Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. LncRNAs in Muscle Development
3. LncRNA and miRNA—Working Together in Muscles
3.1. Cell Cycle and Differentiation Regulators
3.2. Atrophy Regulators
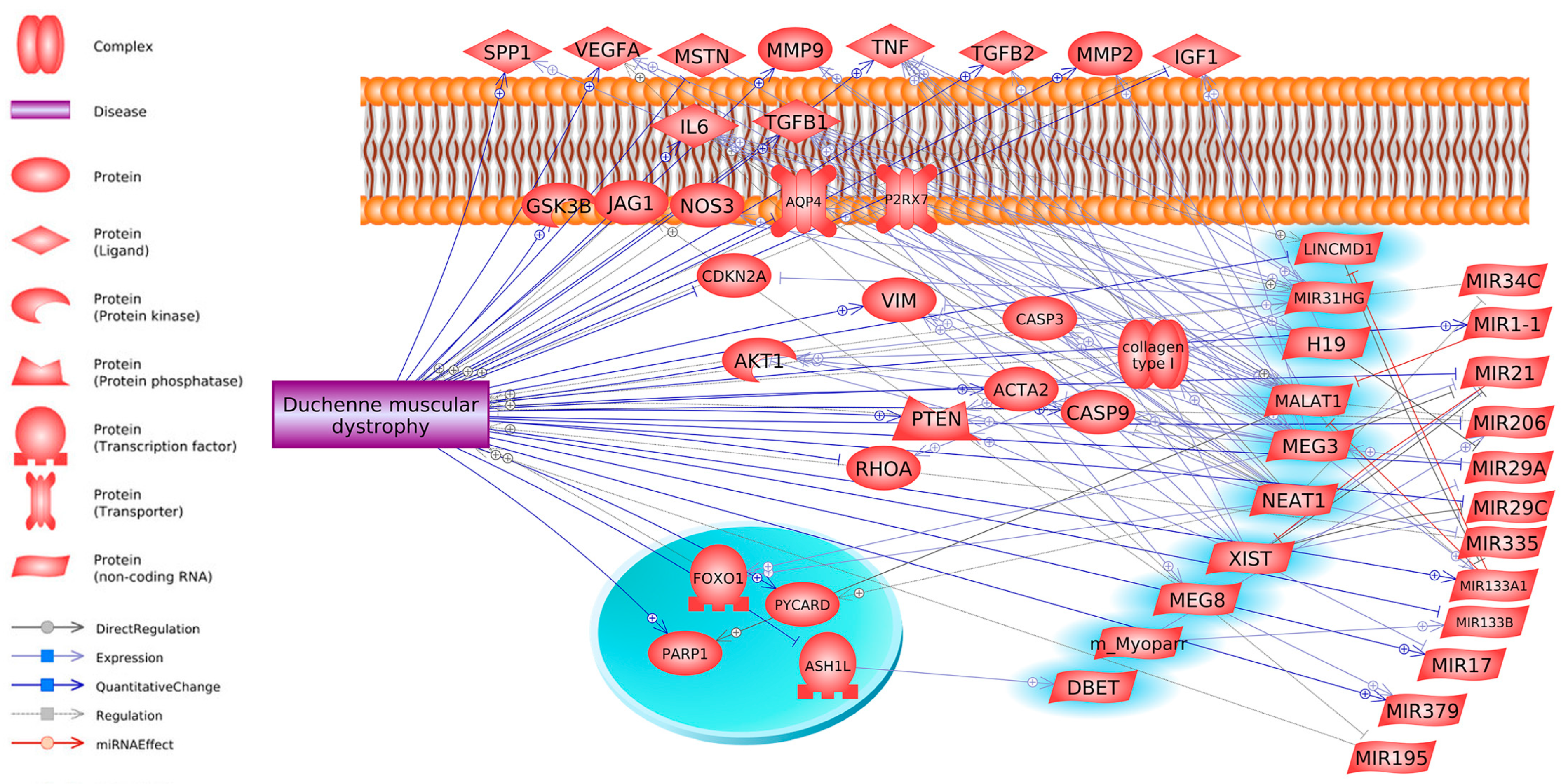
4. LncRNA in DMD Disease
| LncRNA | Chr Location | Effect on Genes | Genes | Chr Location | References | DMD Effect on Gene | References |
|---|---|---|---|---|---|---|---|
| Meg3 | 14 | positive | Igf1 | 12 | [65] | positive | [66] |
| Meg3 | 14 | negative | Akt1 | 14 | [67] | positive | [68] |
| Meg3 | 14 | negative | Il6 | 7 | [69] | positive | [70] |
| Meg3 | 14 | negative | Mmp9 | 20 | [71] | positive | [72] |
| Meg3 | 14 | negative | Tgfb1 | 19 | [73] | positive | [74] |
| Meg3 | 14 | negative | Vegfa | 6 | [75] | positive | [76] |
| Meg3 | 14 | positive | Casp3 | 4 | [77] | - | - |
| Meg3 | 14 | positive | Casp9 | 1 | [78] | positive | [79] |
| Meg3 | 14 | positive | Foxo1 | 13 | [80] | - | - |
| Meg3 | 14 | positive | Mmp2 | 16 | [81] | positive | [82] |
| Meg3 | 14 | positive | Pten | 10 | [83] | positive | [84] |
| Neat1 | 11 | negative | Akt1 | 14 | [85] | positive | [68] |
| Neat1 | 11 | negative | Casp9 | 1 | [86] | positive | [79] |
| Neat1 | 11 | positive | Acta2 | 10 | [87] | positive | [88] |
| Neat1 | 11 | positive | Casp3 | 4 | [89] | - | - |
| Neat1 | 11 | positive | Foxo1 | 13 | [90] | - | - |
| Neat1 | 11 | positive | Il6 | 7 | [91] | positive | [70] |
| Neat1 | 11 | positive | Pycard | 16 | [92] | - | - |
| Neat1 | 11 | positive | Tgfb1 | 19 | [93] | positive | [74] |
| Xist | X | positive | Pten | 10 | [94] | positive | [84] |
| Xist | X | positive | Tgfb1 | 19 | [95] | positive | [74] |
| Xist | X | negative | Tgfb2 | 19 | [96] | positive | [97] |
| Malat1 | 11 | positive | Aqp4 | 18 | [98] | negative | [99] |
| Malat1 | 11 | negative | Mmp2 | 16 | [100] | positive | [82] |
| Malat1 | 11 | positive | Akt1 | 14 | [101] | positive | [68] |
| Malat1 | 11 | positive | Mmp9 | 20 | [102] | positive | [72] |
| Malat1 | 11 | positive | Nos3 | 7 | [103] | positive | [104] |
| Malat1 | 11 | positive | Parp1 | 1 | [105] | positive | [106] |
| Lnc31 | 9 | negative | Gsk3b | 3 | [107] | positive | [108] |
| Lnc31 | 9 | positive | Pten | 10 | [109] | positive | [84] |
| H19 | 11 | positive | Igf1 | 12 | [110] | positive | [66] |
| H19 | 11 | positive | Akt1 | 14 | [111] | positive | [68] |
| H19 | 11 | positive | Il6 | 7 | [112] | positive | [70] |
| H19 | 11 | positive | Vegfa | 6 | [113] | positive | [76] |
| Meg8 | 14 | positive | Jag1 | 20 | [114] | negative | [115] |
| Meg8 | 14 | positive | Vegfa | 6 | [116] | positive | [76] |
| Dbet | 4 | positive | Ash1l | 1 | [117] | negative | [118] |
5. How lncRNAs Can Control DMD Through miRNA and Gene Networks
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMD | Duchenne Muscular Dystrophy |
| lncRNA | Long Non-Coding RNA |
| miRNA | MicroRNA |
| MRFs | Myogenic Regulatory Factors |
| Myf5 | Myogenic Factor 5 |
| MyoD | Myogenic Differentiation Antigen |
| MyoG | Myogenin |
| Mrf4 | Myogenic Regulatory Factor 4 |
| Mhc | Myosin Heavy Chain |
| ORF | Open Reading Frame |
| T2dm | Type 2 Diabetes Mellitus |
| WGCNA | Weighted Gene Co-expression Network Analysis |
| Prc2 | Polycomb Repressive Complex 2 |
| MuSC | Muscle Stem Cells |
| TGF-β | Transforming Growth Factor Beta |
| EMT | Epithelial–Mesenchymal Transition |
| AMPK | AMP-Activated Protein Kinase |
| Akt | Protein Kinase B |
| IGF-1 | Insulin-like Growth Factor 1 |
| Pi3k | Phosphoinositide 3-Kinase |
| P-Akt | Phosphorylated Akt |
| Vim | Vimentin |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor Necrosis Factor Alpha |
| MAPK | Mitogen-Activated Protein Kinase |
| Dusp27 | Dual-Specificity Phosphatase 27 |
| IMP2 | IGF2 mRNA-Binding Protein 2 |
| Mrckα | Myotonic dystrophy kinase-related Cdc42-binding kinase alpha |
| Snca | Alpha-Synuclein |
| Mbnl1 | Muscleblind-Like Splicing Regulator 1 |
| LincRNA | Long Intergenic Non-Coding RNA |
| Ryr2 | Ryanodine Receptor 2 |
| Stx17 | Syntaxin 17 |
| N-Ras | Neuroblastoma RAS Viral (v-ras) Oncogene Homolog |
| c-Myc | MYC Proto-Oncogene |
| TGFBI | Transforming Growth Factor Beta Induced |
| Prrt2 | Proline-Rich Transmembrane Protein 2 |
| ROCK1 | Rho Associated Coiled-Coil Containing Protein Kinase 1 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| VEGFA | Vascular Endothelial Growth Factor A |
| SIRT1 | Sirtuin 1 |
| FOXO1 | Forkhead Box O1 |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CXCR4 | C-X-C Chemokine Receptor Type 4 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SOCS6 | Suppressor of Cytokine Signaling 6 |
| JAK2 | Janus Kinase 2 |
| MEF2C | Myocyte Enhancer Factor 2C |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| NEAT1 | Nuclear Enriched Abundant Transcript 1 |
| MEG3/MEG8 | Maternally Expressed Gene 3/8 |
| H19 | H19 Imprinted Maternally Expressed Transcript |
| Lnc-31 | Long Non-Coding RNA 31 |
| ST2 | Suppression of Tumorigenicity 2 (biomarker) |
| EZH2 | Enhancer of Zeste Homolog 2 |
| BRCA1 | Breast Cancer 1 |
References
- Ousterout, D.G.; Kabadi, A.M.; Thakore, P.I.; Majoros, W.H.; Reddy, T.E.; Gersbach, C.A. Multiplex CRISPR/Cas9-Based Genome Editing for Correction of Dystrophin Mutations That Cause Duchenne Muscular Dystrophy. Nat. Commun. 2015, 6, 6244. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Song, B.; Zheng, C.; Zhong, Y.; Guo, Q.; Zheng, J.; Yin, Y.; Li, J.; Li, F. Dietary Beta-Hydroxy Beta-Methyl Butyrate Supplementation Alleviates Liver Injury in Lipopolysaccharide-Challenged Piglets. Oxidative Med. Cell. Longev. 2021, 2021, e5546843. [Google Scholar] [CrossRef]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular Dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Kong, X. Development of CRISPR-Mediated Systems in the Study of Duchenne Muscular Dystrophy. Hum. Gene Ther. Methods 2019, 30, 71–80. [Google Scholar] [CrossRef]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global Epidemiology of Duchenne Muscular Dystrophy: An Updated Systematic Review and Meta-Analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Weihl, C.C.; Spencer, M.J. Molecular and Cellular Basis of Genetically Inherited Skeletal Muscle Disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 713–732. [Google Scholar] [CrossRef]
- Tajbakhsh, S. Skeletal Muscle Stem Cells in Developmental versus Regenerative Myogenesis. J. Intern. Med. 2009, 266, 372–389. [Google Scholar] [CrossRef]
- Kumar, S.; Williams, D.; Sur, S.; Wang, J.-Y.; Jo, H. Role of Flow-Sensitive microRNAs and Long Noncoding RNAs in Vascular Dysfunction and Atherosclerosis. Vasc. Pharmacol. 2019, 114, 76–92. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; Cruz-Guzmán, O.D.R.; Almeida-Becerril, T.; Solís-Serna, A.D.; Atilano-Miguel, S.; Sánchez-González, J.R.; Barbosa-Cortés, L.; Ruíz-Cruz, E.D.; Huicochea, J.C.; Cárdenas-Conejo, A.; et al. Potential Therapeutic Impact of Omega-3 Long Chain-Polyunsaturated Fatty Acids on Inflammation Markers in Duchenne Muscular Dystrophy: A Double-Blind, Controlled Randomized Trial. Clin. Nutr. 2018, 37, 1840–1851. [Google Scholar] [CrossRef]
- Cheng, J.-T.; Wang, L.; Wang, H.; Tang, F.-R.; Cai, W.-Q.; Sethi, G.; Xin, H.-W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef]
- Martone, J.; Mariani, D.; Desideri, F.; Ballarino, M. Non-Coding RNAs Shaping Muscle. Front. Cell Dev. Biol. 2019, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G. The GENCODE v7 Catalog of Human Long Noncoding RNAs: Analysis of Their Gene Structure, Evolution, and Expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Liu, W.; Kuang, H.; Xia, Y.; Pope, Z.C.; Wang, Z.; Tang, C.; Yin, D. Regular Aerobic Exercise-Ameliorated Troponin I Carbonylation to Mitigate Aged Rat Soleus Muscle Functional Recession. Exp. Physiol. 2019, 104, 715–728. [Google Scholar] [CrossRef]
- Chouvarine, P.; Photiadis, J.; Cesnjevar, R.; Scheewe, J.; Bauer, U.M.M.; Pickardt, T.; Kramer, H.-H.; Dittrich, S.; Berger, F.; Hansmann, G. RNA Expression Profiles and Regulatory Networks in Human Right Ventricular Hypertrophy Due to High Pressure Load. iScience 2021, 24, 102232. [Google Scholar] [CrossRef]
- Kallen, A.N.; Zhou, X.-B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.-S.; Zhang, H. The Imprinted H19 lncRNA Antagonizes Let-7 microRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, K.; Zare, H.; Dell’Orso, S.; Grontved, L.; Gutierrez-Cruz, G.; Derfoul, A.; Hager, G.L.; Sartorelli, V. eRNAs Promote Transcription by Establishing Chromatin Accessibility at Defined Genomic Loci. Mol. Cell 2013, 51, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Volders, P.-J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a Reference Set of Human Long Non-Coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J.; Han, L.; Qi, H.; Wang, Y.; Wang, H.; Chen, S.; Du, L.; Li, S.; Zhang, Y.; et al. The Micropeptide LEMP Plays an Evolutionarily Conserved Role in Myogenesis. Cell Death Dis. 2020, 11, 357. [Google Scholar] [CrossRef]
- Brocker, C.N.; Kim, D.; Melia, T.; Karri, K.; Velenosi, T.J.; Takahashi, S.; Aibara, D.; Bonzo, J.A.; Levi, M.; Waxman, D.J.; et al. Long Non-Coding RNA Gm15441 Attenuates Hepatic Inflammasome Activation in Response to PPARA Agonism and Fasting. Nat. Commun. 2020, 11, 5847. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Guo, Q.; Lu, Q.; Lu, J.; Wang, P.-S.; Dong, Y.; Li, T.; Chen, Y.; Gerhard, G.S.; Yang, X.-F.; et al. Identification of Gm15441, a Txnip Antisense lncRNA, as a Critical Regulator in Liver Metabolic Homeostasis. Cell Biosci. 2021, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, Y.; Yuan, Q.; Gao, Y.; Wang, Y.; Wang, X.; Cui, X.; Xu, P.; Ji, C.; Guo, X.; et al. Dynamic Transcriptome Profile in Db/Db Skeletal Muscle Reveal Critical Roles for Long Noncoding RNA Regulator. Int. J. Biochem. Cell Biol. 2018, 104, 14–24. [Google Scholar] [CrossRef]
- Arvaniti, E.; Moulos, P.; Vakrakou, A.; Chatziantoniou, C.; Chadjichristos, C.; Kavvadas, P.; Charonis, A.; Politis, P.K. Whole-Transcriptome Analysis of UUO Mouse Model of Renal Fibrosis Reveals New Molecular Players in Kidney Diseases. Sci. Rep. 2016, 6, 26235. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Wang, Z.; Shi, X.; Jin, J. Functions and Therapeutic Potentials of Long Noncoding RNA in Skeletal Muscle Atrophy and Dystrophy. J. Cachexia Sarcopenia Muscle 2025, 16, e13747. [Google Scholar] [CrossRef]
- Ling, Y.; Zheng, Q.; Sui, M.; Zhu, L.; Xu, L.; Zhang, Y.; Liu, Y.; Fang, F.; Chu, M.; Ma, Y.; et al. Comprehensive Analysis of LncRNA Reveals the Temporal-Specific Module of Goat Skeletal Muscle Development. Int. J. Mol. Sci. 2019, 20, 3950. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, Y.; Van Arsdell, G.; Nelson, S.F.; Touma, M. Ppp1r1b-lncRNA Inhibits PRC2 at Myogenic Regulatory Genes to Promote Cardiac and Skeletal Muscle Development in Mouse and Human. Rna 2020, 26, 481–491. [Google Scholar] [CrossRef]
- Ge, X.; Sun, T.; Zhang, Y.; Li, Y.; Gao, P.; Zhang, D.; Zhang, B.; Wang, P.; Ma, W.; Lu, S. The Role and Possible Mechanism of the Long Noncoding RNA LINC01260 in Nonalcoholic Fatty Liver Disease. Nutr. Metab. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Kitamura, Y.I.; Funahashi, Y.; Shawber, C.J.; Castrillon, D.H.; Kollipara, R.; DePinho, R.A.; Kitajewski, J.; Accili, D. A Foxo/Notch Pathway Controls Myogenic Differentiation and Fiber Type Specification. J. Clin. Investig. 2007, 117, 2477–2485. [Google Scholar] [CrossRef]
- Kousteni, S. FoxO1, the Transcriptional Chief of Staff of Energy Metabolism. Bone 2012, 50, 437–443. [Google Scholar] [CrossRef]
- Vandewalle, C.; Van Roy, F.; Berx, G. The Role of the ZEB Family of Transcription Factors in Development and Disease. Cell. Mol. Life Sci. CMLS 2009, 66, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.-Y.; Philipps, A.F.; Kelleher, S.L.; Lönnerdal, B. Effects of Zinc Exposure on Zinc Transporter Expression in Human Intestinal Cells of Varying Maturity. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, X.; Yu, S.; Deng, S.; Yan, M.; Sun, F.; Song, J.; Tang, L. Silence of lncRNA MIAT-Mediated Inhibition of DLG3 Promoter Methylation Suppresses Breast Cancer Progression via the Hippo Signaling Pathway. Cell. Signal. 2020, 73, 109697. [Google Scholar] [CrossRef]
- Chen, G.; Chen, H.; Ren, S.; Xia, M.; Zhu, J.; Liu, Y.; Zhang, L.; Tang, L.; Sun, L.; Liu, H.; et al. Aberrant DNA Methylation of mTOR Pathway Genes Promotes Inflammatory Activation of Immune Cells in Diabetic Kidney Disease. Kidney Int. 2019, 96, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, L.I. MALAT1 Knockdown Protects from Bronchial/Tracheal Smooth Muscle Cell Injury via Regulation of microRNA-133a/Ryanodine Receptor 2 Axis. J. Biosci. 2021, 46, 28. [Google Scholar] [CrossRef]
- Fu, X.; Li, S.; Jia, M.; Xu, B.; Yang, L.; Ma, R.; Cheng, H.; Yang, W.; Hu, P. Myogenesis Controlled by a Long Non-Coding RNA 1700113A16RIK and Post-Transcriptional Regulation. Cell Regen. 2022, 11, 13. [Google Scholar] [CrossRef]
- Gao, P.F.; Guo, X.H.; Du, M.; Cao, G.Q.; Yang, Q.C.; Pu, Z.D.; Wang, Z.Y.; Zhang, Q.; Li, M.; Jin, Y.S.; et al. LncRNA Profiling of Skeletal Muscles in Large White Pigs and Mashen Pigs during Development. J. Anim. Sci. 2017, 95, 4239–4250. [Google Scholar] [CrossRef]
- Dey, B.K.; Pfeifer, K.; Dutta, A. The H19 Long Noncoding RNA Gives Rise to microRNAs miR-675-3p and miR-675-5p to Promote Skeletal Muscle Differentiation and Regeneration. Genes Dev. 2014, 28, 491–501. [Google Scholar] [CrossRef]
- Geng, T.; Liu, Y.; Xu, Y.; Jiang, Y.; Zhang, N.; Wang, Z.; Carmichael, G.G.; Taylor, H.S.; Li, D.; Huang, Y. H19 lncRNA Promotes Skeletal Muscle Insulin Sensitivity in Part by Targeting AMPK. Diabetes 2018, 67, 2183–2198. [Google Scholar] [CrossRef]
- García-Padilla, C.; Domínguez, J.N.; Aránega, A.E.; Franco, D. Differential Chamber-Specific Expression and Regulation of Long Non-Coding RNAs during Cardiac Development. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194435. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP Signaling Controls Muscle Mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Winbanks, C.E.; Chen, J.L.; Qian, H.; Liu, Y.; Bernardo, B.C.; Beyer, C.; Watt, K.I.; Thomson, R.E.; Connor, T.; Turner, B.J.; et al. The Bone Morphogenetic Protein Axis Is a Positive Regulator of Skeletal Muscle Mass. J. Cell Biol. 2013, 203, 345–357. [Google Scholar] [CrossRef]
- Neppl, R.L.; Wu, C.-L.; Walsh, K. lncRNA Chronos Is an Aging-Induced Inhibitor of Muscle Hypertrophy. J. Cell Biol. 2017, 216, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, P.C.D.; Farshi, P.; Goldman, D. Dach2-Hdac9 Signaling Regulates Reinnervation of Muscle Endplates. Development 2015, 142, 4038–4048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B.-T. A Newly Identified lncRNA MAR1 Acts as a miR-487b Sponge to Promote Skeletal Muscle Differentiation and Regeneration. J. Cachexia Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Du, M.; Wang, J.; Guo, Y.; Zhang, J.; Zuo, H.; Hou, Y.; Wang, S.; Lv, W.; Bai, W.; et al. Conservative Analysis of Synaptopodin-2 Intron Sense-Overlapping lncRNA Reveals Its Novel Function in Promoting Muscle Atrophy. J. Cachexia Sarcopenia Muscle 2022, 13, 2017–2030. [Google Scholar] [CrossRef]
- Zhang, P.; Du, J.; Guo, X.; Wu, S.; He, J.; Li, X.; Shen, L.; Chen, L.; Li, B.; Zhang, J.; et al. LncMyoD Promotes Skeletal Myogenesis and Regulates Skeletal Muscle Fiber-Type Composition by Sponging miR-370-3p. Genes 2021, 12, 589. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Bao, X.; Zhu, X.; Kwok, Y.K.-Y.; Sun, K.; Chen, X.; Huang, Y.; Jauch, R.; Esteban, M.A.; et al. LncRNA Dum Interacts with Dnmts to Regulate Dppa2 Expression during Myogenic Differentiation and Muscle Regeneration. Cell Res. 2015, 25, 335–350. [Google Scholar] [CrossRef]
- Yu, J.-A.; Wang, Z.; Yang, X.; Ma, M.; Li, Z.; Nie, Q. LncRNA-FKBP1C Regulates Muscle Fiber Type Switching by Affecting the Stability of MYH1B. Cell Death Discov. 2021, 7, 73. [Google Scholar] [CrossRef]
- Wang, S.; Jin, J.; Xu, Z.; Zuo, B. Functions and Regulatory Mechanisms of lncRNAs in Skeletal Myogenesis, Muscle Disease and Meat Production. Cells 2019, 8, 1107. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Hao, S.; Qian, J. Uncovering Novel Landscape of Cardiovascular Diseases and Therapeutic Targets for Cardioprotection via Long Noncoding RNA-miRNA-mRNA Axes. Epigenomics 2018, 10, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Lv, W.; Xia, P.; Xu, Z.Y.; Zheng, A.D.; Wang, X.J.; Wang, S.S.; Zeng, R.; Luo, H.M.; Li, G.L.; et al. Long Noncoding RNA SYISL Regulates Myogenesis by Interacting with Polycomb Repressive Complex 2. Proc. Natl. Acad. Sci. USA 2018, 115, E9802–E9811. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Ramanujan, K.; Clay, I.; Zhang, Y.; Lemire-Brachat, S.; Glass, D.J. A Long Non-Coding RNA, LncMyoD, Regulates Skeletal Muscle Differentiation by Blocking IMP2-Mediated mRNA Translation. Dev. Cell 2015, 34, 181–191. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Tang, H.; Sha, Z.; Chen, R.; Chen, L.; Yu, Y.; Rowe, G.C.; Das, S.; Xiao, J. Inhibition of lncRNA MAAT Controls Multiple Types of Muscle Atrophy by Cis- and Trans-Regulatory Actions. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, L.; Chen, Z.; Cui, C.; Zhang, R.; Qin, L. Magnesium Supplementation Alleviates Corticosteroid-Associated Muscle Atrophy in Rats. Eur. J. Nutr. 2021, 60, 4379–4392. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long Non-Coding RNAs: Insights into Functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Loo, T.H.; Ye, X.; Chai, R.J.; Ito, M.; Bonne, G.; Ferguson-Smith, A.C.; Stewart, C.L. The Mammalian LINC Complex Component SUN1 Regulates Muscle Regeneration by Modulating Drosha Activity. eLife 2019, 8, e49485. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhou, X.; Cao, H.; Zhang, X.; Huang, K.; Li, X.; Yang, G.; Shi, X. miR-324-5p Inhibits C2C12 Cell Differentiation and Promotes Intramuscular Lipid Deposition through lncDUM and PM20D1. Mol. Ther. Nucleic Acids 2020, 22, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Huang, C.; Luo, Y.; He, F.; Zhang, R. Circulating lncRNA NEAT1 Correlates with Increased Risk, Elevated Severity and Unfavorable Prognosis in Sepsis Patients. Am. J. Emerg. Med. 2018, 36, 1659–1663. [Google Scholar] [CrossRef]
- Bovolenta, M.; Erriquez, D.; Valli, E.; Brioschi, S.; Scotton, C.; Neri, M.; Falzarano, M.S.; Gherardi, S.; Fabris, M.; Rimessi, P.; et al. The DMD Locus Harbours Multiple Long Non-Coding RNAs Which Orchestrate and Control Transcription of Muscle Dystrophin mRNA Isoforms. PLoS ONE 2012, 7, e45328. [Google Scholar] [CrossRef]
- Xu, X.; Hao, Y.; Xiong, S.; He, Z. Comprehensive Analysis of Long Non-Coding RNA-Associated Competing Endogenous RNA Network in Duchenne Muscular Dystrophy. Interdiscip. Sci. Comput. Life Sci. 2020, 12, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.G.; Mackey, A.J.; Finn, D.M.; Maguire, P.B.; Ohlendieck, K. Role of Dystrophin Isoforms and Associated Proteins in Muscular Dystrophy (Review). Int. J. Mol. Med. 1998, 2, 639–648. [Google Scholar] [CrossRef]
- Gargaun, E.; Falcone, S.; Solé, G.; Durigneux, J.; Urtizberea, A.; Cuisset, J.M.; Benkhelifa-Ziyyat, S.; Julien, L.; Boland, A.; Sandron, F.; et al. The lncRNA 44s2 Study Applicability to the Design of 45-55 Exon Skipping Therapeutic Strategy for DMD. Biomedicines 2021, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kim, I.-M.; Hamrick, M.; Tang, Y. Uncovering the Gene Regulatory Network of Endothelial Cells in Mouse Duchenne Muscular Dystrophy: Insights from Single-Nuclei RNA Sequencing Analysis. Biology 2023, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Zhang, A.; Yin, S.; Wang, T.; Wang, Y.; Wang, M.; Liu, Y.; Ying, Q.; Sun, J.; et al. Down-Regulation of Long Non-Coding RNA MEG3 Suppresses Osteogenic Differentiation of Periodontal Ligament Stem Cells (PDLSCs) through miR-27a-3p/IGF1 Axis in Periodontitis. Aging 2019, 11, 5334–5350. [Google Scholar] [CrossRef]
- Gehrig, S.M.; Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Insulin-like Growth Factor-I Analogue Protects Muscles of Dystrophic Mdx Mice from Contraction-Mediated Damage. Exp. Physiol. 2008, 93, 1190–1198. [Google Scholar] [CrossRef]
- Jing, X.; Han, J.; Zhang, J.; Chen, Y.; Yuan, J.; Wang, J.; Neo, S.; Li, S.; Yu, X.; Wu, J. Long Non-Coding RNA MEG3 Promotes Cisplatin-Induced Nephrotoxicity through Regulating AKT/TSC/mTOR-Mediated Autophagy. Int. J. Biol. Sci. 2021, 17, 3968–3980. [Google Scholar] [CrossRef]
- Peter, A.K.; Crosbie, R.H. Hypertrophic Response of Duchenne and Limb-Girdle Muscular Dystrophies Is Associated with Activation of Akt Pathway. Exp. Cell Res. 2006, 312, 2580–2591. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Zhang, C.; Wang, M. LncRNA MEG3 Inhibits the Inflammatory Response of Ankylosing Spondylitis by Targeting miR-146a. Mol. Cell. Biochem. 2020, 466, 17–24. [Google Scholar] [CrossRef]
- Messina, S.; Vita, G.L.; Aguennouz, M.; Sframeli, M.; Romeo, S.; Rodolico, C.; Vita, G. Activation of NF-kB Pathway in Duchenne Muscular Dystrophy: Relation to Age. Acta Myol. 2011, 30, 16–23. [Google Scholar]
- Gu, L.; Zhang, J.; Shi, M.; Zhan, Q.; Shen, B.; Peng, C. lncRNA MEG3 Had Anti-Cancer Effects to Suppress Pancreatic Cancer Activity. Biomed. Pharmacother. 2017, 89, 1269–1276. [Google Scholar] [CrossRef]
- Anderson, J.; Seol, H.; Hathout, Y.; Spurney, C. Elevated St2 Serum Levels A Biomarker for Cardiomyopathy in Duchenne Muscular Dystrophy. J. Am. Coll. Cardiol. 2015, 65, A997. [Google Scholar] [CrossRef]
- Zhang, D.; Qin, H.; Leng, Y.; Li, X.; Zhang, L.; Bai, D.; Meng, Y.; Wang, J. LncRNA MEG3 Overexpression Inhibits the Development of Diabetic Retinopathy by Regulating TGF-Β1 and VEGF. Exp. Ther. Med. 2018, 16, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Ishitobi, M.; Haginoya, K.; Zhao, Y.; Ohnuma, A.; Minato, J.; Yanagisawa, T.; Tanabu, M.; Kikuchi, M.; Iinuma, K. Elevated Plasma Levels of Transforming Growth Factor Β1 in Patients with Muscular Dystrophy. NeuroReport 2000, 11, 4033. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Klibanski, A. MEG3 Noncoding RNA: A Tumor Suppressor. J. Mol. Endocrinol. 2012, 48, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yamamoto, Y.; Matsumura, T.; Fujimura, H.; Shinno, S. Serum Levels of Vascular Endothelial Growth Factor Elevated in Patients with Muscular Dystrophy. Brain Dev. 2009, 31, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, Y.; Wang, W.; Zuo, Q.; Jiang, Z.; Sun, M.; De, W.; Sun, L. Down-Regulated Long Non-Coding RNA MEG3 and Its Effect on Promoting Apoptosis and Suppressing Migration of Trophoblast Cells. J. Cell. Biochem. 2015, 116, 542–550. [Google Scholar] [CrossRef]
- Wang, M.; Huang, T.; Luo, G.; Huang, C.; Xiao, X.; Wang, L.; Jiang, G.; Zeng, F. Long Non-Coding RNA MEG3 Induces Renal Cell Carcinoma Cells Apoptosis by Activating the Mitochondrial Pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 541–545. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Liu, W.; Wang, H.; Zhao, L.; Liu, S.; Li, P.; Zhang, S.; Sun, C.; Wu, Y.; et al. microRNA-378 Promotes Autophagy and Inhibits Apoptosis in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2018, 115, E10849–E10858. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Y.-B.; Zhou, J.; Kang, D.-M. Upregulation of lncRNA MEG3 Promotes Hepatic Insulin Resistance via Increasing FoxO1 Expression. Biochem. Biophys. Res. Commun. 2016, 469, 319–325. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Luo, M.; Liu, X.; Luo, Q.; Tao, H.; Wu, D.; Lu, S.; Jin, J.; Zhao, Y.; et al. dNK Derived IFN-γ Mediates VSMC Migration and Apoptosis via the Induction of LncRNA MEG3: A Role in Uterovascular Transformation. Placenta 2017, 50, 32–39. [Google Scholar] [CrossRef] [PubMed]
- von Moers, A.; Zwirner, A.; Reinhold, A.; Brückmann, O.; van Landeghem, F.; Stoltenburg-Didinger, G.; Schuppan, D.; Herbst, H.; Schuelke, M. Increased mRNA Expression of Tissue Inhibitors of Metalloproteinase-1 and -2 in Duchenne Muscular Dystrophy. Acta Neuropathol. 2005, 109, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-Q.; Luo, X.-J.; Zhang, J.; Wang, G.-M.; Guo, J.-M. Crosstalk between Meg3 and miR-1297 Regulates Growth of Testicular Germ Cell Tumor through PTEN/PI3K/AKT Pathway. Am. J. Transl. Res. 2016, 8, 1091–1099. [Google Scholar] [PubMed]
- Yue, F.; Song, C.; Huang, D.; Narayanan, N.; Qiu, J.; Jia, Z.; Yuan, Z.; Oprescu, S.N.; Roseguini, B.T.; Deng, M.; et al. PTEN Inhibition Ameliorates Muscle Degeneration and Improves Muscle Function in a Mouse Model of Duchenne Muscular Dystrophy. Mol. Ther. 2021, 29, 132–148. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Ge, X.; Xu, B.; Peng, W.; Jiang, X.; Shen, L.; Xia, L. Long Noncoding RNA NEAT1 Accelerates the Proliferation and Fibrosis in Diabetic Nephropathy through Activating Akt/mTOR Signaling Pathway. J. Cell. Physiol. 2019, 234, 11200–11207. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Tian, R.; Zhang, H. NEAT1 Promotes Retinoblastoma Progression via Modulating miR-124. J. Cell. Biochem. 2019, 120, 15585–15593. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.-H.; Wu, Y.; Cao, G.-K.; Han, D. LncRNA NEAT1 Regulates Pulmonary Fibrosis through miR-9-5p and TGF-β Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8483–8492. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Zhao, P.; Borup, R.; Hoffman, E.P. Expression Profiling in the Muscular Dystrophies. J. Cell Biol. 2000, 151, 1321–1336. [Google Scholar] [CrossRef]
- Ding, X.-M.; Zhao, L.-J.; Qiao, H.-Y.; Wu, S.-L.; Wang, X.-H. Long Non-Coding RNA-P21 Regulates MPP+-Induced Neuronal Injury by Targeting miR-625 and Derepressing TRPM2 in SH-SY5Y Cells. Chem. Biol. Interact. 2019, 307, 73–81. [Google Scholar] [CrossRef]
- Ma, M.; Hui, J.; Zhang, Q.; Zhu, Y.; He, Y.; Liu, X. Long Non-Coding RNA Nuclear-Enriched Abundant Transcript 1 Inhibition Blunts Myocardial Ischemia Reperfusion Injury via Autophagic Flux Arrest and Apoptosis in Streptozotocin-Induced Diabetic Rats. Atherosclerosis 2018, 277, 113–122. [Google Scholar] [CrossRef]
- Bai, Y.; Lv, Y.; Wang, W.; Sun, G.; Zhang, H. LncRNA NEAT1 Promotes Inflammatory Response and Induces Corneal Neovascularization. J. Mol. Endocrinol. 2018, 61, 231–239. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The lncRNA Neat1 Promotes Activation of Inflammasomes in Macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Tu, J.; Zhao, Z.; Xu, M.; Lu, X.; Chang, L.; Ji, J. NEAT1 Upregulates TGF-Β1 to Induce Hepatocellular Carcinoma Progression by Sponging Hsa-Mir-139-5p. J. Cell. Physiol. 2018, 233, 8578–8587. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Chen, B.; Wang, X.; Wu, K.; Sun, Y. Long Non-Coding RNA XIST Regulates PTEN Expression by Sponging miR-181a and Promotes Hepatocellular Carcinoma Progression. BMC Cancer 2017, 17, 248. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Y.; Yang, X.; Long, Y.; Chen, S.; Lin, X.; Dong, R.; Yuan, J. Silencing of Long Noncoding RNA XIST Protects against Renal Interstitial Fibrosis in Diabetic Nephropathy via microRNA-93-5p-Mediated Inhibition of CDKN1A. Am. J. Physiol.-Ren. Physiol. 2019, 317, F1350–F1358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y. LncRNA XIST Enhanced TGF-Β2 Expression by Targeting miR-141-3p to Promote Pancreatic Cancer Cells Invasion. Biosci. Rep. 2019, 39, BSR20190332. [Google Scholar] [CrossRef]
- McLennan, I.S.; Koishi, K. Cellular Localisation of Transforming Growth Factor-Beta 2 and -Beta 3 (TGF-Beta2, TGF-Beta3) in Damaged and Regenerating Skeletal Muscles. Dev. Dyn. 1997, 208, 278–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, Y.; Wei, J.; Wu, R.; Cai, H. Overexpression of Long Noncoding RNA Malat1 Ameliorates Traumatic Brain Injury Induced Brain Edema by Inhibiting AQP4 and the NF-κB/IL-6 Pathway. J. Cell. Biochem. 2019, 120, 17584–17592. [Google Scholar] [CrossRef]
- Jimi, T.; Wakayama, Y.; Matsuzaki, Y.; Hara, H.; Inoue, M.; Shibuya, S. Reduced Expression of Aquaporin 4 in Human Muscles with Amyotrophic Lateral Sclerosis and Other Neurogenic Atrophies. Pathol. Res. Pract. 2004, 200, 203–209. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Z.; Wu, T.; Huang, Y.; Cheng, Z.; Li, X.; Sun, T.; Xie, X.; Zhou, Y.; Du, Z. Tumor-Suppressive Function of Long Noncoding RNA MALAT1 in Glioma Cells by Downregulation of MMP2 and Inactivation of ERK/MAPK Signaling. Cell Death Dis. 2016, 7, e2123. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, L.; Lv, H.; Pei, C. Quercetin Promotes the Apoptosis of Fibroblast-like Synoviocytes in Rheumatoid Arthritis by Upregulating lncRNA MALAT1. Int. J. Mol. Med. 2016, 38, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-S.; Wang, X.-A.; Wu, W.-G.; Hu, Y.-P.; Li, M.-L.; Ding, Q.; Weng, H.; Shu, Y.-J.; Liu, T.-Y.; Jiang, L.; et al. MALAT1 Promotes the Proliferation and Metastasis of Gallbladder Cancer Cells by Activating the ERK/MAPK Pathway. Cancer Biol. Ther. 2014, 15, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Luo, L.; Li, J. LncRNA MALAT1 Facilitates BM-MSCs Differentiation into Endothelial Cells via Targeting miR-206/VEGFA Axis. Cell Cycle 2020, 19, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Loufrani, L.; Dubroca, C.; You, D.; Li, Z.; Levy, B.; Paulin, D.; Henrion, D. Absence of Dystrophin in Mice Reduces NO-Dependent Vascular Function and Vascular Density: Total Recovery after a Treatment with the Aminoglycoside Gentamicin. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 671–676. [Google Scholar] [CrossRef]
- Ji, D.-G.; Guan, L.-Y.; Luo, X.; Ma, F.; Yang, B.; Liu, H.-Y. Inhibition of MALAT1 Sensitizes Liver Cancer Cells to 5-Flurouracil by Regulating Apoptosis through IKKα/NF-κB Pathway. Biochem. Biophys. Res. Commun. 2018, 501, 33–40. [Google Scholar] [CrossRef]
- Aguennouz, M.; Vita, G.L.; Messina, S.; Cama, A.; Lanzano, N.; Ciranni, A.; Rodolico, C.; Di Giorgio, R.M.; Vita, G. Telomere Shortening Is Associated to TRF1 and PARP1 Overexpression in Duchenne Muscular Dystrophy. Neurobiol. Aging 2011, 32, 2190–2197. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, X.; Wang, X.; Li, J. MIR31HG Promotes Cell Proliferation and Invasion by Activating the Wnt/β-Catenin Signaling Pathway in Non-Small Cell Lung Cancer. Oncol. Lett. 2019, 17, 221–229. [Google Scholar] [CrossRef]
- Feron, M.; Guevel, L.; Rouger, K.; Dubreil, L.; Arnaud, M.-C.; Ledevin, M.; Megeney, L.A.; Cherel, Y.; Sakanyan, V. PTEN Contributes to Profound PI3K/Akt Signaling Pathway Deregulation in Dystrophin-Deficient Dog Muscle. Am. J. Pathol. 2009, 174, 1459–1470. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, H.; Yang, J.; Mao, J.; Wei, G.; Meng, X.; Zang, H. LncRNA MIR31HG Is Induced by Tocilizumab and Ameliorates Rheumatoid Arthritis Fibroblast-like Synoviocyte-Mediated Inflammation via miR-214-PTEN-AKT Signaling Pathway. Aging 2021, 13, 24071–24085. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Liu, Q.; Liu, C.-Z. lncRNA H19 Promotes Matrix Mineralization through Up-Regulating IGF1 by Sponging miR-185-5p in Osteoblasts. BMC Mol. Cell Biol. 2019, 20, 48. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Shen, H.; Zhang, X.; Wang, H.; Wu, G.; Qi, Y.; Wang, L.; Xu, W. Muc5ac Production Inhibited by Decreased lncRNA H19 via PI3K/Akt/NF-kB in Asthma. J. Asthma Allergy 2021, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Xia, S.-W.; Zhang, T.; Wang, Z.-Y.; Yang, X.; Kai, J.; Cheng, X.-D.; Shao, J.-J.; Tan, S.-Z.; Chen, A.-P.; et al. LncRNA-H19 Induces Hepatic Stellate Cell Activation via Upregulating Alcohol Dehydrogenase III-Mediated Retinoic Acid Signals. Int. Immunopharmacol. 2020, 84, 106470. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wu, Q.; Zheng, G.; Long, H.; Wu, H.; Zhou, C.; Guo, T.; Zhong, T.; Wang, L.; et al. Long Noncoding RNA H19 Upregulates Vascular Endothelial Growth Factor A to Enhance Mesenchymal Stem Cells Survival and Angiogenic Capacity by Inhibiting miR-199a-5p. Stem Cell Res. Ther. 2018, 9, 109. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Guo, J.; Zhuo, L.; Chen, Y.; Yuan, H. Knockdown of lncRNA MEG8 Inhibits Cell Proliferation and Invasion, but Promotes Cell Apoptosis in Hemangioma, via miR-203-induced Mediation of the Notch Signaling Pathway. Mol. Med. Rep. 2021, 24, 872. [Google Scholar] [CrossRef]
- Vieira, N.M.; Elvers, I.; Alexander, M.S.; Moreira, Y.B.; Eran, A.; Gomes, J.P.; Marshall, J.L.; Karlsson, E.K.; Verjovski-Almeida, S.; Lindblad-Toh, K.; et al. Jagged 1 Rescues the Duchenne Muscular Dystrophy Phenotype. Cell 2015, 163, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Sun, L.; Zhang, W.; Li, J.; Han, J.; Zheng, J.; Xin, H. LncRNA MEG8 Attenuates Cerebral Ischemia After Ischemic Stroke Through Targeting miR-130a-5p/VEGFA Signaling. Cell. Mol. Neurobiol. 2021, 41, 1311–1324. [Google Scholar] [CrossRef]
- Cabianca, D.S.; Casa, V.; Bodega, B.; Xynos, A.; Ginelli, E.; Tanaka, Y.; Gabellini, D. A Long ncRNA Links Copy Number Variation to a Polycomb/Trithorax Epigenetic Switch in FSHD Muscular Dystrophy. Cell 2012, 149, 819–831. [Google Scholar] [CrossRef]
- Castiglioni, I.; Caccia, R.; Garcia-Manteiga, J.M.; Ferri, G.; Caretti, G.; Molineris, I.; Nishioka, K.; Gabellini, D. The Trithorax Protein Ash1L Promotes Myoblast Fusion by Activating Cdon Expression. Nat. Commun. 2018, 9, 5026. [Google Scholar] [CrossRef]
- Dimartino, D.; Colantoni, A.; Ballarino, M.; Martone, J.; Mariani, D.; Danner, J.; Bruckmann, A.; Meister, G.; Morlando, M.; Bozzoni, I. The Long Non-Coding RNA Lnc-31 Interacts with Rock1 mRNA and Mediates Its YB-1-Dependent Translation. Cell Rep. 2018, 23, 733–740. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Hu, Q.; Egranov, S.D.; Xing, Z.; Zhang, Z.; Liang, K.; Ye, Y.; Pan, Y.; Chatterjee, S.S.; et al. Functional Significance of Gain-of-Function H19 lncRNA in Skeletal Muscle Differentiation and Anti-Obesity Effects. Genome Med. 2021, 13, 137. [Google Scholar] [CrossRef]
- Tran, T.H.T.; Zhang, Z.; Yagi, M.; Lee, T.; Awano, H.; Nishida, A.; Okinaga, T.; Takeshima, Y.; Matsuo, M. Molecular Characterization of an X(P21.2;Q28) Chromosomal Inversion in a Duchenne Muscular Dystrophy Patient with Mental Retardation Reveals a Novel Long Non-Coding Gene on Xq28. J. Hum. Genet. 2013, 58, 33–39. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Qiukai, E.; Shang, Y.; Zhang, X.; Liu, S.; Zhang, X. Long Non-Coding RNA Xist Regulates Oocyte Loss via Suppressing miR-23b-3p/miR-29a-3p Maturation and Upregulating STX17 in Perinatal Mouse Ovaries. Cell Death Dis. 2021, 12, 540. [Google Scholar] [CrossRef]
- You, L.; Wang, N.; Yin, D.; Wang, L.; Jin, F.; Zhu, Y.; Yuan, Q.; De, W. Downregulation of Long Noncoding RNA Meg3 Affects Insulin Synthesis and Secretion in Mouse Pancreatic Beta Cells. J. Cell. Physiol. 2016, 231, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Butchart, L.C.; Terrill, J.R.; Rossetti, G.; White, R.; Filipovska, A.; Grounds, M.D. Expression Patterns of Regulatory RNAs, Including lncRNAs and tRNAs, during Postnatal Growth of Normal and Dystrophic (Mdx) Mouse Muscles, and Their Response to Taurine Treatment. Int. J. Biochem. Cell Biol. 2018, 99, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-Derived Mesenchymal Stromal Cells and Their Exosomes Exert Therapeutic Effects in Duchenne Muscular Dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xia, R.; Zhang, Z.; Xu, C. lncRNA MEG3 Aggravated Neuropathic Pain and Astrocyte Overaction through Mediating miR-130a-5p/CXCL12/CXCR4 Axis. Aging 2021, 13, 23004–23019. [Google Scholar] [CrossRef]
- Luo, R.; Jin, H.; Li, L.; Hu, Y.-X.; Xiao, F. Long Noncoding RNA MEG3 Inhibits Apoptosis of Retinal Pigment Epithelium Cells Induced by High Glucose via the miR-93/Nrf2 Axis. Am. J. Pathol. 2020, 190, 1813–1822. [Google Scholar] [CrossRef]
- Xiao, F.; Li, L.; Fu, J.-S.; Hu, Y.-X.; Luo, R. Regulation of the miR-19b-Mediated SOCS6-JAK2/STAT3 Pathway by lncRNA MEG3 Is Involved in High Glucose-Induced Apoptosis in hRMECs. Biosci. Rep. 2020, 40, BSR20194370. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, L.; Huang, Z.; Zhang, H.; Cheng, C.; Liu, H.; He, J.; Wu, J.; Darwazeh, R.; Wu, Y.; et al. The Long Non-Coding RNA Neat1 Is an Important Mediator of the Therapeutic Effect of Bexarotene on Traumatic Brain Injury in Mice. Brain Behav. Immun. 2017, 65, 183–194. [Google Scholar] [CrossRef]
- Yong, H.; Wu, G.; Chen, J.; Liu, X.; Bai, Y.; Tang, N.; Liu, L.; Wei, J. lncRNA MALAT1 Accelerates Skeletal Muscle Cell Apoptosis and Inflammatory Response in Sepsis by Decreasing BRCA1 Expression by Recruiting EZH2. Mol. Ther. Nucleic Acids 2020, 19, 97–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Q.; Wei, Q.; Li, P.; Zhuang, Z.; Li, J.; Liu, Y.; Zhang, L.; Hong, Z.; He, W.; et al. Long Noncoding RNA XIST Modulates microRNA-135/CREB1 Axis to Influence Osteogenic Differentiation of Osteoblast-like Cells in Mice with Tibial Fracture Healing. Hum. Cell 2022, 35, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Mai, C.; Qi, Y. Long Noncoding RNA XIST/miR-17/PTEN Axis Modulates the Proliferation and Apoptosis of Vascular Smooth Muscle Cells to Affect Stanford Type A Aortic Dissection. J. Cardiovasc. Pharmacol. 2020, 76, 53–62. [Google Scholar] [CrossRef]
- Contreras, O.; Rebolledo, D.L.; Oyarzún, J.E.; Olguín, H.C.; Brandan, E. Connective Tissue Cells Expressing Fibro/Adipogenic Progenitor Markers Increase under Chronic Damage: Relevance in Fibroblast-Myofibroblast Differentiation and Skeletal Muscle Fibrosis. Cell Tissue Res. 2016, 364, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Liu, P.; Yang, J. XIST Promotes Gastric Cancer (GC) Progression through TGF-Β1 via Targeting miR-185. J. Cell. Biochem. 2018, 119, 2787–2796. [Google Scholar] [CrossRef]
- Mo, Y.; He, L.; Lai, Z.; Wan, Z.; Chen, Q.; Pan, S.; Li, L.; Li, D.; Huang, J.; Xue, F.; et al. LINC01287/miR-298/STAT3 Feedback Loop Regulates Growth and the Epithelial-to-Mesenchymal Transition Phenotype in Hepatocellular Carcinoma Cells. J. Exp. Clin. Cancer Res. CR 2018, 37, 149. [Google Scholar] [CrossRef] [PubMed]
- Dill, T.L.; Carroll, A.; Pinheiro, A.; Gao, J.; Naya, F.J. The Long Noncoding RNA Meg3 Regulates Myoblast Plasticity and Muscle Regeneration through Epithelial-Mesenchymal Transition. Development 2021, 148, dev194027. [Google Scholar] [CrossRef]
- Lv, D.; Bi, Q.; Li, Y.; Deng, J.; Wu, N.; Hao, S.; Zhao, M. Long Non-coding RNA MEG3 Inhibits Cell Migration and Invasion of Non-small Cell Lung Cancer Cells by Regulating the miR-21-5p/PTEN Axis. Mol. Med. Rep. 2021, 23, 191. [Google Scholar] [CrossRef]
- Shen, S.; Ma, L.; Shao, F.; Jin, L.; Bian, Z. Long Non-Coding RNA (lncRNA) NEAT1 Aggravates Cerebral Ischemia-Reperfusion Injury by Suppressing the Inhibitory Effect of miR-214 on PTEN. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924781. [Google Scholar] [CrossRef]
- Mammen, A.L.; Sartorelli, V. IL-6 Blockade as a Therapeutic Approach for Duchenne Muscular Dystrophy. EBioMedicine 2015, 2, 274–275. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Wang, H.; Liu, L.; Zhang, S.; Ming, Y.; Zhao, Y.; Cheng, K. Silencing Long Noncoding RNA NEAT1 Alleviates Acute Liver Failure via the EZH2-Mediated microRNA-139/PUMA Axis. Aging 2021, 13, 12537–12551. [Google Scholar] [CrossRef]
- Wei, J.-L.; Wu, C.-J.; Chen, J.-J.; Shang, F.-T.; Guo, S.-G.; Zhang, X.-C.; Liu, H. LncRNA NEAT1 Promotes the Progression of Sepsis-Induced Myocardial Cell Injury by Sponging miR-144-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, L.; He, X.; Wang, S.; Lai, Y.; Wu, W.; Song, H.; Chen, Y.; Yang, Y.; Liao, W.; et al. LncRNA H19 Promotes Vascular Inflammation and Abdominal Aortic Aneurysm Formation by Functioning as a Competing Endogenous RNA. J. Mol. Cell. Cardiol. 2019, 131, 66–81. [Google Scholar] [CrossRef]
- Sun, J.; Song, X.; Su, L.; Cao, S. Long Non-Coding RNA LncHIFCAR Promotes Osteoarthritis Development via Positively Regulating HIF-1α and Activating the PI3K/AKT/mTOR Pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 3000–3009. [Google Scholar] [PubMed]
- Rayavarapu, S.; Coley, W.; Cakir, E.; Jahnke, V.; Takeda, S.; Aoki, Y.; Grodish-Dressman, H.; Jaiswal, J.K.; Hoffman, E.P.; Brown, K.J.; et al. Identification of Disease Specific Pathways Using in Vivo SILAC Proteomics in Dystrophin Deficient Mdx Mouse. Mol. Cell. Proteomics MCP 2013, 12, 1061–1073. [Google Scholar] [CrossRef]
- Guo, Z.; Geng, M.; Huang, Y.; Han, G.; Jing, R.; Lin, C.; Zhang, X.; Zhang, M.; Fan, G.; Wang, F.; et al. Upregulation of Wilms’ Tumor 1 in Epicardial Cells Increases Cardiac Fibrosis in Dystrophic Mice. Cell Death Differ. 2022, 29, 1928–1940. [Google Scholar] [CrossRef]
- Tam, C.; Wong, J.H.; Tsui, S.K.W.; Zuo, T.; Chan, T.F.; Ng, T.B. LncRNAs with miRNAs in Regulation of Gastric, Liver, and Colorectal Cancers: Updates in Recent Years. Appl. Microbiol. Biotechnol. 2019, 103, 4649–4677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niyazi, H.E.X.D.; Zhao, H.R.; Cao, X.P.; Abudula, M.N.S.; Ye, W.J.; Zhang, S.A.; Yiming, R.H.M.; Zhang, Y.; Su, W.P.; et al. Effects of miRNA-143 and the Non-Coding RNA MALAT1 on the Pathogenesis and Metastasis of HeLa Cells. Genet. Mol. Res. GMR 2017, 16, gmr16019269. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.; He, J.; Fang, X.-Q.; Zhu, S.-W.; Xiong, M.-M. Increased Long Noncoding RNA SNHG20 Predicts Poor Prognosis in Colorectal Cancer. BMC Cancer 2016, 16, 655. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, S. Long Non-coding RNA MEG-3 Suppresses Gastric Carcinoma Cell Growth, Invasion and Migration via EMT Regulation. Mol. Med. Rep. 2019, 20, 2685–2693. [Google Scholar] [CrossRef]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A. MicroRNA-486–Dependent Modulation of DOCK3/PTEN/AKT Signaling Pathways Improves Muscular Dystrophy–Associated Symptoms. J. Clin. Investig. 2014, 124, 2651–2667. [Google Scholar] [CrossRef]
- Cacchiarelli, D.; Martone, J.; Girardi, E.; Cesana, M.; Incitti, T.; Morlando, M.; Nicoletti, C.; Santini, T.; Sthandier, O.; Barberi, L.; et al. MicroRNAs Involved in Molecular Circuitries Relevant for the Duchenne Muscular Dystrophy Pathogenesis Are Controlled by the Dystrophin/nNOS Pathway. Cell Metab. 2010, 12, 341–351. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, G.; Chen, Y.; Deng, Y. LncRNA MALAT1 Promotes Migration and Invasion of Non-Small-Cell Lung Cancer by Targeting miR-206 and Activating Akt/mTOR Signaling. Anticancer Drugs 2018, 29, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-206 Promotes Skeletal Muscle Regeneration and Delays Progression of Duchenne Muscular Dystrophy in Mice. J. Clin. Investig. 2012, 122, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yan, B.; Lu, Q.; Lin, Y.; Ma, L. Reciprocal Regulation of Hsa-miR-1 and Long Noncoding RNA MALAT1 Promotes Triple-Negative Breast Cancer Development. Tumour Biol. 2016, 37, 7383–7394. [Google Scholar] [CrossRef] [PubMed]
- Saliani, M.; Mirzaiebadizi, A.; Javadmanesh, A.; Siavoshi, A.; Ahmadian, M.R. KRAS-Related Long Noncoding RNAs in Human Cancers. Cancer Gene Ther. 2022, 29, 418–427. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, L.; Zhang, D.; Yao, X.; Zhang, H.; Wang, Y.-C.; Wang, X.-Y.; Xia, H.; Yan, J.; et al. Circulating Muscle-Specific miRNAs in Duchenne Muscular Dystrophy Patients. Mol. Ther. Nucleic Acids 2014, 3, e177. [Google Scholar] [CrossRef]
- Hu, J.; Kong, M.; Ye, Y.; Hong, S.; Cheng, L.; Jiang, L. Serum miR-206 and Other Muscle-Specific microRNAs as Non-Invasive Biomarkers for Duchenne Muscular Dystrophy. J. Neurochem. 2014, 129, 877–883. [Google Scholar] [CrossRef]
- Jin, C.F.; Li, Y.; Ding, X.B.; Li, X.; Zhang, L.L.; Liu, X.F.; Guo, H. Lnc133b, a Novel, Long Non-Coding RNA, Regulates Bovine Skeletal Muscle Satellite Cell Proliferation and Differentiation by Mediating miR-133b. Gene 2017, 630, 35–43. [Google Scholar] [CrossRef]
- Roohaninasab, M.; Yavari, S.F.; Babazadeh, M.; Hagh, R.A.; Pazoki, M.; Amrovani, M. Evaluating the Role of lncRNAs in the Incidence of Cardiovascular Diseases in Androgenetic Alopecia Patients. Cardiovasc. Toxicol. 2022, 22, 603–619. [Google Scholar] [CrossRef]
- Wang, S.-H.; Zhang, W.-J.; Wu, X.-C.; Zhang, M.-D.; Weng, M.-Z.; Zhou, D.; Wang, J.-D.; Quan, Z.-W. Long Non-Coding RNA Malat1 Promotes Gallbladder Cancer Development by Acting as a Molecular Sponge to Regulate miR-206. Oncotarget 2016, 7, 37857–37867. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Peng, G.; Yan, P.; Qian, C.; Li, F. Long Non-Coding RNA XIST Regulates Hyperglycemia-Associated Apoptosis and Migration in Human Retinal Pigment Epithelial Cells. Biomed. Pharmacother. 2020, 125, 109959. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Q.; Wang, L.; Wang, S.; Sun, F.; Xu, D.; Jiang, J. Knockdown of the Oncogene lncRNA NEAT1 Restores the Availability of miR-34c and Improves the Sensitivity to Cisplatin in Osteosarcoma. Biosci. Rep. 2018, 38, BSR20180375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, J.; Liu, L.; Huang, M.; Li, Y.; Bennett, S.; Xu, J.; Zou, J. The Role of Long Non-Coding RNA, Nuclear Enriched Abundant Transcript 1 (NEAT1) in Cancer and Other Pathologies. Biochem. Genet. 2022, 60, 843–867. [Google Scholar] [CrossRef]
- Yao, C.; Guo, G.; Huang, R.; Tang, C.; Zhu, Q.; Cheng, Y.; Kong, L.; Ren, J.; Fang, M. Manual Therapy Regulates Oxidative Stress in Aging Rat Lumbar Intervertebral Discs through the SIRT1/FOXO1 Pathway. Aging 2022, 14, 2400. [Google Scholar] [CrossRef] [PubMed]


| LncRNA | Regulation | Genes | Function of lncRNA | Organism | References |
|---|---|---|---|---|---|
| Gm15441 | negative | Txnip | decline hepatic glucose production | Mouse | [20,22] |
| 3110045C21Rik | positive | Ddr2 | role in insulin signaling pathway regulation | Mouse | [23] |
| 3110045C21Rik | positive | Cdh1 | to be involved in fibrosis | Mouse | [24] |
| 3110045C21Rik | negative | Acta2 | to be involved in fibrosis | Mouse | [24] |
| 3110045C21Rik | negative | Tgfb1 | to be involved in fibrosis | Mouse | [24] |
| Lnc_011371 | positive | Mb, Clic5 | up-regulation after birth | Anhui white goats (AWG) | [26] |
| Lnc_007561 | positive | Tcf4 | regulates myogenesis | Anhui white goats (AWG) | [26] |
| Lnc_001728 | positive | S100 A4 | promotes cardiomyocyte production, inhibiting apoptosis | Anhui white goats (AWG) | [26] |
| Ppp1r1b | positive | Prc2 | leads to the induction of myogenic transcription factors | C2C12 and Human skeletal muscle myoblast | [27] |
| Miat | negative | Dnmt1, Dnmt3a, and Dnmt3b | silenced MIAT leads to reduced cell proliferation and promotes apoptosis | Human breast cancer | [28] |
| Lnc-1700113A16RIK | positive | Myog, MEF2D | enhance the differentiation of skeletal muscle stem cells | Mouse | [29] |
| Lnc-22988, Lnc-372289 and Lnc-482286 | positive | Acta1, Eno3, Myl1, Myom1, Myoz1, Neb, Ryr1, and Tnnc2 | could directly or indirectly regulate muscle proliferation, differentiation, and development | Pig | [30] |
| H19 | negative | Smad1, Smad5 and Cdc6 | promotion of skeletal muscle differentiation and regeneration | C2C12 Mouse myoblast cell line | [31] |
| H19 | positive | Dusp27 | promote AMPK activity in muscle cells and stimulate glucose uptake and mitochondrial biogenesis | Mouse | [32] |
| Mar1 | positive | Myod, Myog, Mef2c, and Myf5 | positively correlated with muscle differentiation | Mouse | [33] |
| Syisl | negative | Ezh2 | regulate muscle atrophy and sarcopenia | C2C12 | [34] |
| LncMyod (Gm45923) | positive | Imp2 | promoted myoblast proliferation and inhibited myoblast differentiation in the C2C12 cell line | C2C12 | [35] |
| Dum | negative | Dppa2 | promotes myogenic differentiation | C2C12 | [36] |
| FKBP1C | negative | Myh1b | suppress myoblast proliferation and enhance myoblast differentiation in fast and slow muscle fibers. | Chicken | [37] |
| LncRNA | Effect on Genes | miRNA | Genes | References |
|---|---|---|---|---|
| LincMD1 | negative | miR-133A1 | Mef2c and Maml1 | [158] |
| Meg8 | negative | miR-195 | Stat6/Nf-Kβ/Il-31 | [159] |
| Malat1 | negative | miR-133A1 | Ryr2 | [44] |
| Malat1 | negative | miR-206 | Anxa2 and Kras | [160] |
| Xist | negative | miR-17 | Pten | [132] |
| Xist | negative | miR-29A | Stx17 | [122] |
| Xist | negative | miR-21 | Pc/Xist | [161] |
| Neat1 | negative | miR-34C | Cisplatin (DDP) | [162] |
| Neat1 | negative | miR-335 | Abca3 | [163] |
| Meg3 | positive | miR-133A1 | Prrt2 | [164] |
| Meg3 | negative | miR-21 | Rhob and Pten | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorji, A.E.; Roudbari, Z.; Ahmadian, K.; Razban, V.; Shirali, M.; Hasanpur, K.; Sadkowski, T. Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2025, 26, 6032. https://doi.org/10.3390/ijms26136032
Gorji AE, Roudbari Z, Ahmadian K, Razban V, Shirali M, Hasanpur K, Sadkowski T. Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy. International Journal of Molecular Sciences. 2025; 26(13):6032. https://doi.org/10.3390/ijms26136032
Chicago/Turabian StyleGorji, Abdolvahab Ebrahimpour, Zahra Roudbari, Kasra Ahmadian, Vahid Razban, Masoud Shirali, Karim Hasanpur, and Tomasz Sadkowski. 2025. "Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy" International Journal of Molecular Sciences 26, no. 13: 6032. https://doi.org/10.3390/ijms26136032
APA StyleGorji, A. E., Roudbari, Z., Ahmadian, K., Razban, V., Shirali, M., Hasanpur, K., & Sadkowski, T. (2025). Exploring lncRNA-Mediated Mechanisms in Muscle Regulation and Their Implications for Duchenne Muscular Dystrophy. International Journal of Molecular Sciences, 26(13), 6032. https://doi.org/10.3390/ijms26136032









