Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease
Abstract
1. Introduction
2. Results
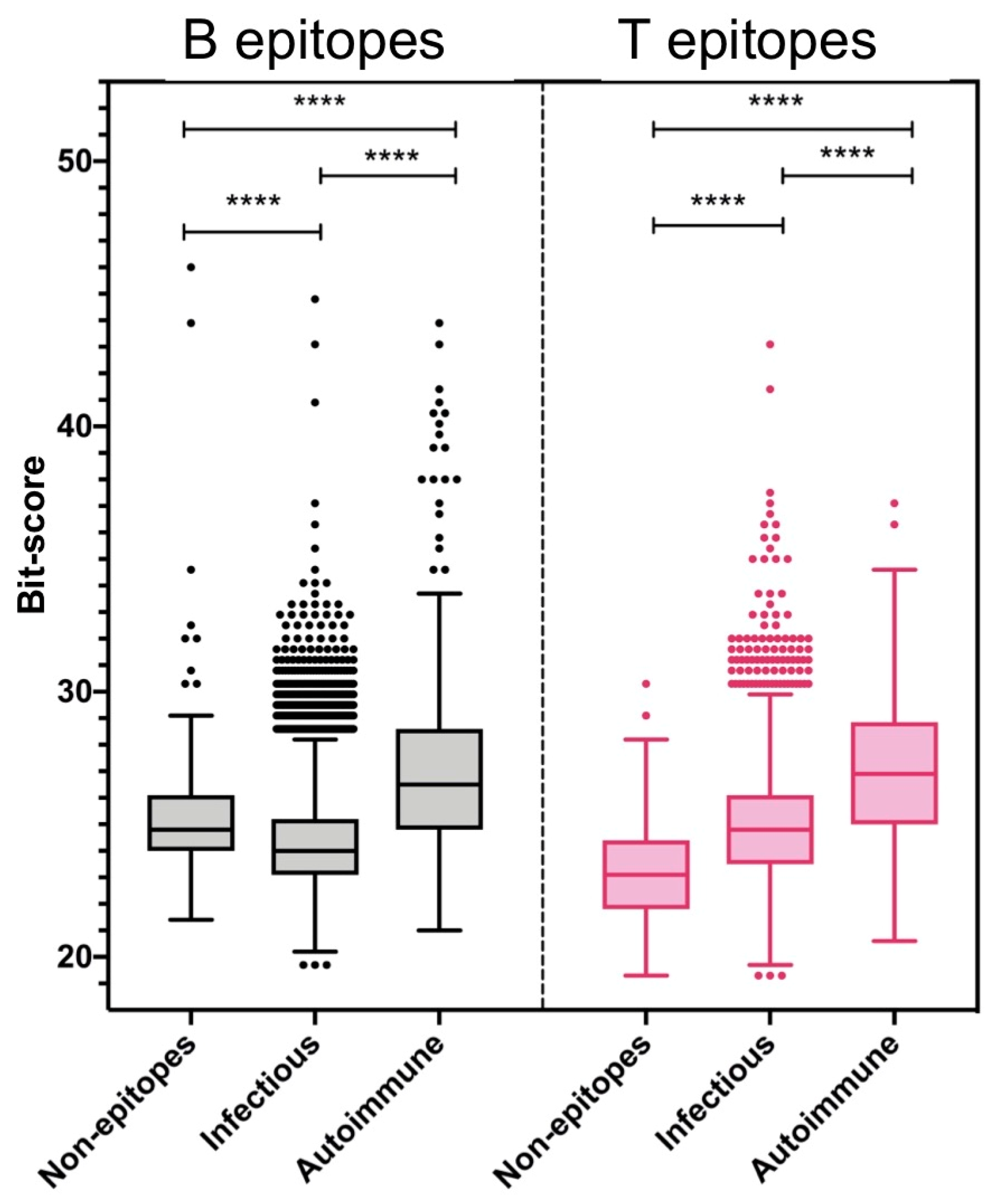
2.1. Molecular Mimicry Between Viral Epitopes and the Human Proteome
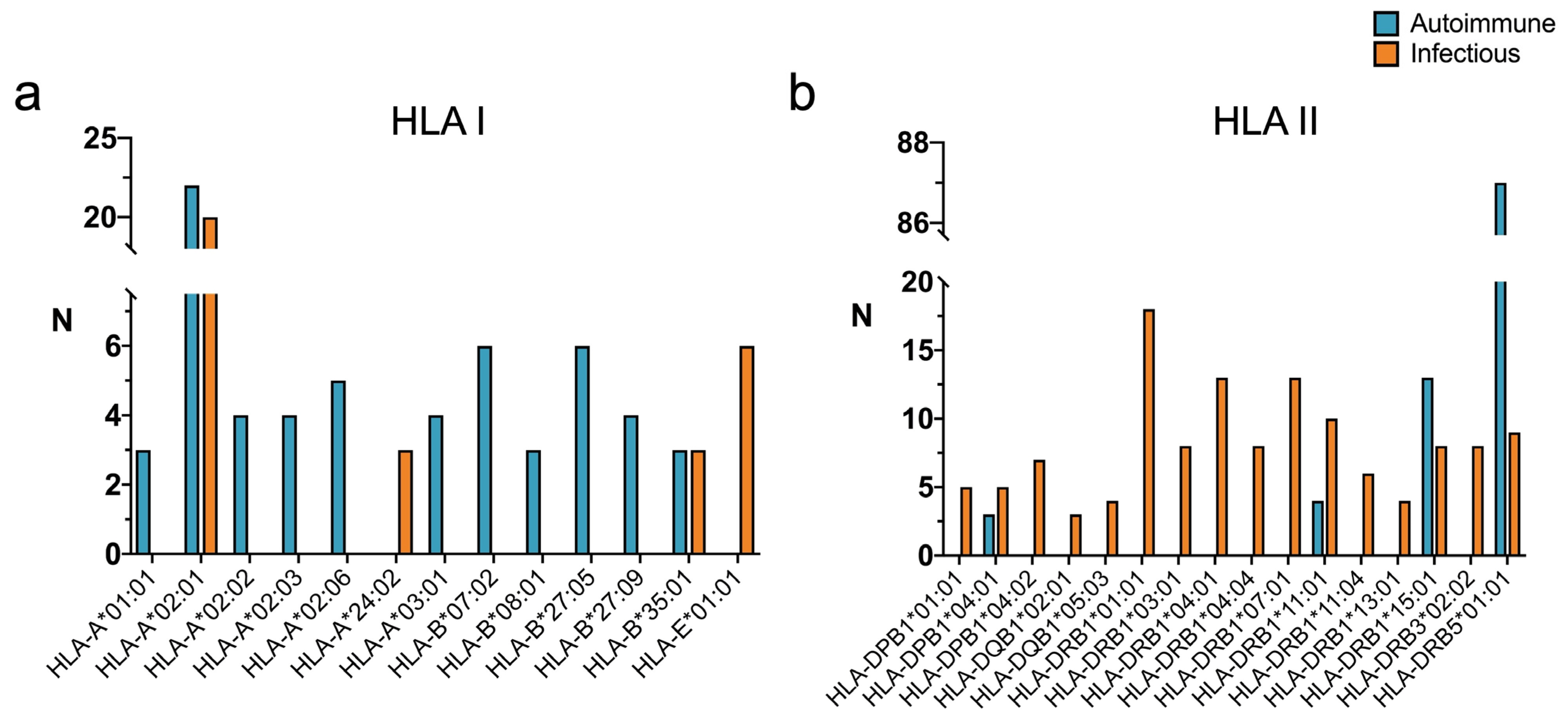
2.2. HLA Restriction of T Cell Epitopes Linked to Infectious Diseases with High Similarity to Human Antigens
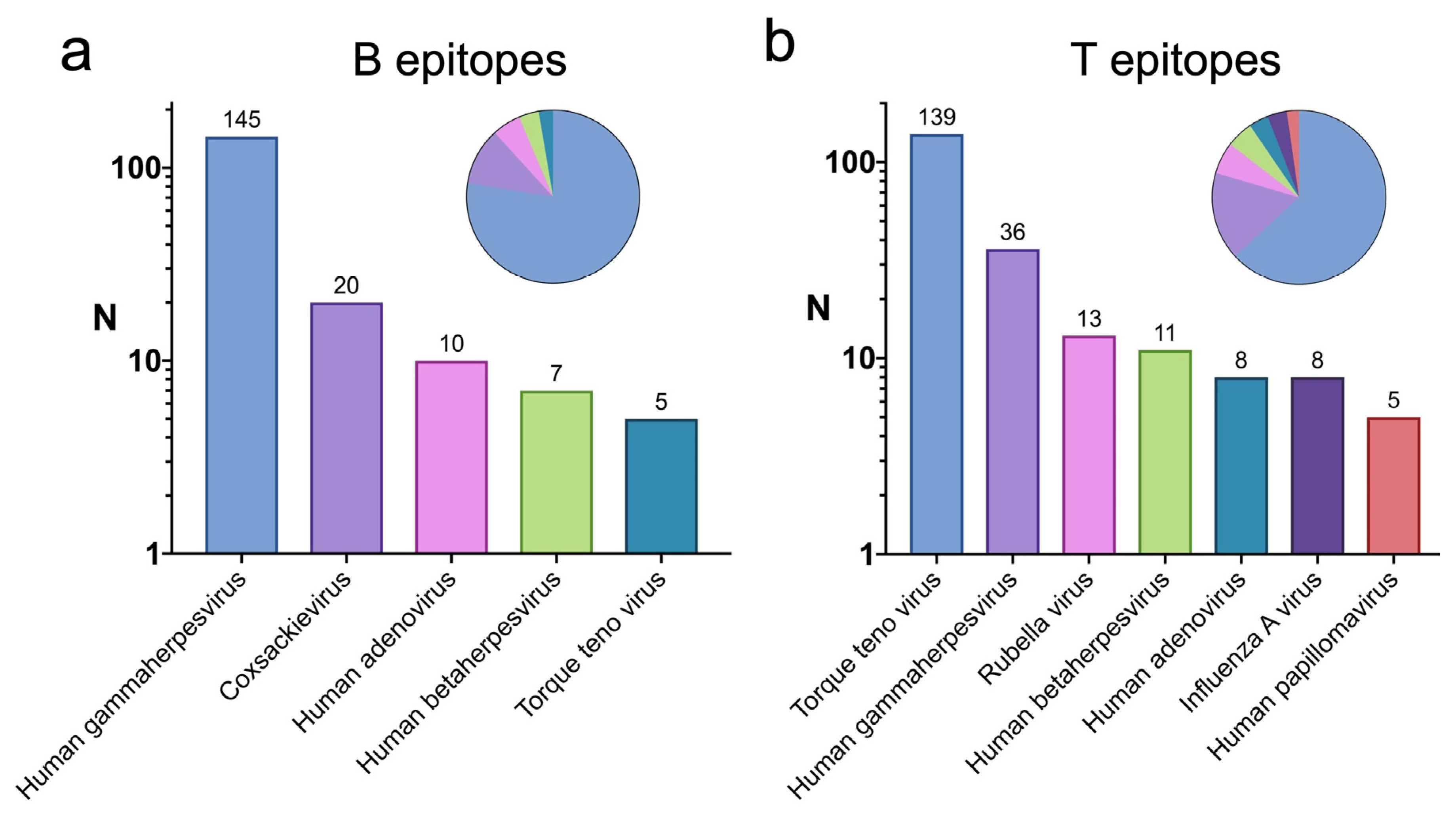
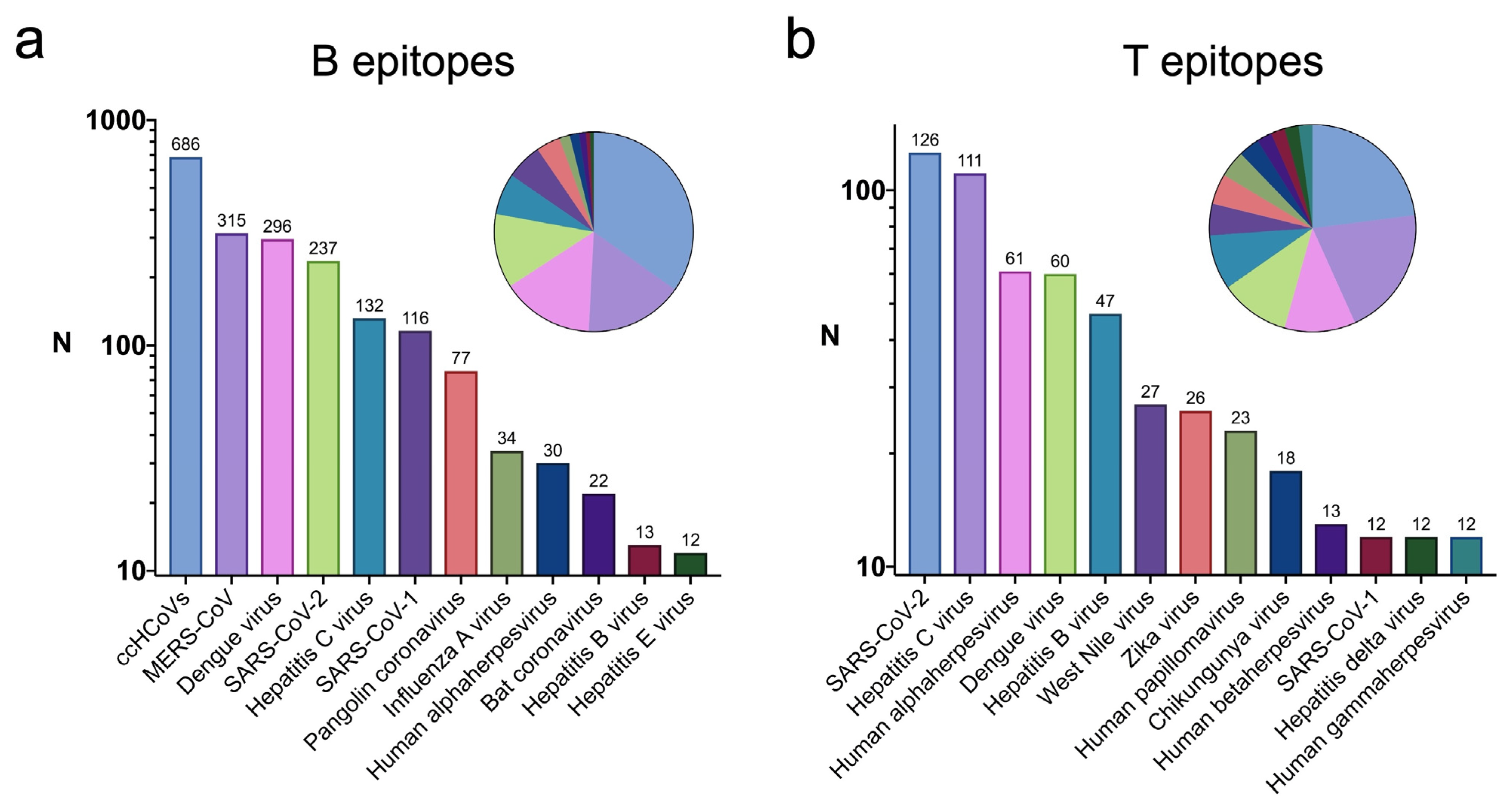
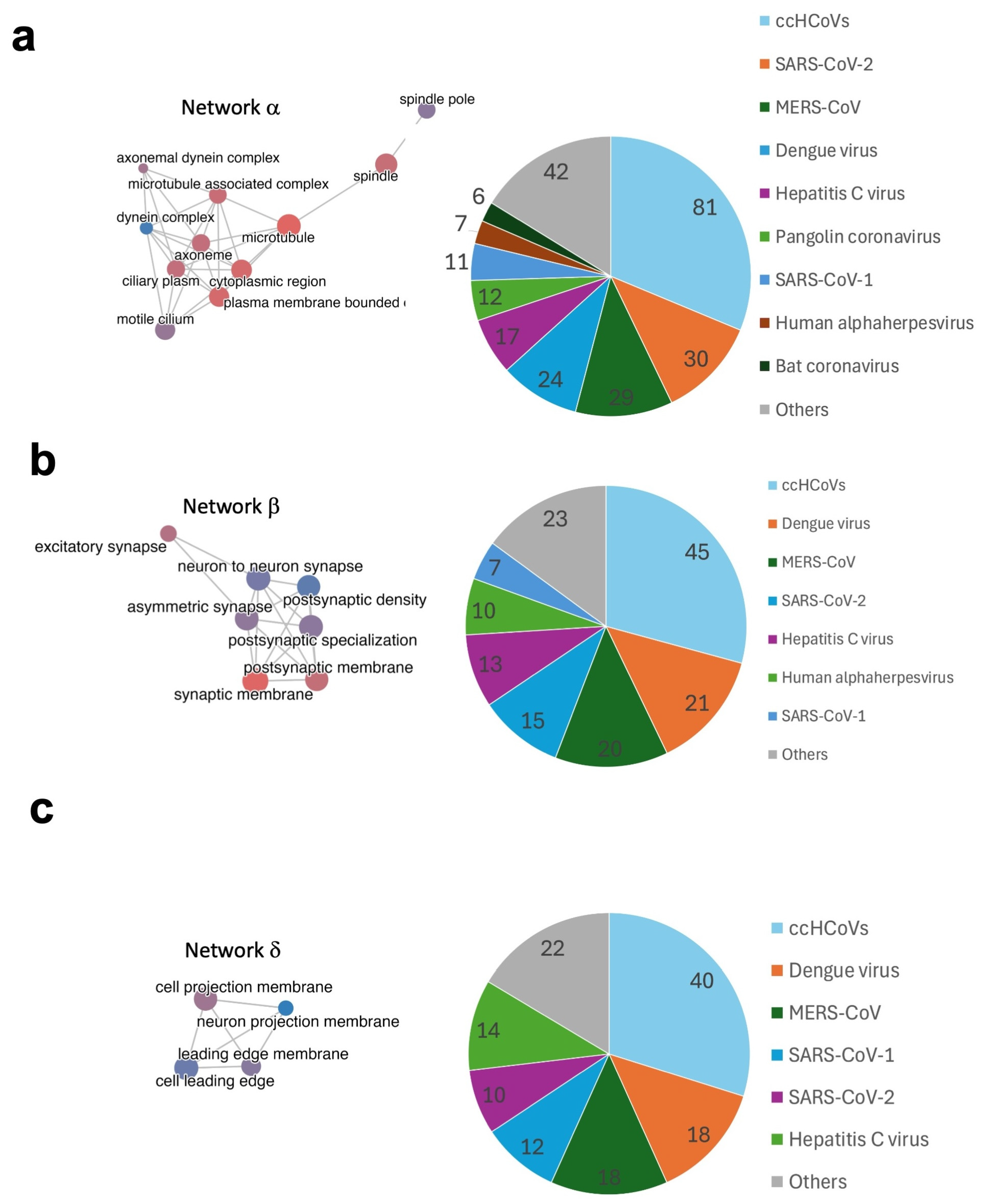
2.3. Viruses with Epitopes Linked to Infectious Diseases with High Similarity to Self-Antigens
2.4. Targets of Potential Cross-Reactive Autoimmunity Due to Molecular Mimicry
3. Discussion
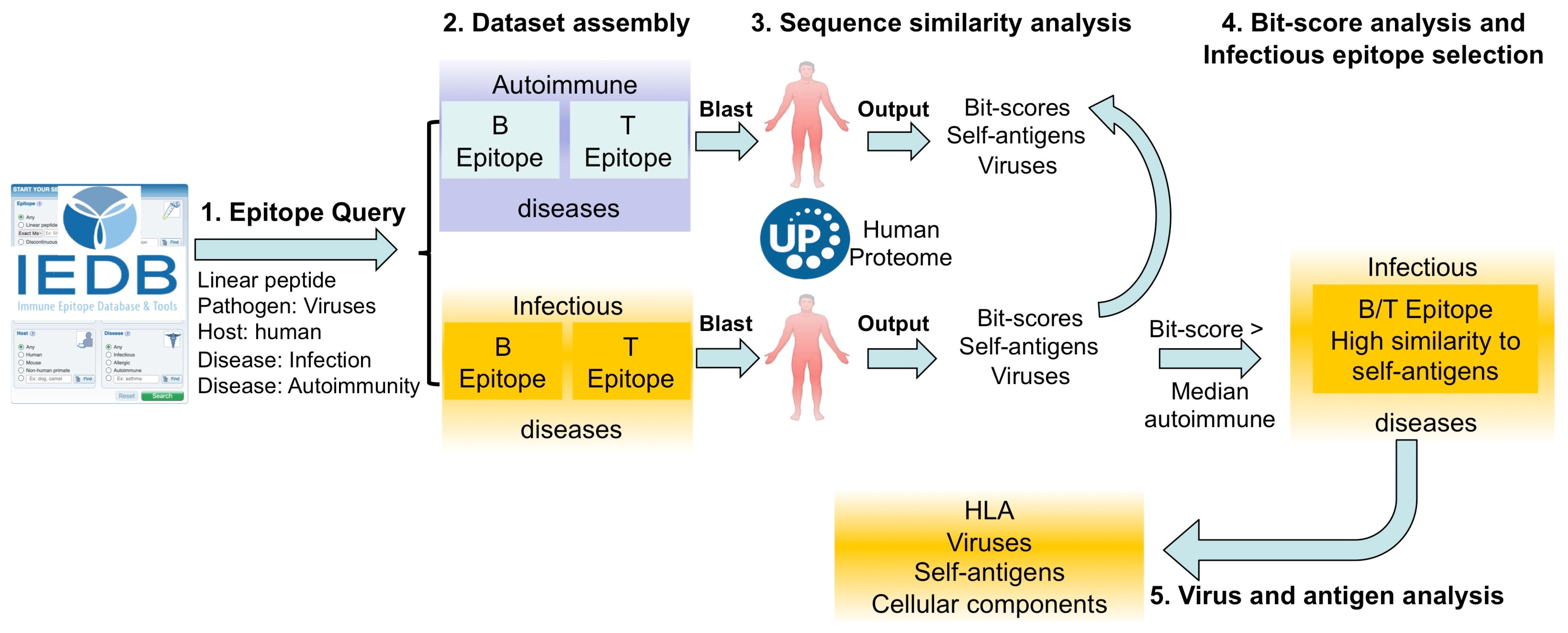
4. Materials and Methods
4.1. Epitope Data Collection and Processing
4.2. Generation of Non-Epitopes from Viruses
4.3. Determining Sequence Similarity to Human Antigens
4.4. Other Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCR | B Cell Receptor |
| BLAST | Basic Local Alignment Search Tool |
| ccHCoVs | Common Cold Human Coronaviruses |
| DENV | Dengue Virus |
| FDR | False Discovery Rate |
| GO | Gene Ontology |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HLA | Human Leukocyte Antigen |
| HPV | Human Papillomavirus |
| IAV | Influenza A Virus |
| IEDB | Immune Epitope Database |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus |
| MHC | Major Histocompatibility Complex |
| NCBI | National Center for Biotechnology Information |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| TCR | T Cell Receptor |
| TTV | Torque Teno Virus |
| WNV | West Nile Virus |
| ZKV | Zika Virus |
References
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J. Immunol. Res. 2017, 2017, 2680160. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L.; Baker, A. Cellular and Molecular Immunology; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780323479783/0323479782/9780323523240/0323523242. [Google Scholar]
- Nemazee, D. Mechanisms of Central Tolerance for B Cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and Negative Selection of the T Cell Repertoire: What Thymocytes See (and Don’t See). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Saouaf, S.J.; Brennan, P.J.; Shen, Y.; Greene, M.I. Mechanisms of Peripheral Immune Tolerance: Conversion of the Immune to the Unresponsive Phenotype. Immunol. Res. 2003, 28, 193–199. [Google Scholar] [CrossRef]
- Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 12149. [Google Scholar] [CrossRef]
- Han, L.; Wu, T.; Zhang, Q.; Qi, A.; Zhou, X. Immune Tolerance Regulation Is Critical to Immune Homeostasis. J. Immunol. Res. 2025, 2025, 5006201. [Google Scholar] [CrossRef]
- Fiyouzi, T.; Pelaez-Prestel, H.F.; Reyes-Manzanas, R.; Lafuente, E.M.; Reche, P.A. Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 7797. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Y.-C.; Ni, Q.-Y.; Chen, Y.; Wan, C.-H.; Liu, S.-Y.; Tao, L.-M.; Jiang, Z.-X.; Ni, J.; Pan, H.-F. Temporal Trends in the Prevalence of Autoimmune Diseases from 1990 to 2019. Autoimmun. Rev. 2023, 22, 103359. [Google Scholar] [CrossRef]
- Miller, F.W. The Increasing Prevalence of Autoimmunity and Autoimmune Diseases: An Urgent Call to Action for Improved Understanding, Diagnosis, Treatment, and Prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of Human Autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Gough, S.C.L. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr. Genom. 2007, 8, 453–465. [Google Scholar] [CrossRef]
- Thorsby, E.; Lie, B.A. HLA Associated Genetic Predisposition to Autoimmune Diseases: Genes Involved and Possible Mechanisms. Transpl. Immunol. 2005, 14, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; Leung, P.S.C.; Gershwin, M.E. Environmental Basis of Autoimmunity. Clin. Rev. Allergy Immunol. 2016, 50, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A. A Potential Link between Environmental Triggers and Autoimmunity. Autoimmune Dis. 2014, 2014, 437231. [Google Scholar] [CrossRef] [PubMed]
- Delogu, L.G.; Deidda, S.; Delitala, G.; Manetti, R. Infectious Diseases and Autoimmunity. J. Infect. Dev. Ctries. 2011, 5, 679–687. [Google Scholar] [CrossRef]
- Ercolini, A.M.; Miller, S.D. The Role of Infections in Autoimmune Disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Sundaresan, B.; Shirafkan, F.; Ripperger, K.; Rattay, K. The Role of Viral Infections in the Onset of Autoimmune Diseases. Viruses 2023, 15, 782. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef]
- Yu, X.; Petersen, F. A Methodological Review of Induced Animal Models of Autoimmune Diseases. Autoimmun. Rev. 2018, 17, 473–479. [Google Scholar] [CrossRef]
- Johnson, D.; Jiang, W. Infectious Diseases, Autoantibodies, and Autoimmunity. J. Autoimmun. 2023, 137, 102962. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.-M. Molecular Mimicry and Autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular Mimicry as a Mechanism of Autoimmune Disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Vanderlugt, C.L.; Miller, S.D. Epitope Spreading in Immune-Mediated Diseases: Implications for Immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Overton, J.A.; Greenbaum, J.A.; Ponomarenko, J.; Clark, J.D.; Cantrell, J.R.; Wheeler, D.K.; Gabbard, J.L.; Hix, D.; Sette, A.; et al. The Immune Epitope Database (IEDB) 3.0. Nucleic Acids Res. 2015, 43, D405–D412. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 Update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Ras-Carmona, A.; Reche, P.A. Analysis of Virus-Specific B Cell Epitopes Reveals Extensive Antigen Degradation Prior to Recognition. Cells 2024, 13, 1076. [Google Scholar] [CrossRef]
- Balbin, C.A.; Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K.; et al. Epitopedia: Identifying Molecular Mimicry of Known Immune Epitopes. BioRxiv 2021. [Google Scholar] [CrossRef]
- Lee, C.H.; Huh, J.; Buckley, P.R.; Jang, M.; Pinho, M.P.; Fernandes, R.A.; Antanaviciute, A.; Simmons, A.; Koohy, H. A Robust Deep Learning Workflow to Predict CD8 + T-Cell Epitopes. Genome Med. 2023, 15, 70. [Google Scholar] [CrossRef]
- Ogishi, M.; Yotsuyanagi, H. Quantitative Prediction of the Landscape of T Cell Epitope Immunogenicity in Sequence Space. Front. Immunol. 2019, 10, 827. [Google Scholar] [CrossRef]
- Yarmarkovich, M.; Warrington, J.M.; Farrel, A.; Maris, J.M. Identification of SARS-CoV-2 Vaccine Epitopes Predicted to Induce Long-Term Population-Scale Immunity. Cell Rep. Med. 2020, 1, 100036. [Google Scholar] [CrossRef]
- Koncz, B.; Balogh, G.M.; Papp, B.T.; Asztalos, L.; Kemény, L.; Manczinger, M. Self-Mediated Positive Selection of T Cells Sets an Obstacle to the Recognition of Nonself. Proc. Natl. Acad. Sci. USA 2021, 118, e2100542118. [Google Scholar] [CrossRef] [PubMed]
- Fridkis-Hareli, M.; Reche, P.A.; Reinherz, E.L. Peptide Variants of Viral CTL Epitopes Mediate Positive Selection and Emigration of Ag-Specific Thymocytes In Vivo. J. Immunol. 2004, 173, 1140–1150. [Google Scholar] [CrossRef]
- Kisielow, P. How Does the Immune System Learn to Distinguish between Good and Evil? The First Definitive Studies of T Cell Central Tolerance and Positive Selection. Immunogenetics 2019, 71, 513–518. [Google Scholar] [CrossRef]
- Janeway, C.A. T-Cell Development: A Role for Self-Peptides in Positive Selection. Curr. Biol. 1999, 9, R342–R345. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, A.; Paul, S.; Schommer, N.; Dillon, M.B.; Bancroft, T.; Greenbaum, J.; Sette, A.; Nielsen, M.; Peters, B. T-Cell Recognition Is Shaped by Epitope Sequence Conservation in the Host Proteome and Microbiome. Immunology 2016, 148, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Miyata, H. Torque Teno Virus (TTV): Current Status. Rev. Med. Virol. 2007, 17, 45–57. [Google Scholar] [CrossRef]
- Gergely, P.; Pullmann, R.; Stancato, C.; Otvos, L.; Koncz, A.; Blazsek, A.; Poor, G.; Brown, K.E.; Phillips, P.E.; Perl, A. Increased Prevalence of Transfusion-Transmitted Virus and Cross-Reactivity with Immunodominant Epitopes of the HRES-1/P28 Endogenous Retroviral Autoantigen in Patients with Systemic Lupus Erythematosus. Clin. Immunol. 2005, 116, 124–134. [Google Scholar] [CrossRef]
- Blazsek, A.; Sillo, P.; Ishii, N.; Gergely, P., Jr.; Poor, G.; Preisz, K.; Hashimoto, T.; Medvecz, M.; Kárpáti, S. Searching for Foreign Antigens as Possible Triggering Factors of Autoimmunity: Torque Teno Virus DNA Prevalence Is Elevated in Sera of Patients with Bullous Pemphigoid. Exp. Dermatol. 2008, 17, 446–454. [Google Scholar] [CrossRef]
- Baker, B.; Guimarães, L.E.; Tomljenovic, L.; Agmon-Levin, N.; Shoenfeld, Y. The Safety of Human Papilloma Virus-Blockers and the Risk of Triggering Autoimmune Diseases. Expert Opin. Drug Saf. 2015, 14, 1387–1394. [Google Scholar] [CrossRef]
- Maximova, N.; Pizzol, A.; Ferrara, G.; Maestro, A.; Tamaro, P. Does Teno Torque Virus Induce Autoimmunity After Hematopoietic Stem Cell Transplantation? A Case Report. J. Pediatr. Hematol. Oncol. 2015, 37, e194–e197. [Google Scholar] [CrossRef]
- Buonocore, S.M.; van der Most, R.G. Narcolepsy and H1N1 Influenza Immunology a Decade Later: What Have We Learned? Front. Immunol. 2022, 13, 902840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, Y.; Liu, X.; Xu, Y.; Wang, Y.; Jiang, D.; Du, P. Zika and Dengue Virus Autoimmunity: An Overview of Related Disorders and Their Potential Mechanisms. Rev. Med. Virol. 2025, 35, e70014. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, P.; Alexopoulos, H.; Sourdi, A.; Papadimitriou, D.; Dimitrakopoulos, A.N.; Moutsopoulos, H.M. West Nile Virus Infection Triggering Autoimmune Encephalitis: Pathophysiological and Therapeutic Implications. Clin. Immunol. 2019, 207, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Singh, M.; Jackson, K.J.L.; Field, M.A.; Peters, T.J.; Angioletti-Uberti, S.; Frenkel, D.; Ravishankar, S.; Gupta, M.; Wang, J.J.; et al. A Triad of Somatic Mutagenesis Converges in Self-Reactive B Cells to Cause a Virus-Induced Autoimmune Disease. Immunity 2025, 58, 412–430.e10. [Google Scholar] [CrossRef]
- Batskikh, S.; Morozov, S.; Vinnitskaya, E.; Sbikina, E.; Borunova, Z.; Dorofeev, A.; Sandler, Y.; Saliev, K.; Kostyushev, D.; Brezgin, S.; et al. May Previous Hepatitis B Virus Infection Be Involved in Etiology and Pathogenesis of Autoimmune Liver Diseases? Adv. Ther. 2022, 39, 430–440. [Google Scholar] [CrossRef]
- Maya, R.; Gershwin, M.E.; Shoenfeld, Y. Hepatitis B Virus (HBV) and Autoimmune Disease. Clin. Rev. Allergy Immunol. 2008, 34, 85–102. [Google Scholar] [CrossRef]
- Hromić-Jahjefendić, A.; Lundstrom, K.; Adilović, M.; Aljabali, A.A.A.; Tambuwala, M.M.; Serrano-Aroca, Á.; Uversky, V.N. Autoimmune Response after SARS-CoV-2 Infection and SARS-CoV-2 Vaccines. Autoimmun. Rev. 2024, 23, 103508. [Google Scholar] [CrossRef]
- Lind, A.; Scherman, M.N.; Hamdan, S.; Agardh, D. Risk of Celiac Disease, Type 1 Diabetes, and Thyroid Disease Autoimmunity during the SARS-CoV-2 Pandemic in South of Sweden: Insights from the TRIAD Study. Autoimmunity 2025, 58, 2490491. [Google Scholar] [CrossRef]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K.; et al. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins with Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2021, 11, 617089. [Google Scholar] [CrossRef]
- Gorse, G.J.; Patel, G.B.; Vitale, J.N.; O’Connor, T.Z. Prevalence of Antibodies to Four Human Coronaviruses Is Lower in Nasal Secretions than in Serum. Clin. Vaccine Immunol. 2010, 17, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Ho, A.; Marques, D.F.P.; McMenamin, J.; Gunson, R.N.; Murcia, P.R. Epidemiology of Seasonal Coronaviruses: Establishing the Context for the Emergence of Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 17–25. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Zhang, P.; LaBaer, J.; Hermjakob, H.; Li, D.; Yu, X. AAgAtlas 1.0: A human autoantigen database. Nucleic Acids Res. 2017, 45, D769–D776. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.L.; Digby, R.J.; Needham, E.J. Neurological Update: COVID-19. J. Neurol. 2021, 268, 4379–4387. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Chakravarty, A. Neurological Complications of Dengue Fever. Curr. Neurol. Neurosci. Rep. 2022, 22, 515–529. [Google Scholar] [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Bassi, G.L.; Pardo, C.A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef]
- Navis, A. A Review of Neurological Symptoms in Long COVID and Clinical Management. Semin. Neurol. 2023, 43, 286–296. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Kuczyńska, K. Psychiatric and Neurological Complications of Long COVID. J. Psychiatr. Res. 2022, 156, 349–360. [Google Scholar] [CrossRef]
- Faccioli, J.; Nardelli, S.; Gioia, S.; Riggio, O.; Ridola, L. Neurological and Psychiatric Effects of Hepatitis C Virus Infection. World J. Gastroenterol. 2021, 27, 4846. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.I.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.; Falcon, S.; Pages, H.; Li, N. org.Hs.eg.db: Genome Wide Annotation for Human, R package version 4.5.0; Bioconductor version 3.21: Boston, MA, USA, 2019; Volume 3, p. 3. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ras-Carmona, A.; Lehmann, A.; Reche, P.A. Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease. Int. J. Mol. Sci. 2025, 26, 6041. https://doi.org/10.3390/ijms26136041
Ras-Carmona A, Lehmann A, Reche PA. Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease. International Journal of Molecular Sciences. 2025; 26(13):6041. https://doi.org/10.3390/ijms26136041
Chicago/Turabian StyleRas-Carmona, Alvaro, Alexander Lehmann, and Pedro A. Reche. 2025. "Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease" International Journal of Molecular Sciences 26, no. 13: 6041. https://doi.org/10.3390/ijms26136041
APA StyleRas-Carmona, A., Lehmann, A., & Reche, P. A. (2025). Similarity to Self-Antigens Shapes Epitope Recognition from Viruses Under Autoimmune and Infectious Disease. International Journal of Molecular Sciences, 26(13), 6041. https://doi.org/10.3390/ijms26136041






