The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Animals
4.1.2. Antibodies
Primary Antibodies
- -
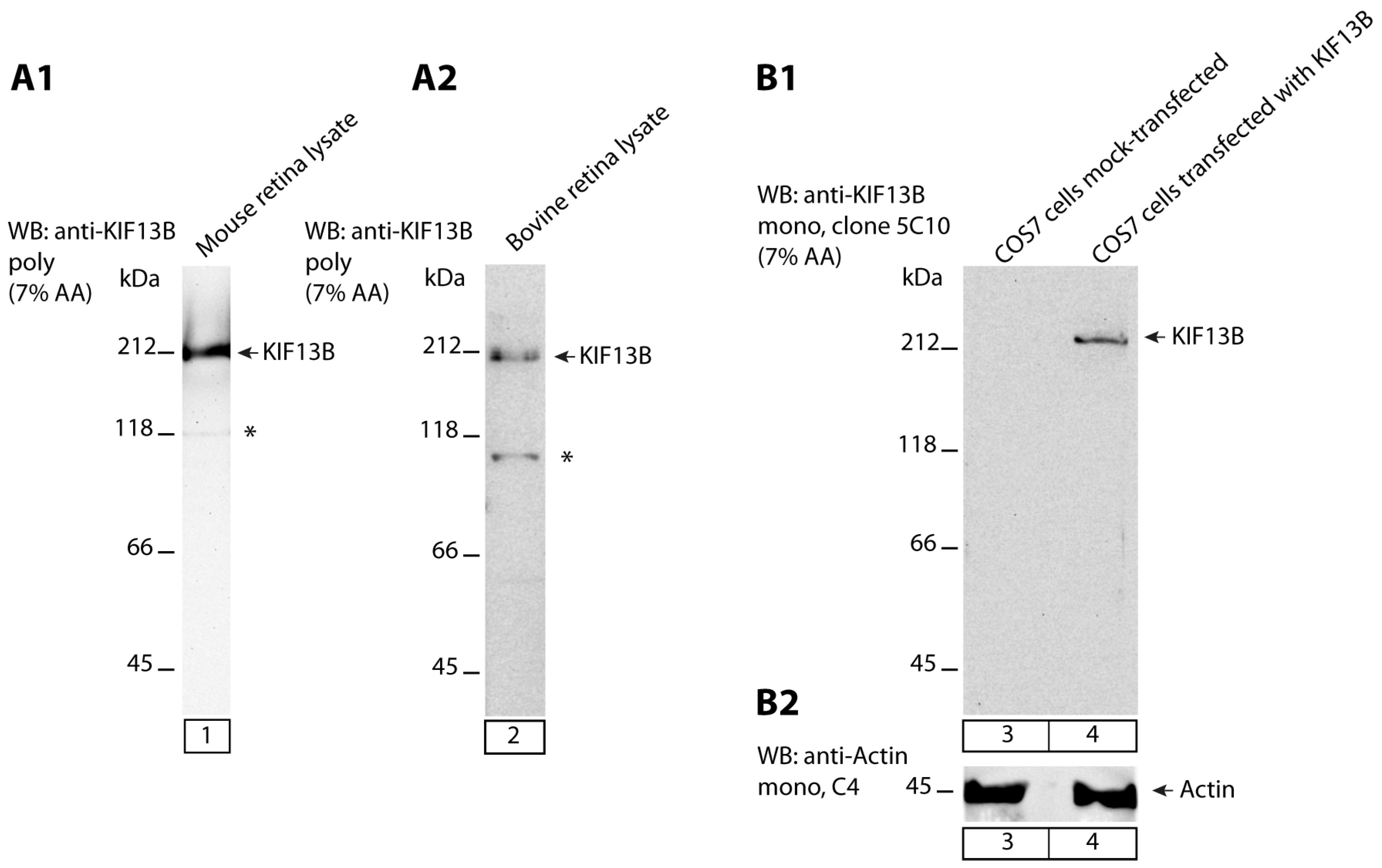
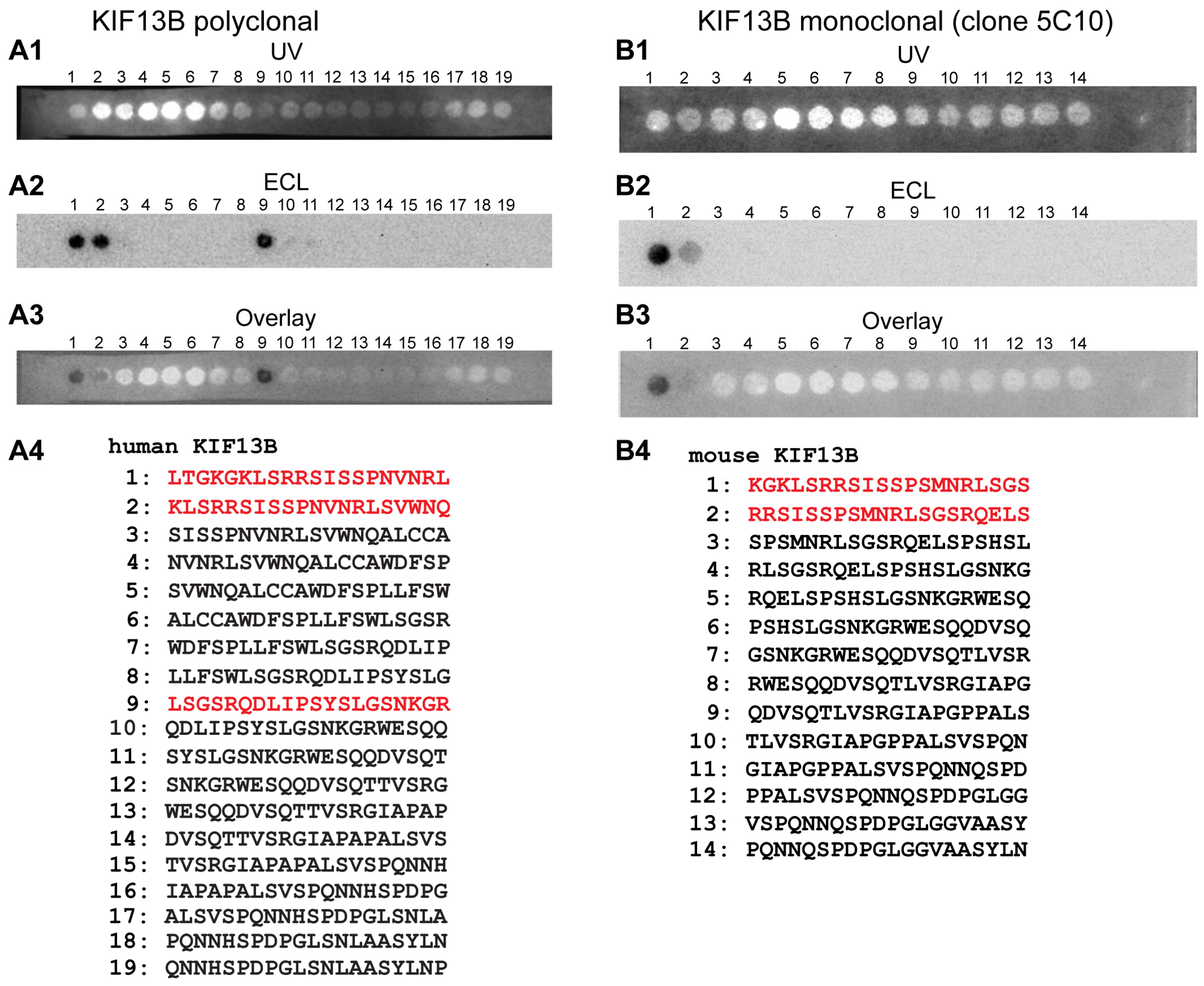
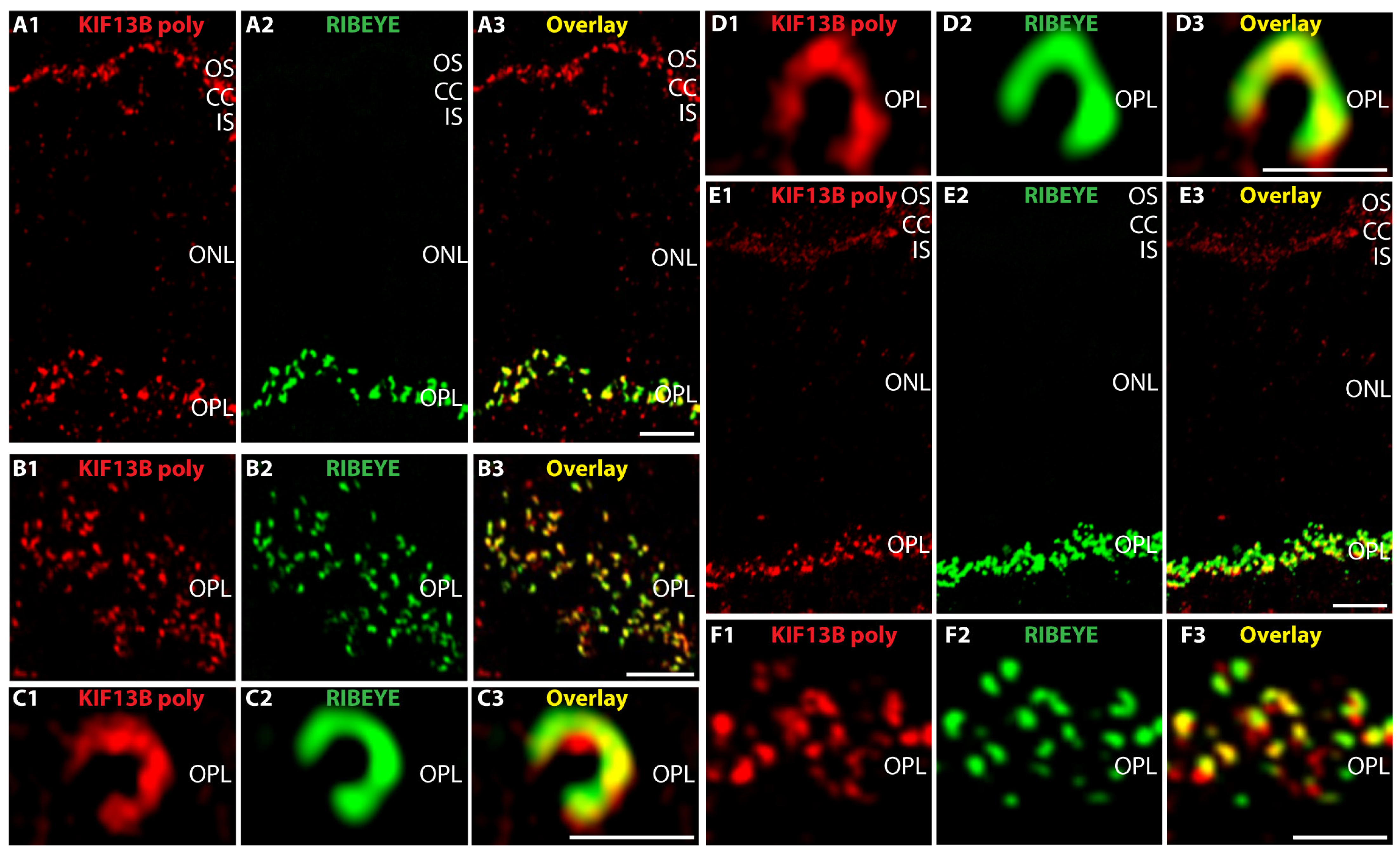
- anti-KIF13B rabbit polyclonal, antigen affinity-purified (commercial HPA025023, Sigma, Taufkirchen, Germany). The antigen affinity-purified antibody was raised against the peptide LTGKGKLSRRSISSPNVNRLSGSRQDLIPSYSLGSNKGRWESQQDVSQTTVSRGIAPAPA LSVSPQNNHSPDPGLSNLAASYLNP deduced from human KIF13B (amino acid (aa)1369-aa1453; Q9NQT8-1; NM_015254.4, encoding a 1826 aa long protein with a predicted mass of ≈203 kDa) [127]. This peptide stretch is highly conserved in mouse KIF13B (86% amino acid identity in comparison to the human aa sequence) and bovine KIF13B (88% amino acid identities in comparison to the human sequence). This polyclonal KIF13B antibody was used for immunofluorescence (IF) microscopy in a 1:500 dilution, for Western blot (WB) analyses in a 1:1000 dilution and for multi-peptide array WB (“Pepspots“) in a 1:100,000 dilution.
- -
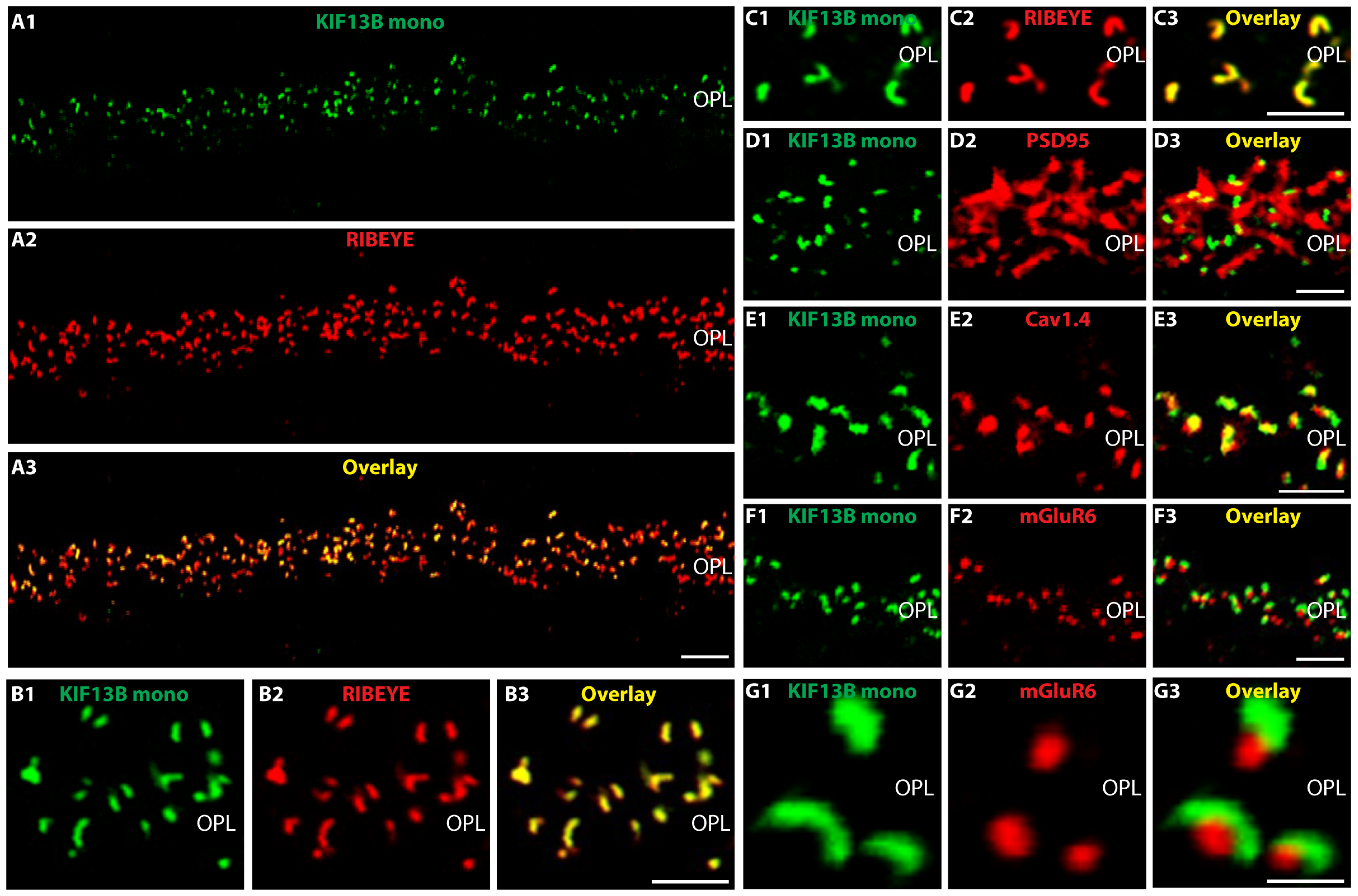
- anti-KIF13B mouse monoclonal: (clone 5C10; IgG2b subtype) was raised against the GST tagged expressed protein of respective KIF13B encoding the sequence stretch KGKLSRRSISSPNVNRLSGSRQDLIPSYSLGSNKGRWESQQDVSQTTVSRGIAPAPALSV SPQNNHSPDPGLSNLAASYLN of mouse KIF13B (aa1371-aa1452 of NM_001081177.3 encoding a 1843 aa long protein) cloned in pGEX-6P-1. The peptide sequence is highly conserved (78% amino acid identities in bovine KIF13B, DAA27057.1; 85% amino acid identities in human KIF13B, EAW63491.1). Cloning of the GST-tagged KIF13B-encoding construct, fusion protein expression and purification, mouse immunization, generation of hybridoma cells, ELISA screening and antibody sub-typing was performed by Absea, Beijing, China. This monoclonal KIF13B antibody was used for IF and EM in a 1:100 dilution, for WB in a 1:1000 dilution and for multi-peptide array WB (“Pepspots”) in a 1:50,000 dilution.
- -
- Additional primary antibodies used in the present study are described in Table 1.
Secondary Antibodies
4.1.3. Plasmids
Eukaryotic KIF13B Expression Plasmid
4.2. Methods
4.2.1. Preparation of Cryostat Section and Immunolabeling of Cryostat Sections
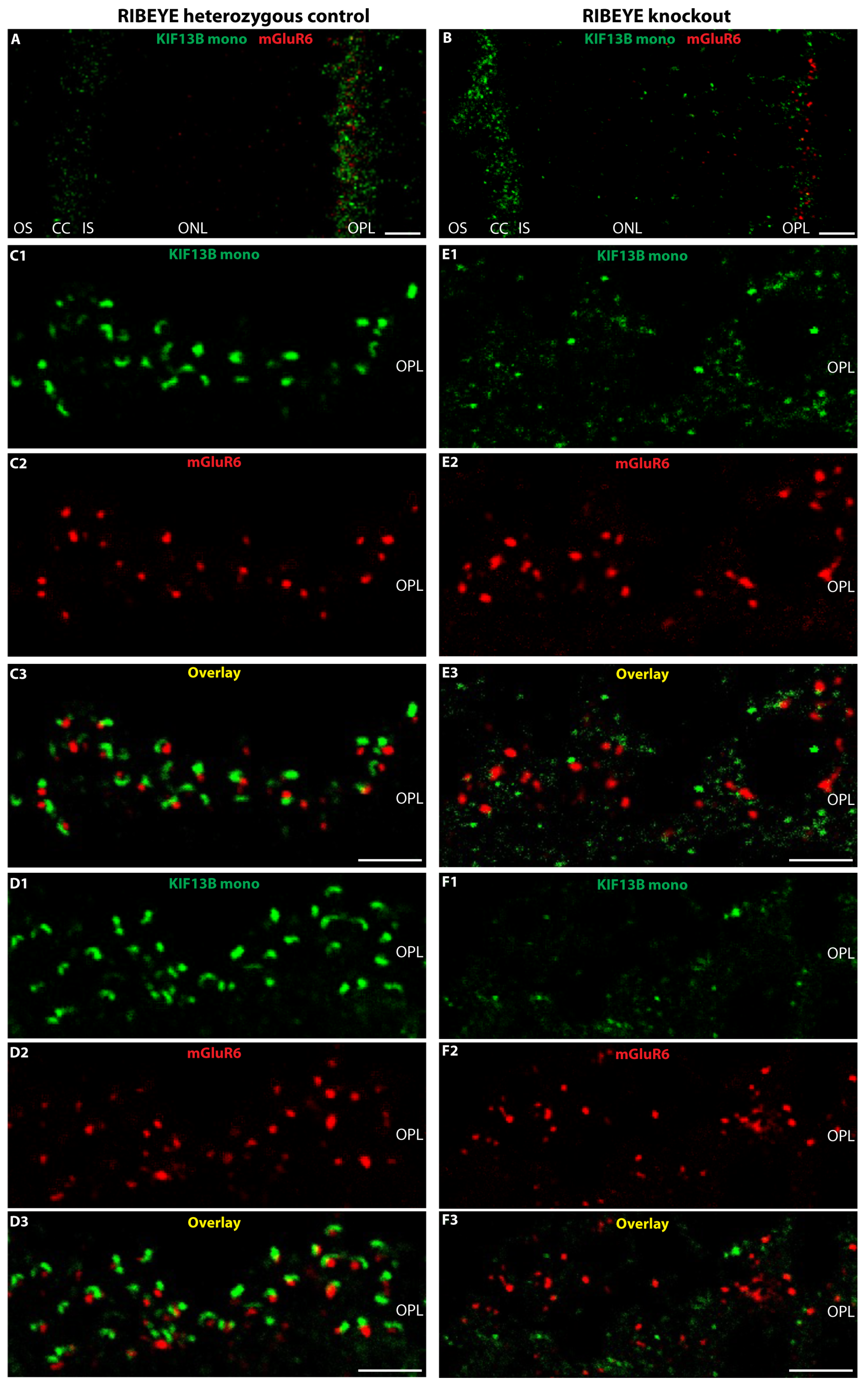
4.2.2. Confocal- and Super-Resolution Structured-Illumination Microscopy (SR-SIM)
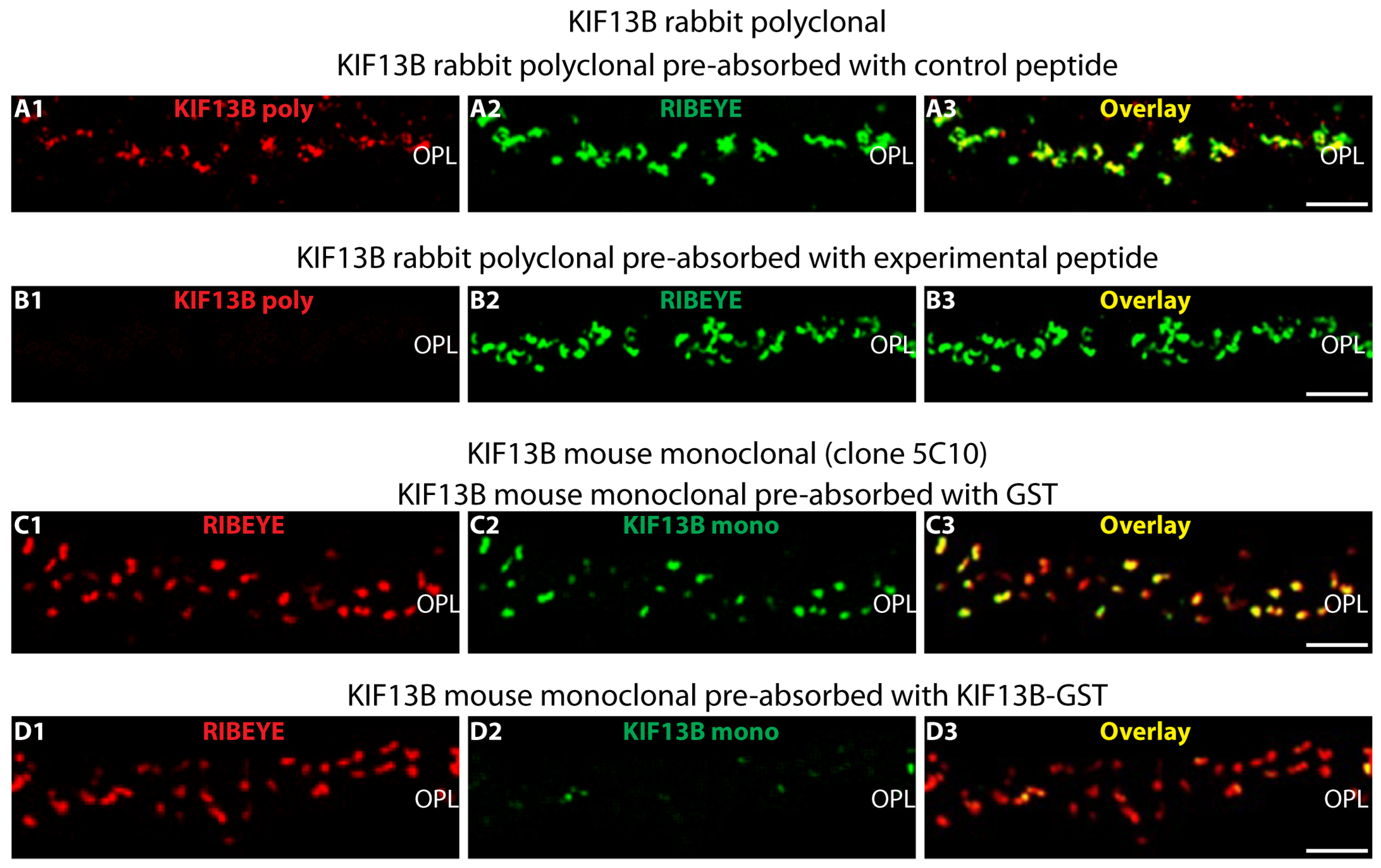
4.2.3. Antibody Pre-Absorption Control Experiments
- -
- KIF13B (Sigma, Taufkirchen, Germany)
- -
- KIF13B (clone 5C10)
4.2.4. Expression and Purification of Recombinant Fusion Protein
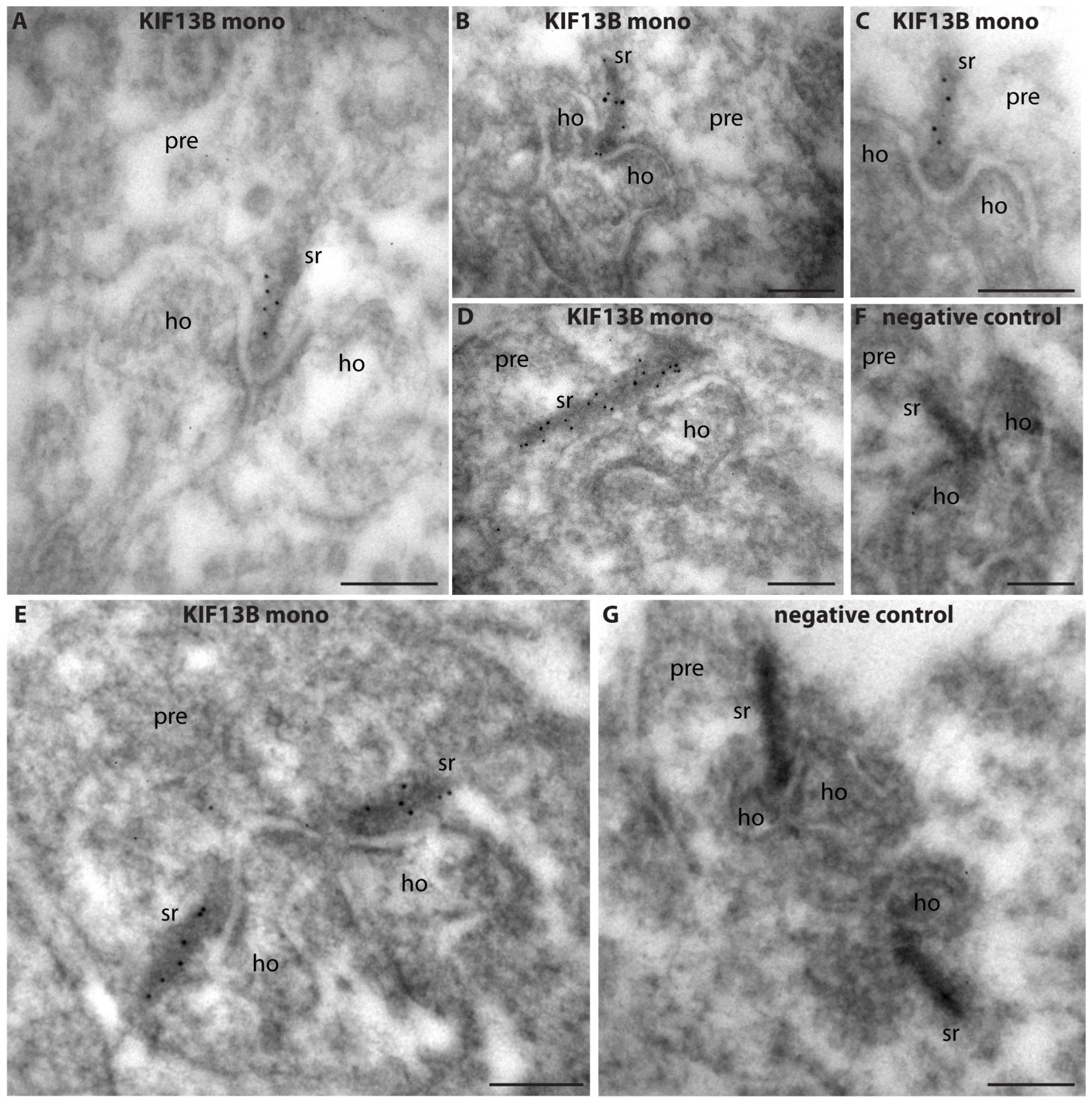
4.2.5. Post-Embedding Immunogold Labeling
4.2.6. Multi-Peptide Arrays (“Pepspots”)
4.2.7. Heterologous Protein Expression in COS7 Cells
4.2.8. SDS-PAGE and Western Blot
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Grabner, C.P.; Schmitz, F. Sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 2020, 100, 103–144. [Google Scholar] [CrossRef]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Carter-Dawson, L.D.; LaVail, M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979, 188, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yau, K.W. Phototransduction in mouse and rod cones. Pflügers Arch. 2007, 454, 805–819. [Google Scholar] [CrossRef]
- Barnes, C.L.; Malhotra, H.; Calvert, P.D. Compartmentalization of Photoreceptor Sensory Cilia. Front. Cell Dev. Biol. 2021, 9, 636737. [Google Scholar] [CrossRef]
- Cohen, A.I. New details of the ultrastructure of the outer segments and ciliary connectives of the rods of human and macaque retinas. Anat. Rec. 1965, 152, 63–79. [Google Scholar] [CrossRef]
- Sung, C.H.; Chuang, J.Z. The cell biology of vision. J. Cell Biol. 2010, 19, 953–963. [Google Scholar] [CrossRef]
- Witzgall, R. Golgi bypass of ciliary proteins. Semin. Cell Dev. Biol. 2018, 83, 51–58. [Google Scholar] [CrossRef]
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Cell Mol. Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef]
- Chen, H.Y.; Kelley, R.A.; Li, T.; Swaroop, A. Primary cilia biogenesis. Sem. Cell Dev. Biol. 2021, 110, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Mill, P.; Christensen, S.T.; Pedersen, L.B. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 2023, 24, 421–441. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chuang, J.Z.; Sung, C.H. Light regulates the ciliary protein transport and outer segment disc renewal of mammalian photoreceptors. Dev. Cell 2015, 32, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Dharmat, R.; Eblimit, A.; Robichaux, M.A.; Zhang, Z.; Nguyen, T.T.; Jung, S.Y.; He, F.; Jain, A.; Li, Y.; Qin, J.; et al. SPATA7 maintains a novel photoreceptor-specific zone in the distal connecting cilium. J. Cell Biol. 2018, 217, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dynlacht, B.D. The regulation of cilium assembly and disassembly. Development 2018, 145, dev151407. [Google Scholar] [CrossRef]
- Furukawa, T.; Ueno, A.; Omori, Y. Molecular mechanisms underlying selective synapse formation of vertebrate retinal photoreceptor cells. Cell. Mol. Life Sci. 2020, 77, 1251–1266. [Google Scholar] [CrossRef]
- Thoreson, W.B. Transmission at rod and cone ribbon synapses in the retina. Pflügers Arch. Eur. J. Physiol. 2021, 473, 1469–1491. [Google Scholar] [CrossRef]
- Zenisek, D.; Steyer, J.A.; Almers, W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 2000, 406, 849–854. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Matthews, G. Visualizing synaptic vesicle turnover and pool refilling driven by calcium nanodomains at presynaptic active zones of ribbon synapses. Proc. Natl. Acad. Sci. USA 2014, 111, 8655–8660. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Henry, D.; Akmentin, W.; Matthews, G. Nanoscale dynamics of synaptic vesicle trafficking and fusion at the presynaptic active zone. eLife 2016, 5, e13245. [Google Scholar] [CrossRef] [PubMed]
- Joselevitch, C.; Zenisek, D. Direct observation of vesicle transport on the synaptic ribbon provides evidence that vesicles are mobilized and prepared for release. J. Neurosci. 2020, 40, 7390–7404. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef]
- Zenisek, D.; Horst, N.K.; Merrifield, C.; Sterling, P.; Matthews, G. Visualizing synaptic ribbons inthe living cell. J. Neurosci. 2004, 24, 9752–9759. [Google Scholar] [CrossRef]
- Schmitz, F. The making of synaptic ribbons: How they are built and what they do. Neuroscientist 2009, 15, 611–624. [Google Scholar] [CrossRef]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Südhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Stewart, W.J.; Akanyeti, O.; Frederick, C.; Zhu, J.; Santos-Sacchi, J.; Sheets, L.; Liao, J.C.; Zenisek, D. Synaptic ribbons require Ribeye for electron density, proper synaptic localization, and recruitment of calcium channels. Cell Rep. 2016, 15, 2784–2795. [Google Scholar] [CrossRef]
- Shankhwar, S.; Schwarz, K.; Katiyar, R.; Jung, M.; Maxeiner, S.; Südhof, T.C.; Schmitz, F. RIBEYE B-domain is essential for A-domain stability and assembly of synaptic ribbons. Front. Mol. Neurosci. 2022, 15, 838311. [Google Scholar] [CrossRef]
- Becker, L.; Schnee, M.E.; Niwa, M.; Sun, W.; Maxeiner, S.; Talaei, S.; Kachar, B.; Rutherford, M.A.; Ricci, A.J. The presynaptic ribbon maintains vesicle populations at the hair cell afferent fiber synapse. eLife 2018, 7, e30241. [Google Scholar] [CrossRef]
- Jean, P.; Lopez de la Morena, D.; Michanski, S.; Jaime Tobon, L.M.; Chakrabarti, R.; Picher, M.M.; Neef, J.; Jung, S.Y.; Gültas, M.; Maxeiner, S.; et al. The synaptic ribbon is critical for sound encoding at high rates and with temporal precision. eLife 2018, 7, e29275. [Google Scholar] [CrossRef]
- Dick, O.; tom Dieck, S.; Altrock, W.D.; Ammermüller, J.; Weiler, R.; Garner, C.C.; Gundelfinger, E.D.; Brandstätter, J.H. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 2003, 37, 775–786. [Google Scholar] [CrossRef] [PubMed]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.-M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstätter, J.H. Molecular dissection of the photoreceptor ribbon synapse. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Ott, C.; Löhner, M.; Atorf, J.; Fuchs, M.; Sedmak, T.; Kremers, J.; Fejtová, A.; Gundelfinger, E.D.; Brandstätter, J.H. Identification and immunocytochemical characterization of piccolino, a novel piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS ONE 2013, 8, e70373. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.M.; Gierke, K.; Joachimsthaler, A.; Sticht, H.; Izsvák, Z.; Hamra, F.K.; Fejtová, A.; Ackermann, F.; Garner, C.C.; Kremers, J.; et al. A multiple Piccolino-RIBEYE interaction supports plate-shaped synaptic ribbons in retinal neurons. J. Neurosci. 2019, 39, 2606–2619. [Google Scholar] [CrossRef]
- Li, P.; Lin, Z.; An, Y.; Lin, J.; Zhang, A.; Wang, S.; Tu, H.; Ran, J.; Wang, J.; Liang, Y.; et al. Piccolo is essential for the maintenance of mouse retina but not cochlear hair cell function. Aging 2021, 13, 11678–11695. [Google Scholar] [CrossRef]
- Won, J.; De Evsiova, C.M.; Smith, R.S.; Hicks, W.L.; Edwards, M.M.; Longo-Guess, C.; Li, T.; Naggert, J.K.; Nishina, P.M. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outre segment formation, and for sperm development. Hum. Mol. Genet. 2011, 20, 482–496. [Google Scholar] [CrossRef]
- Muresan, V.; Lyass, A.; Schnapp, B.J. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J. Neurosci. 1999, 19, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef]
- Suiwal, S.; Dembla, M.; Schwarz, K.; Katiyar, R.; Jung, M.; Carius, Y.; Maxeiner, S.; Lauterbach, M.A.; Lancaster, C.R.D.; Schmitz, F. Ciliary proteins repurposed by the synaptic ribbon: Trafficking myristoylated proteins at rod photoreceptor synapses. Int. J. Mol. Sci. 2022, 23, 7135. [Google Scholar] [CrossRef]
- Schou, K.B.; Mogensen, J.B.; Morthorst, S.K.; Nielsen, B.S.; Aleliunaite, A.; Serra-Marques, A.; Fürstenberg Saunier, S.; Bizet, A.A.; Veland, I.R.; Akhmanova, A.; et al. KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat. Commun. 2017, 8, 14177. [Google Scholar] [CrossRef]
- Hanada, T.; Lin, L.; Tibaldi, E.V.; Reinherz, E.L.; Chishti, A.H. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila disc large tumor suppressor in T lymphocytes. J. Biol. Chem. 2000, 275, 28774–28784. [Google Scholar] [CrossRef] [PubMed]
- Asaba, N.; Hanada, T.; Takeuchi, A.; Chishti, A.H. Direct interaction with a kinesin-related motor mediates transport of mammalian discs large tumor suppressor homologue in epithelial cells. J. Biol. Chem. 2003, 278, 8395–8400. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Hanada, T.; Fukui, Y.; Chishti, A.H. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J. Cell Biol. 2006, 174, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.H.; Hanada, T.; Chishti, A.H. The effector domain of human Dlg tumor suppressor acts as a switch that relieves autoinhibition of kinesin-3 motor GAKIN/KIF13B. Biochemistry 2007, 46, 10039–10045. [Google Scholar] [CrossRef]
- Yamada, K.H.; Nakajima, Y.; Geyer, M.; Wary, K.K.; Ushio-Fukai, M.; Komarova, Y.; Malik, A.B. KIF13B regulates angiogenesis through Golgi to plasma membrane trafficking of VEGFR2. J. Cell Sci. 2014, 127, 4518–4530. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005, 6, 201–214. [Google Scholar] [CrossRef]
- Nakata, T.; Hirokawa, N. Neuronal polarity and the kinesin superfamily proteins. Sci. STKE 2007, 2007, pe6. [Google Scholar] [CrossRef]
- Siddiqui, N.; Straube, A. Intracellular cargo transport by kinesin-3 motors. Biochemistry 2017, 82, 803–815. [Google Scholar] [CrossRef]
- Yang, R.; Bostick, Z.; Garbouchian, A.; Luisi, J.; Banker, G.; Bentley, M. A novel strategy to visualize vesicle-bound kinesins reveals the diversity of kinesin-mediated transport. Traffic 2019, 20, 851–866. [Google Scholar] [CrossRef]
- Serra-Marques, A.; Martin, M.; Katrukha, E.A.; Grigoriev, I.; Peeters, C.A.E.; Liu, Q.; Hooikaas, P.J.; Yao, Y.; Solianova, V.; Smal, I.; et al. Concerted acion of kinesins KIF5B and KIF13B promotes efficient secretory vesicle transport to microtubule plus ends. eLife 2020, 9, e61302. [Google Scholar] [CrossRef]
- Montgomery, A.C.; Mendoza, C.S.; Garbouchian, A.; Quinones, G.B.; Bentley, M. Polarized transport requires AP-1-mediated recruitment of KIF13A and KIF13B at the trans-Golgi. Mol. Biol. Cell 2024, 35, ar61. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A. Mechanism and regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 2024, 26, 86–103. [Google Scholar] [CrossRef]
- Juhl, A.D.; Anvarian, Z.; Kuhns, S.; Berges, J.; Andersen, J.S.; Wüstner, D.; Pedersen, L.B. Transient accumulation and bidirectional movement of KIF13B in primary cilia. J. Cell Sci. 2023, 136, jcs259257. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.G. Microtubules in synapses of the retina. J. Neurocytol. 1976, 5, 361–370. [Google Scholar] [CrossRef]
- Favre, D.; Dememes, D.; Sans, A. Microtubule organization and synaptogenesis in the vestibular sensory cells. Dev. Brain Res. 1986, 25, 137–142. [Google Scholar] [CrossRef]
- Voorn, R.A.; Vogl, C. Molecular Assembly and Structural Plasticity of Sensory Ribbon Synapses-A Presynaptic Perspective. Int. J. Mol. Sci. 2020, 21, 8758. [Google Scholar] [CrossRef]
- Voorn, R.A.; Sternbach, M.; Jarysta, A.; Rankovic, V.; Tarchini, B.; Wolf, F.; Vogl, C. Slow kinesin-dependent microtubular transport facilitates ribbon synapse assembly in developing cochlear inner hair cells. BioRxiv 2024. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, D.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 394. [Google Scholar] [CrossRef]
- Liu, X.; Pacwa, A.; Bresciani, G.; Swierczynska, M.; Dorecka, M.; Smedowski, A. Retinal primary cilia and their dysfunction in retinal neurodegenerative diseases: Beyond ciliopathies. Mol. Methods 2024, 30, 109. [Google Scholar] [CrossRef]
- Dembla, M.; Kesharwani, A.; Natarajan, S.; Fecher-Trost, C.; Fairless, R.; Williams, S.K.; Flockerzi, V.; Diem, R.; Schwarz, K.; Schmitz, F. Early auto-immune targeting of photoreceptor synapses in mouse models of multiple sclerosis. EMBO Mol. Med. 2018, 10, e8926. [Google Scholar] [CrossRef] [PubMed]
- Suiwal, S.; Kiefer, G.; Schmitz, F.; Schwarz, K. An easy, fast and “low-tech”-equipment-requiring alternative method to optimize immunolabelling conditions for pre-embedding immunogold electron microscopy and to correlate light and electron microscopical immunogold labelling results. J. Immunol. Meth. 2017, 444, 7–16. [Google Scholar] [CrossRef]
- Nakajima, Y.; Iwakabe, H.; Akazawa, C.; Nawa, H.; Shigemoto, R.; Mizuno, S.; Nakanishi, S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993, 268, 11868–11873. [Google Scholar] [CrossRef]
- Nomura, A.; Shigemoto, R.; Nakamura, Y.; Okamoto, Y.; Mizuno, N.; Nakanishi, S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 1994, 77, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Masu, M.; Iwakabe, H.; Tagawa, Y.; Miyoshi, T.; Yamashita, M.; Fukuda, Y.; Sasaki, H.; Nakamura, Y.; Shigemoto, R.; Takada, M.; et al. Specific deficit of the ON rsponse in visual transmission by targeted disruption of the mGluR6 gene. Cell 1995, 80, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, C.S.; Barta, L.; Sladek, A.L.; Zenisek, D.; Thoreson, W.B. Eliminating synaptic ribbons from rods and cones halves the relesable vesicle pool and slows down vesicle replenishment. Int. J. Mol. Sci. 2022, 83, 6428. [Google Scholar]
- Uthaiah, R.C.; Hudspeth, A.J. Molecular anatomy of the hair cell’s ribbon synapse. J. Neurosci. 2010, 30, 12387–12399. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; North, M.A.; Berson, E.L.; Dryja, T.P. Recessive mutations in the gene encoding the tubby-like protein Tulp1 in patients with retinitis pigmentosa. Nat. Genet. 1998, 18, 174–176. [Google Scholar] [CrossRef]
- Grossman, G.H.; Pauer, P.J.; Narendra, U.; Peachey, N.S.; Hagstrom, S.A. Early synaptic defects in tulp1-/- mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3074–3083. [Google Scholar] [CrossRef]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, K.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.H.; et al. The disease protein Tulp1 is essential for periactive zone endocytosis in photoreceptor ribbon synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef]
- Ebke, L.A.; Pauer, G.J.; Willard, B.; Hagstrom, S.A. A novel approch to identify photoreceptor compartment—Specific Tulp1 binding partners. Adv. Exp. Med. Biol. 2016, 854, 605–611. [Google Scholar]
- Ebke, L.A.; Sinha, S.; Pauer, G.J.T.; Hagstrom, S.A. Photoreceptor compartment—Specific TULP1 interactomes. Int. J. Mol. Sci. 2021, 22, 8066. [Google Scholar] [CrossRef] [PubMed]
- Higashide, T.; McLaren, M.J.; Inana, G. Localization of HRG4, a photoreceptor protein homologous to unc-119in ribbon synapse. Investig. Ophthalmol. Vis. Sci. 1998, 39, 690–698. [Google Scholar]
- Alpadi, K.; Magupalli, V.G.; Käppel, S.; Köblitz, L.; Schwarz, K.; Seigel, G.M.; Chung, C.H.; Schmitz, F. RIBEYE recruits Munc119, a mammalian orthologue of the of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapes. J. Biol. Chem. 2008, 283, 26461–2626467. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, H.; Zhou, J. Organizations, functions, and mechanisms of the BBSome in development, ciliopathies, and beyond. eLife 2023, 12, e87623. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Garrison, J.E.; Seo, S.; Sheffield, V.C. The absence of BBSome function decreases synaptogenesis and causes ectopic synapse formation in the retina. Sci. Rep. 2020, 10, 8321. [Google Scholar] [CrossRef]
- Morgans, C.W.; Brandstätter, J.H.; Kellermannn, J.; Betz, H.; Wässle, H. A SNARE complex containing syntaxin 3 is present in ribbon synapses in the retina. J. Neurosci. 1996, 16, 6713–6721. [Google Scholar] [CrossRef]
- Morgans, C.W. Presynaptic proteins of ribbon synapses in the retina. Microsc. Res. Tech. 2000, 50, 141–150. [Google Scholar] [CrossRef]
- Chuang, J.Z.; Zhao, Y.; Sung, C.H. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 2007, 130, 535–547. [Google Scholar] [CrossRef]
- Mazelova, J.; Ransom, N.; Astuto-Gribble, L.; Wilson, M.C.; Deretic, D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3-docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the outer segments. J. Cell Sci. 2009, 122, 2003–2013. [Google Scholar] [CrossRef]
- Curtis, L.B.; Doneske, B.; Liu, X.; Thaller, C.; McNew, J.A.; Janz, R. Syntaxin 3B is a T-SNARE specific for ribbon synapses of the retina. J. Comp. Neurol. 2010, 510, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Janecke, A.R.; Liu, X.; Adam, R.; Punuru, S.; Viestenz, A.; Strauß, V.; Laass, M.; Sachez, E.; Adachi, R.; Schatz, M.P.; et al. Pathogenic STX3variants affecting the retinal and intestinal transcripts cause an early-onset severe retinal dystrophy in microvillus inclusion disease subjects. Hum. Genet. 2021, 140, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.; Tebbe, L.; Makia, M.S.; Conley, S.M.; Sherry, D.M.; A-Ubaidi, M.R.; Naash, M.I. Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 20615–20624. [Google Scholar] [CrossRef]
- Zhang, H.; Constantine, R.; Vorobiev, S.; Chen, Y.; Seetharaman, J.; Huang, Y.J.; Xiao, R.; Montelione, G.T.; Gerstner, C.D.; Davis, M.W.; et al. UNC119 is required for G protein trafficking in sensory neurons. Nat. Neurosci. 2011, 14, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Constantine, R.; Zhang, H.; Gerstner, C.D.; Frederick, J.M.; Baehr, W. Uncoordinated (UNC) 119: Coordinating the trafficking of myristoylated proteins. Vis. Res. 2012, 75, 26–32. [Google Scholar] [CrossRef]
- Chaya, T.; Tsutsumi, R.; Varner, L.R.; Maeda, Y.; Yoshida, S.; Furukawa, T. Cul3-Khl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation. EMBO J. 2019, 38, e101409. [Google Scholar] [CrossRef]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825. [Google Scholar] [CrossRef]
- Scholey, J.M. Intraflagellar transport transport motors in cilia moving along the cell’s antenna. J. Cell Biol. 2008, 180, 23–29. [Google Scholar] [CrossRef]
- Lim, Y.S.; Chua, C.E.; Tang, B.L. Rabs and other GTPases in ciliary transport. Biol. Cell 2011, 103, 209–221. [Google Scholar] [CrossRef]
- Malicki, J.J.; Johnson, C.A. The cilium: Cellular antenna and central processing unit. Trends Cell Biol. 2017, 27, 126–140. [Google Scholar] [CrossRef]
- Nievergelt, A.P.; Zykov, I.; Diener, D.; Chhatre, A.; Buchholz, T.O.; Delling, M.; Diez, S.; Jug, F.; Stepanek, L.; Pigino, G. Conversion of anterograde into retrograde trains is an intrinsic property of of intraflagellar transport. Curr. Biol. 2022, 32, 4071–4078. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V. The gymnastics of intraflagellar transport complexes keeps trains running inside cilia. Cell 2022, 185, 4863–4865. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Architecture of intraflagellar transport complexes. Nat. Struct. Mol. Biol. 2023, 30, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Palicharla, V.R.; Mukhopadhyay, S. Molecular and structural perspectives on protein trafficking to the primary cilium membrane. Biochem. Soc. Trans. 2024, 52, 1473–1487. [Google Scholar] [CrossRef]
- Lacey, S.E.; Graziadei, A.; Pigino, G. Extensive structural rearrangements of intraflagellar transport trains underpins bidirectional cargo transport. Cell 2024, 187, 4621–4636. [Google Scholar] [CrossRef]
- Lacey, S.E.; Pigino, G. The intraflagellar transport cycle. Nat. Rev. Mol. Cell. Biol. 2025, 226, 175–192. [Google Scholar] [CrossRef]
- Vaithinathan, T.; Wollmuth, L.P.; Henry, D.; Zenisek, D.; Matthews, G. Tracking newly released synaptic vesicle proteins after exocytosis. iScience 2019, 17, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.M.; Reed, M.; Gerstner, C.D.; Baehr, W. Conditional deletion of dynein heavy chain in postnatal photoreceptors. Investig. Ophthalmol. Vis. Sci. 2021, 62, 23. [Google Scholar] [CrossRef]
- Dahl, T.M.; Baehr, W. Cytoplamic dynein motors in photoreceptors. Mol. Vis. 2021, 27, 506–517. [Google Scholar]
- Kanai, Y.; Wang, D.; Hirokawa, N. KIF13B enhances the endocytosis of LRP1 by recruiting LRP1 to caveolae. J. Cell Biol. 2014, 204, 395–408. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Mogensen, J.B.; Christensen, S.T. Endocytic control of cellular signaling at the primary cilium. Trends Cell Biol. 2016, 41, 784797. [Google Scholar] [CrossRef] [PubMed]
- Boggon, T.J.; Shan, W.S.; Santagata, S.; Myers, S.C.; Shapiro, L. Implication of tubby proteins as transcription factors by structure-based functional analysis. Science 1999, 286, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.; Boggon, T.J.; Baird, C.L.; Gomez, C.A.; Zhao, J.; Shan, W.S.; Myszka, D.G.; Shapiro, L. G-protein signaling through tubby proteins. Science 2001, 292, 2041–2050. [Google Scholar] [CrossRef]
- Xi, Q.; Pauer, G.J.T.; Ball, S.L.; Rayborn, M.; Hollyfield, J.G.; Peachey, N.S.; Crabb, J.W.; Hagstrom, S.A. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2837–2844. [Google Scholar] [CrossRef]
- Heidelberger, R.; Sterling, P.; Matthews, G. Role of ATP in depletion and replenishment of the releasable pool of synaptic vesicles. J. Neurophysiol. 2002, 88, 98–106. [Google Scholar] [CrossRef]
- Graydon, C.W.; Zhang, J.; Oesch, N.W.; Sousa, A.A.; Leapman, R.D.; Diamond, J.S. Passive diffusion as a mechanism underlying ribbon synapse vesicle release and resupply. J. Neurosci. 2014, 34, 8948–8962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wei, Y.; Ronquillo, C.C.; Marc, R.E.; Yoder, B.K.; Frederick, J.M.; Baehr, W. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J. Biol. Chem. 2015, 290, 12765–12778. [Google Scholar] [CrossRef]
- Patel, M.R.; Lehrman, E.K.; Poon, V.Y.; Crump, J.G.; Zhen, M.; Bargmann, C.I.; Shen, K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 2006, 9, 1488–1498. [Google Scholar] [CrossRef]
- Pack-Chung, E.; Kurshanx, P.T.; Dickman, D.K.; Schwarz, T.L. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat. Neurosci. 2007, 10, 980–989. [Google Scholar] [CrossRef]
- Goldstein, A.Y.; Wang, X.; Schwarz, T.L. Axonal transport and the delivery of pre-synaptic components. Curr. Opin. Neurobiol. 2008, 18, 495–503. [Google Scholar] [CrossRef]
- Wagner, O.I.; Esposito, A.; Köhler, B.; Chen, C.W.; Shen, C.P.; Wu, G.H.; Butkevicha, E.; Mandalapua, S.; Wenzel, D.; Wouters, F.S.; et al. Synaptic scaffolding protein SYD-2 clusters and activates UNC-104 in C. elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 19605–19610. [Google Scholar] [CrossRef]
- Sugie, A.; Kakeda-Suzuki, S.; Suzuki, E.; Silies, M.; Mai, S.; Möhl, C.; Suzuki, T.; Tavosanis, G. Molecular remodeling of the presynaptic active zone of drosophila photoreceptors via activity-dependent feedback. Neuron 2015, 86, 711–725. [Google Scholar] [CrossRef]
- Lipton, D.M.; Maeder, C.I.; Shen, K. Rapid assembly of presynaptic materials behind the growth cone in dopaminergic neurons is mediated by precise regulation of axonal transport. Cell Rep. 2018, 24, 2709–2722. [Google Scholar] [CrossRef]
- Guedes-Dias, P.; Holzbaur, E.L.F. Axonal transport: Driving synaptic function. Science 2019, 366, 7. [Google Scholar] [CrossRef]
- Guedes-Dias, P.; Nirschl, J.; Abreu, N.; Tokito, M.K.; Janke, C.; Magiera, M.M.; Holzbaur, E.L.F. Kinesin-3 responds to local microtubule dynamics to target synaptic cargo delivery to the presynapse. Curr. Biol. 2020, 29, 268–282. [Google Scholar] [CrossRef]
- Oliver, D.; Ramachandran, S.; Philbrook, A.; Lambert, C.M.; Nguyen, K.C.Q.; Hall, D.H.; Francis, M.M. Kinesin-3 mediated axonal delivery of presynaptic neurexin stabilizes dendritic spines and postsynaptic components. PLoS Genet. 2022, 18, e1010016. [Google Scholar]
- Bayansan, O.; Bhan, P.; Chang, C.Y.; Barmaver, S.N.; Shen, C.P.; Wagner, O.I. UNC-10/SYD-2 linke kinesin-3 to Rab-3-containing vesicles in the absence of the motor’s PH domain. Neurobiol. Dis. 2025, 204, 106766. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, Y.; Xia, Y.; Zhang, R.; Zhang, M. An Atypical MAGUK GK Target Recognition Mode Revealed by the Interaction between DLG and KIF13B. Structure 2016, 24, 1876–1885. [Google Scholar] [CrossRef]
- Koulen, P.; Fletcher, E.L.; Craven, S.E.; Bredt, D.S.; Wässle, H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J. Neurosci. 1998, 18, 10136–10149. [Google Scholar] [CrossRef]
- David, S.; Pinter, K.; Nguyen, K.K.; Lee, D.S.; Lei, Z.; Sokolova, Y.; Sheets, L. Kindt KS Kif1a and intact microtubules maintain synaptic-vesicle populations at ribbon synapses in zebrafish hair cells. J. Physiol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Peris, L.; Thery, M.; Faure, J.; Saoudi, Y.; Lafanechere, L.; Chilton, J.K.; Gordon-Weeks, P.; Galjart, N.; Bornens, M.; Wordeman, L.; et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus end. J. Cell Biol. 2006, 174, 839–849. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell. Biol. 2008, 9, 309–322. [Google Scholar] [CrossRef]
- Galjart, N. Plus-end-tracking proteins and their interactions at microtubule ends. Curr. Biol. 2010, 20, R528–R537. [Google Scholar] [CrossRef]
- Barra, H.S.; Arce, C.A.; Argarana, C.E. Posttranslational tyrosination/detyrosination of tubulin. Mol. Neurobiol. 1988, 2, 133–153. [Google Scholar] [CrossRef]
- Fan, X.; McKenney, R.J. Control and motor landing and processivity by the CAP-Gly domain in the KIF13B tail. Nat. Commun. 2023, 14, 4715. [Google Scholar] [CrossRef]
- Olbrich, H.; Fliegauf, M.; Hoefele, J.; Kispert, A.; Otto, E.; Volz, A.; Wolf, M.T.; Sasmaz, G.; Trauer, U.; Reinhard, R.; et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapetoretinal degeneration and hepatic firbrosis. Nat. Genet. 2003, 34, 455–459. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Terabayashi, T.; Miki, H. Par1b/MARK2 phosphorylates kinesin-like motor protein GAKIN/KIF13B to regulate axon formation. Mol. Biol. Cell 2010, 30, 2206–2209. [Google Scholar] [CrossRef]
- Lessard, J.L. Two monoclonal antibodies to actin: One muscle selective and one generally reactive. Cell Motil. Cytoskelet. 1988, 10, 349–362. [Google Scholar] [CrossRef]
- Irie, M.; Hata, Y.; Takeuchi, M.; Ichtchenko, K.; Toyoda, A.; Hirao, K.; Takai, Y.; Rosahl, T.W.; Südhof, T.C. Binding of neuroligins to PSD-95. Science 1997, 277, 1511–1515. [Google Scholar] [CrossRef]
- Mukherjee, A.; Katiyar, R.; Dembla, E.; Dembla, M.; Kumar, P.; Belkacemi, A.; Jung, M.; Beck, A.; Flockerzi, V.; Schwarz, K.; et al. Disturbed presynaptic Ca2+ signaling in photoreceptors in the EAE mouse model of multiple sclerosis. iScience 2020, 23, 101830. [Google Scholar] [CrossRef]
- Dembla, E.; Dembla, M.; Maxeiner, S.; Schmitz, F. Synaptic ribbons foster active zone stability and illumination-dependent active zone enrichment of RIM2 and Cav1.4 in photoreceptor synapses. Sci. Rep. 2020, 10, 5957. [Google Scholar] [CrossRef]
- Suiwal, S.; Wartenberg, P.; Boehm, U.; Schmitz, F.; Schwarz, K. A novel Cre recombinase mouse strain for cell-specific deletion of floxed genes in ribbon synapse-forming retinal neurons. Int. J. Mol. Sci. 2024, 25, 1916. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Katiyar, R.; Schmitz, F. A Local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons. J. Neurosci. 2013, 33, 10278–10300. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Schwarz, K.; Dembla, E.; Dembla, M.; Schmitz, F. Early changes in exo- and endocytosis in the EAE mouse model of multiple sclerosis correlate with decreased synaptic ribbon size and reduced ribbon-associated vesicle pools in rod photoreceptor synapses. Int. J. Mol. Sci. 2021, 22, 10789. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.; Ramesh, G.; Jung, M.; Schmitz, F. Rabconnectin-3a/DMXL2 is locally enriched at the synaptic ribbon of rod photoreceptor synapses. Cells 2023, 12, 1665. [Google Scholar] [CrossRef]
- El Samad, A.; Jaffal, J.; Ibrahim, D.R.; Schwarz, K.; Schmitz, F. Decreased expression of the EAAT5 glutamate transporter at photoreceptor synapses in early, pre-clinical experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. Biomedicines 2024, 12, 2545. [Google Scholar] [CrossRef]
- Ibrahim, D.R.; Schwarz, K.; Suiwal, S.; Maragkou, S.; Schmitz, F. Early synapse-specific alterations of photoreceptor mitochondria in the EAE mouse model of multiple sclerosis. Cells 2025, 14, 206. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Harsman, A.; Kopp, A.; Wagner, R.; Zimmermann, R.; Jung, M. Calmodulin regulation of the calcium-leak channel sec61 is unique to vertebrates. Channels 2011, 5, 293–298. [Google Scholar] [CrossRef]

| Primary Antibodies | References/Source | Dilution |
|---|---|---|
| anti-RIBEYE mouse monoclonal antibody (2D9) against the C-terminus of RIBEYE(B)-domain/CtBP2 | [28,61] | 1:1000 (IF) 1:1000 (WB) 1:1000 (EM) |
| anti-actin (clone C4) mouse monoclonal antibody | Millipore, Burlington, MA, USA, MAB1501 [128] | 1:1000 (WB) |
| anti-RIBEYE rabbit polyclonal antibody (U2656) against RIBEYE(B)-domain | [23] | 1:1000 (IF) |
| anti-PSD95 (post-synaptic density protein 95) rabbit polyclonal antibody (L667) | [129] | 1:1000 (IF) |
| anti-mGluR6 rabbit polyclonal antibody (1205) | [26,130] | 1:2000 (IF) |
| anti-Cav1.4 Cterm rabbit polyclonal antibody | [131] | 1:1000 (IF) |
| Antibody | Source | Dilution |
|---|---|---|
| Chicken anti-mouse Alexa488 | Invitrogen Molecular Probes, Eugene, OR, USA, A-21200 | 1:1000 (IF) |
| Donkey anti-mouse Alexa488 | Invitrogen Molecular Probes, A-21202 | 1:1000 (IF) |
| Donkey anti-rabbit Alexa568 | Invitrogen, Molecular Probes, A-10042 | 1:1000 (IF) |
| Chicken anti-rabbit Alexa488 | Invitrogen, Molecular Probes, A-21441 | 1:1000 (IF) |
| Donkey anti-mouse Alexa568 | Invitrogen, Molecular Probes, A-10037 | 1:1000 (IF) |
| Goat anti-mouse Alexa647 | Invitrogen, Molecular Probes, A-21236 | 1:1000 (IF) |
| Donkey anti-mouse Alexa647 | Invitrogen, Molecular Probes, A-31571 | 1:1000 (IF) |
| Goat anti-rabbit peroxidase-conjugated (POX) IgG | Sigma, A-6154 | 1:3000 (WB) 1:10,000 (Pepspots) |
| Goat anti-mouse peroxidase-conjugated (POX) IgG | Sigma, A-3673 | 1:3000 (WB) 1:10,000 (Pepspots) |
| Goat anti-mouse secondary antibody conjugated to 5 nm gold particles | Sigma, G7527 | 1:100 (EM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suiwal, S.; Schwarz, K.; Maxeiner, S.; Schmitz, F. The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex. Int. J. Mol. Sci. 2025, 26, 6044. https://doi.org/10.3390/ijms26136044
Suiwal S, Schwarz K, Maxeiner S, Schmitz F. The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex. International Journal of Molecular Sciences. 2025; 26(13):6044. https://doi.org/10.3390/ijms26136044
Chicago/Turabian StyleSuiwal, Shweta, Karin Schwarz, Stephan Maxeiner, and Frank Schmitz. 2025. "The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex" International Journal of Molecular Sciences 26, no. 13: 6044. https://doi.org/10.3390/ijms26136044
APA StyleSuiwal, S., Schwarz, K., Maxeiner, S., & Schmitz, F. (2025). The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex. International Journal of Molecular Sciences, 26(13), 6044. https://doi.org/10.3390/ijms26136044







