Targeting Prostate Cancer Metabolism Through Transcriptional and Epigenetic Modulation: A Multi-Target Approach to Therapeutic Innovation
Abstract
1. Introduction
2. Metabolic Reprogramming-Based Treatments for Prostate Cancer: The Classic and the Chemosensitization Approaches
| Metabolic Reprogramming Mechanism | Target | Drug | Experimental Model | Methods | Main Results | Reference |
|---|---|---|---|---|---|---|
| Citrate metabolism via Krebs Cycle | ACO2 | Camptothecin | LNCaP and PC3 cell lines | WB and mitochondrial enzymatic activity assay | Upregulation of p53, decreased ACO2 expression | [54] |
| Zinc metabolism regulation | ZIP | 5-azacytidine | RWPE-1 normal prostate epithelium cell line; LNCaP and DU-145 prostate carcinoma cell lines | qRT-PCR, chromatin immunoprecipitation (ChIP) | Reduced cell growth, reactivation of silenced ZIP1 and ZIP3 | [55] |
| Glucose metabolism | GLUTs inhibitors | Genistein, phloretin, apigenin, and daidzein | PNT1A normal prostate cells, LNCaP and PC3 cells, LNCaP and PC3 androgen insensitive and androgen sensitive clones | WB, cellular location confirmation by ICC | Apigenin and phloretin modified GLUT1 and GLUT4 expression, reducing cell proliferation in androgen independent PCa cells | [56] |
| PFK1 | Citrate | PC3, LNCaP and WPMY-1 cell lines | Cell proliferation assays, apoptosis determination by Annexin-V-FITC/PI Double Staining; WB | Citrate triggers autophagic cell death in prostate cancer cells by inhibiting the CaMKII/AKT/mTOR pathway, which may be linked to reduced PFK1 activity | [22] | |
| Lipid metabolism | FASN | TVB-2640 | LNCaP and C4-2 prostate carcinoma cell lines | WB for protein confirmation. STRING, GSEA, RNA-Seq, and K-M analyses using database search for FASN expression and correlations with clinical parameters | Increases Lipin1 and ASCL1 expression, both FASN interactors, with increased lipid accumulation. Cell cycle arrest | [57] |
| TVB-3166 | 22Rv1 cell line | β-Tubulin Confocal Immunofluorescence, WB | Reduced β-tubulin mRNA expression | [58] | ||
| IPI-9119 | LNCaP, LNCaP-95, 22Rv1, C4-2, xenograft implant, and MSK-PCa3 organoids (advanced mCRPC) | Real time qPCR, RNA-Seq and metabolomic profiling | Decreased cyclin A2 expression, down-regulation of pathways associated with amino acid and protein translation (LNCaP), and purine and pyrimidine synthesis (22Rv1 and LNCaP 95). Reduced expression and transcriptional activity of AR-FL and AR-V7 | [59] | ||
| Triclosan | Panel of prostate cancer cells (LNCaP, C4-2B, PC-3, 22RV1, RWPE-1 LAPC4, BPH-1, WPMY-1, and 3T3 cells) | Live imaging, qRT-PCR, WB | Increased cytotoxicity in PCa cell lines. Significant reduction of FASN gene expression, increased PLA2G6 expression, reduced lipid content in LNCaP cell lines | [60] | ||
| ACLY | miR-22 | RWPE-1 normal prostate epithelium cell line and PC3 PCa cell lines | qRT-PCR, WB | Disrupted ACLY post transcriptional control, decreased cell proliferation and invasion, downregulation of FASN and HMGCR | [61] | |
| ACC | Soraphen A | LNCaP, PC3-M (PC-3M-luc-C6), and BPH-1 cells | Fatty acid oxidation measurement, acetate incorporation assay, WB | At nanomolar concentrations, soraphen A blocks fatty acid synthesis, stimulating fatty acid oxidation in LNCaP and PC-3M cells | [62] | |
| PF-05175157 | Prostate-derived explants, LNCaP cell line | Metabolomic profiling (MALDI-MSI), RNA-Seq, IHC | Reduced fatty acid elongation, decreased expression of Ki67, cleaved caspase-3, and increased p-ACC expression in LNCaP cells | [63] | ||
| SCD1 | BZ36 | PNT2 prostate epithelial cell line, LNCaP and C4-2 cell lines, human prostate tissue samples, xenograph implant | qRT-PCR, WB, IHC | Decreased de novo lipogenesis, AKT/PIP3 and GSK3α/β/β-catenin signaling pathways, cell growth arrest | [64] | |
| SREBP | Fatostatin | LNCaP and C4-2B cell lines, and xenograph implant | qRT-PCR, WB, IHC | Inhibition of SREBP processing and transcriptional activity, decreased Ki67 expression and increased cleaved PARP | [65] | |
| Production of chemokines CCL2, CXCL12, receptor CXCR4, and pro-inflammatory cytokines TNF-α and IFN-γ, pro angiogenic regulators (VEGF, CXCL8, angiogenin) and MMP-9 | ALCAR | In vitro: PC-3, DU-145, LNCaP, 22Rv1 and benign prostate hyperplasia cell line (BPH); In vivo xenograph model | Flow cytometry, WB | Reduced expression of VEGF, CXCL8, CCL2, angiogenin and metalloprotease MMP-9. Inhibited expression of CXCR4, CXCR1, CXCR2 and CCR2 | [66] | |
| ACSL1, ACC, ACeCS1, FASN, Lipin 1 | Tannic acid | C4− 2, DU145 and PC-3 cells | RNA extraction and mRNA microarrays, bioinformatic iPathway guide analysis | Induced ROS and endoplasmic reticulum (ER) stress, nuclear disorganization, apoptosis | [67] | |
| ACC, ACLY, FASN, CPT1A | Withaferin A | LNCaP and 22Rv1 cell lines | RNA-Seq, KEGG pathway analysis, qRT-PCR, xenograph implant | Downregulation of ACC, ACLY, FASN, CPT1A | [68] | |
| Glutaminolysis | GLS1/2 | CB-839 | PC3 and PC3M | Reverse-phase protein array (RPPA) and WB | Metabolic differences in metastatic cell line, including increased glutamine utilization corroborated by differences in levels of phosphorylated AKT (pS473P) and mTOR in PC3M | [69] |
| LNCaP, PC3, enzalutamide-resistant C4-2MDVR cells | Tissue microarrays and IHC for biomarker validation, qPCR for transcript validation, LC/MS for metabolite assessment | GLS1 up regulated by AR signaling and glutaminase C (GAC) activation. Pharmacological inhibition of GAC show better treatment effect for castration resistant PCa | [35] | |||
| AR | Proxalutamide | AR-positive (22RV1 and LNCaP) and AR-negative cells (PC3 and DU145) | LC-Q/TOF-MS for metabolite assessment | Inhibition of glutamine metabolism, redox homeostasis, and de novo pyrimidine synthesis in AR-positive PCa cells | [70] | |
| Mitochondria and oxidative stress | Mitochondria | Synthetic non-glycoside analogs from sugar conjugates of 1,4-naphthoquinone urchin pigments spinochromes | PC-3, DU145, 22Rv1, and LNCaP, as well as human prostate non-cancer cell lines RWPE-1 and PNT2 | Apoptosis determination by Annexin-V-FITC/PI Double Staining; WB, Tandem Mass Spectrometry, Bioinformatic Ingenuity Pathway Analysis (IPA) | Mitochondria membrane permeabilization, ROS upregulation and release of cytotoxic mitochondrial proteins (AIF and cytochrome C), apoptosis | [71] |
| Metabolic Pathway | Treatment | Target | Experimental Model | Methods | Main Results | Reference |
|---|---|---|---|---|---|---|
| Lipid metabolism | miR-33a plus statins decreasing CPT1A and HADHB | CPT1A and HADHB | LNCaP and VCaP cells | MicroRNA transfection, cell proliferation test, Matrigel invasion test, soft agar colony test | Decreased cellular progression | [72] |
| Combinations of BMS-303141 and SB-204990 with enzalutamide | ACLY | C4-2, LNCaP and LNCaP abl (long-term androgen-free incubation), PC3 and 3T3-L1, focused on CRPC model. Combination treatments with ACLY inhibitor, BMS-303141 (ACLYi) | GS-MS metabolite measurements, qRT-PCR, immunoblotting, RNA-Seq | ACLYi and enzalutamide suppresses AR target gene expression in DHT treated and androgen depleted cells | [73] | |
| TVB-3166 and paclitaxel | FASN | 22Rv1 | β-Tubulin Confocal Immunofluorescence, WB | Reduced b-tubulin mRNA expression | [58] | |
| Etomoxir, perhexiline, ranolazine and enzalutamide | CPT1A | 22Rv1, LNCaP-MDV resistant and TRAMPC1 cells | RNA-Seq, RT PCR, CalcuSyn for CI determination, and xenograph implant | AR-related genes upregulation due to CPT1A KD. Decreased tumor growth with enzalutamide combinations | [46] | |
| Perhexiline and AUY922 (HSP90 inhibitor) | HSP90 | Patient derived explants, LNCaP, C4-2B, and 22RV1 cell lines | MS, flow cytometry and qRT-PCR | Increased protein expression of fatty acid oxidation and oxidative phosphorylation pathways following AUY922 treatment. Increased cell cycle arrest and apoptosis following cotreatment with perhexiline | [74] | |
| Glutaminolysis | CB-839 with talazoparib (PARP inhibitor) | GSL1 | DU145 cell line | CalcuSyn for CI determination, flow cytometry, immunofluorescence, xenograph implant | Synergistic effects of CB-839 and talazoparib, cell growth inhibition, decreased tumor volume. No results for metabolic reprogramming | [75] |
| Metabolic-related pathways with cellular signaling modulation | Valproic acid and simvastatin, in combination with docetaxel | Mevalonate pathway and AMPK | PC3, 22Rv1, DU145, DU145R80, and LNCaP. Creation of a 22Rv1 docetaxel-resistant cell line | CalcuSyn for CI determination, clonogenic agarassay, WB, RT-PCR, spheroid forming assay, and xenograph implant | Valproic acid and simvastatin combination inhibited YAP oncogene activity in a mCRPC model, increased AMPK fosforilation and downstream HMG-CoA reductase inhibitory phosphorylation. Synergistic potentiated effects with docetaxel. | [47] |
3. The Crosstalk Between Transcription and Epigenetics to Influence Metabolic Reprogramming in Prostate Cancer
3.1. AR
3.2. p53
3.3. MYC
3.4. HIF-1
3.5. Nrf2
3.6. PPARγ
4. The Role of Signaling Pathways as Metabolic Activators
5. Multi-Target Compounds: Therapeutic Implications of Transcriptional and Epigenetic Targeting in Metabolic Reprogramming of Prostate Cancer
| Molecule Name and Specific Chemical Type | Chemical Structure | In Vitro Data | In Vivo Data | Multi-Target Effects |
|---|---|---|---|---|
| Ursolic acid * (pentacyclic triterpenoid) |  DB: 15588 DB: 15588CID: 64945 | IC50: 35 μM (PC3), 47 μM (LNCaP), and 80 μM (DU145) at 24 h [182] | Reduction in tumor growth (VCaP) with 0.1% diet (w/w) [109] | SAM induction and effects of extracellular matrix remodeling, angiogenesis and cell adhesion in PCa. Epigenetic modification in PCa by modulating PTEN suppressive response |
| Evodiamine * (indolic alkaloid) |  DB: - DB: -CID: 442088 | Cell viability of 50% with 10 μM (PC3 and DU145) at 48 h [183] | Decreased tumor weight with 20 mg/kg dose (PC3) [184] | Increased expression of semaphorin 3A, and repression of HIF-1 α, H3K18la, GPX4 and PD-L1 |
| Genistein * (isoflavone) |  DB: 01645 DB: 01645CID: 5280961 | IC50: 15.1 μM (LNCaP), and 35.3 μM (PC3) at 24 h [56] | Tumor reduction with 250 mg/kg diet (high dose) [185] | GLUT and DNMT inhibitor. Epigenetic effects include demethylation of promoter region of metabolism-related tumor suppressor genes such as RAR β, and O6-methylguanine methyltransferase |
| Juglone * (1,4-naftoquinone) |  DB: - DB: -CID: 3806 | IC50: 32.2 μM (LNCaP) at 24 h [186] | HK, PFK, PK, and OXPHOS activity inhibition | |
| β-elemene *† (sesquiterpene) |  DB: 18097 DB: 18097CID: 6918391 | For elemene *† IC50: 146.8 μM (LNCaP) and 215.3 μM (PC3) For β-elemene IC50: 342.5 μM (LNCaP) and 318.1 μM (PC3) | p53 and FZR1upregulation Downregulation of PFKFB3 | |
| Tannic acid * (polyphenol) | 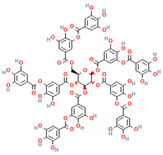 DB: 09372 DB: 09372CID: 16129878 | IC50: 29.1 μM (LNCaP), and 35.3 μM (PC3) [187] Decreased cell viability of ~70% with 10 μM at 24 h, and ~50% with 20 μM at 48 h, for C4-2, DU145, and PC3 [67] | Inhibitory effect on ACSL1, ACC, ACeCS1, FASN, Lipin 1 | |
| Withaferin A * (withanolide, naturally occurring steroid derived from ergostane skeleton) | 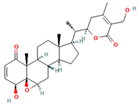 DB: - DB: -CID: 265237 | Cell proliferation below 50% at 48 h with 1 and 2 μM (LNCaP and 22Rv1) [68] | ~67% decrease in carcinoma in situ with 0.1 mg/mouse dose [68] | Inhibitory effect on ACC, ACLY, FASN, CPT1A |
| Sulforaphane * (isothiocyanate) |  DB: 12422 DB: 12422CID: 5350 | IC50: 10 μM (DU145) at 24 h [188]; 40 to 60% cell viability (DU145 and PC3) following 30 μM for 72 h [189]; 10 μM (LNCaP) reverse high AR expression [190] | Slower tumor development rate with high sulforaphane diet (15% broccoli sprouts) [191]; 6 μmol/mouse dose increase 60–70% downregulation of lipid metabolism related enzymes [99] | c-Myc suppression Nrf2 activation Lowered expression of HK2, PK2, LDHA. Lowered expression of ACC, FASN, and CPT1A |
| T0070907 ** (2-Chloro-5-nitro-N-4-pyridinylbenzamide) | 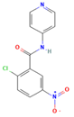 DB: - DB: -CID: 2777391 | Increased citotoxicity at 100 nM (PC3, LNCaP with high AR and PPARγ and LAPC4) [102] | 15 mg/kg dose reduce volume of high AR and PPARγ expression LNCaP xenographs [102] | Impaired PPARγ activity Decreased ACC, AR, and FASN expression |
6. Targeting Metabolic Reprogramming and Epigenetics in Prostate Cancer and Therapeutic Opportunities
7. Clinical Trials
8. Key Insights and Perspectives
- Enzyme Inhibition: This may occur through direct binding to the enzyme itself or indirectly by modifying allosteric regulation. A compound acting as an inhibitor might also disrupt metabolic signaling by affecting a pivotal enzyme. Evidence suggests that glycolysis, lipid oxidation, and lipogenesis are particularly sensitive to multi-target effects for PCa. Due to their interwoven relationship, transcription factors such as MYC or p53 might be crucial to extend the phenotypic reversal of PCa. Omics approaches, with a focus on metabolic flux analysis, may be required to fully elucidate the specific reprogrammed pathways in the case of phenotypic reversal.
- Direct Transcription Factor Binding: Compounds may directly bind to disrupted transcription factors, altering their conformation and promoting a reversal in metabolic reprogramming response.
- Pathway Modulation: By targeting upstream regulators or interacting pathways, the compound can indirectly affect the activity of transcription factors. For PCa, there is particular interest in acetyl-CoA modulation, which requires further research.
- Epigenetic Changes: The compound could induce epigenetic modifications that influence the accessibility of transcription factors to DNA. This might include affecting the activity of DNMT, modulation of ncRNAs, and, in particular,, SAM regulation, which seems to induce a favorable metabolic reprogramming in PCa and is related to other important processes of tumor progression.
- Protein–Protein Interactions: The compound might disrupt or enhance interactions between transcription factors and their co-factors or inhibitors. More comprehensive studies using omics data are needed to fully understand the correlation with metabolic reprogramming in PCa.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, E.O.-T.; Liu, X.; Lok, V.; Ngai, C.H.; Zhang, L.; Xu, W.; Zheng, Z.-J.; Chiu, P.K.-F.; Vasdev, N.; et al. Global Trends of Prostate Cancer by Age, and Their Associations with Gross Domestic Product (GDP), Human Development Index (HDI), Smoking, and Alcohol Drinking. Clin. Genitourin. Cancer 2023, 21, e261–e270.e250. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S. Chemotherapy and its evolving role in the management of advanced prostate cancer. Asian J. Androl. 2014, 16, 334–340. [Google Scholar] [CrossRef]
- Saad, F.; Aprikian, A.; Finelli, A.; Fleshner, N.E.; Gleave, M.; Kapoor, A.; Niazi, T.; North, S.A.; Pouliot, F.; Rendon, R.A.; et al. 2022 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guideline: Management of castration-resistant prostate cancer (CRPC). Can. Urol. Assoc. J. 2022, 16, e506–e515. [Google Scholar] [CrossRef]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B.; Grünwald, V. Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers 2023, 15, 461. [Google Scholar] [CrossRef]
- Baillif, B.; Wichard, J.; Méndez-Lucio, O.; Rouquié, D. Exploring the Use of Compound-Induced Transcriptomic Data Generated from Cell Lines to Predict Compound Activity Toward Molecular Targets. Front. Chem. 2020, 8, 296. [Google Scholar] [CrossRef]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In silico methods and tools for drug discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef]
- Türkmenoğlu, B. Investigation of novel compounds via in silico approaches of EGFR inhibitors as anticancer agents. J. Indian Chem. Soc. 2022, 99, 100601. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Nong, S.; Han, X.; Xiang, Y.; Qian, Y.; Wei, Y.; Zhang, T.; Tian, K.; Shen, K.; Yang, J.; Ma, X. Metabolic reprogramming in cancer: Mechanisms and therapeutics. MedComm 2023, 4, e218. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Ortega, Á.; Santeliz, R.; Garrido, B.; Chacín, M.; Galban, N.; Vera, I.; De Sanctis, J.B.; Bermúdez, V. Metabolic Reprogramming in Cancer Cells: Emerging Molecular Mechanisms and Novel Therapeutic Approaches. Pharmaceutics 2022, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.C.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef]
- Lasorsa, F.; di Meo, N.A.; Rutigliano, M.; Ferro, M.; Terracciano, D.; Tataru, O.S.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 910. [Google Scholar] [CrossRef]
- Singh, K.K.; Desouki, M.M.; Franklin, R.B.; Costello, L.C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer 2006, 5, 14. [Google Scholar] [CrossRef]
- Xue, Y.N.; Liu, Y.N.; Su, J.; Li, J.L.; Wu, Y.; Guo, R.; Yu, B.B.; Yan, X.Y.; Zhang, L.C.; Sun, L.K.; et al. Zinc cooperates with p53 to inhibit the activity of mitochondrial aconitase through reactive oxygen species accumulation. Cancer Med. 2019, 8, 2462–2473. [Google Scholar] [CrossRef]
- Karunasinghe, N. Zinc in Prostate Health and Disease: A Mini Review. Biomedicines 2022, 10, 3206. [Google Scholar] [CrossRef]
- Caiazza, C.; D’Agostino, M.; Passaro, F.; Faicchia, D.; Mallardo, M.; Paladino, S.; Pierantoni, G.M.; Tramontano, D. Effects of Long-Term Citrate Treatment in the PC3 Prostate Cancer Cell Line. Int. J. Mol. Sci. 2019, 20, 2613. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, J.; Yan, X.; Bi, X.; Liang, J.; Lu, S.; Luo, L.; Zhou, D.; Yin, Z. Citrate activates autophagic death of prostate cancer cells via downregulation CaMKII/AKT/mTOR pathway. Life Sci. 2021, 275, 119355. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Coquerel, A.; Wu, Z.; Gligorov, J.; Fuks, D.; Fournel, L.; Lincet, H.; Simula, L. Understanding the Central Role of Citrate in the Metabolism of Cancer Cells and Tumors: An Update. Int. J. Mol. Sci. 2021, 22, 6587. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, V.; Infantino, V. Citrate—New functions for an old metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Gray, K.; Pennington, C.J.; Edwards, D.R.; Riddick, A.C.; Ross, J.A.; Habib, F.K. Analysis of hypoxia-associated gene expression in prostate cancer: Lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol. Rep. 2008, 20, 1561–1567. [Google Scholar] [CrossRef]
- Reinicke, K.; Sotomayor, P.; Cisterna, P.; Delgado, C.; Nualart, F.; Godoy, A. Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue. J. Cell Biochem. 2012, 113, 553–562. [Google Scholar] [CrossRef]
- White, M.A.; Tsouko, E.; Lin, C.; Rajapakshe, K.; Spencer, J.M.; Wilkenfeld, S.R.; Vakili, S.S.; Pulliam, T.L.; Awad, D.; Nikolos, F.; et al. GLUT12 promotes prostate cancer cell growth and is regulated by androgens and CaMKK2 signaling. Endocr. Relat. Cancer 2018, 25, 453–469. [Google Scholar] [CrossRef]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef]
- Vaz, C.V.; Alves, M.G.; Marques, R.; Moreira, P.I.; Oliveira, P.F.; Maia, C.J.; Socorro, S. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int. J. Biochem. Cell Biol. 2012, 44, 2077–2084. [Google Scholar] [CrossRef]
- Sun, J.; Bok, R.A.; DeLos Santos, J.; Upadhyay, D.; DeLos Santos, R.; Agarwal, S.; Van Criekinge, M.; Vigneron, D.B.; Aggarwal, R.; Peehl, D.M.; et al. Resistance to Androgen Deprivation Leads to Altered Metabolism in Human and Murine Prostate Cancer Cell and Tumor Models. Metabolites 2021, 11, 139. [Google Scholar] [CrossRef]
- Fidelito, G.; Watt, M.J.; Taylor, R.A. Personalized Medicine for Prostate Cancer: Is Targeting Metabolism a Reality? Front. Oncol. 2021, 11, 778761. [Google Scholar] [CrossRef]
- Peitzsch, C.; Gorodetska, I.; Klusa, D.; Shi, Q.; Alves, T.C.; Pantel, K.; Dubrovska, A. Metabolic regulation of prostate cancer heterogeneity and plasticity. Semin. Cancer Biol. 2022, 82, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Centenera, M.M.; Scott, J.S.; Machiels, J.; Nassar, Z.D.; Miller, D.C.; Zinonos, I.; Dehairs, J.; Burvenich, I.J.G.; Zadra, G.; Chetta, P.M.; et al. ELOVL5 Is a Critical and Targetable Fatty Acid Elongase in Prostate Cancer. Cancer Res. 2021, 81, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Zhao, X.; Hao, S.; Li, F.; Wang, Y.; Liu, B.; Zhang, D.; Wang, Y.; Zhou, H. Key events in cancer: Dysregulation of SREBPs. Front. Pharmacol. 2023, 14, 1130747. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Ung, P.M.; Schlessinger, A. SLC Transporters: Structure, Function, and Drug Discovery. MedChemComm 2016, 7, 1069–1081. [Google Scholar] [CrossRef]
- Xu, L.; Yin, Y.; Li, Y.; Chen, X.; Chang, Y.; Zhang, H.; Liu, J.; Beasley, J.; McCaw, P.; Zhang, H.; et al. A glutaminase isoform switch drives therapeutic resistance and disease progression of prostate cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2012748118. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, J.H. MTHFR polymorphism and the risk of prostate cancer: A meta-analysis of case-control studies. Prostate Cancer Prostatic Dis. 2012, 15, 244–249. [Google Scholar] [CrossRef]
- Sreenath, T.L.; Dobi, A.; Petrovics, G.; Srivastava, S. Oncogenic activation of ERG: A predominant mechanism in prostate cancer. J. Carcinog. 2011, 10, 37. [Google Scholar] [CrossRef]
- Ta, T.; Chen, M.; Jiang, R.R.; Guan, H.; Huang, Y.Q.; Su, H.; Hu, Q.; Han, X.; Xiao, J. Involvement of EZH2 in aerobic glycolysis of prostate cancer through miR-181b/HK2 axis. Oncol. Rep. 2017, 37, 1430–1436. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, S. Metabolic reprogramming and cancer precision medicine: A narrative review. Precis. Cancer Med. 2021, 4, 35. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Gori, S.S.; Tsukamoto, T.; Rais, R.; Slusher, B.S. Clinical development of metabolic inhibitors for oncology. J. Clin. Investig. 2022, 132, e148550. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Manne, R.K.; Anas, M.; Penugurti, V.; Chen, T.; Pan, B.S.; Hsu, C.C.; Lin, H.K. Deregulated transcription factors in cancer cell metabolisms and reprogramming. Semin. Cancer Biol. 2022, 86, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur. J. Pharmacol. 2019, 859, 172513. [Google Scholar] [CrossRef]
- Flaig, T.W.; Salzmann-Sullivan, M.; Su, L.J.; Zhang, Z.; Joshi, M.; Gijón, M.A.; Kim, J.; Arcaroli, J.J.; Van Bokhoven, A.; Lucia, M.S.; et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 2017, 8, 56051–56065. [Google Scholar] [CrossRef]
- Iannelli, F.; Roca, M.S.; Lombardi, R.; Ciardiello, C.; Grumetti, L.; De Rienzo, S.; Moccia, T.; Vitagliano, C.; Sorice, A.; Costantini, S.; et al. Synergistic antitumor interaction of valproic acid and simvastatin sensitizes prostate cancer to docetaxel by targeting CSCs compartment via YAP inhibition. J. Exp. Clin. Cancer Res. 2020, 39, 213. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, J.; Zhang, X.; Yuan, J.; Shi, Y.; Qiao, L. Inhibition of lipogenesis and induction of apoptosis by valproic acid in prostate cancer cells via the C/EBPα/SREBP-1 pathway. Acta Biochim. Biophys. Sin. 2021, 53, 354–364. [Google Scholar] [CrossRef]
- Kortenhorst, M.S.; Isharwal, S.; van Diest, P.J.; Chowdhury, W.H.; Marlow, C.; Carducci, M.A.; Rodriguez, R.; Veltri, R.W. Valproic acid causes dose- and time-dependent changes in nuclear structure in prostate cancer cells in vitro and in vivo. Mol. Cancer Ther. 2009, 8, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Kortenhorst, M.S.; Wissing, M.D.; Rodríguez, R.; Kachhap, S.K.; Jans, J.J.; Van der Groep, P.; Verheul, H.M.; Gupta, A.; Aiyetan, P.O.; van der Wall, E.; et al. Analysis of the genomic response of human prostate cancer cells to histone deacetylase inhibitors. Epigenetics 2013, 8, 907–920. [Google Scholar] [CrossRef]
- Sidana, A.; Wang, M.; Shabbeer, S.; Chowdhury, W.H.; Netto, G.; Lupold, S.E.; Carducci, M.; Rodriguez, R. Mechanism of growth inhibition of prostate cancer xenografts by valproic acid. J. Biomed. Biotechnol. 2012, 2012, 180363. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.; Burfeind, P.; von Hardenberg, S.; Opitz, L.; Salinas-Riester, G.; Bremmer, F.; Schweyer, S.; Thelen, P.; Neesen, J.; Kaulfuss, S. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis 2013, 34, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Biancolella, M.; Amati, F.; Gravina, P.; Miano, R.; Chillemi, G.; Farcomeni, A.; Bueno, S.; Vespasiani, G.; Desideri, A.; et al. Valproic acid induces neuroendocrine differentiation and UGT2B7 up-regulation in human prostate carcinoma cell line. Drug Metab. Dispos. 2007, 35, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Feng, T.H.; Lin, Y.F.; Chang, P.L.; Juang, H.H. p53 downregulates the gene expression of mitochondrial aconitase in human prostate carcinoma cells. Prostate 2011, 71, 62–70. [Google Scholar] [CrossRef]
- Makhov, P.B.; Golovine, K.V.; Kutikov, A.; Canter, D.J.; Rybko, V.A.; Roshchin, D.A.; Matveev, V.B.; Uzzo, R.G.; Kolenko, V.M. Reversal of epigenetic silencing of AP-2alpha results in increased zinc uptake in DU-145 and LNCaP prostate cancer cells. Carcinogenesis 2011, 32, 1773–1781. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Menendez, P.; Hevia, D.; Rodriguez-Garcia, A.; Mayo, J.C.; Sainz, R.M. Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology 2014, 155, 3238–3250. [Google Scholar] [CrossRef]
- Chianese, U.; Papulino, C.; Ali, A.; Ciardiello, F.; Cappabianca, S.; Altucci, L.; Carafa, V.; Benedetti, R. FASN multi-omic characterization reveals metabolic heterogeneity in pancreatic and prostate adenocarcinoma. J. Transl. Med. 2023, 21, 32. [Google Scholar] [CrossRef]
- Heuer, T.S.; Ventura, R.; Mordec, K.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G. FASN Inhibition and Taxane Treatment Combine to Enhance Anti-tumor Efficacy in Diverse Xenograft Tumor Models through Disruption of Tubulin Palmitoylation and Microtubule Organization and FASN Inhibition-Mediated Effects on Oncogenic Signaling and Gene Expression. eBioMedicine 2017, 16, 51–62. [Google Scholar] [CrossRef]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 631–640. [Google Scholar] [CrossRef]
- Sadowski, M.C.; Pouwer, R.H.; Gunter, J.H.; Lubik, A.A.; Quinn, R.J.; Nelson, C.C. The fatty acid synthase inhibitor triclosan: Repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 2014, 5, 9362–9381. [Google Scholar] [CrossRef]
- Xin, M.; Qiao, Z.; Li, J.; Liu, J.; Song, S.; Zhao, X.; Miao, P.; Tang, T.; Wang, L.; Liu, W.; et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: Evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 2016, 7, 44252–44265. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; Organe, S.; Timmermans, L.; Scheys, K.; Peeters, A.; Brusselmans, K.; Verhoeven, G.; Swinnen, J.V. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007, 67, 8180–8187. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Mah, C.Y.; Machiels, J.; Vincent, A.D.; Irani, S.; Mutuku, S.M.; Spotbeen, X.; Bagadi, M.; Waltregny, D.; Moldovan, M.; et al. Lipidomic Profiling of Clinical Prostate Cancer Reveals Targetable Alterations in Membrane Lipid Composition. Cancer Res. 2021, 81, 4981–4993. [Google Scholar] [CrossRef] [PubMed]
- Fritz, V.; Benfodda, Z.; Rodier, G.; Henriquet, C.; Iborra, F.; Avancès, C.; Allory, Y.; de la Taille, A.; Culine, S.; Blancou, H.; et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther. 2010, 9, 1740–1754. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.T.; Hu, P.; Huang, W.C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Cascini, C.; Gallazzi, M.; Mortara, L.; Sessa, F.; Pelosi, G.; Albini, A.; Noonan, D.M. Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: Rationale for prevention and interception strategies. J. Exp. Clin. Cancer Res. 2019, 38, 464. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef]
- Kim, S.H.; Hahm, E.R.; Singh, K.B.; Shiva, S.; Stewart-Ornstein, J.; Singh, S.V. RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis 2020, 41, 778–789. [Google Scholar] [CrossRef]
- Zacharias, N.M.; McCullough, C.; Shanmugavelandy, S.; Lee, J.; Lee, Y.; Dutta, P.; McHenry, J.; Nguyen, L.; Norton, W.; Jones, L.W.; et al. Metabolic Differences in Glutamine Utilization Lead to Metabolic Vulnerabilities in Prostate Cancer. Sci. Rep. 2017, 7, 16159. [Google Scholar] [CrossRef]
- Qu, F.; Gu, Y.; Wang, Q.; He, M.; Zhou, F.; Sun, J.; Wang, G.; Peng, Y. Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells. Investig. New Drugs 2020, 38, 1292–1302. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Sabutskii, Y.E.; Khmelevskaya, E.A.; Krisp, C.; Kaune, M.; Venz, S.; Borisova, K.L.; Busenbender, T.; et al. Inspired by Sea Urchins: Warburg Effect Mediated Selectivity of Novel Synthetic Non-Glycoside 1,4-Naphthoquinone-6S-Glucose Conjugates in Prostate Cancer. Mar. Drugs 2020, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.F.; Ittmann, M.M. MiR-33a and statins collaboratively reduce the proliferative capacity of prostate cancer cells. Eur. Res. J. 2018, 4, 266–274. [Google Scholar] [CrossRef][Green Version]
- Shah, S.; Carriveau, W.J.; Li, J.; Campbell, S.L.; Kopinski, P.K.; Lim, H.W.; Daurio, N.; Trefely, S.; Won, K.J.; Wallace, D.C.; et al. Targeting ACLY sensitizes castration-resistant prostate cancer cells to AR antagonism by impinging on an ACLY-AMPK-AR feedback mechanism. Oncotarget 2016, 7, 43713–43730. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Z.D.; Mah, C.Y.; Centenera, M.M.; Irani, S.; Sadowski, M.C.; Scott, J.S.; Nguyen, E.V.; Nagarajan, S.R.; Moldovan, M.; Lynn, D.J.; et al. Fatty Acid Oxidation Is an Adaptive Survival Pathway Induced in Prostate Tumors by HSP90 Inhibition. Mol. Cancer Res. 2020, 18, 1500–1511. [Google Scholar] [CrossRef]
- Emberley, E.D.; Bennett, M.; Chen, J.; Gross, M.; Huang, T.; Makkouk, A.; Marguier, G.; Pan, A.; Spurlock, S.M.; Steggerda, S.; et al. Abstract 3509: The glutaminase inhibitor CB-839 synergizes with CDK4/6 and PARP inhibitors in pre-clinical tumor models. Cancer Res. 2018, 78, 3509. [Google Scholar] [CrossRef]
- Huang, X.Q.; Cao, J.S.; Zu, X.Y. Tumor-associated macrophages: An important player in breast cancer progression. Thorac. Cancer 2022, 13, 269–276. [Google Scholar] [CrossRef]
- Varshney, P.; Sharma, V.; Yadav, D.; Kumar, Y.; Singh, A.; Kagithala, N.R.; Sharma, P.K.; Porwal, O.; Fuloria, N.K.; Sharma, P.K.; et al. The Impacts and Changes Related to the Cancer Drug Resistance Mechanism. Curr. Drug Metab. 2023, 24, 787–802. [Google Scholar] [CrossRef]
- Martín-Martín, N.; Carracedo, A.; Torrano, V. Metabolism and Transcription in Cancer: Merging Two Classic Tales. Front. Cell Dev. Biol. 2017, 5, 119. [Google Scholar] [CrossRef]
- Coffey, K.; Blackburn, T.J.; Cook, S.; Golding, B.T.; Griffin, R.J.; Hardcastle, I.R.; Hewitt, L.; Huberman, K.; McNeill, H.V.; Newell, D.R.; et al. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS ONE 2012, 7, e45539. [Google Scholar] [CrossRef]
- Chen, L.; Ahmad, N.; Liu, X. Combining p53 stabilizers with metformin induces synergistic apoptosis through regulation of energy metabolism in castration-resistant prostate cancer. Cell Cycle 2016, 15, 840–849. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Zou, L.; Chen, W.; Yang, T.; Luo, J.; Wu, K.; Shu, F.; Tan, X.; Yang, Y.; Cen, S.; et al. Flubendazole, FDA-approved anthelmintic, elicits valid antitumor effects by targeting P53 and promoting ferroptosis in castration-resistant prostate cancer. Pharmacol. Res. 2021, 164, 105305. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Laurent, K.; Giuliano, S.; Larbret, F.; Ponzio, G.; Gounon, P.; Le Marchand-Brustel, Y.; Giorgetti-Peraldi, S.; Cormont, M.; Bertolotto, C.; et al. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010, 70, 2465–2475. [Google Scholar] [CrossRef]
- Kido, L.A.; Hahm, E.R.; Kim, S.H.; Baseggio, A.M.; Cagnon, V.H.A.; Singh, S.V.; Maróstica, M.R., Jr. Prevention of Prostate Cancer in Transgenic Adenocarcinoma of the Mouse Prostate Mice by Yellow Passion Fruit Extract and Antiproliferative Effects of Its Bioactive Compound Piceatannol. J. Cancer Prev. 2020, 25, 87–99. [Google Scholar] [CrossRef]
- Shangguan, X.; Ma, Z.; Yu, M.; Ding, J.; Xue, W.; Qi, J. Squalene Epoxidase Metabolic Dependency Is a Targetable Vulnerability in Castration-Resistant Prostate Cancer. Cancer Res. 2022, 82, 3032–3044. [Google Scholar] [CrossRef]
- Dong, X.M.; Chen, L.; Wu, P.; Cheng, L.H.; Wang, Y.; Yang, Y.; Zhang, Y.; Tang, W.Y.; Xie, T.; Zhou, J.L. Targeted metabolomics reveals PFKFB3 as a key target for elemene-mediated inhibition of glycolysis in prostate cancer cells. Phytomedicine 2024, 123, 155185. [Google Scholar] [CrossRef]
- Chappell, W.H.; Candido, S.; Abrams, S.L.; Akula, S.M.; Steelman, L.S.; Martelli, A.M.; Ratti, S.; Cocco, L.; Cervello, M.; Montalto, G.; et al. Influences of TP53 and the anti-aging DDR1 receptor in controlling Raf/MEK/ERK and PI3K/Akt expression and chemotherapeutic drug sensitivity in prostate cancer cell lines. Aging (Albany NY) 2020, 12, 10194–10210. [Google Scholar] [CrossRef]
- Vyas, A.R.; Moura, M.B.; Hahm, E.R.; Singh, K.B.; Singh, S.V. Sulforaphane Inhibits c-Myc-Mediated Prostate Cancer Stem-Like Traits. J. Cell Biochem. 2016, 117, 2482–2495. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.R.; Alumkal, J.J.; Foley, L.M.; Hitchens, T.K.; Shiva, S.S.; Parikh, R.A.; Jacobs, B.L.; Singh, S.V. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis 2019, 40, 1545–1556. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Huang, Y.; Chen, Y.; Luo, Z.; Huang, H.; West, R.E., 3rd; Nolin, T.D.; Wang, Z.; Li, S. Improved antitumor activity against prostate cancer via synergistic targeting of Myc and GFAT-1. Theranostics 2023, 13, 578–595. [Google Scholar] [CrossRef]
- Itkonen, H.M.; Gorad, S.S.; Duveau, D.Y.; Martin, S.E.; Barkovskaya, A.; Bathen, T.F.; Moestue, S.A.; Mills, I.G. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget 2016, 7, 12464–12476. [Google Scholar] [CrossRef]
- Barfeld, S.J.; Fazli, L.; Persson, M.; Marjavaara, L.; Urbanucci, A.; Kaukoniemi, K.M.; Rennie, P.S.; Ceder, Y.; Chabes, A.; Visakorpi, T.; et al. Myc-dependent purine biosynthesis affects nucleolar stress and therapy response in prostate cancer. Oncotarget 2015, 6, 12587–12602. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, P.; Du, W.; Xu, R.; Yan, G.; Deng, Y.; Li, X.; Chen, Y. Metabolically stable diphenylamine derivatives suppress androgen receptor and BET protein in prostate cancer. Biochem. Pharmacol. 2020, 177, 113946. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Alborzinia, H.; Khan, S.J.; Büttner, M.; Wölfl, S. 1,25(OH)2D3 disrupts glucose metabolism in prostate cancer cells leading to a truncation of the TCA cycle and inhibition of TXNIP expression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, J.; Wang, Z.; Cui, C.; Zhang, T.; Zhang, S.; Gao, P.; Hou, Z.; Liu, H.; Guo, J.; et al. Prostate-specific oncogene OTUD6A promotes prostatic tumorigenesis via deubiquitinating and stabilizing c-Myc. Cell Death Differ. 2022, 29, 1730–1743. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, A.K.; Woytash, J.; Inigo, J.R.; Gokhale, A.A.; Bshara, W.; Attwood, K.; Wang, J.; Spernyak, J.A.; Rath, E.; et al. A mitochondrial unfolded protein response inhibitor suppresses prostate cancer growth in mice via HSP60. J. Clin. Investig. 2022, 132, e149906. [Google Scholar] [CrossRef]
- Baker, L.C.; Boult, J.K.; Walker-Samuel, S.; Chung, Y.L.; Jamin, Y.; Ashcroft, M.; Robinson, S.P. The HIF-pathway inhibitor NSC-134754 induces metabolic changes and anti-tumour activity while maintaining vascular function. Br. J. Cancer 2012, 106, 1638–1647. [Google Scholar] [CrossRef]
- Qin, L.; Chung, Y.M.; Berk, M.; Naelitz, B.; Zhu, Z.; Klein, E.; Chakraborty, A.A.; Sharifi, N. Hypoxia-Reoxygenation Couples 3βHSD1 Enzyme and Cofactor Upregulation to Facilitate Androgen Biosynthesis and Hormone Therapy Resistance in Prostate Cancer. Cancer Res. 2022, 82, 2417–2430. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Vega, M.I.; León-Contreras, J.C.; Hernández-Pando, R.; Medina-Campos, O.N.; Rodríguez, E.; Tapia, E.; Pedraza-Chaverri, J. Sulforaphane induces differential modulation of mitochondrial biogenesis and dynamics in normal cells and tumor cells. Food Chem. Toxicol. 2017, 100, 90–102. [Google Scholar] [CrossRef]
- Singh, K.B.; Kim, S.H.; Hahm, E.R.; Pore, S.K.; Jacobs, B.L.; Singh, S.V. Prostate cancer chemoprevention by sulforaphane in a preclinical mouse model is associated with inhibition of fatty acid metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef]
- Chen, J.; Qin, P.; Tao, Z.; Ding, W.; Yao, Y.; Xu, W.; Yin, D.; Tan, S. Anticancer Activity of Methyl Protodioscin against Prostate Cancer by Modulation of Cholesterol-Associated MAPK Signaling Pathway via FOXO1 Induction. Biol. Pharm. Bull. 2023, 46, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Elix, C.C.; Salgia, M.M.; Otto-Duessel, M.; Copeland, B.T.; Yoo, C.; Lee, M.; Tew, B.Y.; Ann, D.; Pal, S.K.; Jones, J.O. Peroxisome proliferator-activated receptor gamma controls prostate cancer cell growth through AR-dependent and independent mechanisms. Prostate 2020, 80, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Tew, B.Y.; Hong, T.B.; Otto-Duessel, M.; Elix, C.; Castro, E.; He, M.; Wu, X.; Pal, S.K.; Kalkum, M.; Jones, J.O. Vitamin K epoxide reductase regulation of androgen receptor activity. Oncotarget 2017, 8, 13818–13831. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lu, Y.L.; Chen, H.Y.; Hu, M.L. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem. 2012, 23, 1155–1162. [Google Scholar] [CrossRef]
- Zadra, G.; Photopoulos, C.; Tyekucheva, S.; Heidari, P.; Weng, Q.P.; Fedele, G.; Liu, H.; Scaglia, N.; Priolo, C.; Sicinska, E.; et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol. Med. 2014, 6, 519–538. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Marcinko, K.; Tsakiridis, E.; Zacharidis, P.G.; Villani, L.; Lally, J.S.V.; Menjolian, G.; Maharaj, D.; Mathurin, T.; Smoke, M.; et al. Salicylate enhances the response of prostate cancer to radiotherapy. Prostate 2019, 79, 489–497. [Google Scholar] [CrossRef]
- Hao, Q.; Diaz, T.; Verduzco, A.D.R.; Magyar, C.E.; Zhong, J.; Elshimali, Y.; Rettig, M.B.; Henning, S.M.; Vadgama, J.V.; Wang, P. Arctigenin inhibits prostate tumor growth in high-fat diet fed mice through dual actions on adipose tissue and tumor. Sci. Rep. 2020, 10, 1403. [Google Scholar] [CrossRef]
- Guo, S.; Ma, B.; Jiang, X.; Li, X.; Jia, Y. Astragalus Polysaccharides Inhibits Tumorigenesis and Lipid Metabolism Through miR-138-5p/SIRT1/SREBP1 Pathway in Prostate Cancer. Front. Pharmacol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Li, S.; Wu, R.; Wang, L.; Dina Kuo, H.C.; Sargsyan, D.; Zheng, X.; Wang, Y.; Su, X.; Kong, A.N. Triterpenoid ursolic acid drives metabolic rewiring and epigenetic reprogramming in treatment/prevention of human prostate cancer. Mol. Carcinog. 2022, 61, 111–121. [Google Scholar] [CrossRef]
- Tan, K.N.; Avery, V.M.; Carrasco-Pozo, C. Metabolic Roles of Androgen Receptor and Tip60 in Androgen-Dependent Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 6622. [Google Scholar] [CrossRef]
- Liu, X.; Feng, C.; Liu, J.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Jiao, J.; Niu, Y. Androgen receptor and heat shock protein 27 co-regulate the malignant potential of molecular apocrine breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.E.; Ozanne, D.M.; Gaughan, L.; Waite, I.; Cook, S.; Neal, D.E.; Robson, C.N. Tip60 is a nuclear hormone receptor coactivator. J. Biol. Chem. 1999, 274, 17599–17604. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Liu, Y.; Zou, J.; Franklin, R.B. Mitochondrial aconitase gene expression is regulated by testosterone and prolactin in prostate epithelial cells. Prostate 2000, 42, 196–202. [Google Scholar] [CrossRef]
- Naftalin, R.J.; Afzal, I.; Cunningham, P.; Halai, M.; Ross, C.; Salleh, N.; Milligan, S.R. Interactions of androgens, green tea catechins and the antiandrogen flutamide with the external glucose-binding site of the human erythrocyte glucose transporter GLUT1. Br. J. Pharmacol. 2003, 140, 487–499. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Ulrix, W.; Heyns, W.; Verhoeven, G. Coordinate regulation of lipogenic gene expression by androgens: Evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12975–12980. [Google Scholar] [CrossRef]
- Heemers, H.; Maes, B.; Foufelle, F.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Androgens stimulate lipogenic gene expression in prostate cancer cells by activation of the sterol regulatory element-binding protein cleavage activating protein/sterol regulatory element-binding protein pathway. Mol. Endocrinol. 2001, 15, 1817–1828. [Google Scholar] [CrossRef]
- Tousignant, K.D.; Rockstroh, A.; Taherian Fard, A.; Lehman, M.L.; Wang, C.; McPherson, S.J.; Philp, L.K.; Bartonicek, N.; Dinger, M.E.; Nelson, C.C.; et al. Lipid Uptake Is an Androgen-Enhanced Lipid Supply Pathway Associated with Prostate Cancer Disease Progression and Bone Metastasis. Mol. Cancer Res. 2019, 17, 1166–1179. [Google Scholar] [CrossRef]
- Wang, Q.; Hardie, R.A.; Hoy, A.J.; van Geldermalsen, M.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef]
- Reisman, D.; Takahashi, P.; Polson, A.; Boggs, K. Transcriptional Regulation of the p53 Tumor Suppressor Gene in S-Phase of the Cell-Cycle and the Cellular Response to DNA Damage. Biochem. Res. Int. 2012, 2012, 808934. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Fu, Y.M.; Lin, H.; Liu, X.; Fang, W.; Meadows, G.G. Cell death of prostate cancer cells by specific amino acid restriction depends on alterations of glucose metabolism. J. Cell Physiol. 2010, 224, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chao, Z.; Wang, Q.; Zou, F.; Song, T.; Xu, L.; Ning, J.; Cheng, F. EXO1/P53/SREBP1 axis-regulated lipid metabolism promotes prostate cancer progression. J. Transl. Med. 2024, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.E.; Rahimi, S.; Zarandi, B.; Chegeni, R.; Safa, M. MYC: A multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J. Hematol. Oncol. 2021, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Dozmorov, M.G.; Hurst, R.E.; Culkin, D.J.; Kropp, B.P.; Frank, M.B.; Osban, J.; Penning, T.M.; Lin, H.K. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate 2009, 69, 1077–1090. [Google Scholar] [CrossRef]
- Qu, X.; Sun, J.; Zhang, Y.; Li, J.; Hu, J.; Li, K.; Gao, L.; Shen, L. c-Myc-driven glycolysis via TXNIP suppression is dependent on glutaminase-MondoA axis in prostate cancer. Biochem. Biophys. Res. Commun. 2018, 504, 415–421. [Google Scholar] [CrossRef]
- Khodayari Moez, E.; Pyne, S.; Dinu, I. Association between bivariate expression of key oncogenes and metabolic phenotypes of patients with prostate cancer. Comput. Biol. Med. 2018, 103, 55–63. [Google Scholar] [CrossRef]
- Valencia, T.; Kim, J.Y.; Abu-Baker, S.; Moscat-Pardos, J.; Ahn, C.S.; Reina-Campos, M.; Duran, A.; Castilla, E.A.; Metallo, C.M.; Diaz-Meco, M.T.; et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell 2014, 26, 121–135. [Google Scholar] [CrossRef]
- Ulz, P.; Belic, J.; Graf, R.; Auer, M.; Lafer, I.; Fischereder, K.; Webersinke, G.; Pummer, K.; Augustin, H.; Pichler, M.; et al. Whole-genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat. Commun. 2016, 7, 12008. [Google Scholar] [CrossRef]
- Crowell, P.D.; Giafaglione, J.M.; Jones, A.E.; Nunley, N.M.; Hashimoto, T.; Delcourt, A.M.L.; Petcherski, A.; Agrawal, R.; Bernard, M.J.; Diaz, J.A.; et al. MYC is a regulator of androgen receptor inhibition-induced metabolic requirements in prostate cancer. Cell Rep. 2023, 42, 113221. [Google Scholar] [CrossRef]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef]
- Priolo, C.; Pyne, S.; Rose, J.; Regan, E.R.; Zadra, G.; Photopoulos, C.; Cacciatore, S.; Schultz, D.; Scaglia, N.; McDunn, J.; et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res. 2014, 74, 7198–7204. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Hahm, E.-R.; Kim, S.-H.; Wendell, S.G.; Singh, S.V. A novel metabolic function of Myc in regulation of fatty acid synthesis in prostate cancer. Oncogene 2021, 40, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.F.; Liu, C.; Huang, T.; Liang, C.Z.; Fan, Y.D. Identifying the role of apolipoprotein A-I in prostate cancer. Asian J. Androl. 2021, 23, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, Q.; Hicks, J.L.; Trabzonlu, L.; Ozbek, B.; Jones, T.; Vaghasia, A.; Larman, T.C.; Wang, R.; Markowski, M.C.; et al. MYC-driven increases in mitochondrial DNA copy number occur early and persist throughout prostatic cancer progression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Holubova, M.; Axmanova, M.; Gumulec, J.; Raudenska, M.; Sztalmachova, M.; Babula, P.; Adam, V.; Kizek, R.; Masarik, M. KRAS NF-κB is involved in the development of zinc resistance and reduced curability in prostate cancer. Metallomics 2014, 6, 1240–1253. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Zinc supplement use and risk of aggressive prostate cancer: A 30-year follow-up study. Eur. J. Epidemiol. 2022, 37, 1251–1260. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, K. Epigenetic regulation of prostate cancer: The theories and the clinical implications. Asian J. Androl. 2019, 21, 279–290. [Google Scholar] [CrossRef]
- Chen, M.K.; Xiao, Z.Y.; Huang, Z.P.; Xue, K.Y.; Xia, H.; Zhou, J.W.; Liao, D.Y.; Liang, Z.J.; Xie, X.; Wei, Q.Z.; et al. Glycine Decarboxylase (GLDC) Plays a Crucial Role in Regulating Energy Metabolism, Invasion, Metastasis and Immune Escape for Prostate Cancer. Int. J. Biol. Sci. 2023, 19, 4726–4743. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Qian, Y.C.; Yang, W.J.; Ye, L.H.; Guo, G.D.; Lv, W.; Huan, M.X.; Feng, X.Y.; Wang, K.; Yang, Z.; et al. CHD1 deletion stabilizes HIF1α to promote angiogenesis and glycolysis in prostate cancer. Asian J. Androl. 2023, 25, 152–157. [Google Scholar] [CrossRef]
- Ragnum, H.B.; Røe, K.; Holm, R.; Vlatkovic, L.; Nesland, J.M.; Aarnes, E.K.; Ree, A.H.; Flatmark, K.; Seierstad, T.; Lilleby, W.; et al. Hypoxia-independent downregulation of hypoxia-inducible factor 1 targets by androgen deprivation therapy in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, Q.; Peng, F.; Wang, J.; Zhao, W.; Guo, G. Expression and Clinical Significance of HKII and HIF-1α in Grade Groups of Prostate Cancer. Front. Genet. 2021, 12, 680928. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Zheng, X.; Xie, X.; Chen, Y.; Li, Y.; He, W. MMP11 and MMP14 contribute to the interaction between castration-resistant prostate cancer and adipocytes. Am. J. Cancer Res. 2023, 13, 5934–5949. [Google Scholar] [PubMed]
- Laurent, V.; Toulet, A.; Attané, C.; Milhas, D.; Dauvillier, S.; Zaidi, F.; Clement, E.; Cinato, M.; Le Gonidec, S.; Guérard, A.; et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. 2019, 17, 821–835. [Google Scholar] [CrossRef]
- Palayoor, S.T.; Mitchell, J.B.; Cerna, D.; Degraff, W.; John-Aryankalayil, M.; Coleman, C.N. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int. J. Cancer 2008, 123, 2430–2437. [Google Scholar] [CrossRef]
- Geng, H.; Ko, H.K.; Pittsenbarger, J.; Harvey, C.T.; Xue, C.; Liu, Q.; Wiens, S.; Kachhap, S.K.; Beer, T.M.; Qian, D.Z. HIF1 and ID1 Interplay Confers Adaptive Survival to HIF1α-Inhibition. Front. Cell Dev. Biol. 2021, 9, 724059. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, F.; Meng, D.; Zhang, X.; Feng, Y.; Yin, G.; Liang, P.; Chen, S.; Liu, H. Mechanism of Action and Related Natural Regulators of Nrf2 in Nonalcoholic Fatty Liver Disease. Curr. Drug Deliv. 2024, 21, 1300–1319. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, J.-H. Recent advances in understanding Nrf2 agonism and its potential clinical application to metabolic and inflammatory diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Chen, L.; Wu, Y.; Li, Y. NRF2 in age-related musculoskeletal diseases: Role and treatment prospects. Genes Dis. 2024, 11, 101180. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of NRF2/KEAP1 Signaling by Phytotherapeutics in Periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, W.D.; Wang, H. Friend or foe: Xenobiotic activation of Nrf2 in disease control and cardioprotection. Pharm. Res. 2021, 38, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.Q.; Bai, Y.; Gu, J.; Fan, B.Y. Stanniocalcin1 knockdown induces ferroptosis and suppresses glycolysis in prostate cancer via the Nrf2 pathway. Neoplasma 2022, 69, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Beaver, L.M.; Kuintzle, R.; Buchanan, A.; Wiley, M.W.; Glasser, S.T.; Wong, C.P.; Johnson, G.S.; Chang, J.H.; Löhr, C.V.; Williams, D.E.; et al. Long noncoding RNAs and sulforaphane: A target for chemoprevention and suppression of prostate cancer. J. Nutr. Biochem. 2017, 42, 72–83. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Goudarzi, M.; Berk, M.; Chung, Y.M.; Zhang, R.; Li, J.; Klein, E.; Sharifi, N. 5-Hydroxyeicosatetraenoic Acid Controls Androgen Reduction in Diverse Types of Human Epithelial Cells. Endocrinology 2022, 164, bqac191. [Google Scholar] [CrossRef]
- Hartley, A.; Ahmad, I. The role of PPARγ in prostate cancer development and progression. Br. J. Cancer 2023, 128, 940–945. [Google Scholar] [CrossRef]
- Poutanen, M.; Hagberg Thulin, M.; Härkönen, P. Targeting sex steroid biosynthesis for breast and prostate cancer therapy. Nat. Rev. Cancer 2023, 23, 686–709. [Google Scholar] [CrossRef]
- Song, X.; Yan, G.; Wang, H.; Lou, D. Septin 4 activates PPARγ/LXRα signaling by upregulating ABCA1 and ABCG1 expression to inhibit the formation of THP-1 macrophage-derived foam cells. Exp. Ther. Med. 2021, 22, 763. [Google Scholar] [CrossRef]
- Akinyeke, T.O.; Stewart, L.V. Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism. Cancer Biol. Ther. 2011, 11, 1046–1058. [Google Scholar] [CrossRef]
- Bolden, A.; Bernard, L.; Jones, D.; Akinyeke, T.; Stewart, L.V. The PPAR Gamma Agonist Troglitazone Regulates Erk 1/2 Phosphorylation via a PPARγ-Independent, MEK-Dependent Pathway in Human Prostate Cancer Cells. PPAR Res. 2012, 2012, 929052. [Google Scholar] [CrossRef]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N.; Anto, R.J. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, J.B.; Shi, Y.; Han, J.J.; Tsouko, E.; White, M.A.; Burns, A.R.; Zhang, A.; Xia, X.; Ilkayeva, O.R.; Xin, L.; et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch. Oncogene 2014, 33, 5251–5261. [Google Scholar] [CrossRef] [PubMed]
- Zingales, V.; Distefano, A.; Raffaele, M.; Zanghi, A.; Barbagallo, I.; Vanella, L. Metformin: A Bridge between Diabetes and Prostate Cancer. Front. Oncol. 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 8540. [Google Scholar] [CrossRef]
- Yenmis, G.; Sarac, E.Y.; Besli, N.; Soydas, T.; Tastan, C.; Kancagi, D.D.; Yilanci, M.; Senol, K.; Karagulle, O.O.; Ekmekci, C.G. Anti-cancer effect of metformin on the metastasis and invasion of primary breast cancer cells through mediating NF-kB activity. Acta Histochem. 2021, 123, 151709. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Zhou, J.-R.; Parhar, I. Metformin in colorectal cancer: Molecular mechanism, preclinical and clinical aspects. J. Exp. Clin. Cancer Res. 2019, 38, 491. [Google Scholar] [CrossRef]
- Henderson, D.; Frieson, D.; Zuber, J.; Solomon, S.S. Metformin has positive therapeutic effects in colon cancer and lung cancer. Am. J. Med. Sci. 2017, 354, 246–251. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Wu, Y.; Shi, K.; Bing, L.; Hao, J. Wnt/β-catenin signaling pathway upregulates c-Myc expression to promote cell proliferation of P19 teratocarcinoma cells. Anat. Rec. 2012, 295, 2104–2113. [Google Scholar] [CrossRef]
- Huang, L.; Liu, D.; Wang, N.; Ling, S.; Tang, Y.; Wu, J.; Hao, L.; Luo, H.; Hu, X.; Sheng, L.; et al. Integrated genomic analysis identifies deregulated JAK/STAT-MYC-biosynthesis axis in aggressive NK-cell leukemia. Cell Res. 2018, 28, 172–186. [Google Scholar] [CrossRef]
- Xie, K.; Tan, K.; Naylor, M.J. Transcription Factors as Novel Therapeutic Targets and Drivers of Prostate Cancer Progression. Front. Oncol. 2022, 12, 854151. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.H. Targeting Transcription Factors for Cancer Treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.S.; Tambwe, N.; Mahfouz, D.H.; Wium, M.; Cacciatore, S.; Paccez, J.D.; Zerbini, L.F. Transcription Factors in Prostate Cancer: Insights for Disease Development and Diagnostic and Therapeutic Approaches. Genes 2024, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Gach-Janczak, K.; Drogosz-Stachowicz, J.; Janecka, A.; Wtorek, K.; Mirowski, M. Historical Perspective and Current Trends in Anticancer Drug Development. Cancers 2024, 16, 1878. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Pop, M.S.; Stransky, N.; Garvie, C.W.; Theurillat, J.P.; Hartman, E.C.; Lewis, T.A.; Zhong, C.; Culyba, E.K.; Lin, F.; Daniels, D.S.; et al. A small molecule that binds and inhibits the ETV1 transcription factor oncoprotein. Mol. Cancer Ther. 2014, 13, 1492–1502. [Google Scholar] [CrossRef]
- Rahim, S.; Beauchamp, E.M.; Kong, Y.; Brown, M.L.; Toretsky, J.A.; Üren, A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PLoS ONE 2011, 6, e19343. [Google Scholar] [CrossRef]
- Winters, B.; Brown, L.; Coleman, I.; Nguyen, H.; Minas, T.Z.; Kollath, L.; Vasioukhin, V.; Nelson, P.; Corey, E.; Üren, A.; et al. Inhibition of ERG Activity in Patient-derived Prostate Cancer Xenografts by YK-4-279. Anticancer Res. 2017, 37, 3385–3396. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Lomenick, B.; Shi, H.; Huang, J.; Chen, C. Identification and characterization of β-sitosterol target proteins. Bioorg. Med. Chem. Lett. 2015, 25, 4976–4979. [Google Scholar] [CrossRef]
- Ullah, M.F.; Usmani, S.; Shah, A.; Abuduhier, F.M. Dietary molecules and experimental evidence of epigenetic influence in cancer chemoprevention: An insight. Semin. Cancer Biol. 2022, 83, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kwon, H.-Y.; Sohn, E.J.; Kim, K.A.; Kim, B.; Jeong, S.-J.; Song, J.h.; Koo, J.S.; Kim, S.-H. Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol. Rep. 2013, 65, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.T.; Um, J.Y.; Chinnathambi, A.; Alharbi, S.A.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Ahn, K.S. Evodiamine Mitigates Cellular Growth and Promotes Apoptosis by Targeting the c-Met Pathway in Prostate Cancer Cells. Molecules 2020, 25, 1320. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, X.; Liang, C.; Zhang, P. Evodiamine impairs HIF1A histone lactylation to inhibit Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in prostate cancer. Eur. J. Pharmacol. 2023, 957, 176007. [Google Scholar] [CrossRef]
- Harper, C.E.; Cook, L.M.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Arabshahi, A.; Shirai, T.; Lamartiniere, C.A. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate 2009, 69, 1668–1682. [Google Scholar] [CrossRef]
- Fang, F.; Chen, S.; Ma, J.; Cui, J.; Li, Q.; Meng, G.; Wang, L. Juglone suppresses epithelial-mesenchymal transition in prostate cancer cells via the protein kinase B/glycogen synthase kinase-3β/Snail signaling pathway. Oncol. Lett. 2018, 16, 2579–2584. [Google Scholar] [CrossRef]
- Karakurt, S.; Adali, O. Tannic Acid Inhibits Proliferation, Migration, Invasion of Prostate Cancer and Modulates Drug Metabolizing and Antioxidant Enzymes. Anti-Cancer Agents Med. Chem. 2016, 16, 781–789. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.; Blaheta, R.A. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef]
- Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Freire-Garabal, M. Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. Int. J. Mol. Sci. 2019, 20, 4977. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Rodriguez, T.; Avery, V.M. The Molecular Effects of Sulforaphane and Capsaicin on Metabolism upon Androgen and Tip60 Activation of Androgen Receptor. Int. J. Mol. Sci. 2019, 20, 5384. [Google Scholar] [CrossRef]
- Beaver, L.M.; Löhr, C.V.; Clarke, J.D.; Glasser, S.T.; Watson, G.W.; Wong, C.P.; Zhang, Z.; Williams, D.E.; Dashwood, R.H.; Shannon, J.; et al. Broccoli Sprouts Delay Prostate Cancer Formation and Decrease Prostate Cancer Severity with a Concurrent Decrease in HDAC3 Protein Expression in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Mice. Curr. Dev. Nutr. 2018, 2, nzy002. [Google Scholar] [CrossRef] [PubMed]
- Temre, M.K.; Kumar, A.; Singh, S.M. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors. Front. Pharmacol. 2022, 13, 1035510. [Google Scholar] [CrossRef] [PubMed]
- Chudhary, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Hamrouni, L. Chapter 49—Walnut. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 671–684. [Google Scholar]
- Hu, C.; Xu, H.Y.; Li, Z.H.; Liu, D.D.; Zhang, S.Q.; Fang, F.; Wang, L.G. Juglone promotes antitumor activity against prostate cancer via suppressing glycolysis and oxidative phosphorylation. Phytother. Res. 2023, 37, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Gu, X.; Jia, R.; Ge, S.; Chai, P.; Zhuang, A.; Fan, X. Crosstalk between metabolic reprogramming and epigenetics in cancer: Updates on mechanisms and therapeutic opportunities. Cancer Commun. 2022, 42, 1049–1082. [Google Scholar] [CrossRef]
- Lee, J.V.; Carrer, A.; Shah, S.; Snyder, N.W.; Wei, S.; Venneti, S.; Worth, A.J.; Yuan, Z.F.; Lim, H.W.; Liu, S.; et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014, 20, 306–319. [Google Scholar] [CrossRef]
- Zhao, S.; Torres, A.; Henry, R.A.; Trefely, S.; Wallace, M.; Lee, J.V.; Carrer, A.; Sengupta, A.; Campbell, S.L.; Kuo, Y.M.; et al. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep. 2016, 17, 1037–1052. [Google Scholar] [CrossRef]
- Chen, J.; Guccini, I.; Di Mitri, D.; Brina, D.; Revandkar, A.; Sarti, M.; Pasquini, E.; Alajati, A.; Pinton, S.; Losa, M.; et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat. Genet. 2018, 50, 219–228. [Google Scholar] [CrossRef]
- Fan, J.P.; Kim, H.S.; Han, G.D. Induction of apoptosis by l-carnitine through regulation of two main pathways in Hepa1c1c 7 cells. Amino Acids 2008, 36, 365. [Google Scholar] [CrossRef]
- Jayaraman, A.; Kumar, P.; Marin, S.; de Atauri, P.; Mateo, F.; Thomson, T.M.; Centelles, J.J.; Graham, S.F.; Cascante, M. Untargeted metabolomics reveals distinct metabolic reprogramming in endothelial cells co-cultured with CSC and non-CSC prostate cancer cell subpopulations. PLoS ONE 2018, 13, e0192175. [Google Scholar] [CrossRef]
- Nasca, C.; Bigio, B.; Lee, F.S.; Young, S.P.; Kautz, M.M.; Albright, A.; Beasley, J.; Millington, D.S.; Mathé, A.A.; Kocsis, J.H.; et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc. Natl. Acad. Sci. USA 2018, 115, 8627–8632. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Linares, J.F.; Duran, A.; Cordes, T.; L’Hermitte, A.; Badur, M.G.; Bhangoo, M.S.; Thorson, P.K.; Richards, A.; Rooslid, T.; et al. Increased Serine and One-Carbon Pathway Metabolism by PKCλ/ι Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell 2019, 35, 385–400.e9. [Google Scholar] [CrossRef] [PubMed]
- Stielow, B.; Zhou, Y.; Cao, Y.; Simon, C.; Pogoda, H.M.; Jiang, J.; Ren, Y.; Phanor, S.K.; Rohner, I.; Nist, A.; et al. The SAM domain-containing protein 1 (SAMD1) acts as a repressive chromatin regulator at unmethylated CpG islands. Sci. Adv. 2021, 7, eabf2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Sarwar, M.S.; Chou, P.; Wang, Y.; Su, X.; Kong, A.T. PTEN-knockout regulates metabolic rewiring and epigenetic reprogramming in prostate cancer and chemoprevention by triterpenoid ursolic acid. FASEB J. 2022, 36, e22626. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Z.; Yu, Y.; Zhang, P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef]
- Yang, C.S.; Fang, M.; Lambert, J.D.; Yan, P.; Huang, T.H. Reversal of hypermethylation and reactivation of genes by dietary polyphenolic compounds. Nutr. Rev. 2008, 66 (Suppl. S1), S18–S20. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, H.; Kanesaka, M.; Imamura, Y.; Sakamoto, S.; Ichikawa, T.; Kaneda, A. Epigenetic modifications in prostate cancer. Int. J. Urol. 2021, 28, 140–149. [Google Scholar] [CrossRef]
- Ngum, J.A.; Tatang, F.J.; Toumeni, M.H.; Nguengo, S.N.; Simo, U.S.F.; Mezajou, C.F.; Kameni, C.; Ngongang, N.N.; Tchinda, M.F.; Dongho Dongmo, F.F.; et al. An overview of natural products that modulate the expression of non-coding RNAs involved in oxidative stress and inflammation-associated disorders. Front. Pharmacol. 2023, 14, 1144836. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Imai-Sumida, M.; Majid, S.; Dasgupta, P.; Kulkarni, P.; Saini, S.; Bhagirath, D.; Kato, T.; Maekawa, S.; Hashimoto, Y.; Shiina, M.; et al. Abstract 3449: Genistein inhibits renal cancer progression through long non-coding RNA HOTAIR suppression. Cancer Res. 2017, 77, 3449. [Google Scholar] [CrossRef]
- Hung, C.L.; Wang, L.Y.; Yu, Y.L.; Chen, H.W.; Srivastava, S.; Petrovics, G.; Kung, H.J. A long noncoding RNA connects c-Myc to tumor metabolism. Proc. Natl. Acad. Sci. USA 2014, 111, 18697–18702. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, M.; Toney, N.J.; Donahue, R.N.; Wroblewski, S.; Zibelman, M.; Ghatalia, P.; Ross, E.A.; Karzai, F.; Madan, R.A.; Dahut, W.L.; et al. A randomized phase 2 study of bicalutamide with or without metformin for biochemical recurrence in overweight or obese prostate cancer patients (BIMET-1). Prostate Cancer Prostatic Dis. 2022, 25, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Pujalte Martin, M.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B.; et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Alghandour, R.; Ebrahim, M.A.; Elshal, A.M.; Ghobrial, F.; Elzaafarany, M.; MA, E.L. Repurposing metformin as anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED). Urol. Oncol. 2021, 39, 831.e1–831.e10. [Google Scholar] [CrossRef]
- Mahalingam, D.; Hanni, S.; Serritella, A.V.; Fountzilas, C.; Michalek, J.; Hernandez, B.; Sarantopoulos, J.; Datta, P.; Romero, O.; Pillai, S.M.A.; et al. Utilizing metformin to prevent metabolic syndrome due to androgen deprivation therapy (ADT): A randomized phase II study of metformin in non-diabetic men initiating ADT for advanced prostate cancer. Oncotarget 2023, 14, 622–636. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Davis, E.W., 2nd; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868. [Google Scholar]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003, 92, 375–378; discussion 378. [Google Scholar] [CrossRef]
- Clark, P.E.; Hall, M.C.; Borden, L.S., Jr.; Miller, A.A.; Hu, J.J.; Lee, W.R.; Stindt, D.; D’Agostino, R., Jr.; Lovato, J.; Harmon, M.; et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006, 67, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, C.; Ubrig, B.; Thürmann, P.; Eggersmann, C.; Roth, S. Lycopene for advanced hormone refractory prostate cancer: A prospective, open phase II pilot study. J. Urol. 2009, 181, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Gann, P.H.; Deaton, R.J.; Rueter, E.E.; van Breemen, R.B.; Nonn, L.; Macias, V.; Han, M.; Ananthanarayanan, V. A Phase II Randomized Trial of Lycopene-Rich Tomato Extract Among Men with High-Grade Prostatic Intraepithelial Neoplasia. Nutr. Cancer 2015, 67, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, E.; Uchio, E.; Lilly, M.; Zi, X.; Fruehauf, J.P. A phase II study of docetaxel plus lycopene in metastatic castrate resistant prostate cancer. Biomed. Pharmacother. 2021, 143, 112226. [Google Scholar] [CrossRef]
- Gross, C.; Stamey, T.; Hancock, S.; Feldman, D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J. Urol. 1998, 159, 2035–2039; discussion 2039–2040. [Google Scholar] [CrossRef]
- Beer, T.M.; Eilers, K.M.; Garzotto, M.; Egorin, M.J.; Lowe, B.A.; Henner, W.D. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J. Clin. Oncol. 2003, 21, 123–128. [Google Scholar] [CrossRef]
- Beer, T.M.; Garzotto, M.; Katovic, N.M. High-dose calcitriol and carboplatin in metastatic androgen-independent prostate cancer. Am. J. Clin. Oncol. 2004, 27, 535–541. [Google Scholar] [CrossRef]
- Trump, D.L.; Potter, D.M.; Muindi, J.; Brufsky, A.; Johnson, C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006, 106, 2136–2142. [Google Scholar] [CrossRef]
- Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507. [Google Scholar] [CrossRef]
- deVere White, R.W.; Hackman, R.M.; Soares, S.E.; Beckett, L.A.; Li, Y.; Sun, B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology 2004, 63, 259–263. [Google Scholar] [CrossRef]
- Xu, L.; Ding, Y.; Catalona, W.J.; Yang, X.J.; Anderson, W.F.; Jovanovic, B.; Wellman, K.; Killmer, J.; Huang, X.; Scheidt, K.A.; et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009, 101, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W.; et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, P.T.; Murtola, T.J.; Talala, K.; Taari, K.; Tammela, T.L.; Auvinen, A. Warfarin use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scand. J. Urol. 2016, 50, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.U.; Haq, I.U.; Bungau, S. An Extensive Pharmacological Evaluation of New Anti-Cancer Triterpenoid (Nummularic Acid) from Ipomoea batatas through In Vitro, In Silico, and In Vivo Studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef]
- Majid, M.; Farhan, A.; Baig, M.W.; Khan, M.T.; Kamal, Y.; Hassan, S.S.U.; Bungau, S.; Haq, I.U. Ameliorative Effect of Structurally Divergent Oleanane Triterpenoid, 3-Epifriedelinol from Ipomoea batatas against BPA-Induced Gonadotoxicity by Targeting PARP and NF-κB Signaling in Rats. Molecules 2022, 28, 290. [Google Scholar] [CrossRef]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-isothiocyanate conjugate suppresses growth of prostate cancer cells irrespective of androgen receptor status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef]
- Mathes, A.; Duman, M.B.; Neumann, A.; Dobreva, G.; Schmidt, T. S-adenosylmethionine treatment affects histone methylation in prostate cancer cells. Gene 2024, 893, 147915. [Google Scholar] [CrossRef]
- Schmidt, T. S-Adenosylmethionine affects ERK1/2 and STAT3 pathway in androgen-independent prostate cancer cells. Mol. Biol. Rep. 2022, 49, 4805–4817. [Google Scholar] [CrossRef]
- Diaz, J.E.; Ahsen, M.E.; Schaffter, T.; Chen, X.; Realubit, R.B.; Karan, C.; Califano, A.; Losic, B.; Stolovitzky, G. The transcriptomic response of cells to a drug combination is more than the sum of the responses to the monotherapies. eLife 2020, 9, e52707. [Google Scholar] [CrossRef]
- Matuszczak, M.; Schalken, J.A.; Salagierski, M. Prostate Cancer Liquid Biopsy Biomarkers’ Clinical Utility in Diagnosis and Prognosis. Cancers 2021, 13, 3373. [Google Scholar] [CrossRef] [PubMed]
- Pun, F.W.; Ozerov, I.V.; Zhavoronkov, A. AI-powered therapeutic target discovery. Trends Pharmacol. Sci. 2023, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Turanli, B.; Grøtli, M.; Boren, J.; Nielsen, J.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Drug Repositioning for Effective Prostate Cancer Treatment. Front. Physiol. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Marín de Mas, I.; Aguilar, E.; Zodda, E.; Balcells, C.; Marin, S.; Dallmann, G.; Thomson, T.M.; Papp, B.; Cascante, M. Model-driven discovery of long-chain fatty acid metabolic reprogramming in heterogeneous prostate cancer cells. PLoS Comput. Biol. 2018, 14, e1005914. [Google Scholar] [CrossRef]
- Turanli, B.; Zhang, C.; Kim, W.; Benfeitas, R.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Discovery of therapeutic agents for prostate cancer using genome-scale metabolic modeling and drug repositioning. eBioMedicine 2019, 42, 386–396. [Google Scholar] [CrossRef]
- Eidelman, E.; Twum-Ampofo, J.; Ansari, J.; Siddiqui, M.M. The Metabolic Phenotype of Prostate Cancer. Front. Oncol. 2017, 7, 131. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
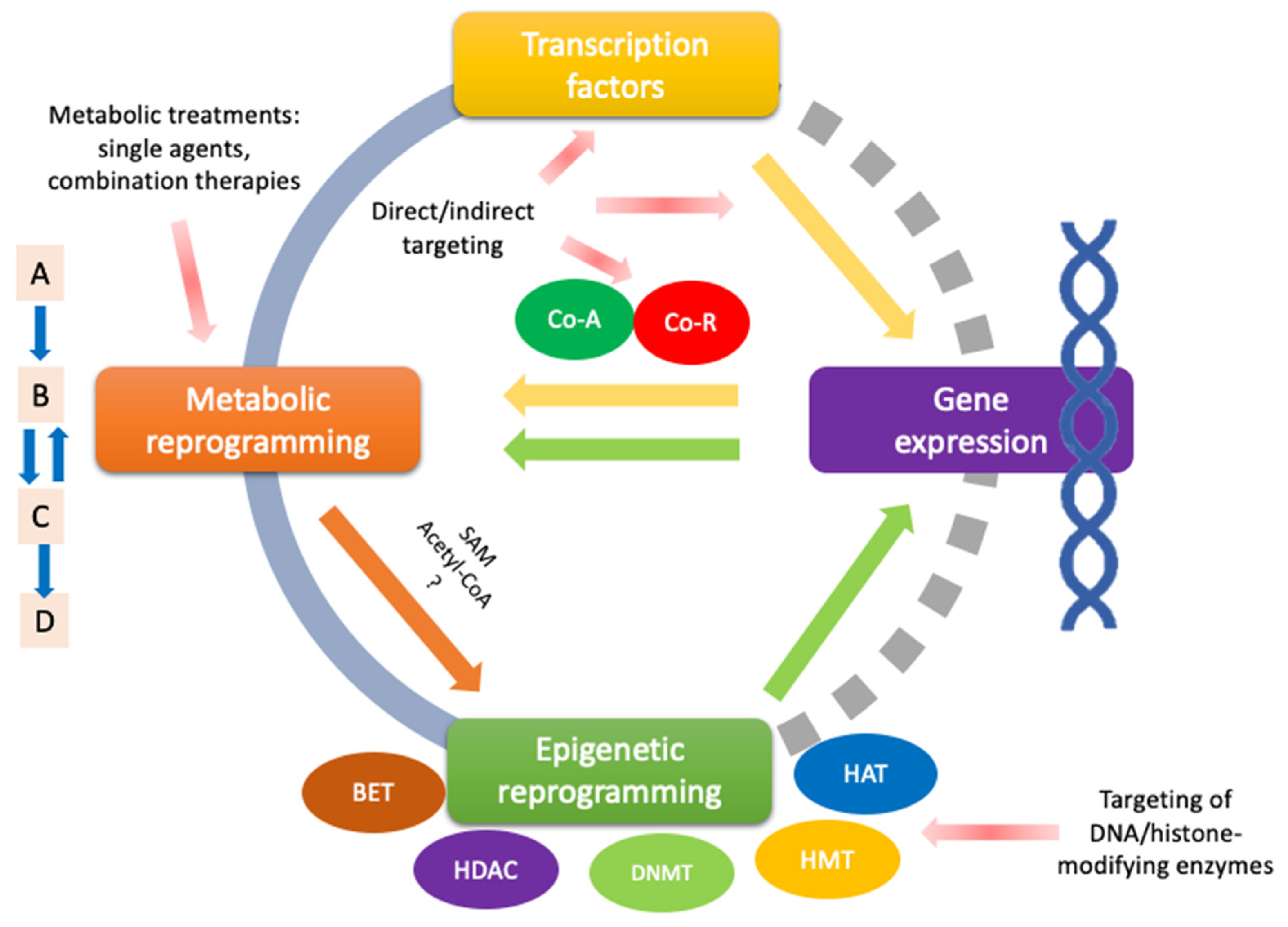
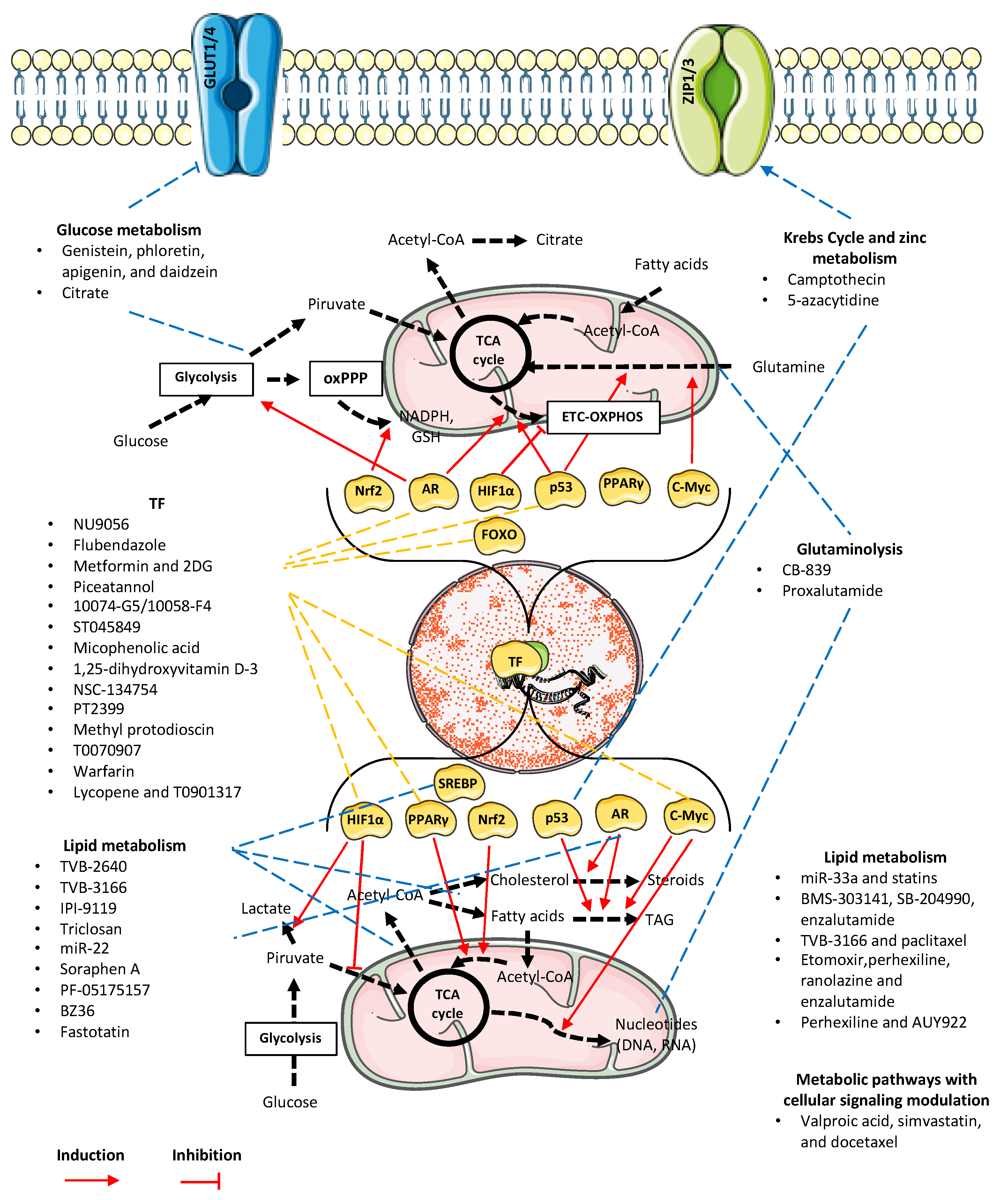
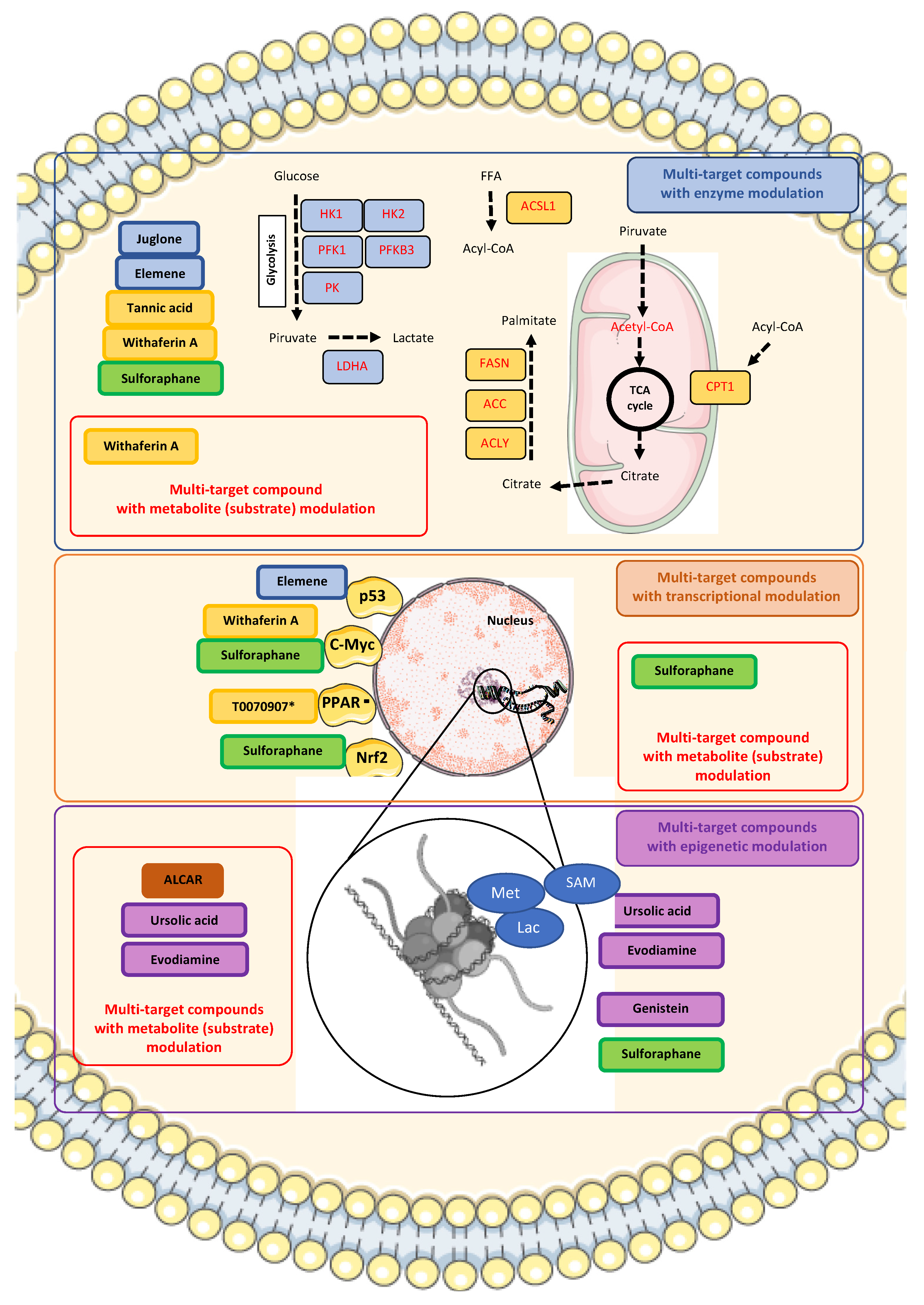
| Transcription Factor/Signaling Pathway or Metabolic Regulator/Substrate for Chromatin Modification | Type of Intervention | Main Results | Research Phase | Reference |
|---|---|---|---|---|
| AR | 1,2-bis(isothiazol-5-yl) disulfane, or NU9056/single treatment | Decreased expression of AR and PSA in PCa cells. | In vitro | [79] |
| p53 | Tenovin-1 and BI2536 in combo with metformin/combined treatment | Enhanced inhibition of OXPHOS in PCa cells with inactivated p53 WT. Chemosensitization, particularly in C4-2 cells (CRPC). | In vitro | [80] |
| Flubendazole/single treatment | Upregulation of p53 and increased ferroptosis CRPC. Dysregulation of SLC7A11. | In vitro | [81] | |
| Metformin and 2DG/combined treatment | Increased p53-regulated apoptosis via AMPK. | In vitro | [82] | |
| Piceatannol/single treatment | No modulation of glucose metabolism, but p53 upregulation. | In vitro, In vivo | [83] | |
| FR194738/single treatment | Inhibition of SQLE with FR194738 alters CRPC program. | In vitro | [84] | |
| Beta-elemene from Curcuma wenyujin/single treatment | Glycolysis inhibition in PCa cells by downregulating PFKFB3 expression. Increased p53 and FZR1 expression. | In vitro, In vivo | [85] | |
| Biomarker focalized study/promissory target: DDR1 | Restoration of p53 and DDR1 transcriptomic activity enhances the response to signals that “reverse” the cancerous phenotype in prostate cancer. | In vitro | [86] | |
| MYC | Sulforaphane/single treatment | Suppression of c-Myc expression and stem-like characteristics in PCa. | In vitro, In vivo | [87] |
| Suppression of c-Myc metabolic activity, lowered expression of HK2, PK2M2 and or LDHA, in models of c-Myc overexpression without affecting plasma lactate levels measured in plasma. | In vitro, In vivo | [88] | ||
| 10074-G5/10058-F4 and 6-diazo-5-oxo-L-norleucine/combined treatment | Induction of GFAT1 by MYC inhibitors, with additional GFAT1 inhibition with 6-diazo-5-oxo-L-norleucine showed synergistic effects by inhibiting proliferation of PCa. | In vitro, In vivo | [89] | |
| ST045849 and alanine aminotransferase inhibitors/combined treatment | OGT and GPT2 inhibitors decrease cell viability and growth of PCa. OGT inhibition leads to c-Myc loss. | In vitro | [90] | |
| Mycophenolic acid/combined treatment | IMPDH inhibition, combined with antiandrogens, decreases GTP and stabilizes p53. | In vitro | [91] | |
| Diphenylamines derivatives, compound 7d/single treatment | Compound 7d terminates coregulatory function of BET and AR domain, transactivation of wild-type AR, enzalutamide resistance mutants, downregulation of c-Myc expression by BET inhibition. Terminates VCaP cells with AR-V7 variant in vivo. | In vitro, In vivo | [92] | |
| 1,25-dihydroxyvitamin D-3/single treatment | Low glycolysis, dysregulated TCA, low c-Myc expression. | In vitro | [93] | |
| Biomarker focalized study/promissory target: OTUD6A | OTUD6A acts as a physiological deubiquitinase for c-Myc, stabilizing it. | In vitro, In vivo | [94] | |
| Biomarker focalized study/promissory target: HSP90-CIpP | HSP60 regulates CIpP expression via c-Myc, restoring mitochondrial functions in advanced PCa. | In vitro, In vivo | [95] | |
| HIF-1 | NSC-134754/single treatment | Decreased HIF1α and GLUT1 expression. Low glucose intake in PCa. | In vitro, In vivo | [96] |
| PT2399/single treatment | Inhibition of HIF2α. Decreased PCa proliferation. | In vitro | [97] | |
| Sulforaphane/single treament | Decreased HIF1α nuclear translocation. Increased mitochondrial biogenesis and activity | In vitro | [98] | |
| Nrf2 | Sulforaphane/single treament | Low expression of ACC1 and FASN in androgen-dependent and androgen-independent PCa. Low CPT1A expression. | In vitro, In vivo | [99] |
| Increased Nrf2 nuclear translocation. Increased mitochondrial biogenesis and activity | In vitro | [98] | ||
| FOXO | Apigenin/single treatment | Decreased tumor size in TRAMP mice model. Loss of FOXO3a phosphorylation generates its activation, by decreasing 14-3-3 binding. Reduced PCa proliferation | In vivo | [100] |
| Methyl protodioscin (furostanol saponin)/single treatment | Reduced MAPK signaling activity. Induced FOXO1 expression, which increased apoptosis in PCa model in vivo. | In vitro, In vivo | [101] | |
| PPARγ | T0070907/single treatment | Disruption in cell growth by impaired PPARγ signaling, following decreasing ACC, AR, and FASN expression in PCa cell lines | In vitro, In vivo | [102] |
| Warfarin/single treatment | PPARγ inhibition and low expression of RA target genes. | In vitro | [103] | |
| Lycopene and T0901317/combined treatment | Increased PPARγ-LXRα-ABCA1 expression, increased cholesterol eflux, synergistic effect of increased PPARγ-LXRα-ABCA1 following lycopene and T0901317 combined treatment | In vitro | [104] | |
| AMPK | MT 63–78 and AR signaling inhibitors/combined treatment | AMPK activation, mTORC blockade, increased growth inhibitory effect of AR signaling inhibitors MDV3100 and abiraterone | In vitro | [105] |
| Salicylate and radiotherapy/combined treatment | Salicylate enhanced the effects of radiotherapy on AMPK and ACC but blocked markers of mTOR activation | In vitro, In vivo | [106] | |
| mTOR | Arctigenin/single treatment | Inhibition of proliferation, decreased AR, decreased circulating FFAs, IGF-1, VEGF, MCP-1, and increased Nkx3.1 | In vitro, In vivo | [107] |
| SIRT1 | Astragalus Polysaccharides/single treatment | Decreased SIRT1 expression, decreased proliferation and invasion of PCa cells and decreased cellular triglyceride and cholesterol levels | In vitro, In vivo | [108] |
| Acetyl CoA and SAM | Ursolic acid/single treatment | Increased SAM, Nrf2-mediated oxidative stress response, CXCR4 signaling, TGF-β signaling. CpG methylation sites and anti-metastatic reprogramming | In vitro, In vivo | [109] |
| Drug | Trial Registration and/or Reference | Intervention/Treatment | Main Outcomes/Adverse Events |
|---|---|---|---|
| Metformin | NCT01215032 | Metformin taken orally twice daily each 28-day cycle, for 12 cycles, in men with CRPC | Lack of efficacy. No serious adverse events |
| NCT02614859 [213] | Metformin (1000 mg) twice a day plus bicalutamide (50 mg) daily for 24 weeks in overweight or obese PCa patients | Metformin induced modest PSA declines in 40% of patients after 8 weeks. No serious adverse events. | |
| NCT01796028 [214] | Docetaxel (75 mg/m2) plus metformin (850 mg) oral twice a day on a continuous daily dosing schedule in men with metastatic hormone-refractory PCa | Metformin addition failed to improve the standard regimen | |
| NCT03137186 [215] | Metformin (850 mg) once or twice daily combined with the standard treatment of locally advanced PCa patients | Combining with ADT, CRPC-free survival was significantly improved with metformin mainly in locally advanced or metastatic PCa. No significant adverse events apart from grade II diarrhea. | |
| NCT:01620593 [216] | Metformin (500 mg) twice or three times a day after castration for 28 weeks in non-diabetic patients with biochemically relapsed or advanced PCa | Metformin added to ADT did not show differences in PSA response. Adverse events overall were increased in the metformin cohort compared to placebo | |
| Sulforaphane | NCT01228084 [217] | Sulforaphane (200 μmol) daily for 20 weeks in men with recurrent PCa | 5% of patients achieved ≥50% decline in PSA. No serious adverse events. |
| NCT01265953 [218] | Sulforaphane (200 μmol) daily for 4 weeks in men scheduled for a prostate biopsy | No significant difference in prostate HDAC activity or Ki67. No serious adverse events. | |
| NCT00946309 | Sulforaphane (100 umol), broccoli sprout extract) every other day for 5 weeks in men with low or intermediate-grade PCa | No serious adverse events. | |
| NCT01950143 [219] | Glucoraphanin-rich broccoli soup weekly for 12 months in men on active surveillance | Attenuation of changes in prostate gene expression and associated oncogenic pathways | |
| Lycopene | [220] | Lycopene (15 mg) twice daily for 3 weeks before radical prostatectomy in patients with a diagnosis of PCa | Decrease in plasma PSA level and tumor volume. No adverse events reported |
| [221] | Lycopene (2 mg) twice daily plus orchidectomy in patients confirmed with metastatic PCa | More reliable and consistent decrease in serum PSA level in lycopene patients still alive after 2 years. No adverse reactions | |
| [222] | Dose-escalating (15–120 mg/day for 1 year) trial of lycopene in patients with biochemical relapse of PCa after definitive local therapy | No serum PSA responses were observed. Lycopene supplementation was safe and well tolerated | |
| [223] | Lycopene supplementation (15 mg) daily for 6 months in patients with progressive hormone refractory PCa | No clinically relevant benefits were observed | |
| NCT00416325 [224] | Tomato extract capsules containing lycopene (30 mg) once, twice, or three times daily for 3 months in patients who are at high risk of developing PCa | No treatment effects were apparent on either the serum or benign tissue endpoints | |
| NCT01882985 [225] | Docetaxel on day 2 and lycopene (30 mg) once daily on days 1–21 for at least 4 courses in chemotherapy-naïve PCa patients | PSA response rate of 76.9% and median survival of 35.1 months versus 45% PSA response rate and 17.4 months median survival reported for docetaxel plus prednisone (TAX 327 trial). No patients experienced grade 3 or above anemia. | |
| 1,25-dihydroxyvitamin D3 | [226] | Calcitriol (0.5–2.5 µg) daily for 6 to 15 months in patients with recurrence indicated by rising serum PSA levels after primary treatment with radiation or surgery | Rate of PSA rise during significantly decreased in 6 of 7 patients versus before calcitriol therapy. Dose-dependent calciuric side effects |
| [227] | Calcitriol (0.5 µg/kg) on day 1 followed by docetaxel (36 mg/m2) on day 2, repeated weekly for 6 weeks of an 8-week cycle in patients with metastatic androgen-independent PCa | PSA, time to progression and survival are promising when compared with contemporary phase II studies of single-agent docetaxel. | |
| [228] | Calcitriol (0.5 µg/kg) on day 1 and carboplatin (AUC 7 or AUC 6 in patients with prior radiation) on day 2, repeated every 4 weeks in patients with metastatic androgen-independent PCa | No increase in the response rate when compared with the activity of carboplatin alone. Toxicity was mild and generally similar to that expected with single-agent carboplatin | |
| [229] | High-dose, intermittent calcitriol (8 -12 µg) plus dexamethasone 4 mg in patients whom prostate cancer was progressive despite androgen deprivation | Response rate was not found to be clearly higher than expected with dexamethasone alone. Combination appears to be safe | |
| NCT00741364 [230] | Vitamin D (400 IU, 10 000 IU or 40 000 IU) once per day, for a 3- to 8-week period ending the day before radical prostatectomy | Vitamin D3 modestly lowered PSA and PTH. All reported side effects were classified as grade 1 | |
| Genistein | [231] | Genistein-rich extract three times daily for six months in patients with histologically proven PCa | Genistein-rich extract did not reduce PSA levels by 50% or more in 51 of 52 subjects |
| NCT00058266 [232] | Genistein (150 mg) once daily for 1–2 months in patients that undergo radical prostatectomy, and then continue oral genistein once daily for 1–2 months afterward (3 months of therapy) | MMP-2 transcript level in normal prostate epithelial cells was higher in the untreated group than in the genistein-treated group | |
| [233] | Synthetic genistein (30 mg) daily for 3–6 weeks before radical prostatectomy in patients with localized PCa | PSA decreased by 7.8% in the genistein arm and increased by 4.4% in the placebo arm. Adverse events were few and mild. | |
| Warfarin | [234] | All warfarin uses among 78,615 men during 1995–2009 was analyzed and estimated PCa risk overall, and by tumor grade and stage. | A similar risk of PCa was found among warfarin users and the general population |
| 2-Deoxyglucose | NCT00633087 | 2-deoxyglucose (30 mg/kg) daily for two weeks of a three-week (21 day) cycle in patients with advanced and hormone refractory PCa | 75% (9/12) of 5-year progression-free survival; 58.3% (7/12) of 5-year overall survival; 25% (3/12) of serious adverse events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Pérez, P.J.; Espitia-Perez, L.M.; Negrette-Guzmán, M. Targeting Prostate Cancer Metabolism Through Transcriptional and Epigenetic Modulation: A Multi-Target Approach to Therapeutic Innovation. Int. J. Mol. Sci. 2025, 26, 6013. https://doi.org/10.3390/ijms26136013
Espitia-Pérez PJ, Espitia-Perez LM, Negrette-Guzmán M. Targeting Prostate Cancer Metabolism Through Transcriptional and Epigenetic Modulation: A Multi-Target Approach to Therapeutic Innovation. International Journal of Molecular Sciences. 2025; 26(13):6013. https://doi.org/10.3390/ijms26136013
Chicago/Turabian StyleEspitia-Pérez, Pedro Juan, Lyda Marcela Espitia-Perez, and Mario Negrette-Guzmán. 2025. "Targeting Prostate Cancer Metabolism Through Transcriptional and Epigenetic Modulation: A Multi-Target Approach to Therapeutic Innovation" International Journal of Molecular Sciences 26, no. 13: 6013. https://doi.org/10.3390/ijms26136013
APA StyleEspitia-Pérez, P. J., Espitia-Perez, L. M., & Negrette-Guzmán, M. (2025). Targeting Prostate Cancer Metabolism Through Transcriptional and Epigenetic Modulation: A Multi-Target Approach to Therapeutic Innovation. International Journal of Molecular Sciences, 26(13), 6013. https://doi.org/10.3390/ijms26136013








