Targeting Topoisomerase I and DNA with LCS1269 Drives Glioblastoma Cell Death Despite ATM/Chk1/BRCA1/RAD51 Signaling Pathway Activation
Abstract
1. Introduction
2. Results
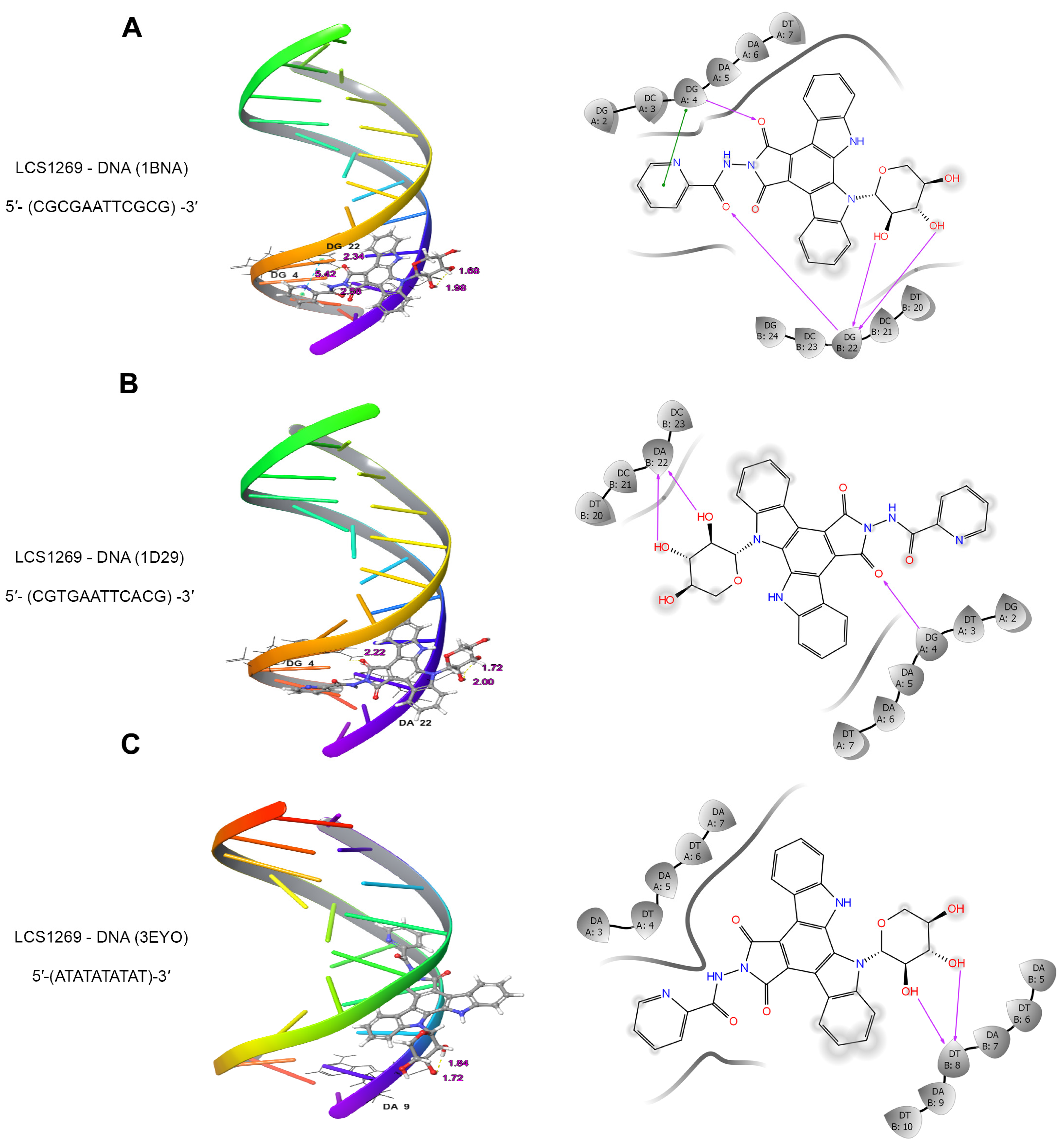
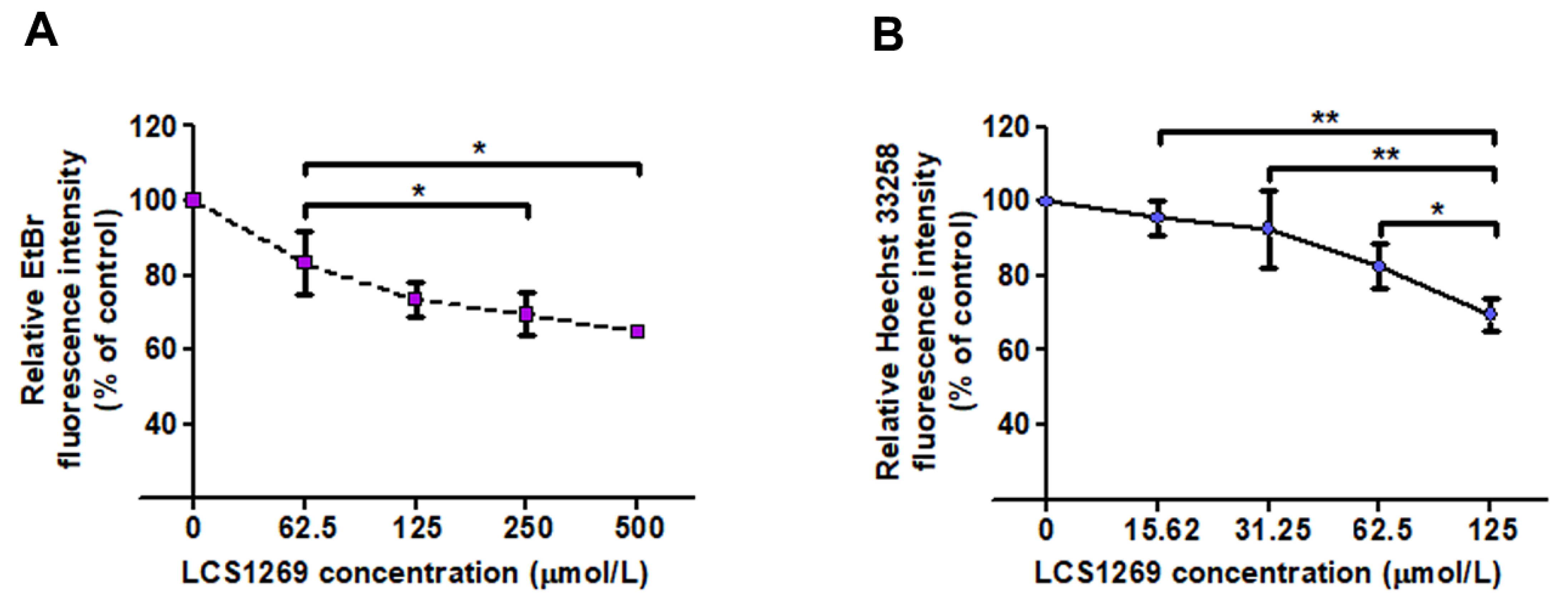
2.1. LCS1269 Interacts with DNA Through Minor Groove Binding Rather than in an Intercalative Manner
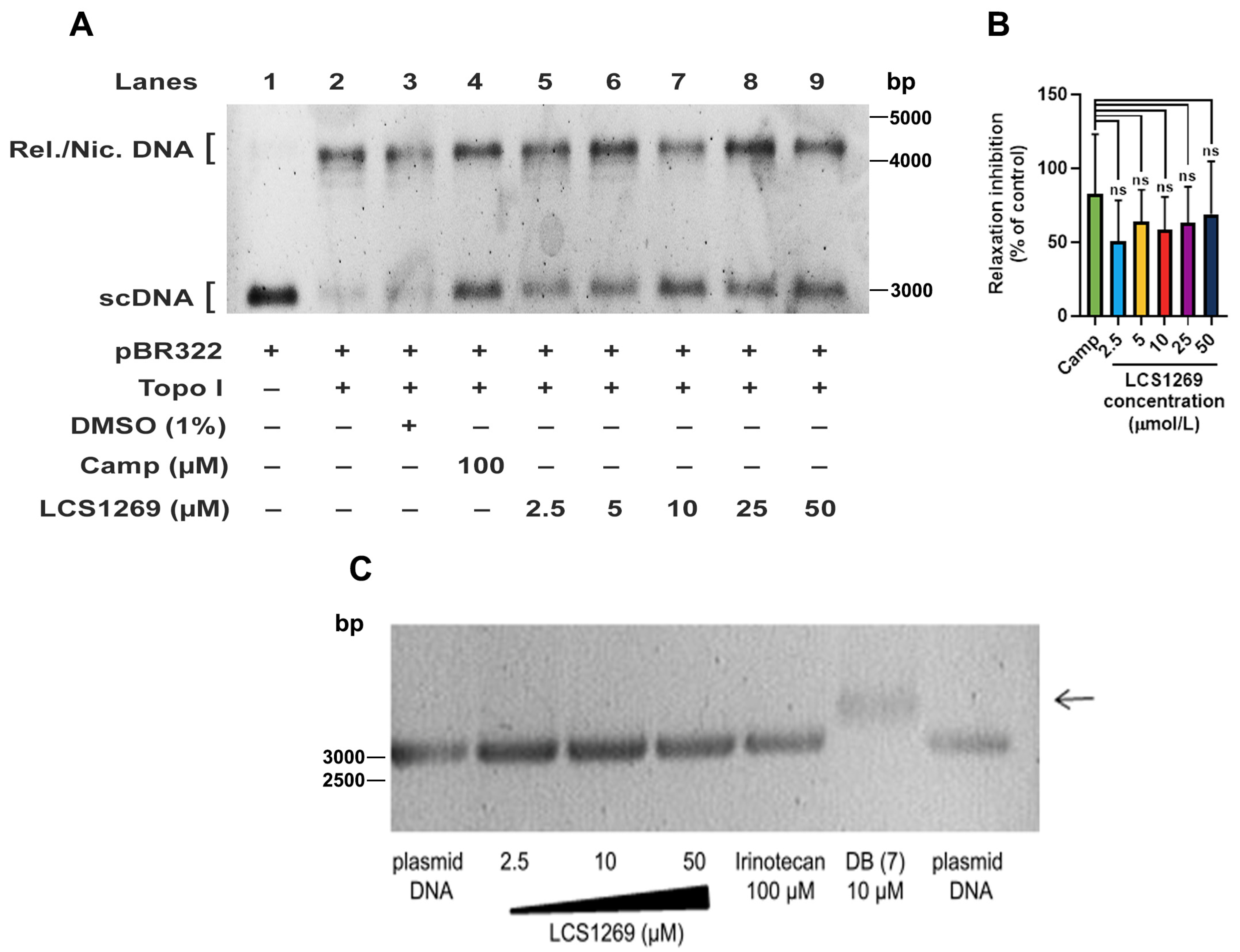
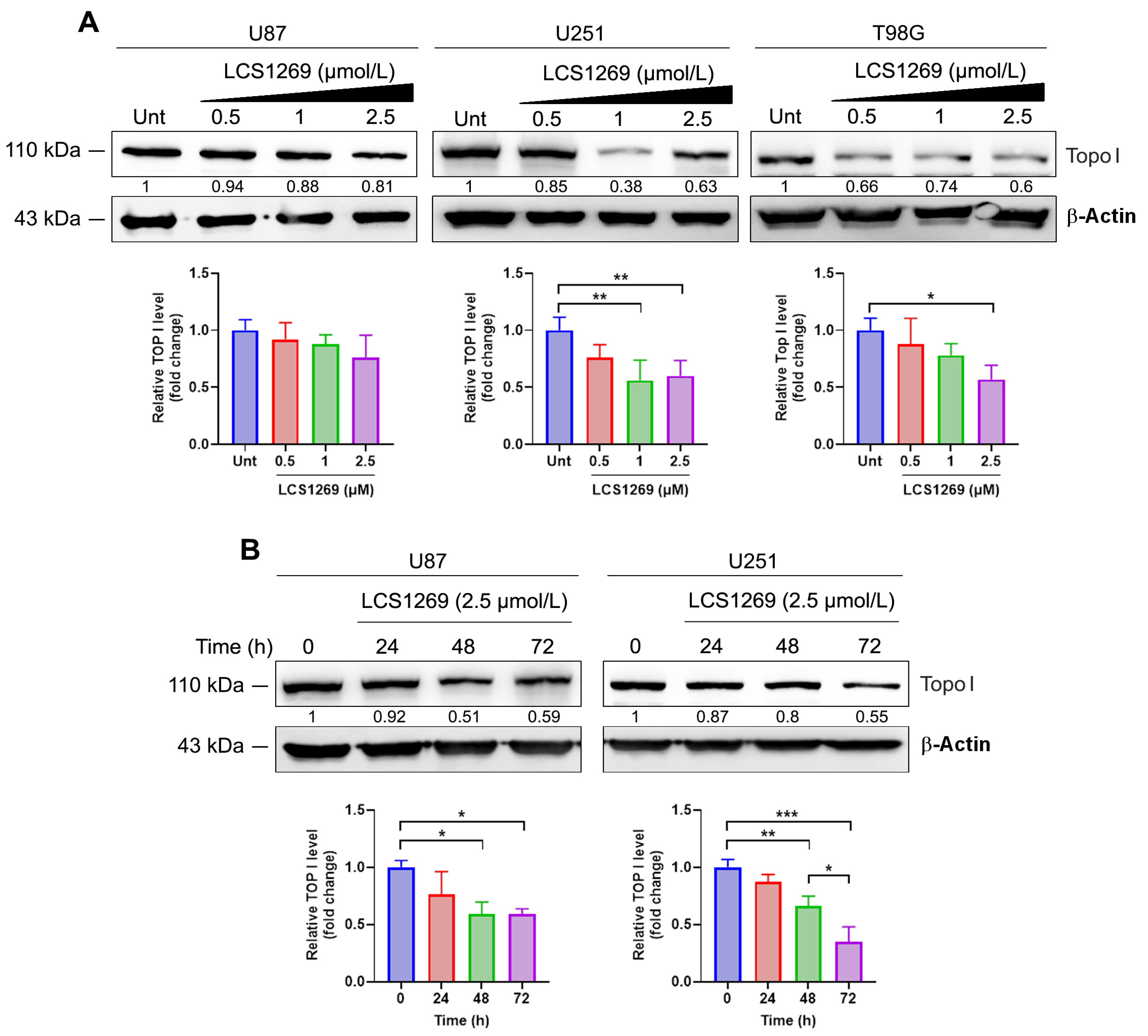
2.2. LCS1269 Inhibits Enzyme Activity and Alleviates Top I Protein Expression
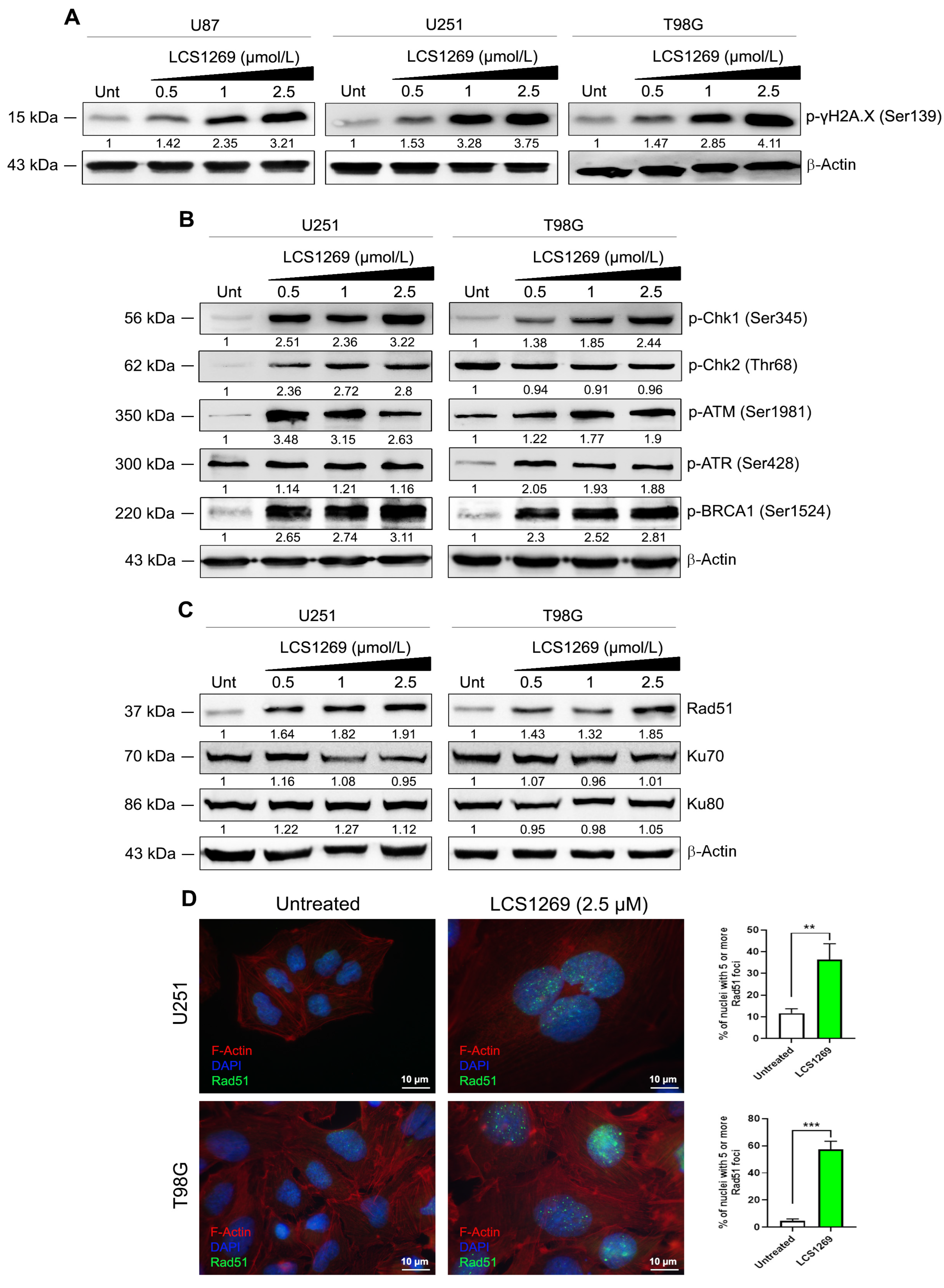
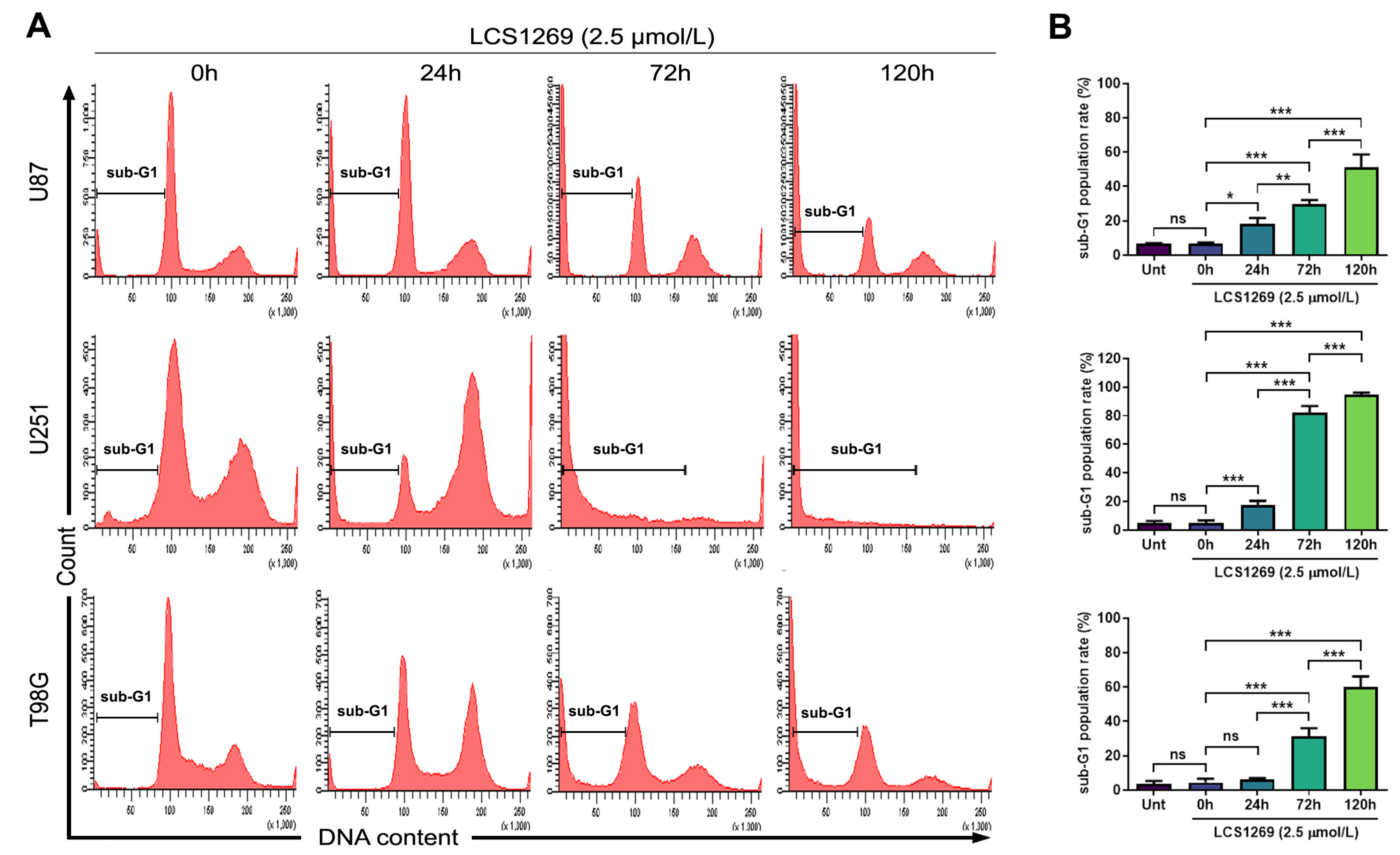
2.3. LCS1269 Triggers DNA Damage Propagation Followed by Cell Death in Glioblastoma Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Cell Cycle Analysis and Sub-G1 Content Evaluation
4.3. Western Blotting
4.4. Plasmid DNA Relaxation Assay
4.5. DNA Competition Studies
4.6. pBR322 Gel Migration Assay
4.7. Rad51 Foci Formation
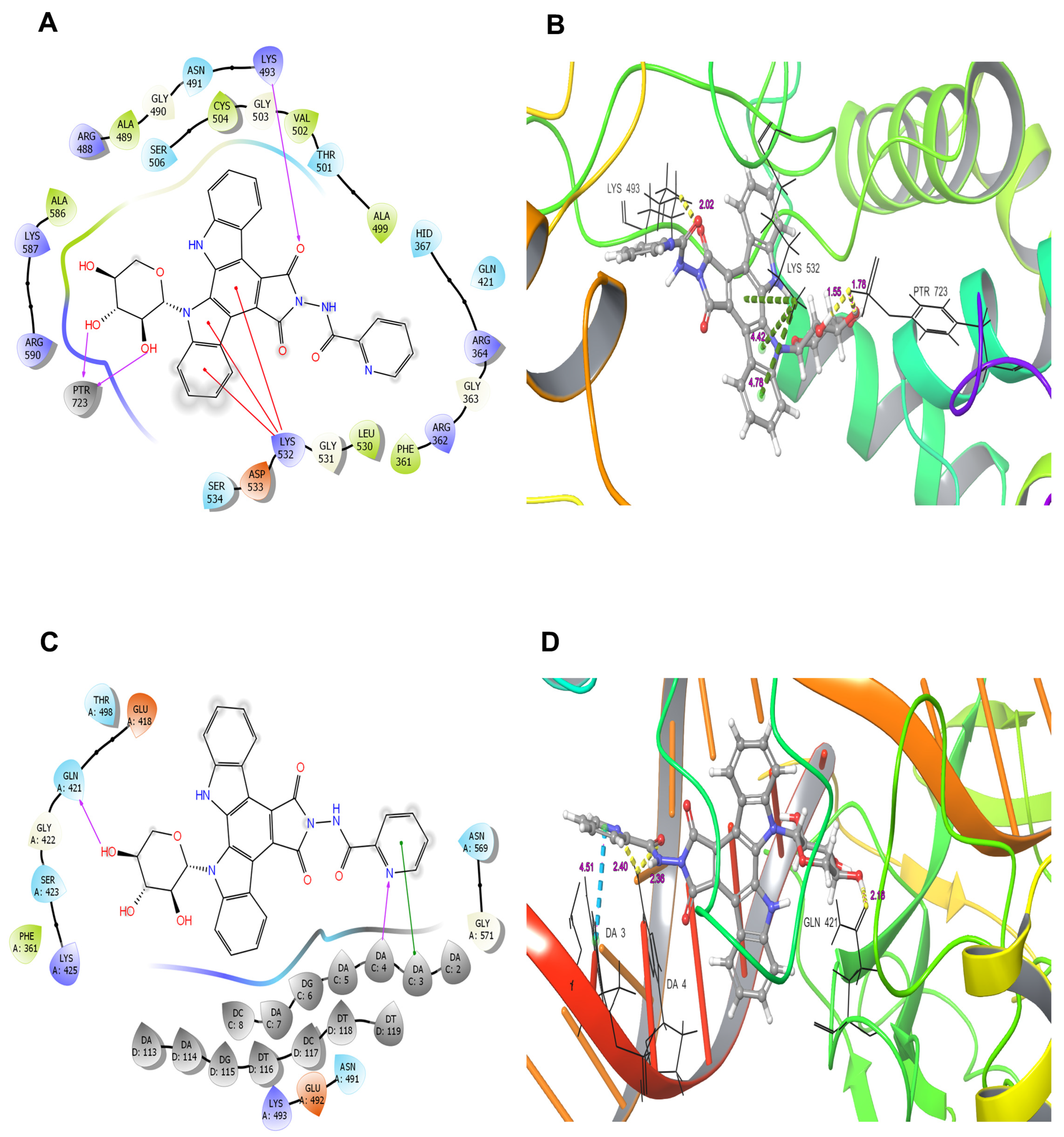
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Ramón Y Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical implications of intratumor heterogeneity: Challenges and opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef]
- Garnier, D.; Renoult, O.; Alves-Guerra, M.C.; Paris, F.; Pecqueur, C. Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front. Oncol. 2019, 9, 118. [Google Scholar] [CrossRef]
- Prager, B.C.; Bhargava, S.; Mahadev, V.; Hubert, C.G.; Rich, J.N. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer 2020, 6, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Ivens, E.; Cominetti, M.M.D.; Searcey, M. Junctions in DNA: Underexplored targets for therapeutic intervention. Bioorg. Med. Chem. 2022, 69, 116897. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Reinhold, W.C.; Mergny, J.L.; Liu, H.; Ryan, M.; Pfister, T.D.; Kinders, R.; Parchment, R.; Doroshow, J.; Weinstein, J.N.; Pommier, Y. Exon array analyses across the NCI-60 reveal potential regulation of TOP1 by transcription pausing at guanosine quartets in the first intron. Cancer Res. 2010, 70, 2191–2203. [Google Scholar] [CrossRef]
- Friedman, H.S.; Petros, W.P.; Friedman, A.H.; Schaaf, L.J.; Kerby, T.; Lawyer, J.; Parry, M.; Houghton, P.J.; Lovell, S.; Rasheed, K.; et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J. Clin. Oncol. 1999, 17, 1516–1525. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Filka, E.; Kuhn, J.; Nelson, G.; Kabbinavar, F.; Friedman, H.; Miller, L.L.; Elfring, G.L. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer 2003, 97, 2381–2386. [Google Scholar] [CrossRef]
- Sasine, J.P.; Savaraj, N.; Feun, L.G. Topoisomerase I inhibitors in the treatment of primary CNS malignancies: An update on recent trends. Anticancer Agents Med. Chem. 2010, 10, 683–696. [Google Scholar] [CrossRef]
- Fisher, B.; Won, M.; Macdonald, D.; Johnson, D.W.; Roa, W. Phase II study of topotecan plus cranial radiation for glioblastoma multiforme: Results of Radiation Therapy Oncology Group 9513. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.W.; Altscher, R.; Brandtner, M.; Haeusser-Mischlich, H.; Chiricuta, I.C.; Siegmann, A.D.; Engenhart-Cabillic, R. Open-label simultaneous radio-chemotherapy of glioblastoma multiforme with topotecan in adults. Clin. Neurol. Neurosurg. 2005, 107, 207–213. [Google Scholar] [CrossRef]
- Bernier-Chastagner, V.; Grill, J.; Doz, F.; Bracard, S.; Gentet, J.C.; Marie-Cardine, A.; Luporsi, E.; Margueritte, G.; Lejars, O.; Laithier, V.; et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: Results of a French Society of Paediatric Oncology Phase II Study. Cancer 2005, 104, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Klautke, G.; Schütze, M.; Bombor, I.; Benecke, R.; Piek, J.; Fietkau, R. Concurrent chemoradiotherapy and adjuvant chemotherapy with Topotecan for patients with glioblastoma multiforme. J. Neurooncol. 2006, 77, 199–205. [Google Scholar] [CrossRef]
- Feun, L.; Savaraj, N. Topoisomerase I inhibitors for the treatment of brain tumors. Expert. Rev. Anticancer Ther. 2008, 8, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kalitin, N.N.; Ektova, L.V.; Kostritsa, N.S.; Sivirinova, A.S.; Kostarev, A.V.; Smirnova, G.B.; Borisova, Y.A.; Golubeva, I.S.; Ermolaeva, E.V.; Vergun, M.A.; et al. A novel glycosylated indolocarbazole derivative LCS1269 effectively inhibits growth of human cancer cells in vitro and in vivo through driving of both apoptosis and senescence by inducing of DNA damage and modulating of AKT/mTOR/S6K and ERK pathways. Chem. Biol. Interact. 2022, 364, 110056. [Google Scholar] [CrossRef]
- Vartanian, A.; Baryshnikova, M.; Burova, O.; Afanasyeva, D.; Misyurin, V.; Belyavsky, A.; Shprakh, Z. Inhibitor of vasculogenic mimicry restores sensitivity of resistant melanoma cells to DNA-damaging agents. Melanoma Res. 2017, 27, 8–16. [Google Scholar] [CrossRef]
- Lantsova, A.; Golubeva, I.; Borisova, L.; Nikolaeva, L.; Ektova, L.; Dmitrieva, M.; Orlova, O. A new indolocarbazole derivative in melanoma and carcinoma lung in vivo treatment. BMC Complement. Med. Ther. 2021, 21, 117. [Google Scholar] [CrossRef]
- Kalitin, N.; Koroleva, N.; Lushnikova, A.; Babaeva, M.; Samoylenkova, N.; Savchenko, E.; Smirnova, G.; Borisova, Y.; Kostarev, A.; Karamysheva, A.; et al. N-Glycoside of Indolo[2,3-a]pyrrolo[3,4-c]carbazole LCS1269 Exerts Anti-Glioblastoma Effects by G2 Cell Cycle Arrest and CDK1 Activity Modulation: Molecular Docking Studies, Biological Investigations, and ADMET Prediction. Pharmaceuticals 2024, 17, 1642. [Google Scholar] [CrossRef]
- Monchaud, D.; Allain, C.; Teulade-Fichou, M.P. Development of a fluorescent intercalator displacement assay (G4-FID) for establishing quadruplex-DNA affinity and selectivity of putative ligands. Bioorg. Med. Chem. Lett. 2006, 16, 4842–4845. [Google Scholar] [CrossRef]
- Zenkov, R.; Vlasova, O.; Maksimova, V.; Fetisov, T.; Karpechenko, N.; Ektova, L.; Eremina, V.; Popova, V.; Usalka, O.; Lesovaya, E.; et al. Molecular Mechanisms of Anticancer Activity of N-Glycosides of Indolocarbazoles LCS-1208 and LCS-1269. Molecules 2021, 26, 7329. [Google Scholar] [CrossRef] [PubMed]
- Yevdokimov, Y.; Skuridin, S.; Nechipurenko, Y.; Zakharov, M.; Salyanov, V.; Kurnosov, A.; Kuznetsov, V.; Nikiforov, V. Nanoconstructions based on double-stranded nucleic acids. Int. J. Biol. Macromol. 2005, 36, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.A.; Kopka, M.L.; Dickerson, R.E. Crystal structure analysis of the B-DNA dodecamer CGTGAATTCACG. Biochemistry 1991, 30, 4443–4449. [Google Scholar] [CrossRef]
- Moreno, T.; Pous, J.; Subirana, J.A.; Campos, J.L. Coiled-coil conformation of a pentamidine-DNA complex. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.-Y.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Liu, S.; Shiotani, B.; Lahiri, M.; Maréchal, A.; Tse, A.; Leung, C.C.Y.; Glover, J.N.; Yang, X.H.; Zou, L. ATR autophosphorylation as a molecular switch for checkpoint activation. Mol. Cell 2011, 43, 192–202. [Google Scholar] [CrossRef]
- Menolfi, D.; Zha, S. ATM, ATR and DNA-PKcs kinases—The lessons from the mouse models: Inhibition≠deletion. Cell Biosci. 2020, 10, 8. [Google Scholar] [CrossRef]
- Cortez, D.; Wang, Y.; Qin, J.; Elledge, S.J. Requirement of ATM-dependent phosphorylation of BRCA1 in the DNA damage response to double-strand breaks. Science 1999, 286, 1162–1166. [Google Scholar] [CrossRef]
- Lieber, M.R. The mechanism of human non-homologous DNA end joining. J. Biol. Chem. 2008, 283, 1–5. [Google Scholar] [CrossRef] [PubMed]
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 2008, 77, 229–257. [Google Scholar] [CrossRef]
- Tashiro, S.; Kotomura, N.; Shinohara, A.; Tanaka, K.; Ueda, K.; Kamada, N. S phase specific formation of the human Rad51 protein nuclear foci in lymphocytes. Oncogene 1996, 12, 2165–2170. [Google Scholar] [PubMed]
- Pannunzio, N.R.; Watanabe, G.; Lieber, M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10512–10523. [Google Scholar] [CrossRef]
- Gullo, C.; Au, M.; Feng, G.; Teoh, G. The biology of Ku and its potential oncogenic role in cancer. Biochim. Biophys. Acta 2006, 1765, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Teh, M.T.; Gemenetzidis, E.; Chaplin, T.; Young, B.D.; Philpott, M.P. Upregulation of FOXM1 induces genomic instability in human epidermal keratinocytes. Mol. Cancer 2010, 9, 45. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.S.; Doetsch, P.W.; Werner, E. Replication stress and FOXM1 drive radiation induced genomic instability and cell transformation. PLoS ONE 2020, 15, e0235998. [Google Scholar] [CrossRef]
- Gheghiani, L.; Wang, L.; Zhang, Y.; Moore, X.T.R.; Zhang, J.; Smith, S.C.; Tian, Y.; Wang, L.; Turner, K.; Jackson-Cook, C.K.; et al. PLK1 Induces Chromosomal Instability and Overrides Cell-Cycle Checkpoints to Drive Tumorigenesis. Cancer Res. 2021, 8, 1293–1307. [Google Scholar] [CrossRef]
- Kandala, S.; Ramos, M.; Voith von Voithenberg, L.; Diaz-Jimenez, A.; Chocarro, S.; Keding, J.; Brors, B.; Imbusch, C.D.; Sotillo, R. Chronic chromosome instability induced by Plk1 results in immune suppression in breast cancer. Cell Rep. 2023, 42, 113266. [Google Scholar] [CrossRef]
- Buzun, K.; Bielawska, A.; Bielawski, K.; Gornowicz, A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2020, 35, 1781–1799. [Google Scholar] [CrossRef]
- Bondarev, A.D.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Lagunas-Rangel, F.A.; Schiöth, H.B. Recent developments of topoisomerase inhibitors: Clinical trials, emerging indications, novel molecules and global sales. Pharmacol. Res. 2024, 209, 107431. [Google Scholar] [CrossRef]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J. Enzym. Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef]
- Kozlov, S.V.; Graham, M.E.; Jakob, B.; Tobias, F.; Kijas, A.W.; Tanuji, M.; Chen, P.; Robinson, P.J.; Taucher-Scholz, G.; Suzuki, K.; et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J. Biol. Chem. 2011, 286, 9107–9119. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Jazayeri, A.; Falck, J.; Lukas, C.; Bartek, J.; Smith, G.C.; Lukas, J.; Jackson, S.P. ATM- and cell cycle dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.S.; Watanabe, N.; Morisaki, H.; Akita, H.; Fujimoto, A.; Tominaga, K.; Terasawa, M.; Tachibana, A.; Ikeda, K.; Nakanishi, M. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene 1999, 18, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell Biol. 2001, 21, 4129–4139. [Google Scholar] [CrossRef]
- Buisson, R.; Boisvert, J.L.; Benes, C.H.; Zou, L. Distinct but concerted roles of ATR, DNA-PK, and Chk1 in countering replication stress during S phase. Mol. Cell 2015, 59, 1011–1024. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; van den Top, A.; Bockaj, I.; Beijnen, J.H.; Wurdinger, T.; van Tellingen, O. The G2 checkpoint—A node-based molecular switch. FEBS Open Bio 2017, 7, 439–455. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Matsuoka, S.; Rotman, G.; Ogawa, A.; Shiloh, Y.; Tamai, K.; Elledge, S.J. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 10389–10394. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Cortez, D.; Bowers, B.; Elledge, S.J.; Gellert, M. Direct DNA binding by Brca1. Proc. Natl. Acad. Sci. USA 2001, 98, 6086–6091. [Google Scholar] [CrossRef]
- Karanam, K.; Kafri, R.; Loewer, A.; Lahav, G. Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. Mol. Cell 2012, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kalitin, N.N.; Karamysheva, A.F. RARα mediates all-trans-retinoic acid-induced VEGF-C, VEGF-D, and VEGFR3 expression in lung cancer cells. Cell Biol. Int. 2016, 40, 456–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalitin, N.; Savchenko, E.; Samoylenkova, N.; Koroleva, N.; Lushnikova, A.; Karamysheva, A.; Pavlova, G. Targeting Topoisomerase I and DNA with LCS1269 Drives Glioblastoma Cell Death Despite ATM/Chk1/BRCA1/RAD51 Signaling Pathway Activation. Int. J. Mol. Sci. 2025, 26, 6014. https://doi.org/10.3390/ijms26136014
Kalitin N, Savchenko E, Samoylenkova N, Koroleva N, Lushnikova A, Karamysheva A, Pavlova G. Targeting Topoisomerase I and DNA with LCS1269 Drives Glioblastoma Cell Death Despite ATM/Chk1/BRCA1/RAD51 Signaling Pathway Activation. International Journal of Molecular Sciences. 2025; 26(13):6014. https://doi.org/10.3390/ijms26136014
Chicago/Turabian StyleKalitin, Nikolay, Ekaterina Savchenko, Nadezhda Samoylenkova, Natalia Koroleva, Anna Lushnikova, Aida Karamysheva, and Galina Pavlova. 2025. "Targeting Topoisomerase I and DNA with LCS1269 Drives Glioblastoma Cell Death Despite ATM/Chk1/BRCA1/RAD51 Signaling Pathway Activation" International Journal of Molecular Sciences 26, no. 13: 6014. https://doi.org/10.3390/ijms26136014
APA StyleKalitin, N., Savchenko, E., Samoylenkova, N., Koroleva, N., Lushnikova, A., Karamysheva, A., & Pavlova, G. (2025). Targeting Topoisomerase I and DNA with LCS1269 Drives Glioblastoma Cell Death Despite ATM/Chk1/BRCA1/RAD51 Signaling Pathway Activation. International Journal of Molecular Sciences, 26(13), 6014. https://doi.org/10.3390/ijms26136014





