Proteomic Characterization of Human Peripheral Blood Mononuclear Cells Exposed to a 50 Hz Magnetic Field
Abstract
1. Introduction
2. Results
2.1. ELF-MF Does Not Affect Human PBMC Proliferation, Cell Death, or Cell Cycle Progression
2.2. Alteration of the Proteomic Profile in PBMCs Exposed to ELF-MF
2.3. Cell Signaling Pathways Involved in the PBMC Response to ELF-MF Exposure
3. Discussion
4. Materials and Methods
4.1. Donors and Blood Samples
4.2. PBMC Isolation and Culture
4.3. PBMC Exposure to ELF-MF
4.4. Cell Counting
4.5. Flow Cytometry
4.6. Protein Extraction, Digestion, and Peptide Preparation for Mass Spectrometry-Based Proteomics
4.7. Mass Spectrometry Analysis
4.8. Bioinformatics Analyses
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frahm, J.; Lantow, M.; Lupke, M.; Weiss, D.G.; Simkó, M. Alteration in cellular functions in mouse macrophages after exposure to 50 Hz magnetic fields. J. Cell. Biochem. 2006, 99, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, R.; Eléxpuru-Zabaleta, M.; Piva, F.; Giulietti, M.; Fulgenzi, G.; Tartaglione, M.F.; Zingaretti, L.; Tagliabracci, A.; Valentino, M.; Santarelli, L.; et al. Effects of extremely low-frequency magnetic fields on human MDA-MB-231 breast cancer cells: Proteomic characterization. Ecotoxicol. Environ. Saf. 2023, 253, 114650. [Google Scholar] [CrossRef]
- Li, H.; Zeng, Q.; Weng, Y.; Lu, D.; Jiang, H.; Xu, Z. Effects of ELF magnetic fields on protein expression profile of human breast cancer cell MCF7. Sci. China C Life Sci. 2005, 48, 506–514. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.O. Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: Possible immune cell activation. J. Cell. Biochem. 2004, 93, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Zhang, Y.; Wan, B.; Zhang, J.; He, W.; Hu, D.; Yang, Y.; Lai, J.; He, M.; et al. Exposure to a 50 Hz magnetic field at 100 µT exerts no DNA damage in cardiomyocytes. Biol. Open 2019, 8, bio041293. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.; Mattsson, M.O.; Simkó, M. Exposure to ELF magnetic fields modulate redox related protein expression in mouse macrophages. Toxicol. Lett. 2010, 192, 330–336. [Google Scholar] [CrossRef]
- Benassi, B.; Filomeni, G.; Montagna, C.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Consales, C. Extremely Low Frequency Magnetic Field (ELF-MF) Exposure Sensitizes SH-SY5Y Cells to the Pro-Parkinson’s Disease Toxin MPP(.). Mol. Neurobiol. 2016, 53, 4247–4260. [Google Scholar] [CrossRef]
- Du, L.; Fan, H.; Miao, H.; Zhao, G.; Hou, Y. Extremely low frequency magnetic fields inhibit adipogenesis of human mesenchymal stem cells. Bioelectromagnetics 2014, 35, 519–530. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chuang, C.Y.; Shu, W.Y.; Chang, C.W.; Chen, C.R.; Fan, T.C.; Hsu, I.C. Distinct epidermal keratinocytes respond to extremely low-frequency electromagnetic fields differently. PLoS ONE 2014, 9, e113424. [Google Scholar] [CrossRef]
- Elexpuru-Zabaleta, M.; Lazzarini, R.; Tartaglione, M.F.; Piva, F.; Ciarapica, V.; Marinelli Busilacchi, E.; Poloni, A.; Valentino, M.; Santarelli, L.; Bracci, M. A 50 Hz magnetic field influences the viability of breast cancer cells 96 h after exposure. Mol. Biol. Rep. 2023, 50, 1005–1017. [Google Scholar] [CrossRef]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: Possible involvement of a redox mechanism. Biochim. Biophys. Acta 2005, 1743, 120–129. [Google Scholar] [CrossRef]
- Shah, A.D.; Goode, R.J.A.; Huang, C.; Powell, D.R.; Schittenhelm, R.B. LFQ-Analyst: An Easy-To-Use Interactive Web Platform To Analyze and Visualize Label-Free Proteomics Data Preprocessed with MaxQuant. J. Proteome Res. 2020, 19, 204–211. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Costantini, A.; Viola, N.; Costantini, B.; Olivieri, J.; Butini, L.; Mancini, G.; Scortechini, I.; Chiarucci, M.; Poiani, M.; et al. Immunomodulatory Effects of Tyrosine Kinase Inhibitor In Vitro and In Vivo Study. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 267–275. [Google Scholar] [CrossRef]
- Delli Muti, N.; Salvio, G.; Ciarloni, A.; Perrone, M.; Tossetta, G.; Lazzarini, R.; Bracci, M.; Balercia, G. Can extremely low frequency magnetic field affect human sperm parameters and male fertility? Tissue Cell 2023, 82, 102045. [Google Scholar] [CrossRef]
- Nezamtaheri, M.S.; Goliaei, B.; Shariatpanahi, S.P.; Ansari, A.M. Differential biological responses of adherent and non-adherent (cancer and non-cancerous) cells to variable extremely low frequency magnetic fields. Sci. Rep. 2022, 12, 14225. [Google Scholar] [CrossRef] [PubMed]
- López de Mingo, I.; Rivera González, M.X.; Maestú Unturbe, C. The Cellular Response Is Determined by a Combination of Different ELF-EMF Exposure Parameters: A Scope Review. Int. J. Mol. Sci. 2024, 25, 5074. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Simkó, M.; Mattsson, M.O.; Pioli, C. Immune-Modulating Perspectives for Low Frequency Electromagnetic Fields in Innate Immunity. Front. Public Health 2018, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Makinistian, L.; Marková, E.; Belyaev, I. A high throughput screening system of coils for ELF magnetic fields experiments: Proof of concept on the proliferation of cancer cell lines. BMC Cancer 2019, 19, 188. [Google Scholar] [CrossRef]
- Martínez, M.A.; Úbeda, A.; Cid, M.A.; Trillo, M. The proliferative response of NB69 human neuroblastoma cells to a 50 Hz magnetic field is mediated by ERK1/2 signaling. Cell. Physiol. Biochem. 2012, 29, 675–686. [Google Scholar] [CrossRef]
- Martínez, M.A.; Úbeda, A.; Moreno, J.; Trillo, M. Power Frequency Magnetic Fields Affect the p38 MAPK-Mediated Regulation of NB69 Cell Proliferation Implication of Free Radicals. Int. J. Mol. Sci. 2016, 17, 510. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, K.W.; Ni, D.X.; Fang, H.J.; Jang, L.S.; Chen, C.H. Effect of extremely low frequency electromagnetic field parameters on the proliferation of human breast cancer. Electromagn. Biol. Med. 2021, 40, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Capri, M.; Mesirca, P.; Remondini, D.; Carosella, S.; Pasi, S.; Castellani, G.; Franceschi, C.; Bersani, F. 50 Hz sinusoidal magnetic fields do not affect human lymphocyte activation and proliferation in vitro. Phys. Biol. 2004, 1, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Shinmura, Y.; Mizoe, H.; Yoshizawa, H.; Yoshida, A.; Kanao, S.; Sumitani, H.; Hasebe, S.; Motomura, T.; Yamakawa, T.; et al. No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro. Bioelectromagnetics 2003, 24, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gobba, F.; Bargellini, A.; Scaringi, M.; Bravo, G.; Borella, P. Extremely low frequency-magnetic fields (ELF-EMF) occupational exposure and natural killer activity in peripheral blood lymphocytes. Sci. Total Environ. 2009, 407, 1218–1223. [Google Scholar] [CrossRef]
- Glück, B.; Güntzschel, V.; Berg, H. Inhibition of proliferation of human lymphoma cells U937 by a 50 Hz electromagnetic field. Cell. Mol. Biol. 2001, 47, OL115-7. [Google Scholar]
- Radeva, M.; Berg, H. Differences in lethality between cancer cells and human lymphocytes caused by LF-electromagnetic fields. Bioelectromagnetics 2004, 25, 503–507. [Google Scholar] [CrossRef]
- Cadossi, R.; Bersani, F.; Cossarizza, A.; Zucchini, P.; Emilia, G.; Torelli, G.; Franceschi, C. Lymphocytes and low-frequency electromagnetic fields. FASEB J. 1992, 6, 2667–2674. [Google Scholar] [CrossRef]
- Cossarizza, A.; Monti, D.; Bersani, F.; Cantini, M.; Cadossi, R.; Sacchi, A.; Franceschi, C. Extremely low frequency pulsed electromagnetic fields increase cell proliferation in lymphocytes from young and aged subjects. Biochem. Biophys. Res. Commun. 1989, 160, 692–698. [Google Scholar] [CrossRef]
- Lyle, D.B.; Schechter, P.; Adey, W.R.; Lundak, R.L. Suppression of T-lymphocyte cytotoxicity following exposure to sinusoidally amplitude-modulated fields. Bioelectromagnetics 1983, 4, 281–292. [Google Scholar] [CrossRef]
- Vignati, M.; Giuliani, L. Radiofrequency exposure near high-voltage lines. Environ. Health Perspect. 1997, 105 (Suppl. 6), 1569–1573. [Google Scholar] [CrossRef]
- Kawamata, H.; Tiranti, V.; Magrané, J.; Chinopoulos, C.; Manfredi, G. adPEO mutations in ANT1 impair ADP-ATP translocation in muscle mitochondria. Hum. Mol. Genet. 2011, 20, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Jaroslawska, J.; Ghosh Dastidar, R.; Carlberg, C. In vivo vitamin D target genes interconnect key signaling pathways of innate immunity. PLoS ONE 2024, 19, e0306426. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, T.; Lu, Y.; Shi, T.; Yang, Q. The role of lactylation in plasma cells and its impact on rheumatoid arthritis pathogenesis: Insights from single-cell RNA sequencing and machine learning. Front. Immunol. 2024, 15, 1453587. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Thompson, C.B. Activation and inhibition of lymphocytes by costimulation. J. Clin. Investig. 2002, 109, 295–299. [Google Scholar] [CrossRef]
- Grapentine, S.; Singh, R.K.; Basu, P.; Sivanesan, S.; Mattos, G.; Oresajo, O.; Cheema, J.; Demeke, W.; Dolinsky, V.W.; Bakovic, M. Pcyt2 deficiency causes age-dependant development of nonalcoholic steatohepatitis and insulin resistance that could be attenuated with phosphoethanolamine. Sci. Rep. 2022, 12, 1048. [Google Scholar] [CrossRef]
- Lim, S.A.; Su, W.; Chapman, N.M.; Chi, H. Lipid metabolism in T cell signaling and function. Nat. Chem. Biol. 2022, 18, 470–481. [Google Scholar] [CrossRef]
- Petkevicius, K.; Virtue, S.; Bidault, G.; Jenkins, B.; Çubuk, C.; Morgantini, C.; Aouadi, M.; Dopazo, J.; Serlie, M.J.; Koulman, A.; et al. Accelerated phosphatidylcholine turnover in macrophages promotes adipose tissue inflammation in obesity. eLife 2019, 8, e47990. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Fahy, E.; Guan, Z.; Kelly, S.; Li, X.; McDonald, J.G.; Milne, S.; Myers, D.; Park, H.; Ryan, A.; et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010, 51, 2785–2797. [Google Scholar] [CrossRef]
- Snider, S.A.; Margison, K.D.; Ghorbani, P.; LeBlond, N.D.; O’Dwyer, C.; Nunes, J.R.C.; Nguyen, T.; Xu, H.; Bennett, S.A.L.; Fullerton, M.D. Choline transport links macrophage phospholipid metabolism and inflammation. J. Biol. Chem. 2018, 293, 11600–11611. [Google Scholar] [CrossRef]
- Tian, Y.; Pate, C.; Andreolotti, A.; Wang, L.; Tuomanen, E.; Boyd, K.; Claro, E.; Jackowski, S. Cytokine secretion requires phosphatidylcholine synthesis. J. Cell Biol. 2008, 181, 945–957. [Google Scholar] [CrossRef]
- Pavlovic, Z.; Bakovic, M. Regulation of Phosphatidylethanolamine Homeostasis–The Critical Role of CTP:Phosphoethanolamine Cytidylyltransferase (Pcyt2). Int. J. Mol. Sci. 2013, 14, 2529–2550. [Google Scholar] [CrossRef]
- Hao, T.; Fang, W.; Xu, D.; Chen, Q.; Liu, Q.; Cui, K.; Cao, X.; Li, Y.; Mai, K.; Ai, Q. Phosphatidylethanolamine alleviates OX-LDL-induced macrophage inflammation by upregulating autophagy and inhibiting NLRP1 inflammasome activation. Free Radic. Biol. Med. 2023, 208, 402–417. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, X.; Liu, Q.; Zhan, R.; Tang, Y.; Bu, X.; Li, W.; Du, J.; Li, Y.; Mai, K.; et al. Phosphatidylethanolamine exerts anti-inflammatory action by regulating mitochondrial function in macrophages of large yellow croaker (Larimichthys crocea). FASEB J. 2024, 38, e70180. [Google Scholar] [CrossRef]
- Gupta, S.S.; Wang, J.; Chen, M. Metabolic Reprogramming in CD8(+) T Cells During Acute Viral Infections. Front. Immunol. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Vasan, K.; Chandel, N.S. Mitochondrial Metabolism Regulation of T Cell-Mediated Immunity. Annu. Rev. Immunol. 2021, 39, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Guy, C.S.; Chapman, N.M.; Palacios, G.; Wei, J.; Zhou, P.; Long, L.; Wang, Y.D.; Qian, C.; Dhungana, Y.; et al. Metabolic control of T(FH) cells and humoral immunity by phosphatidylethanolamine. Nature 2021, 595, 724–729. [Google Scholar] [CrossRef]
- Kipmen-Korgun, D.; Bilmen-Sarikcioglu, S.; Altunbas, H.; Demir, R.; Korgun, E.T. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand. J. Clin. Lab. Investig. 2009, 69, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.; Habtemichael, E.N.; Julca, O.; Sales, C.I.; Westergaard, X.O.; DeVries, S.G.; Ruiz, D.; Sayal, B.; Bogan, J.S. GLUT4 Storage Vesicles: Specialized Organelles for Regulated Trafficking. Yale J. Biol. Med. 2019, 92, 453–470. [Google Scholar]
- Srivastava, P.; Uddin, M.W.; Diwakar, K.; Biswal, S.; Senapati, A. Homozygous Mutation in the QARS1 Gene Causing Developmental Epileptic Encephalopathy in Siblings in the Southeast Asian Region: An Interesting Case Report and Discussion. Cureus 2025, 17, e78333. [Google Scholar] [CrossRef]
- Zhang, X.; Ling, J.; Barcia, G.; Jing, L.; Wu, J.; Barry, B.J.; Mochida, G.H.; Hill, R.S.; Weimer, J.M.; Stein, Q.; et al. Mutations in QARS, encoding glutaminyl-tRNA synthetase, cause progressive microcephaly, cerebral-cerebellar atrophy, and intractable seizures. Am. J. Hum. Genet. 2014, 94, 547–558. [Google Scholar] [CrossRef]
- Shao, Y.; Qi, Y.; Huang, Y.; Liu, Z.; Ma, Y.; Guo, X.; Jiang, S.; Sun, Z.; Ruan, Q. Human cytomegalovirus-encoded miR-US4-1 promotes cell apoptosis and benefits discharge of infectious virus particles by targeting QARS. J. Biosci. 2016, 41, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Deng, Z.; Zhu, T. Membrane protein trafficking in the anti-tumor immune response: Work of endosomal-lysosomal system. Cancer Cell Int. 2022, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Crisman, L.; Dou, X.; Winborn, C.S.; Wan, C.; Puscher, H.; Yin, Q.; Kennedy, M.J.; Shen, J. Regulation of cargo exocytosis by a Reps1-Ralbp1-RalA module. Sci. Adv. 2023, 9, eade2540. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-ionizing radiation, Part 1: Static and extremely low-frequency (ELF) electric and magnetic fields. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 80, 1–395. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Gaps in Knowledge Relevant to the “Guidelines for Limiting Exposure to Time-Varying Electric and Magnetic Fields (1 Hz–100 kHz)”. Health Phys. 2020, 118, 533–542. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Health Criteria 238: Extremely Low Frequency Fields; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Gobba, F.; Bargellini, A.; Bravo, G.; Scaringi, M.; Cauteruccio, L.; Borella, P. Natural killer cell activity decreases in workers occupationally exposed to extremely low frequency magnetic fields exceeding 1 microT. Int. J. Immunopathol. Pharmacol. 2009, 22, 1059–1066. [Google Scholar] [CrossRef]
- Mahaki, H.; Tanzadehpanah, H.; Jabarivasal, N.; Sardanian, K.; Zamani, A. A review on the effects of extremely low frequency electromagnetic field (ELF-EMF) on cytokines of innate and adaptive immunity. Electromagn. Biol. Med. 2019, 38, 84–95. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emidio, G. Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxidative Med. Cell. Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef]
- Lin, K.W.; Yang, C.J.; Lian, H.Y.; Cai, P. Exposure of ELF-EMF and RF-EMF Increase the Rate of Glucose Transport and TCA Cycle in Budding Yeast. Front. Microbiol. 2016, 7, 1378. [Google Scholar] [CrossRef]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A.; Elias-Viñas, D.; Verdugo-Diaz, L. Effects of whole body exposure to extremely low frequency electromagnetic fields (ELF-EMF) on serum and liver lipid levels, in the rat. Lipids Health Dis. 2007, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Adair, R.K. Constraints on biological effects of weak extremely-low-frequency electromagnetic fields. Phys. Rev. A 1991, 43, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Fleischmann, M.; Preparata, G.; Talpo, G. On the “unreasonable” effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 2002, 23, 522–530. [Google Scholar] [CrossRef]
- Zhadin, M.N.; Bakharev, B.V.; Bobkova, N.V. Mechanism of action of combined extremely weak magnetic field on aqueous solution of amino acid. Biofizika 2014, 59, 829–832. [Google Scholar] [CrossRef]
- Zhadin, M.; Giuliani, L. Some problems in modern bioelectromagnetics. Electromagn. Biol. Med. 2006, 25, 227–243. [Google Scholar] [CrossRef]
- DIRECTIVE 2013/35/EU Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/dir/2013/35/oj/eng (accessed on 15 April 2025).
- Modenese, A.; Gobba, F. Occupational Exposure to Electromagnetic Fields and Health Surveillance According to the European Directive 2013/35/EU. Int. J. Environ. Res. Public Health 2021, 18, 1730. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.J. Parametric design of tri-axial nested Helmholtz coils. Rev. Sci. Instrum. 2015, 86, 054701. [Google Scholar] [CrossRef]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef]
- Mittal, L.; Camarillo, I.G.; Varadarajan, G.S.; Srinivasan, H.; Aryal, U.K.; Sundararajan, R. High-throughput, Label-Free Quantitative Proteomic Studies of the Anticancer Effects of Electrical Pulses with Turmeric Silver Nanoparticles: An in vitro Model Study. Sci. Rep. 2020, 10, 7258. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Cox, J. Perseus: A Bioinformatics Platform for Integrative Analysis of Proteomics Data in Cancer Research. Methods Mol. Biol. 2018, 1711, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]

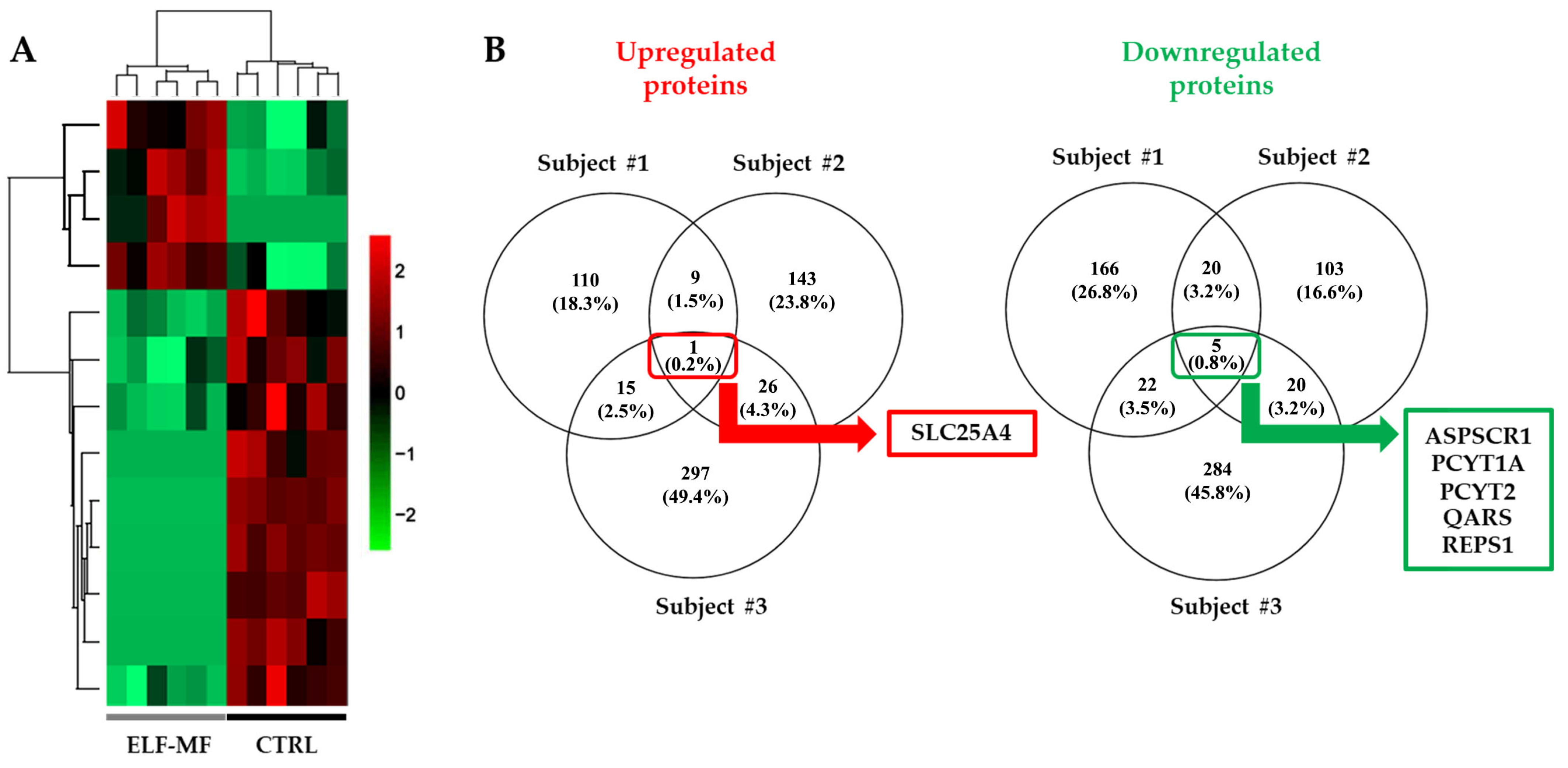
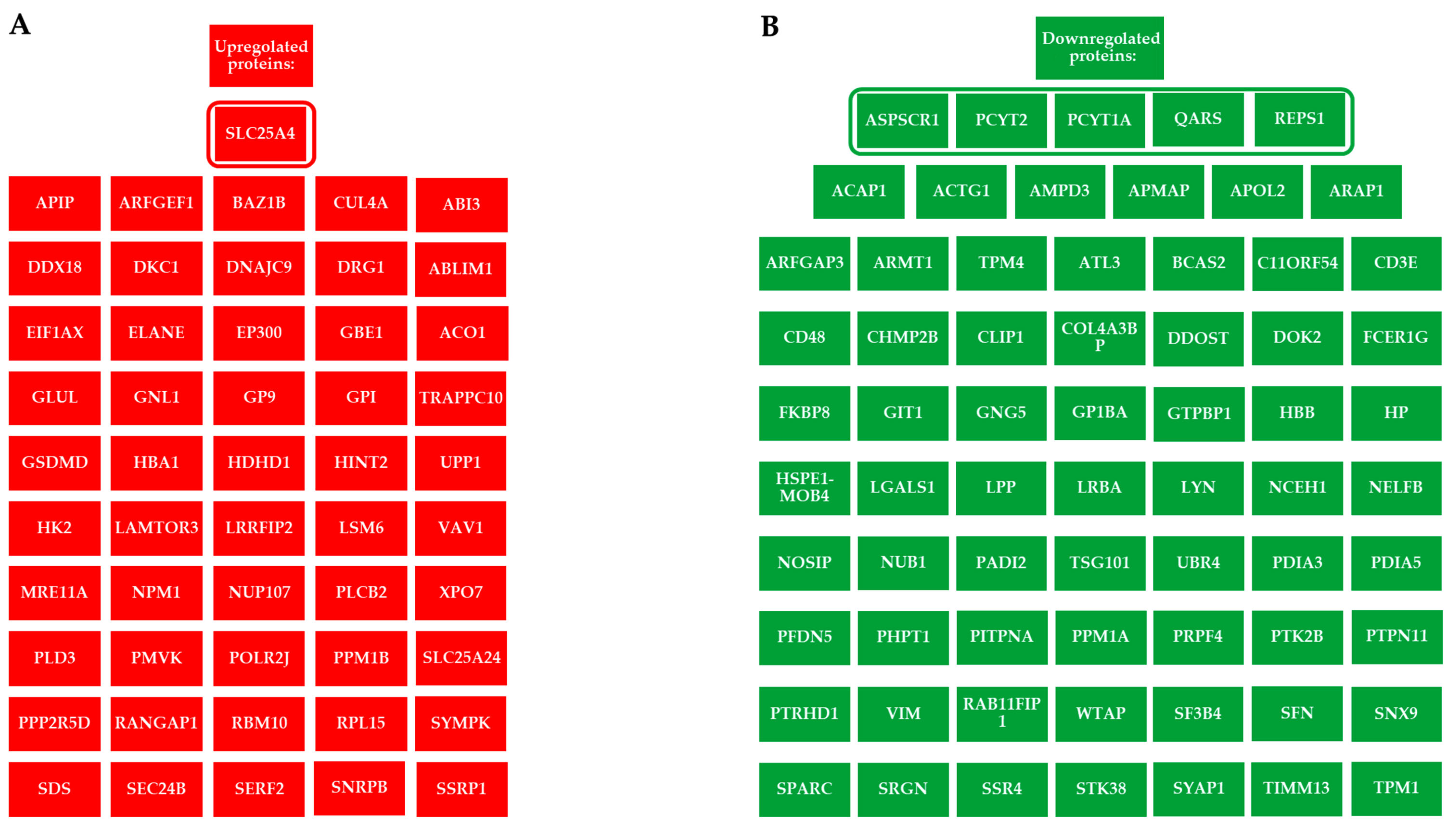
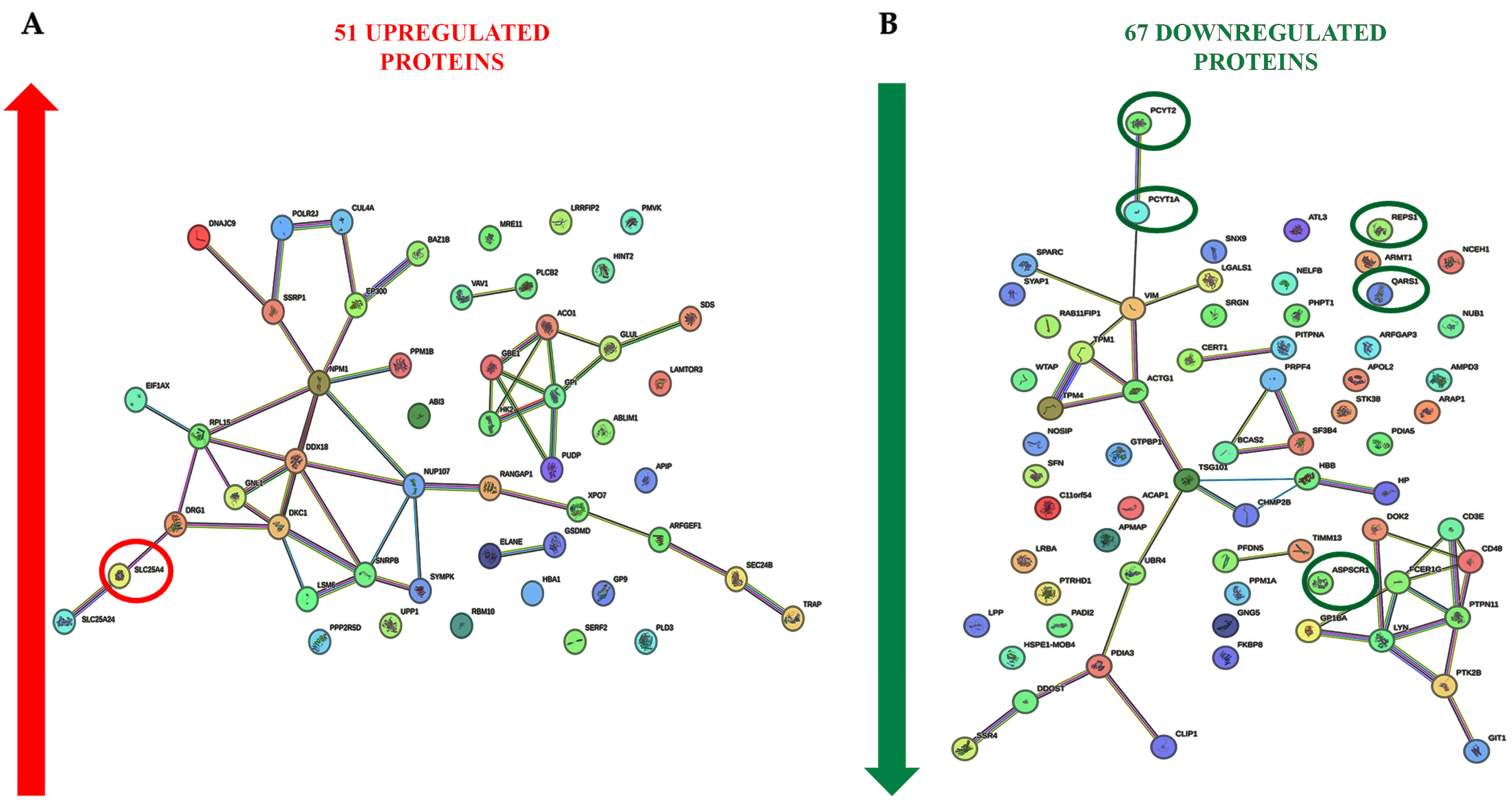
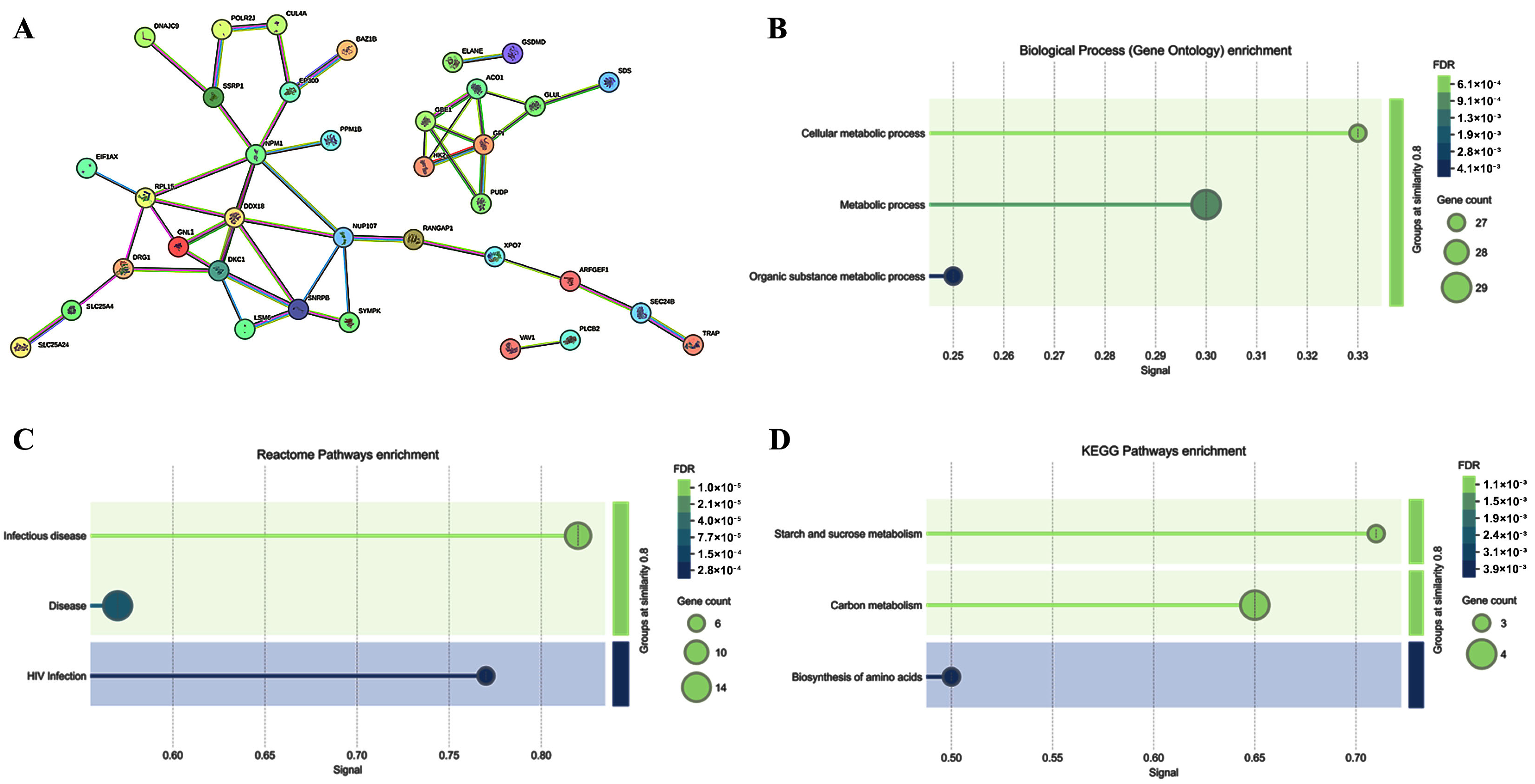

| Subject #1 | Subject #2 | Subject #3 | |
|---|---|---|---|
| Age (years) | 30 | 43 | 32 |
| Sex | Male | Male | Male |
| Ethnic background | European | European | European |
| BMI (kg/m2) | 23.9 | 23.5 | 26.3 |
| Systolic blood pressure (mmHg) | 130 | 110 | 120 |
| Diastolic blood pressure (mmHg) | 70 | 80 | 75 |
| Fasting blood glucose (mg/dL) | 88 | 92 | 85 |
| Total cholesterol (mg/dL) | 172 | 198 | 188 |
| Triglycerides (mg/dL) | 134 | 142 | 117 |
| Alcohol consumption (grams/week) | 24 | 36 | 12 |
| Leisure-time physical activity (min/week) | 120 | 60 | 180 |
| Smoker | No | No | No |
| Family history of obesity | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracci, M.; Lazzarini, R.; Piva, F.; Giulietti, M.; Marinelli Busilacchi, E.; Rossi, E.; Di Criscio, F.; Santarelli, L.; Poloni, A. Proteomic Characterization of Human Peripheral Blood Mononuclear Cells Exposed to a 50 Hz Magnetic Field. Int. J. Mol. Sci. 2025, 26, 6035. https://doi.org/10.3390/ijms26136035
Bracci M, Lazzarini R, Piva F, Giulietti M, Marinelli Busilacchi E, Rossi E, Di Criscio F, Santarelli L, Poloni A. Proteomic Characterization of Human Peripheral Blood Mononuclear Cells Exposed to a 50 Hz Magnetic Field. International Journal of Molecular Sciences. 2025; 26(13):6035. https://doi.org/10.3390/ijms26136035
Chicago/Turabian StyleBracci, Massimo, Raffaella Lazzarini, Francesco Piva, Matteo Giulietti, Elena Marinelli Busilacchi, Elisa Rossi, Fabio Di Criscio, Lory Santarelli, and Antonella Poloni. 2025. "Proteomic Characterization of Human Peripheral Blood Mononuclear Cells Exposed to a 50 Hz Magnetic Field" International Journal of Molecular Sciences 26, no. 13: 6035. https://doi.org/10.3390/ijms26136035
APA StyleBracci, M., Lazzarini, R., Piva, F., Giulietti, M., Marinelli Busilacchi, E., Rossi, E., Di Criscio, F., Santarelli, L., & Poloni, A. (2025). Proteomic Characterization of Human Peripheral Blood Mononuclear Cells Exposed to a 50 Hz Magnetic Field. International Journal of Molecular Sciences, 26(13), 6035. https://doi.org/10.3390/ijms26136035









