly productive rabbits to dietary sulphur amino acid content for reproduction and growth. Reprod. Nutr. Dev. 1996, 36, 191–203. [Google Scholar] [CrossRef]
- Berchiche, M.; Lebas, F. Supplémentation en Méthionine dun aliment a base de féverole: Effets sur la croissance, le rendement a l’abattage et la composition de la carcasse chez le lapin. World Rabbit. Sci. 2010, 2, 135–140. [Google Scholar] [CrossRef]
- Adamson, I.; Fisher, H. Amino Acid Requirement of the Growing Rabbit: An Estimate of Quantitative Needs. J. Nutr. 1973, 103, 1306–1310. [Google Scholar] [CrossRef]
- Marín-García, P.J.; López-Luján, M.C.; Ródenas, L.; Martínez-Paredes, E.; Cambra-López, M.; Blas, E.; Pascual, J.J. Do Growing Rabbits with a High Growth Rate Require Diets with High Levels of Essential Amino Acids? A Choice-Feeding Trial. Animals 2021, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, E.; Xiang, X.; Su, Y.; Zhu, W. Dynamic Changes in Serum Metabolomic Profiles of Growing Pigs Induced by Intravenous Infusion of Sodium Butyrate. Metabolites 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Wang, W.; Jiang, Z.; Ma, X.; Wang, F. Comparison of Global Metabolite for Growing Pigs Fed at Metabolizable Energy Requirement for Maintenance. Front. Vet. Sci. 2022, 9, 917033. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Siegerstetter, S.-C.; Magowan, E.; Lawlor, P.G.; O’connell, N.E.; Zebeli, Q. Feed Restriction Reveals Distinct Serum Metabolome Profiles in Chickens Divergent in Feed Efficiency Traits. Metabolites 2019, 9, 38. [Google Scholar] [CrossRef]
- Katafuchi, A.; Shimamoto, S.; Kawaguchi, M.; Tomonaga, S.; Nakashima, K.; Ishihara, S.; Ohtsuka, A.; Ijiri, D. Effects of Delaying Post-hatch Feeding on the Plasma Metabolites of Broiler Chickens Revealed by Targeted and Untargeted Metabolomics. J. Poult. Sci. 2023, 60, 2023032. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. BioSyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Sun, G.; Xue, M.; Sun, S.; Huang, T.; Zhou, J.; Loor, J.J.; Li, M. Transcriptome Analysis of the Effects of Fasting Caecotrophy on Hepatic Lipid Metabolism in New Zealand Rabbits. Animals 2019, 9, 648. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Huang, T.; Wang, Y.; Xue, M.; Sun, S.; Yan, D.; Song, G.; Sun, G.; Li, M. Influence of cecotrophy on fat metabolism mediated by caecal microorganisms in New Zealand white rabbits. Anim. Physiol. Nutr. 2020, 104, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Bellier, R.; Gidenne, T.; Vernay, M.; Colin, M. In vivo study of circadian variations of the cecal fermentation pattern in postweaned and adult rabbits. J. Anim. Sci. 1995, 73, 128. [Google Scholar] [CrossRef]
- Prud’Hon, M.; Chérubin, M.; Goussopoulos, J.; Carles, Y. Évolution, au cours de la croissance, des caractéristiques de la consommation d’aliments solide et liquide du lapin domestique nourri ad libitum. Ann. Zootech. 1975, 24, 289–298. [Google Scholar] [CrossRef]
- Hirakawa, H. Coprophagy in leporids and other mammalian herbivores. Mammal Rev. 2001, 31, 61–80. [Google Scholar] [CrossRef]
- Wang, Z.; He, H.; Chen, M.; Ni, M.; Yuan, D.; Cai, H.; Chen, Z.; Li, M.; Xu, H. Impact of coprophagy prevention on the growth performance, serum biochemistry, and intestinal microbiome of rabbits. BMC Microbiol. 2023, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Romero, C.G.; Rebollar, P.; Dal Bosco, A.; Castellini, C.; Cardinali, R. Dietary effect of short-chain organic acids on growth performance, mortality and development of intestinal lymphoid tissues in young non-medicated rabbits. World Rabbit. Sci. 2011, 19, 133–142. [Google Scholar] [CrossRef]
- Smith, A.H.; Meyer, C.E. The influence of diet on the endogenous production of citric acid. J. Biol. Chem. 1939, 131, 45–55. [Google Scholar] [CrossRef]
- Lillefosse, H.H.; Clausen, M.R.; Yde, C.C.; Ditlev, D.B.; Zhang, X.; Du, Z.Y.; Bertram, H.C.; Madsen, L.; Kristiansen, K.; Liaset, B. Urinary Loss of Tricarboxylic Acid Cycle Intermediates As Revealed by Metabolomics Studies: An Underlying Mechanism to Reduce Lipid Accretion by Whey Protein Ingestion? J. Proteome Res. 2014, 13, 2560–2570. [Google Scholar] [CrossRef]
- Axelrod, D.R. Ascorbic Acid and Urinary pH. JAMA 1985, 254, 1310. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; De Magistris, L.; et al. Slow intestinal transit contributes to elevate urinary p -cresol level in I talian autistic children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef]
- Gidenne, T.; Feugier, A. Feed restriction strategy in the growing rabbit. 1. Impact on digestion, rate of passage and microbial activity. Animal 2009, 3, 501–508. [Google Scholar] [CrossRef]
- Roager, H.M.; Hansen, L.B.S.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gøbel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H.; et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef]
- Cummings, J.; Hill, M.; Bone, E.; Branch, W.; Jenkins, D.J.A. The effect of meat protein and dietary fiber on colonic function and metabolism II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 1979, 32, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Letertre, M.P.; Myridakis, A.; Whiley, L.; Camuzeaux, S.; Lewis, M.R.; Chappell, K.E.; Thaikkatil, A.; Dumas, M.-E.; Nicholson, J.K.; Swann, J.R.; et al. A targeted ultra performance liquid chromatography—Tandem mass spectrometric assay for tyrosine and metabolites in urine and plasma: Application to the effects of antibiotics on mice. J. Chromatogr. B 2021, 1164, 122511. [Google Scholar] [CrossRef] [PubMed]
- Portman, O.W.; Alexander, M. Influence of lysophosphatidylcholine on the metabolism of plasma lipoproteins. Biochim. Et. Biophys. Acta (BBA)—Lipids Lipid Metab. 1976, 450, 322–334. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, B.H.; Kim, S.Y.; Chung, B.C. A rapid and sensitive method for quantitation of nucleosides in human urine using liquid chromatography/mass spectrometry with direct urine injection. Rapid Commun. Mass. Spectrom. 2004, 18, 973–977. [Google Scholar] [CrossRef]
- Colonna, A.; Russo, T.; Esposito, F.; Salvatore, F.; Cimino, F. Determination of pseudouridine and other nucleosides in human blood serum by high-performance liquid chromatography. Anal. Biochem. 1983, 130, 19–26. [Google Scholar] [CrossRef]
- Hamma, T.; Ferré-D’Amaré, A.R. Pseudouridine Synthases. Chem. Biol. 2006, 13, 1125–1135. [Google Scholar] [CrossRef]
- Brito, A.F.; Zang, Y. A Review of Lignan Metabolism, Milk Enterolactone Concentration, and Antioxidant Status of Dairy Cows Fed Flaxseed. Molecules 2018, 24, 41. [Google Scholar] [CrossRef]
- Marín-García, P.; Llobat, L.; Aguayo-Adán, J.A.; Larsen, T.; Cambra-López, M.; Blas, E.; Pascual, J.J.; Rouco, C. The Nutritional Strategy of European Rabbits Is Affected by Age and Sex: Females Eat More and Have Better Nutrient Optimisation. Anim. Physiol. Nutr. 2023, jpn.13826. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L.; Aguayo-Adán, J.A.; Franch, J.; Cambra-López, M.; Blas, E.; Pascual, J.J.; Rouco, C. Nutritional Ecology of European Rabbit ( Oryctolagus Cuniculus ): Factors Affecting Chemical Composition of Gastric Content. Anim. Physiol. Nutr. 2023, jpn.13849. [Google Scholar] [CrossRef]
- Llobat, L.; Marín-García, P.J. Application of Protein Nutrition in Natural Ecosystem Management for European Rabbit (Oryctolagus Cuniculus) Conservation. Biodivers Conserv 2022, 31, 1435–1444. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado. Real Decreto 53/2013, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. BOE 2013, 34, 11370–11421. [Google Scholar]
- Marín-García, P.J.; Ródenas, L.; Martínez-Paredes, E.; Moya, V.J.; Cambra-López, M.; Blas, E.; Pascual, J.J. Effect of Increasing the Methionine Level and Reducing the Threonine Level in the Diet of Fast-Growing Rabbits. Animals 2023, 13, 1471. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of the Association of Official Analytical Chemists. AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Batey, I.L. Starch Analysis Using Thermostable alpha-Amylases. Starch/Stärke 1982, 34, 125–128. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fibre in feed with refluxing beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bosch, L.; Alegría, A.; Farré, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to the RP-HPLC determination of amino acids in infant foods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 176–183. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
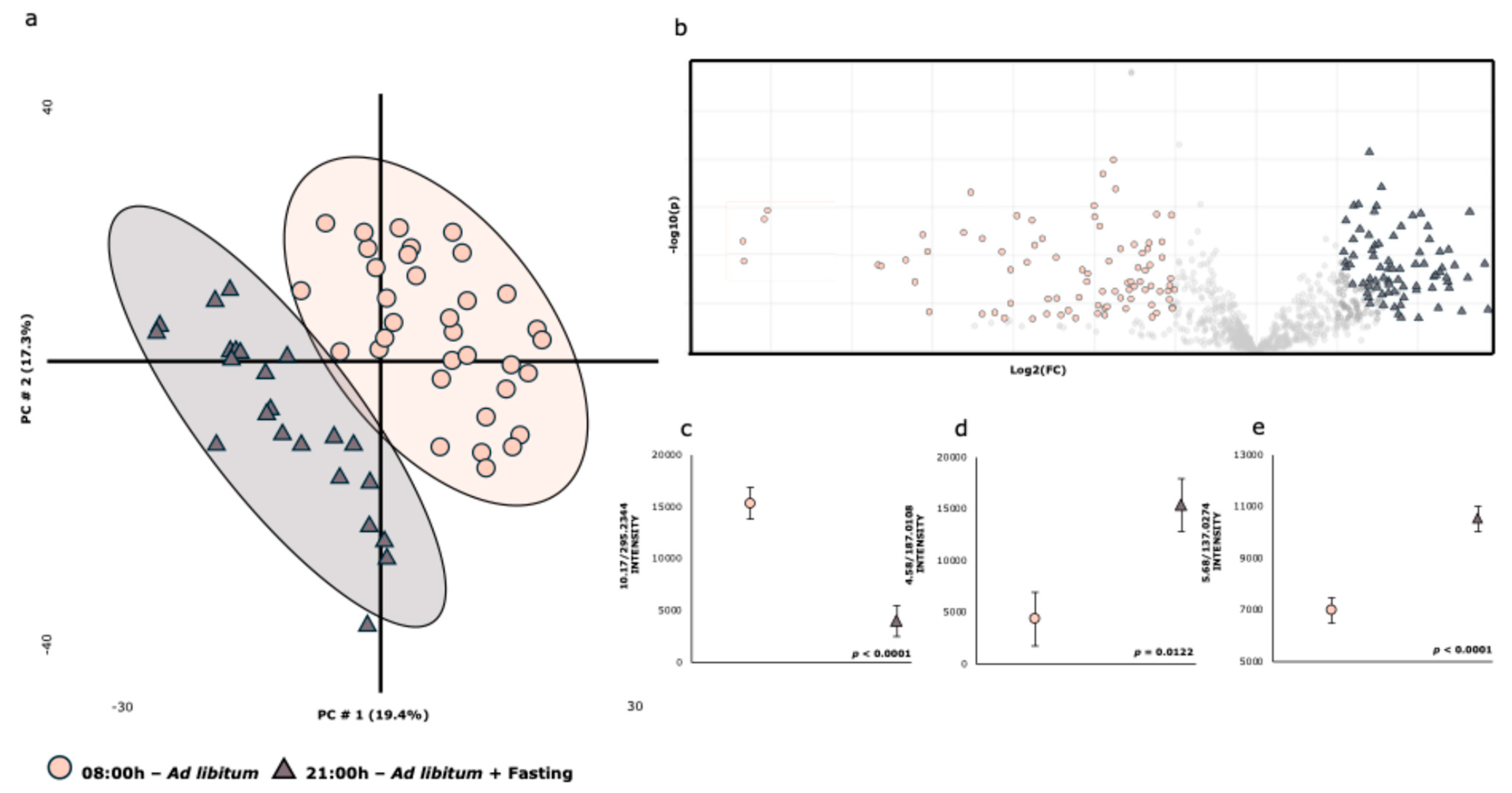
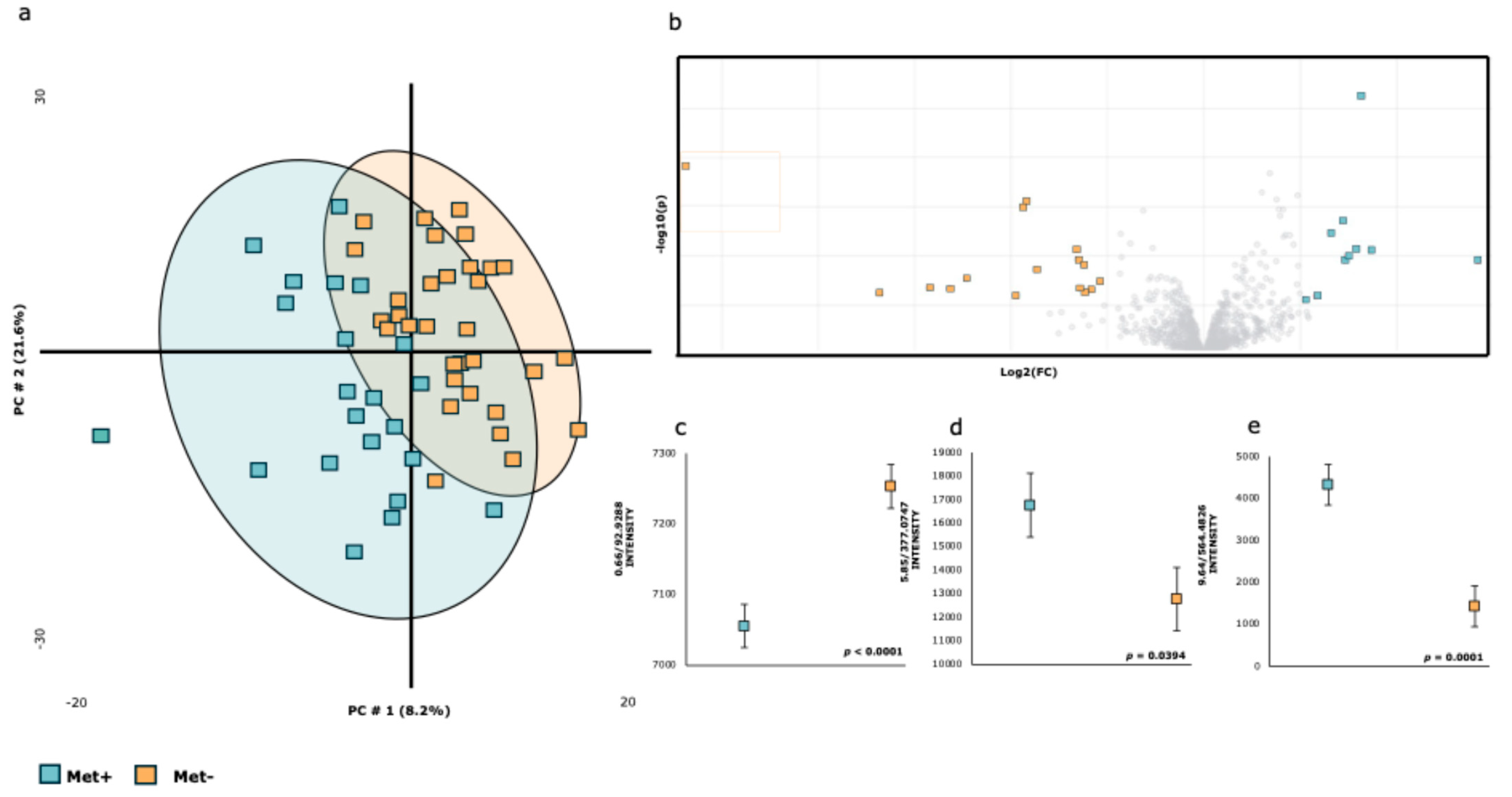
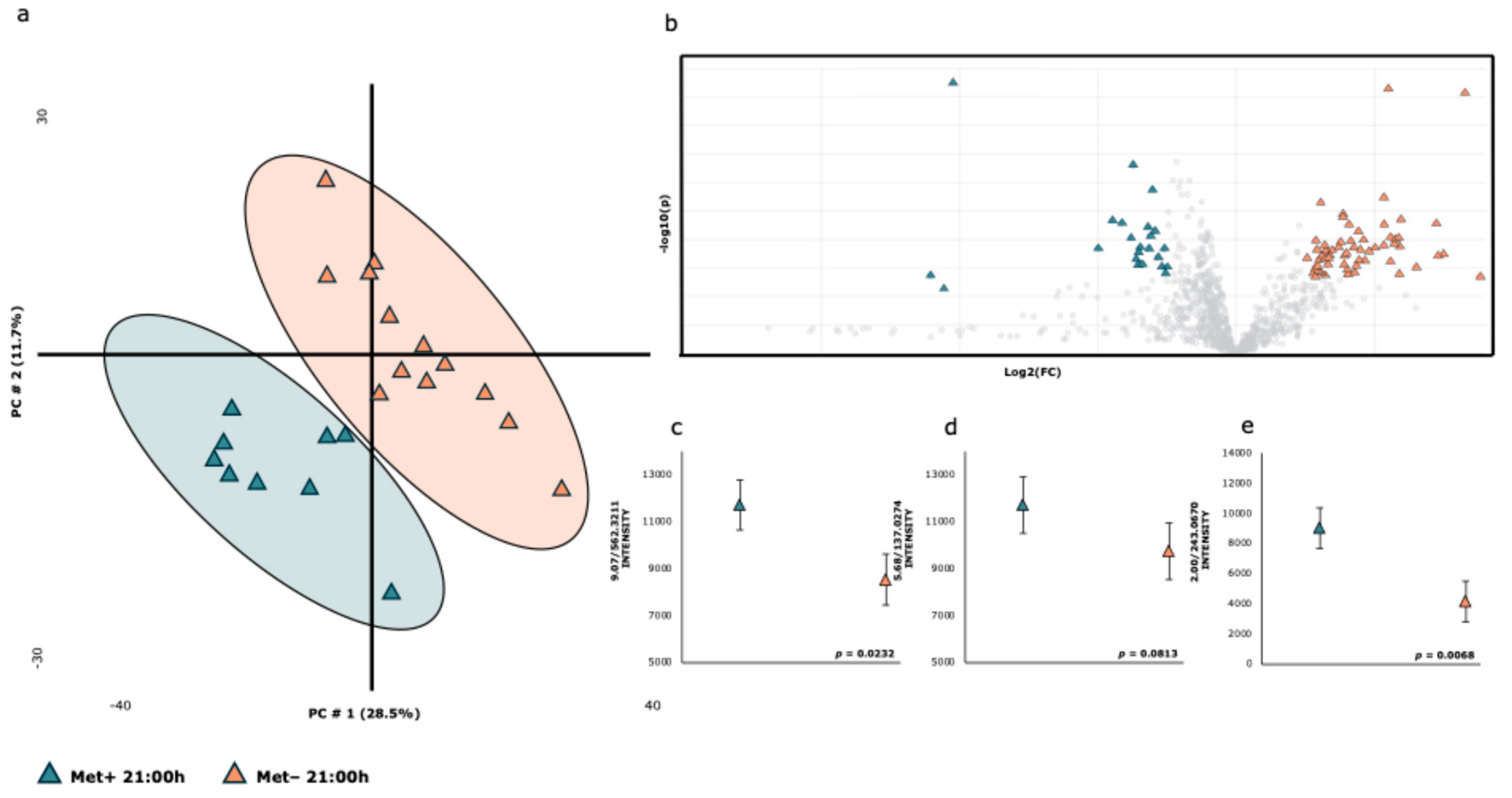

| 08:00 h | 21:00 h | |||||||
|---|---|---|---|---|---|---|---|---|
| RT-m/z | ION | Metabolite ** | X | SE | X | SE | Fold | p-Value |
| 1.05/243.0655 | [M-H]- | Pseudouridine | 3832 | ±148 | 3414 | ±193 | 1.122 | 0.0926 |
| 1.30/191.0235 | [M-H]- | Citric acid | 79,074 | ±5111 | 51,472 | ±6664 | 1.536 | 0.0018 |
| 2.00/243.0670 | [M-H]- | Uridine | 7559 | ±772 | 5592 | ±1006 | 1.351 | 0.1271 |
| 3.20/218.1077 | [M-H]- | Panthothenic acid | 5469 | ±196 | 4412 | ±256 | 1.240 | 0.0019 |
| 3.99/212.0073 | [M-H]- | Indoxylsulfate | 4270 | ±684 | 8175 | ±892 | 0.522 | 0.0011 |
| 4.13/178.0550 | [M-H]- | Hippuric acid | 44,145 | ±1561 | 39,954 | ±2036 | 1.105 | 0.1083 |
| 5.38/187.1013 | [M-H]- | Azelaic acid | 12,267 | ±1057 | 7407 | ±1379 | 1.656 | 0.0072 |
| 5.68/137.0274 | [M-H]- | 4-Hydroxybenzoic acid | 6990 | ±487 | 10,529 | ±634 | 0.664 | <0.0001 |
| 6.94/329.2392 | [M-H]- | Octadecenoic acid *** | 97,105 | ±21,496 | 28,878 | ±28,027 | 3.363 | 0.0589 |
| 7.23/229.1472 | [M-H]- | Dodecanedioic acid | 7407 | ±469 | 4837 | ±611 | 1.531 | 0.0016 |
| 10.36/566.3580 | [M+HCOO]- | LysoPC(18:1(11Z)/0:0) | 168,409 | ±8863 | 116,908 | ±11,557 | 1.441 | 0.0009 |
| 8.57/391.2913 | [M-H]- | Deoxycholic acid | 9823 | ±579 | 6434 | ±754 | 1.527 | 0.0008 |
| 10.17/295.2344 | [M-H]- | 9-Hydroxylinoleic acid | 15,357 | ±1517 | 4008 | ±1978 | 3.832 | <0.001 |
| 9.64/564.3369 | [M-H]- | LysoPC(18:2/0:0) | 304,386 | ±13,309 | 227,654 | ±17,353 | 1.337 | 0.0009 |
| 9.64/564.4826 | [M-H]- | LysoPC | 2787 | ±411 | 4445 | ±536 | 0.623 | 0.0175 |
| 9.07/562.3211 | [M+HCOO]- | LysoPC(18:3(9Z,12Z,15Z)/0:0) | 14,797 | ±812 | 9477 | ±1059 | 1.561 | 0.0002 |
| 8.01/407.2851 | [M-H]- | Allocholic acid | 14,915 | ±1598 | 2135 | ±2084 | 6.986 | <0.0001 |
| 11.25/568.3632 | [M+Fa-H]- | LysoPC(18:0/0:0) | 263,130 | ±12,369 | 214,175 | ±16,127 | 1.229 | 0.0196 |
| 4.58/187.0108 | [M-H]- | P-cresol sulfate | 4350 | ±2583 | 15,376 | ±3368 | 0.283 | 0.0122 |
| 11.25/508.3527 | [M-H]- | LysoPC(18:0/0:0)—Fragment | 61,711 | ±3043 | 47,903 | ±3967 | 1.288 | 0.0079 |
| 5.85/377.0747 | [M-H]- | Enterolactone sulfate | 14,640 | ±920 | 8749 | ±1200 | 1.673 | 0.0003 |
| 0.66/92.9288 | [M-H]- | Chloroacetic acid | 7160 | ±53 | 6664 | ±69 | 1.074 | <0.0001 |
| 9.18/311.2288 | [M-H]- | 11-HpODE **** | 13,833 | ±3042 | 3091 | ±3966 | 4.475 | 0.0363 |
| Met+ | Met− | |||||||
|---|---|---|---|---|---|---|---|---|
| RT-m/z | ION | Metabolite * | X | SE | X | SE | Fold | p-Value |
| 1.05/243.0655 | [M-H]- | Pseudouridine | 3726 | ±215 | 3926 | ±203 | 0.949 | 0.5043 |
| 1.30/191.0235 | [M-H]- | Citric acid | 72,695 | ±7373 | 84,743 | ±6952 | 0.858 | 0.2432 |
| 2.00/243.0670 | [M-H]- | Uridine | 7543 | ±1219 | 7574 | ±1150 | 0.996 | 0.9857 |
| 3.20/218.1077 | [M-H]- | Panthothenic acid | 5434 | ±274 | 5501 | ±258 | 0.988 | 0.8599 |
| 3.99/212.0073 | [M-H]- | Indoxylsulfate | 4767 | ±746 | 3829 | ±703 | 1.245 | 0.3669 |
| 4.13/178.0550 | [M-H]- | Hippuric acid | 45,038 | ±1972 | 43,353 | ±1860 | 1.039 | 0.5384 |
| 5.38/187.1013 | [M-H]- | Azelaic acid | 12,329 | ±1909 | 12,213 | ±1800 | 1.009 | 0.9650 |
| 5.68/137.0274 | [M-H]- | 4-Hydroxybenzoic acid | 7252 | ±669 | 6758 | ±632 | 1.073 | 0.5984 |
| 6.94/329.2392 | [M-H]- | Octadecenoic acid ** | 86,309 | ±37,309 | 106,702 | ±35,175 | 0.809 | 0.6935 |
| 7.23/229.1472 | [M-H]- | Dodecanedioic acid | 8083 | ±733 | 6808 | ±691 | 1.187 | 0.2147 |
| 10.36/566.3580 | [M+HCOO]- | LysoPC(18:1(11Z)/0:0) | 157,480 | ±14,462 | 178,124 | ±13,635 | 0.884 | 0.3068 |
| 8.57/391.2913 | [M-H]- | Deoxycholic acid | 9689 | ±964 | 9943 | ±909 | 0.974 | 0.8493 |
| 10.17/295.2344 | [M-H]- | 9-Hydroxylinoleic acid | 14,240 | ±2670 | 17,238 | ±2545 | 0.826 | 0.2893 |
| 9.64/564.3369 | [M-H]- | LysoPC(18:2/0:0) | 287,263 | ±21,681 | 319,608 | ±20,441 | 0.899 | 0.2858 |
| 9.64/564.4826 | [M-H]- | LysoPC | 4328 | ±490 | 1418 | ±462 | 3.052 | 0.0001 |
| 9.07/562.3211 | [M+HCOO]- | LysoPC(18:3(9Z,12Z,15Z)/0:0) | 13,964 | ±1381 | 15,538 | ±1303 | 0.899 | 0.4137 |
| 8.01/407.2851 | [M-H]- | Allocholic acid | 15,682 | ±2957 | 14,281 | ±2787 | 1.098 | 0.7423 |
| 11.25/568.3632 | [M+Fa-H]- | LysoPC(18:0/0:0) | 238,330 | ±20,434 | 285,174 | ±19,265 | 0.836 | 0.1051 |
| 4.58/187.0108 | [M-H]- | P-cresol sulfate | 4008 | ±1584 | 4654 | ±1493 | 0.861 | 0.7685 |
| 11.25/508.3527 | [M-H]- | LysoPC(18:0/0:0)—Fragment | 55,592 | ±4856 | 67,151 | ±4579 | 0.828 | 0.0929 |
| 5.85/377.0747 | [M-H]- | Enterolactone sulfate | 16,751 | ±1351 | 12,763 | ±1274 | 1.312 | 0.0394 |
| 0.66/92.9288 | [M-H]- | Chloroacetic acid | 7055 | ±31 | 7253 | ±29 | 0.973 | <0.001 |
| 9.18/311.2288 | [M-H]- | 11-HpODE *** | 12,554 | ±5395 | 14,969 | ±5086 | 0.839 | 0.7467 |
| Met+ | Met− | |||||||
|---|---|---|---|---|---|---|---|---|
| RT-m/z | ION | Metabolite * | X | SE | X | SE | Fold | p-Value |
| 1.05/243.0655 | [M-H]- | Pseudouridine | 3405 | ±372 | 3418 | ±243 | 0.996 | 0.9762 |
| 1.30/191.0235 | [M-H]- | Citric acid | 40,552 | ±12,211 | 56,153 | ±7994 | 0.722 | 0.2992 |
| 2.00/243.0670 | [M-H]- | Uridine | 9008 | ±1335 | 4129 | ±874 | 2.182 | 0.0068 |
| 3.20/218.1077 | [M-H]- | Panthothenic acid | 4959 | ±501 | 41,777 | ±328 | 0.119 | 0.2087 |
| 3.99/212.0073 | [M-H]- | Indoxylsulfate | 9418 | ±2200 | 7642 | ±1440 | 1.232 | 0.5080 |
| 4.13/178.0550 | [M-H]- | Hippuric acid | 45,581 | ±4327 | 37,541 | ±2833 | 1.214 | 0.1375 |
| 5.38/187.1013 | [M-H]- | Azelaic acid | 8137 | ±70,983 | 7093 | ±647 | 1.147 | 0.3884 |
| 5.68/137.0274 | [M-H]- | 4-Hydroxybenzoic acid | 12,399 | ±1210 | 9728 | ±792 | 1.275 | 0.0813 |
| 6.94/329.2392 | [M-H]- | Octadecenoic acid ** | 8914 | ±30,025 | 37,435 | ±19,656 | 0.238 | 0.4371 |
| 7.23/229.1472 | [M-H]- | Dodecanedioic acid | 6075 | ±895 | 4306 | ±586 | 1.411 | 0.1157 |
| 10.36/566.3580 | [M+HCOO]- | LysoPC(18:1(11Z)/0:0) | 139,923 | ±14,803 | 99,346 | ±7634 | 0.131 | 0.0083 |
| 8.57/391.2913 | [M-H]- | Deoxycholic acid | 7883 | ±951 | 5813 | ±623 | 1.356 | 0.0854 |
| 10.17/295.2344 | [M-H]- | 9-Hydroxylinoleic acid | 4108 | ±1370 | 3963 | ±897 | 1.037 | 0.9306 |
| 9.64/564.3369 | [M-H]- | LysoPC(18:2/0:0) | 261,742 | ±22,293 | 202,718 | ±12,460 | 1.229 | 0.0164 |
| 9.64/564.4826 | [M-H]- | LysoPC | 965 | ±389 | 1583 | ±255 | 0.610 | 0.2010 |
| 9.07/562.3211 | [M+HCOO]- | LysoPC(18:3(9Z,12Z,15Z)/0:0) | 11,709 | ±1075 | 8521 | ±704 | 1.374 | 0.0232 |
| 8.01/407.2851 | [M-H]- | Allocholic acid | 2091 | ±505 | 2154 | ±331 | 0.971 | 0.9188 |
| 11.25/568.3632 | [M+Fa-H]- | LysoPC(18:0/0:0) | 226,973 | ±18,439 | 208,690 | ±12,071 | 1.088 | 0.4176 |
| 4.58/187.0108 | [M-H]- | P-cresol sulfate | 9371 | ±9720 | 17,949 | ±6363 | 0.522 | 0.4698 |
| 11.25/508.3527 | [M-H]- | LysoPC(18:0/0:0)—Fragment | 50,689 | ±5351 | 46,709 | ±3503 | 1.085 | 0.5416 |
| 5.85/377.0747 | [M-H]- | Enterolactone sulfate | 12,891 | ±1611 | 6974 | ±1055 | 1.848 | 0.0066 |
| 0.66/92.9288 | [M-H]- | Chloroacetic acid | 6771 | ±194 | 6618 | ±127 | 1.023 | 0.5180 |
| 9.18/311.2288 | [M-H]- | 11-HpODE *** | 538 | ±3542 | 4186 | ±2319 | 0.129 | 0.4003 |
| Ingredients | Chemical Composition | Met+ | Met− | |
|---|---|---|---|---|
| Wheat gran | 300 | Dry matter 2 | 907 | 907 |
| DDGS corn | 50 | Ash 2 | 104 | 104 |
| Bakery by-product | 30 | Crude protein 2 | 155 | 155 |
| Sunflower meal | 36 | Crude fat 2 | 29.7 | 29.7 |
| Alfalfa meal | 334 | Neutral detergent fiber (NDF) 2 | 455 | 455 |
| Beet pulp | 80 | Acid detergent fiber (FAD) 2 | 29.7 | 29.7 |
| Straw | 136 | Acid detergent lignin (ADL) 2 | 455 | 455 |
| Beet molasses | 13.9 | Digestible energy 2 | 262 | 262 |
| L-Arginine | 3.1 | Amino acid composition 3: | ||
| L-Histidine | 1.5 | Aspartic acid | 12.95 | 12.95 |
| Calcium carbonate | 6.5 | Serine | 5.74 | 5.74 |
| Sodium clorhide | 4 | Glutamic acid | 22.96 | 22.96 |
| Vitamine/mineral 1 | 5 | Glycine | 6.53 | 6.53 |
| Histidine | 3.57 | 3.57 | ||
| Arginine | 9.28 | 9.28 | ||
| Threonine | 6.90 | 6.90 | ||
| Alanine | 6.69 | 6.69 | ||
| Proline | 8.04 | 8.04 | ||
| Cystine | 2.37 | 2.37 | ||
| Tyrosine | 2.90 | 2.90 | ||
| Valine | 7.03 | 7.03 | ||
| Methionine 4 + Cystine | 6.60 | 4.90 | ||
| Isoleucine | 5.07 | 5.07 | ||
| Lysine | 8.10 | 8.10 | ||
| Leucine | 9.70 | 9.70 | ||
| Phenylalanine | 5.58 | 5.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-García, P.J.; Mateo-López, J.; Cortés-García, C.; Llobat, L.; Huertas-Herrera, A.; Toro-Manríquez, M.; Cambra-López, M.; Pascual, J.J.; Hedemann, M.S. Untargeted Metabolomics to Harness Ideal Protein Concept and Mitigate Environmental Impact in Rabbit Models. Int. J. Mol. Sci. 2025, 26, 6047. https://doi.org/10.3390/ijms26136047
Marín-García PJ, Mateo-López J, Cortés-García C, Llobat L, Huertas-Herrera A, Toro-Manríquez M, Cambra-López M, Pascual JJ, Hedemann MS. Untargeted Metabolomics to Harness Ideal Protein Concept and Mitigate Environmental Impact in Rabbit Models. International Journal of Molecular Sciences. 2025; 26(13):6047. https://doi.org/10.3390/ijms26136047
Chicago/Turabian StyleMarín-García, Pablo Jesús, Jorge Mateo-López, César Cortés-García, Lola Llobat, Alejandro Huertas-Herrera, Mónica Toro-Manríquez, María Cambra-López, Juan José Pascual, and Mette Skou Hedemann. 2025. "Untargeted Metabolomics to Harness Ideal Protein Concept and Mitigate Environmental Impact in Rabbit Models" International Journal of Molecular Sciences 26, no. 13: 6047. https://doi.org/10.3390/ijms26136047
APA StyleMarín-García, P. J., Mateo-López, J., Cortés-García, C., Llobat, L., Huertas-Herrera, A., Toro-Manríquez, M., Cambra-López, M., Pascual, J. J., & Hedemann, M. S. (2025). Untargeted Metabolomics to Harness Ideal Protein Concept and Mitigate Environmental Impact in Rabbit Models. International Journal of Molecular Sciences, 26(13), 6047. https://doi.org/10.3390/ijms26136047











