Characterization of Polylactic Acid Membranes for Local Release of Tramadol
Abstract
1. Introduction
2. Results
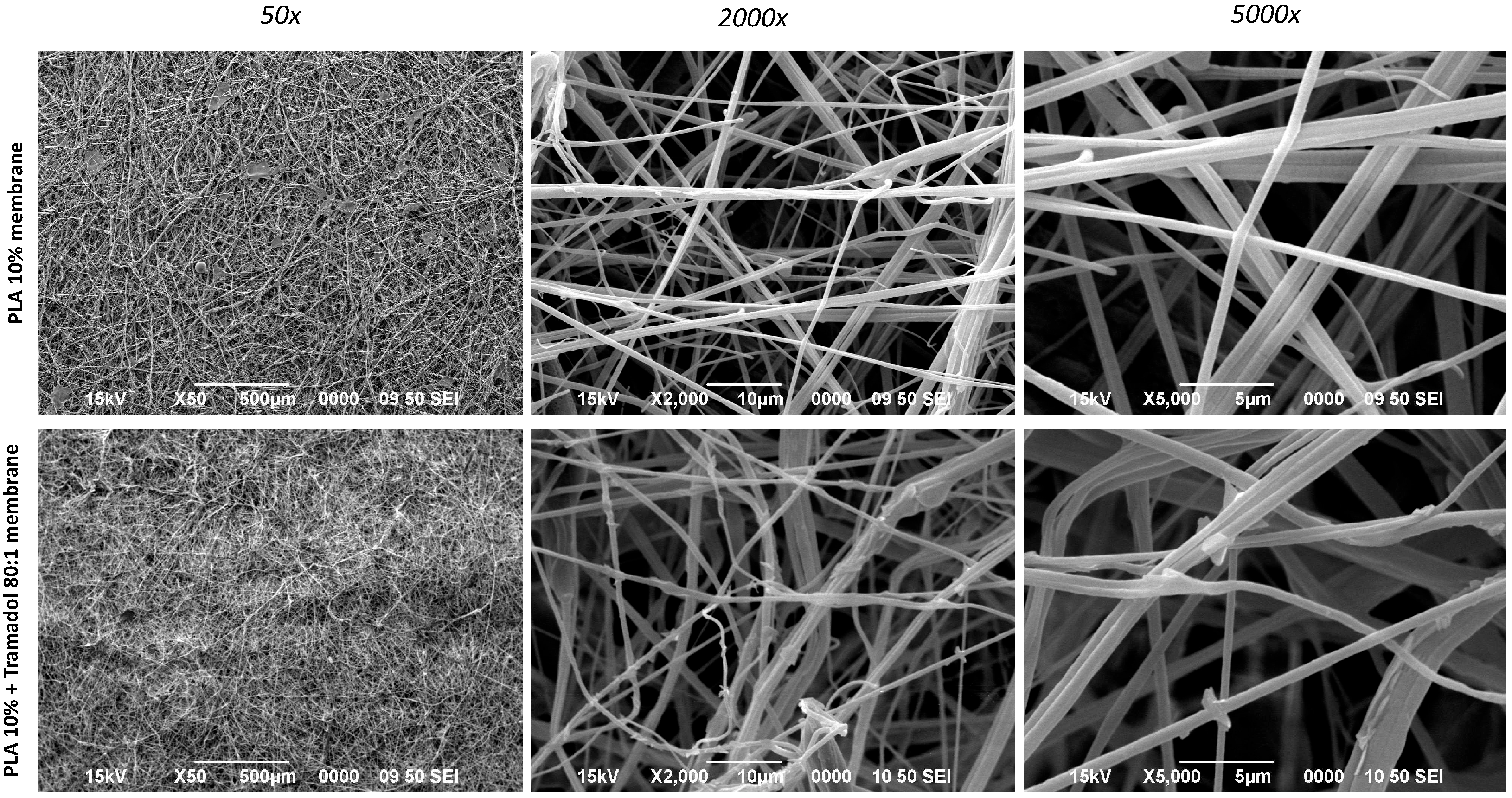
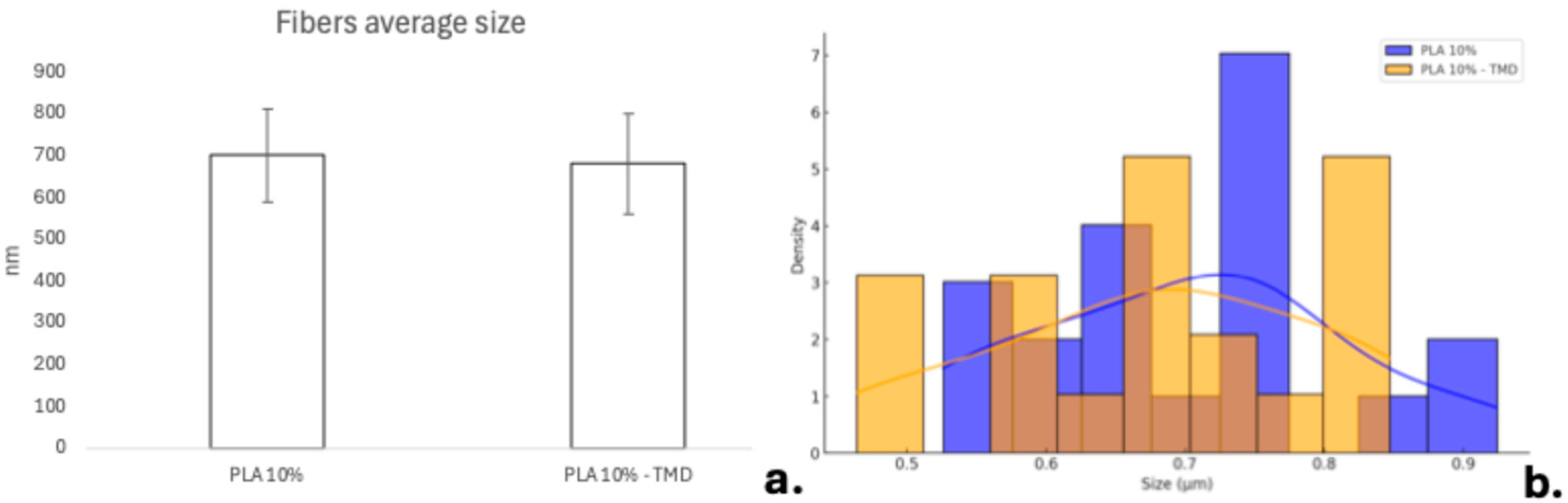
2.1. Macro and Microphotograph Analysis
2.2. Analysis of Thermodynamic Profile
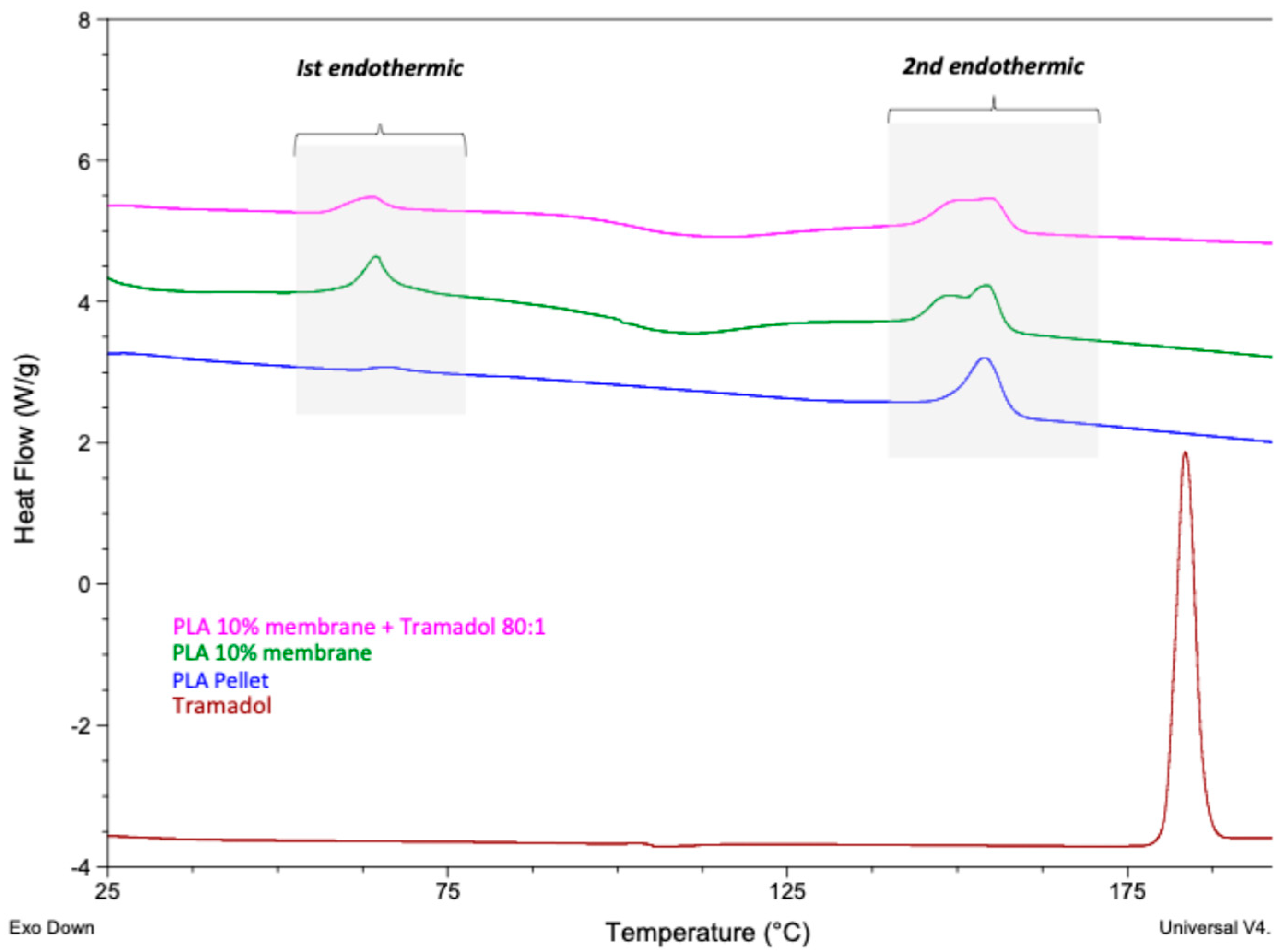
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Thermogravimetric Analysis (TGA)
2.3. Chemical Composition
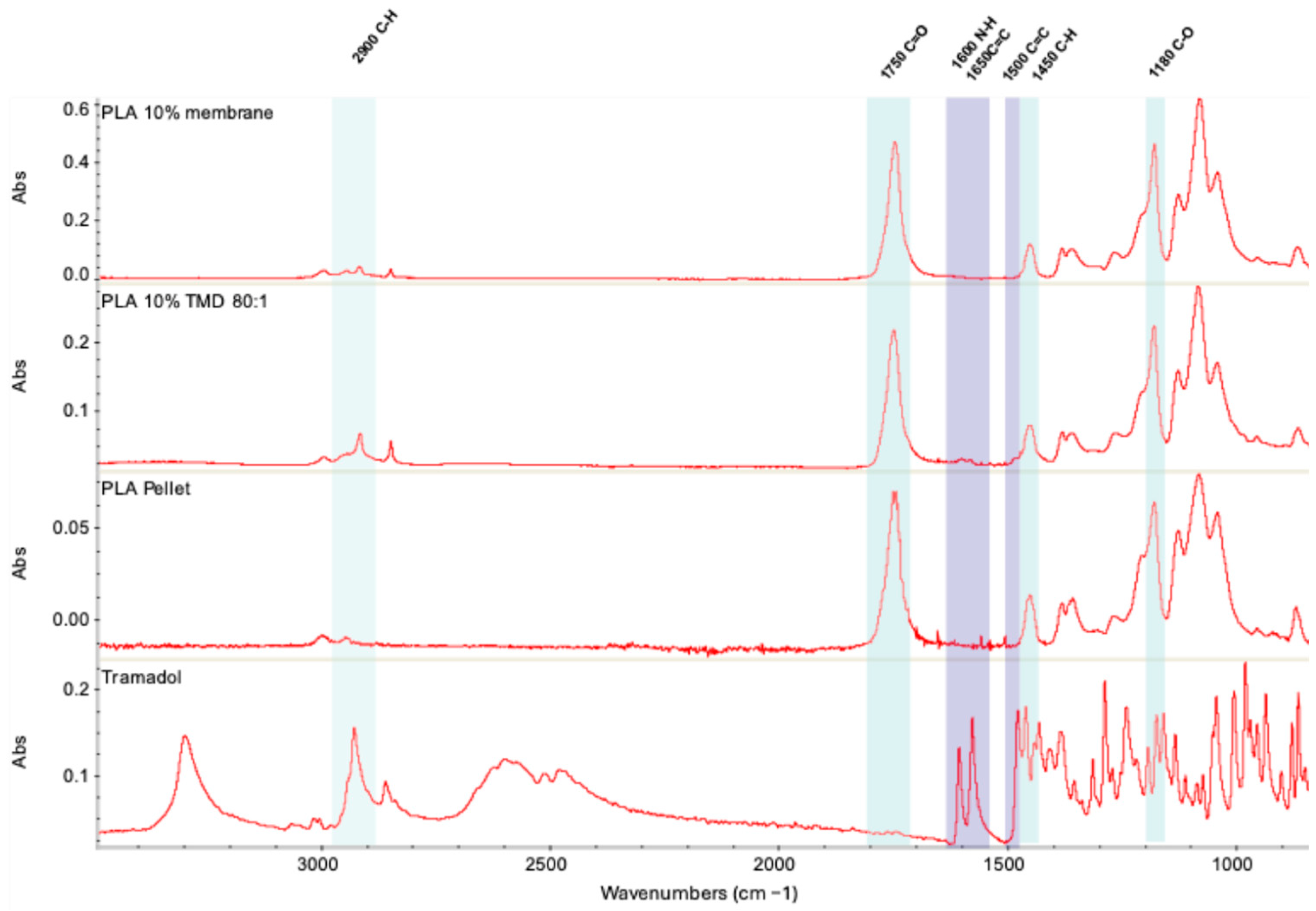
2.3.1. FT-IR Analysis
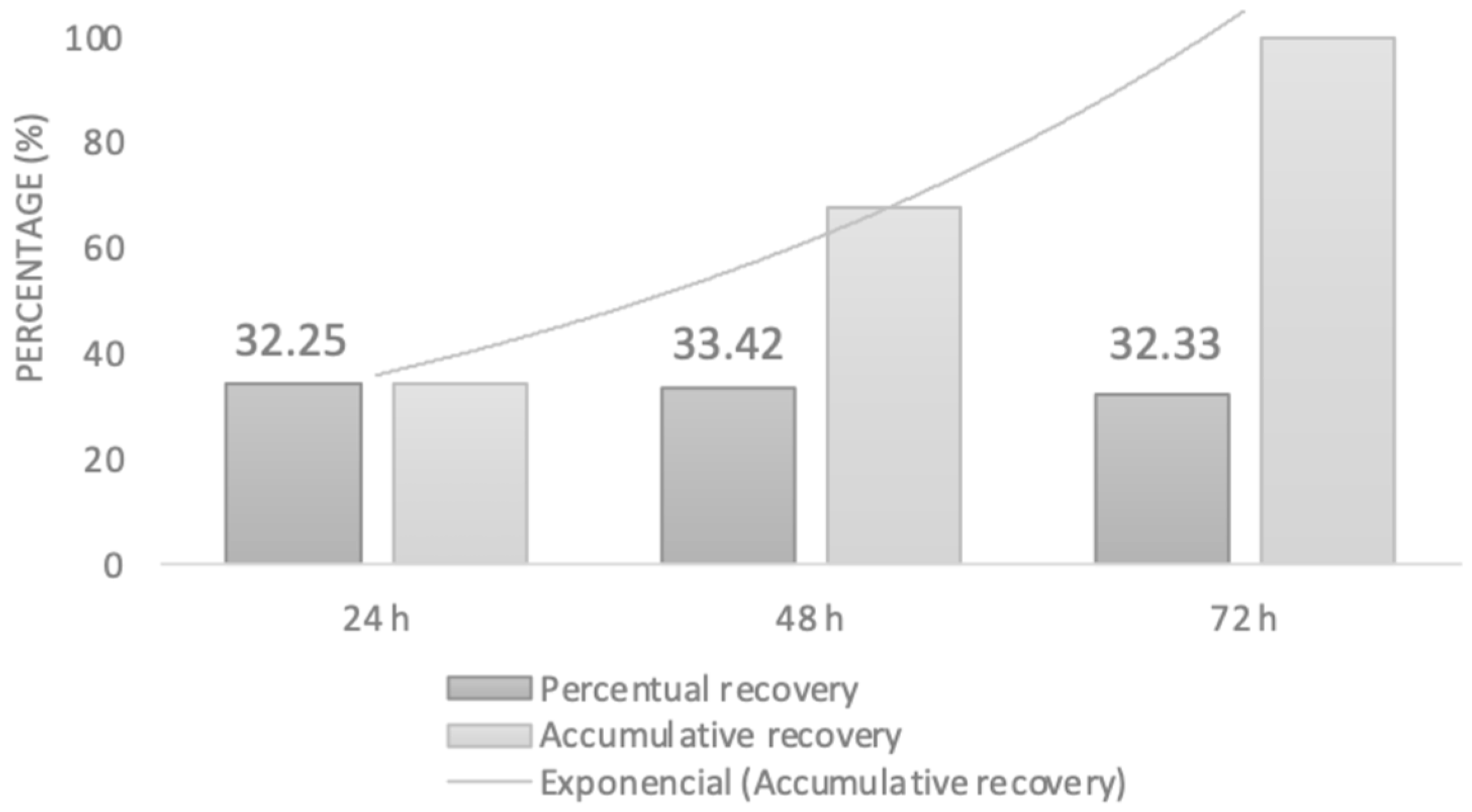
2.3.2. UV-VIS Spectrometric Analysis
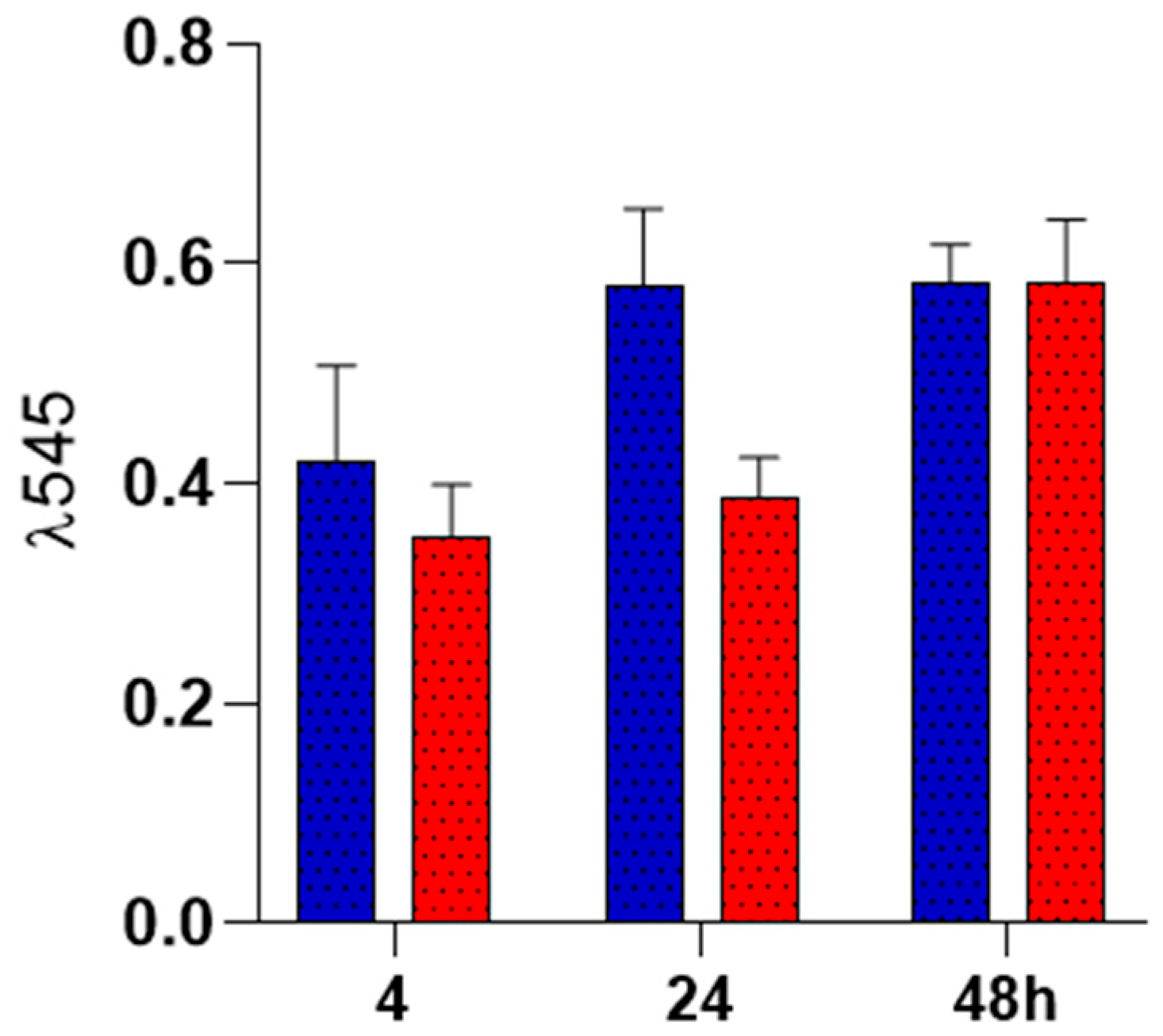
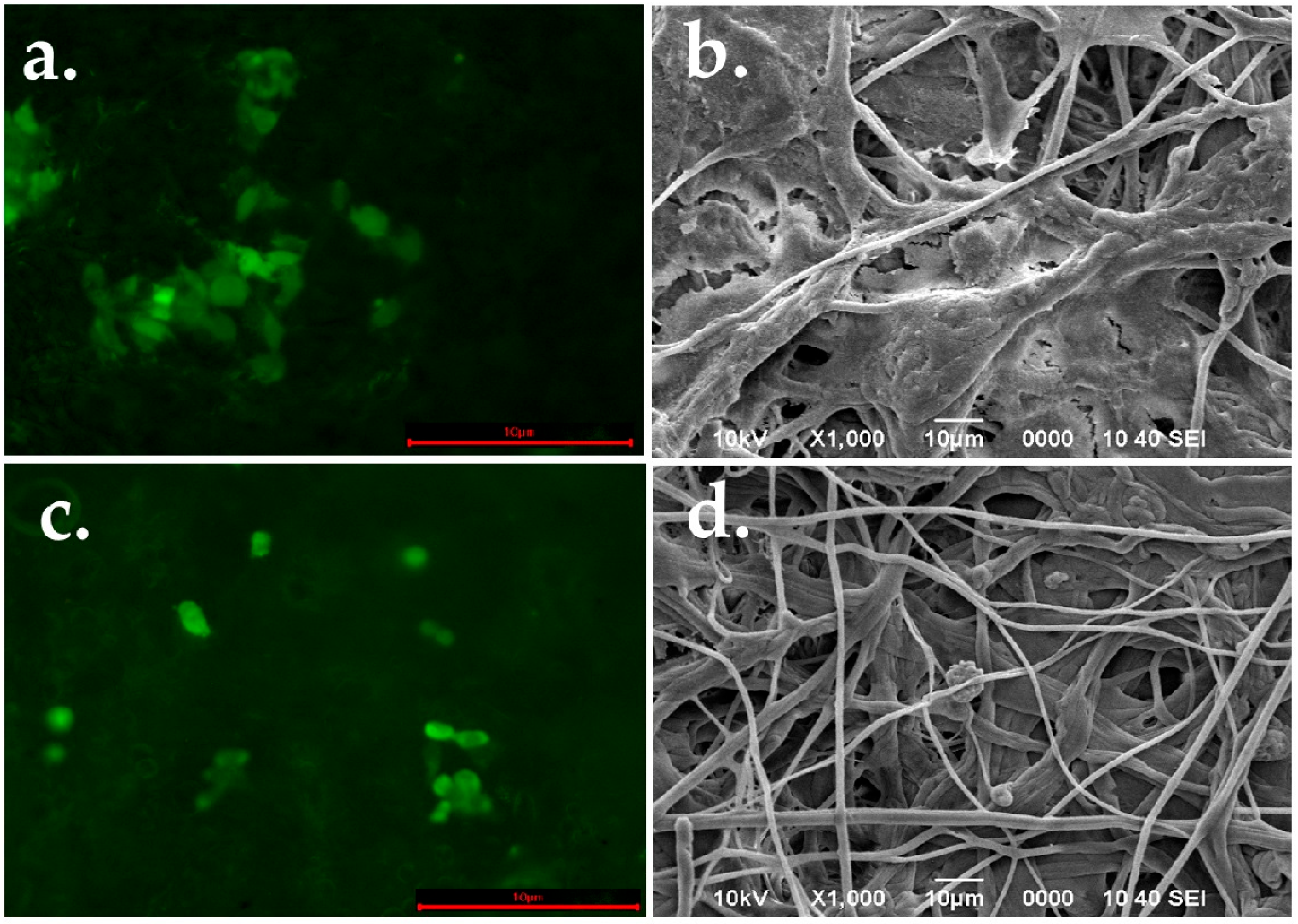
2.4. Biocompatibility Analysis
Colonization of PLA Spun Membrane
3. Discussion
4. Materials and Methods
4.1. Polymer Fiber Fabrication of PLA Loaded with Tramadol
4.2. PLA Loaded with Tramadol Fiber Scaffold Characterization
4.3. In Vitro Studies
4.4. Statistic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLA | Polylactic acid |
| TMD | Tramadol |
| AJS | Air jet spinning |
| SEM | Scanning electron microscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| ANOVA | Analysis of variance |
| ECM | Extracellular matrix |
| MW | Molecular weight |
References
- Woolf, C.J. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann. Intern. Med. 2004, 140, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A. Revisiting tramadol: A multi-modal agent for pain management. CNS Drugs 2019, 33, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J. Topical and peripherally acting analgesics. Pharmacol. Rev. 2003, 55, 1–20. [Google Scholar] [CrossRef]
- Pozos-Guillén, A.J.; Aguirre-Bañuelos, P.; Arellano-Guerrero, A.; Castañeda-Hernández, G.; Hoyo-Vadillo, C.; Pérez-Urizar, J. Isobolographic analysis of the dual-site synergism in the antinociceptive response of tramadol in the formalin test in rats. Life Sci. 2006, 79, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Ege, B.; Ege, M.; Koparal, M.; Alan, H. Comparison of the anesthetic efficiency of lidocaine and tramadol hydrochloride in orthodontic extractions: A split-mouth, prospective, randomized, double-blind study. J. Oral Maxillofac. Surg. 2020, 78, 52–62. [Google Scholar] [CrossRef]
- Chavarria-Bolaños, D.; Pozos-Guillén, A. Peripheral tramadol in dentistry: A new use for an old drug. Odovtos-Int. J. Dent. Sci. 2016, 18, 10–14. [Google Scholar] [CrossRef][Green Version]
- Pozos., A.J.; Martinez, R.; Aguirre, P.; Perez, J. The effects of tramadol added to articaine on anesthesia duration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 614–617. [Google Scholar] [CrossRef]
- Rodríguez-Wong, L.; Pozos-Guillen, A.; Silva-Herzog, D.; Chavarría-Bolaños, D. Efficacy of mepivacaine-tramadol combination on the success of inferior alveolar nerve blocks in patients with symptomatic irreversible pulpitis: A randomized clinical trial. Int. Endod. J. 2016, 49, 325–333. [Google Scholar] [CrossRef]
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V. Membranes for periodontal regeneration-A materials perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar] [PubMed]
- Suarez-Franco, J.L.; Vázquez-Vázquez, F.C.; Pozos-Guillen, A.; Montesinos, J.J.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Influence of diameter of fiber membrane scaffolds on the biocompatibility of hPDL mesenchymal stromal cells. Dent. Mater. J. 2018, 37, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D.; Hardwick, W.R.; Dahlin, C. Healing pattern of bone regeneration in membrane-protected defects: A histologic study in the canine mandible. Int. J. Oral Maxillofac. Implants 1994, 9, 13–29. [Google Scholar]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.R. Poly (lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Stojanovska, E.; Canbay, E.; Pampal, E.S.; Calisir, M.D.; Agma, O.; Polat, Y.; Kilic, A. A review on non-electro nanofibre spinning techniques. RSC Adv. 2016, 6, 83783–83801. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Lim, J.K. Air jet spinning of hydroxyapatite/poly (lactic acid) hybrid nanocomposite membrane mats for bone tissue engineering. Colloids Surf. B Biointerfaces 2013, 102, 635–643. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Liu, S.J. Nanofibers used for the delivery of analgesics. Nanomedicine 2015, 10, 1785–1800. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149. [Google Scholar] [CrossRef] [PubMed]
- Granados-Hernández, M.V.; Serrano-Bello, J.; Montesinos, J.J.; Alvarez-Gayosso, C.; Medina-Velázquez, L.A.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. In vitro and in vivo biological characterization of poly (lactic acid) fiber scaffolds synthesized by air jet spinning. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H. Solution blow spinning: A new method to produce micro-and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Lou, H.; Han, W.; Wang, X. Numerical study on the solution blowing annular jet and its correlation with fiber morphology. Ind. Eng. Chem. Res. 2014, 53, 2830–2838. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Tutak, W.; Sarkar, S.; Lin-Gibson, S.; Farooque, T.M.; Jyotsnendu, G.; Wang, D.; Simon, C.G., Jr. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013, 34, 2389–2398. [Google Scholar] [CrossRef]
- Hoffman, K.; Skrtic, D.; Sun, J.; Tutak, W. Airbrushed composite polymer Zr-ACP nanofiber scaffolds with improved cell penetration for bone tissue regeneration. Tissue Eng. Part C Methods 2015, 21, 284–291. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, J.; Liu, H.; Yang, Y.; Hou, Y.; Li, S. Tramadol loading, release and iontophoretic characteristics of ion-exchange fiber. Int. J. Pharm. 2014, 465, 102–111. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, I. Formulation and characterization of tramadol-loaded IPN microgels of alginate and gelatin: Optimization using response surface methodology. Acta Pharm. 2010, 60, 295. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; Chavarría-Bolaños, D.; Pozos-Guillén, A.D.J.; Escobar-Barrios, V.A.; Cerda-Cristerna, B.I. Synthesis of a PVA drug delivery system for controlled release of a Tramadol-Dexketoprofen combination. J. Mater. Sci. Mater. Med. 2021, 32, 56. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Quinn, F.X. Thermal Analysis: Fundamentals and Applications to Polymer Science, 2nd ed.; WILLEY: Hoboken, NJ, USA, 1999. [Google Scholar]
- Mabrouk, M.; Beherei, H.H.; ElShebiney, S.; Tanaka, M. Newly developed controlled release subcutaneous formulation for tramadol hydrochloride. Saudi Pharm. J. 2018, 26, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.N.; Ahmad, M. Production and stability evaluation of modified-release microparticles for the delivery of drug combinations. AAPS PharmSciTech 2010, 11, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ahad, H.A.; Ishaq, B.M.; Shaik, M.; Bandagisa, F. Designing and characterizing of tramadol hydrochloride transdermal patches prepared with Ficus carica fruit mucilage and povidone. Pak. J. Pharm. Sci. 2016, 29, 945–951. [Google Scholar] [PubMed]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, optimization and in-vitro characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef]
- Kücük, A.; Kadioğlu, Y. Determination of tramadol hydrochloride in ampoule dosage forms by using UV spectrophotometric and HPLC-DAD methods in methanol and water media. Il Farmaco 2005, 60, 163–169. [Google Scholar] [CrossRef]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid core-shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef]
- Chanes-Cuevas, O.A.; Arellano-Sánchez, U.; Alvarez-Gayosso, C.A.; Suaste-Olmos, F.; Villarreal-Ramirez, E.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Synthesis of PLA/SBA-15 Composite Scaffolds for Bone Tissue Engineering. Mater. Res. 2020, 23, e20200211. [Google Scholar] [CrossRef]
- Osorio-Arciniega, R.; García-Hipólito, M.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Composite Fiber Spun Mat Synthesis and In Vitro Biocompatibility for Guide Tissue Engineering. Molecules 2021, 26, 7597. [Google Scholar] [CrossRef]
- Vazquez-Vazquez, F.C.; Chavarria-Bolaños, D.; Ortiz-Magdaleno, M.; Guarino, V.; Alvarez-Perez, M.A. 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering 2022, 9, 189. [Google Scholar] [CrossRef]
- Pereira, M.I.A.; Monteiro, C.A.P.; Oliveira, W.F.D.; Santos, B.S.; Fontes, A.; Cabral Filho, P.E. Resazurin-based assay to evaluate cell viability after quantum dot interaction. Methods Mol. Biol. 2020, 2135, 213–221. [Google Scholar]
- Dos Santos, A.C.M.; Akkari, A.C.S.; Ferreira, I.R.S.; Maruyama, C.R.; Pascoli, M.; Guilherme, V.A.; de Araujo, D.R. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: Sol-gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int. J. Nanumea. 2015, 10, 2391. [Google Scholar]


| Onset Point (To) °C | Inflection Point (Tp) °C | |
| Tramadol | 234.39 | 270.57 |
| PLA 10% membrane | 310.24 | 343.35 |
| PLA 10% membrane + Tramadol 80:1 | 302.33 | 334.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Minotre, L.; Montero-Aguilar, M.; Vázquez-Vázquez, F.C.; Serrano-Bello, J.; Vega-Baudrit, J.; Pereira-Reyes, R.; Pozos-Guillén, A.; Chavarría-Bolaños, D. Characterization of Polylactic Acid Membranes for Local Release of Tramadol. Int. J. Mol. Sci. 2025, 26, 6018. https://doi.org/10.3390/ijms26136018
Fernández-Minotre L, Montero-Aguilar M, Vázquez-Vázquez FC, Serrano-Bello J, Vega-Baudrit J, Pereira-Reyes R, Pozos-Guillén A, Chavarría-Bolaños D. Characterization of Polylactic Acid Membranes for Local Release of Tramadol. International Journal of Molecular Sciences. 2025; 26(13):6018. https://doi.org/10.3390/ijms26136018
Chicago/Turabian StyleFernández-Minotre, Lafitte, Mauricio Montero-Aguilar, Febe Carolina Vázquez-Vázquez, Janeth Serrano-Bello, José Vega-Baudrit, Reinaldo Pereira-Reyes, Amaury Pozos-Guillén, and Daniel Chavarría-Bolaños. 2025. "Characterization of Polylactic Acid Membranes for Local Release of Tramadol" International Journal of Molecular Sciences 26, no. 13: 6018. https://doi.org/10.3390/ijms26136018
APA StyleFernández-Minotre, L., Montero-Aguilar, M., Vázquez-Vázquez, F. C., Serrano-Bello, J., Vega-Baudrit, J., Pereira-Reyes, R., Pozos-Guillén, A., & Chavarría-Bolaños, D. (2025). Characterization of Polylactic Acid Membranes for Local Release of Tramadol. International Journal of Molecular Sciences, 26(13), 6018. https://doi.org/10.3390/ijms26136018








