Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of the Study Population
2.2. Histopathological Characteristics of Renal Allograft Biopsies
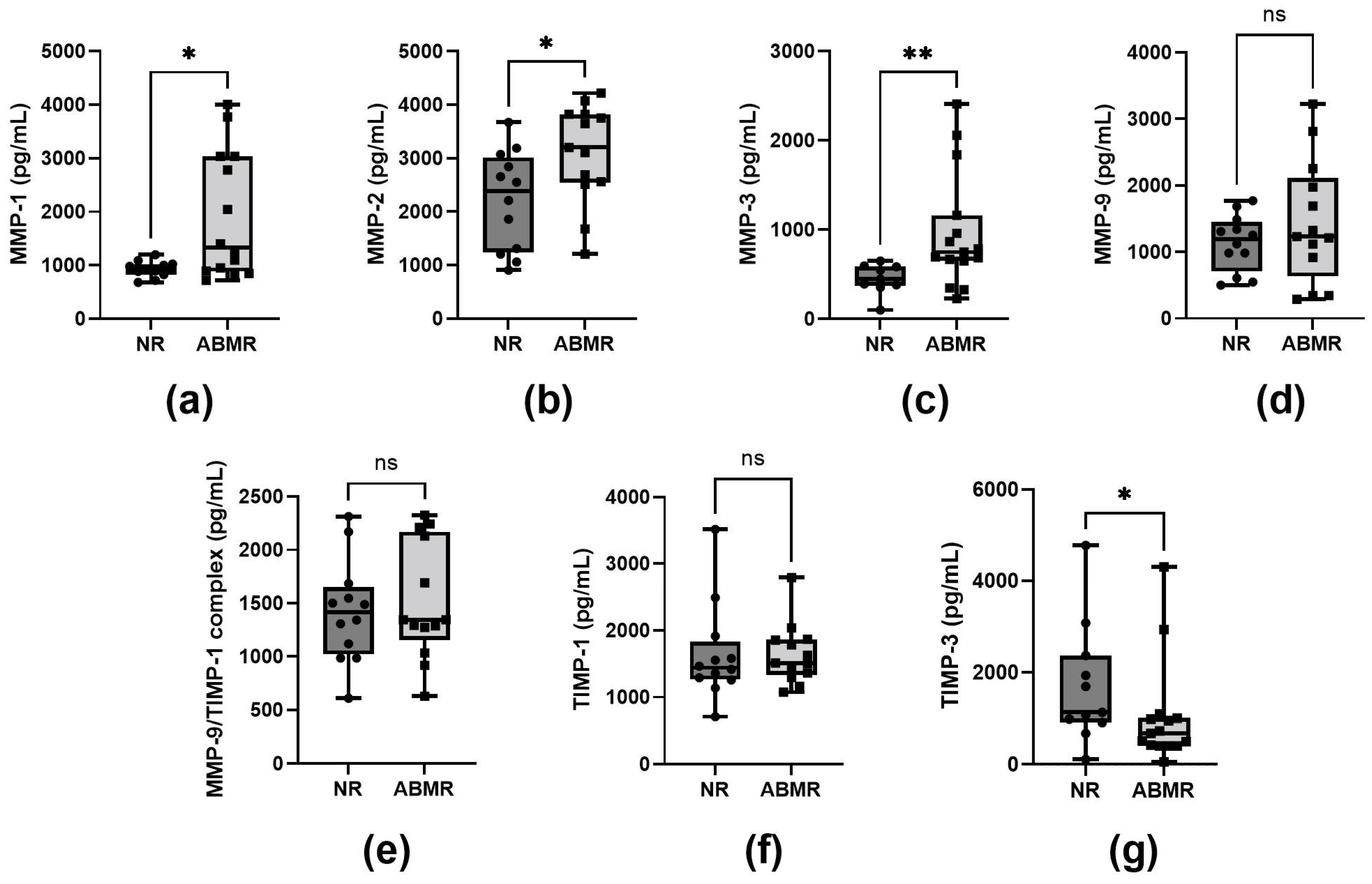
2.3. Plasma MMP-1, MMP-2, and MMP-3 Levels in Patients with Antibody-Mediated Rejection (ABMR)
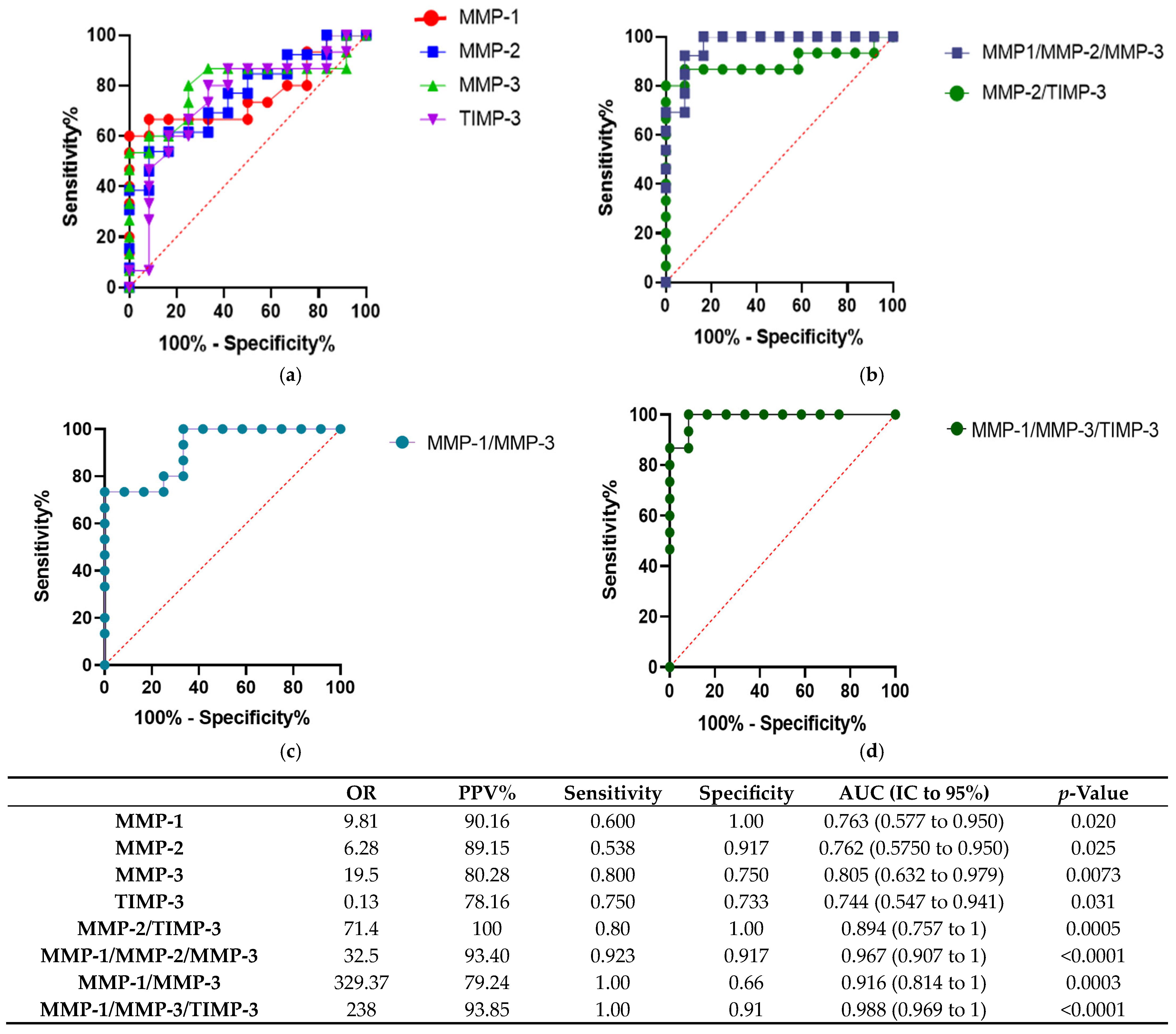
2.4. Plasma Concentrations of MMP-1, MMP-2, MMP-3, TIMP-3 Can Predict ABMR
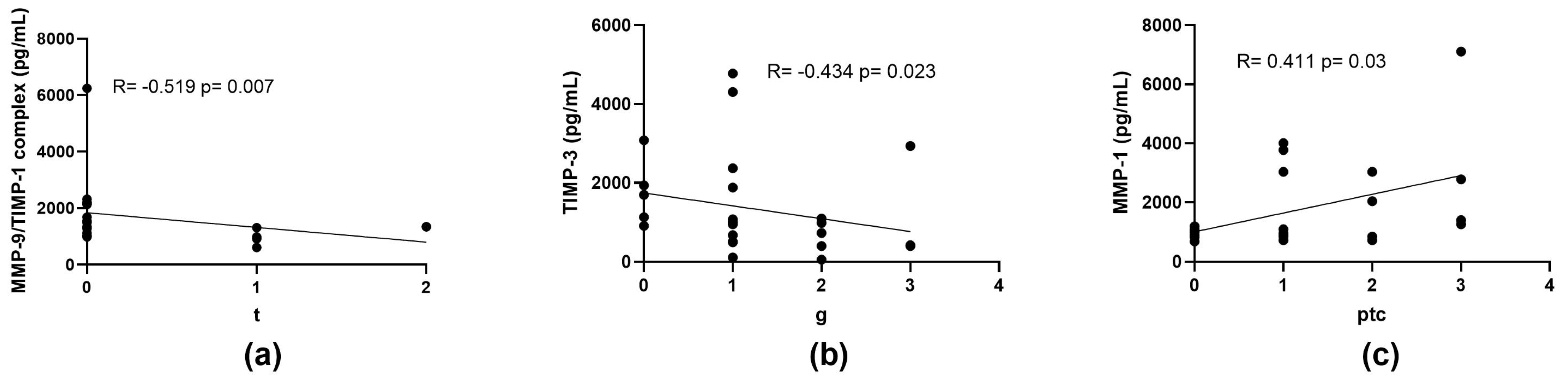
2.5. Correlation of MMPs and TIMPs Concentrations with the Severity of Histopathological Findings
3. Discussion
4. Materials and Methods
4.1. Clinical Parameters
4.2. Clinical-Pathological Diagnosis
4.3. Measurement of Circulating Anti-HLA Antibodies
4.4. Determination of MMPs and TIMPs Concentrations
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABMR | Acute Antibody-Mediated Rejection |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| BSA | Bovine Serum Albumin |
| CDC-XM | Complement-Dependent Cytotoxicity Crossmatch |
| CF-XM | Flow Cytometry Crossmatch |
| CI | Confidence Interval |
| C4d | C4d deposition in peritubular capillaries |
| Cg | Chronic Glomerulopathy |
| Ci | Interstitial Fibrosis |
| Ct | Tubular Atrophy |
| Cv | Vascular fibrous intimal thickening |
| DNDSA | De Novo Donor-Specific Antibodies |
| DSA | Donor-Specific Antibodies |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic Acid |
| EGFR | Estimated Glomerular Filtration Rate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMT | Epithelial–Mesenchymal Transition |
| G | Glomerulitis |
| HLA | Human Leukocyte Antigen |
| HRP | Horseradish Peroxidase |
| I | Interstitial inflammation |
| i-IFTA | Inflammation in areas of interstitial fibrosis and tubular atrophy |
| IL-1β | Interleukin-1 Beta |
| IQR | Interquartile Range |
| MFI | Mean Fluorescence Intensity |
| MGN | Membranous Glomerulonephritis |
| MMP | Matrix Metalloproteinase |
| MM | Mesangial Matrix Expansion |
| NR | No-Rejection |
| OR | Odds Ratio |
| PBS | Phosphate-Buffered Saline |
| PPV | Positive Predictive Value |
| PRA-SA | Panel Reactive Antibody by Solid Assay |
| PTC | Peritubular Capillaritis |
| Rho | Spearman’s Rho |
| ROC | Receiver Operating Characteristic |
| SPSS | Statistical Package for the Social Sciences |
| T | Tubulitis |
| TGF-β | Transforming Growth Factor Beta |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TMB | Tetramethylbenzidine |
| TNF-α | Tumor Necrosis Factor Alpha |
| V | Intimal arteritis |
References
- Truong, L.D.; Barrios, R.; Adrogue, H.E.; Gaber, L.W. Acute antibody-mediated rejection of renal transplant: Pathogenetic and diagnostic considerations. Arch. Pathol. Lab. Med. 2007, 131, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.M.; Valujskikh, A.; Fairchild, R.L. Mechanisms of antibody-mediated acute and chronic rejection of kidney allografts. Curr. Opin. Organ. Transplant. 2016, 21, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sellares, J.; Tinel, C.; Anglicheau, D.; Bestard, O.; Friedewald, J.J. European Society of Organ Transplantation Consensus Statement on Testing for Non-Invasive Diagnosis of Kidney Allograft Rejection. Transplant. Int. 2024, 36, 12115. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 2012, 302, F1351–F1361. [Google Scholar] [CrossRef] [PubMed Central]
- Yan, Q.; Sui, W.; Wang, B.; Zou, H.; Zou, G.; Luo, H. Expression of MMP-2 and TIMP-1 in Renal Tissue of Patients with Chronic Active Antibody-mediated Renal Graft Rejection. Diagn. Pathol. 2012, 7, 141. [Google Scholar] [CrossRef] [PubMed Central]
- Wong, W.; DeVito, J.; Nguyen, H.; Sarracino, D.; Porcheray, F.; Dargon, I.; Pelle, P.D.; Collins, A.B.; Tolkoff-Rubin, N.; Smith, R.N.; et al. Chronic humoral rejection of human kidney allografts is associated with mmp-2 accumulation in podocytes and its release in the urine. Am. J. Transplant. 2010, 10, 2463–2471. [Google Scholar] [CrossRef]
- Kollar, B.; Uffing, A.; Borges, T.J.; Shubin, A.V.; Aoyama, B.T.; Dagot, C.; Haug, V.; Kauke, M.; Safi, A.-F.; Talbot, S.G.; et al. MMP3 Is a Non-invasive Biomarker of Rejection in Skin-Bearing Vascularized Composite Allotransplantation: A Multicenter Validation Study. Front. Immunol. 2019, 10, 2771. [Google Scholar] [CrossRef]
- Cheng, Z.; Limbu, M.H.; Wang, Z.; Liu, J.; Liu, L.; Zhang, X.; Chen, P.; Liu, B. MMP-2 and 9 in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russa, A.L.; Serra, R.; Faga, T.; Crugliano, G.; Bonelli, A.; Coppolino, G.; Bolignano, D.; Battaglia, Y.; Ielapi, N.; Costa, D.; et al. Kidney Fibrosis and Matrix Metalloproteinases (MMPs). Front. Biosci. (Landmark Ed.) 2024, 29, 192. [Google Scholar] [CrossRef] [PubMed]
- Kassiri, Z.; Oudit, G.Y.; Kandalam, V.; Awad, A.; Wang, X.; Ziou, X.; Maeda, N.; Herzenberg, A.M.; Scholey, J.W. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J. Am. Soc. Nephrol. 2009, 20, 1223–1235. [Google Scholar] [CrossRef]
- Klimm, W.; Szamotulska, K.; Karwański, M.; Bartoszewicz, Z.; Witkowski, W.; Rozmyslowicz, T.; Niemczyk, S. Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury After Massive Burns. Med. Sci. Monit. 2024, 30, e943500-1–e943500-14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampieri, C.L.; Orozco-Ortega, R.A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in chronic kidney disease and acute kidney injury: A systematic review of the literature. Hippokratia 2018, 22, 99–104. [Google Scholar] [PubMed] [PubMed Central]
- Eikmans, M.; Gielis, E.M.; Ledeganck, K.J.; Yang, J.; Abramowicz, D.; Claas, F.F.J. Non-invasive Biomarkers of Acute Rejection in Kidney Transplantation: Novel Targets and Strategies. Front. Med. 2019, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A.; Mol, P.; Transl, B.; Author, S. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed Central]
- Karanovic, D.; Grujic-Milanovic, J.; Miloradovic, Z.; Ivanov, M.; Jovovic, D.; Vajic, U.J.; Zivotic, M.; Markovic-Lipkovski, J.; Mihailovic-Stanojevic, N. Effects of Single and Combined Losartan and Tempol Treatments on Oxidative Stress, Kidney Structure and Function in Spontaneously Hypertensive Rats with Early Course of Proteinuric Nephropathy. PLoS ONE 2016, 11, e0161706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, W.; Dang, Y.; Liu, X.; Li, Y.; Peng, X.; Ye, X. Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J. Diabetes Res. 2013, 2013, 463740. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, N.; Jacobs-Cachá, C.; Mora-Gutiérrez, J.M.; Vergara, A.; Orbe, J.; Soler, M.J. Matrix Metalloproteinases in Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatt, M. P1613influence of Genetic Polymorphism in Mmps and Timps on Allograft Outcome in Renal Transplant Recipients. Nephrol. Dial. Transplant. 2020, 35 (Suppl. 3), gfaa142.P1613. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Zhang, Y.; Li, L.; Chen, P. Role of MMP-2 and CD147 in kidney fibrosis. Open Life Sci. 2022, 17, 1182–1190. [Google Scholar] [CrossRef] [PubMed Central]
- Rodrigo, E.; López-Hoyos, M.; Escallada, R.; Fernández-Fresnedo, G.; Ruiz, J.C.; Piñera, C.; Cotorruelo, J.G.; Zubimendi, J.A.; de Francisco, A.L.M.; Arias, M. Circulating levels of matrix metalloproteinases MMP-3 and MMP-2 in renal transplant recipients with chronic transplant nephropathy. Nephrol. Dial. Transplant. 2000, 15, 2041–2045. [Google Scholar] [CrossRef][Green Version]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Shimizu, A.; Mii, A.; Fujita, E.; Fujino, T.; Kunugi, S.; Du, X.; Akimoto, T.; Tsuruoka, S.; Ohashi, R.; et al. Role of matrix metalloproteinase-2 in recovery after tubular damage in acute kidney injury in mice. Nephron Exp. Nephrol. 2012, 122, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, P.A.; Noelken, M.E.; Suzuki, K.; Hudson, B.G.; Nagase, H. Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem. J. 1988, 256, 413–419. [Google Scholar] [CrossRef] [PubMed Central]
- Adamidis, K.N.; Kopaka, M.E.; Petraki, C.; Charitaki, E.; Apostolou, T.; Christodoulidou, C.; Nikolopoulou, N.; Giatromanolaki, A.; Vargemesis, V.; Passadakis, P. Glomerular expression of matrix metalloproteinases in systemic lupus erythematosus in association with activity index and renal function. Ren. Fail. 2019, 41, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed Central]
- Wang, Z.; Famulski, K.; Lee, J.; Das, S.K.; Wang, X.; Halloran, P.; Oudit, G.Y.; Kassiri, Z. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int. 2014, 85, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Nee, L.E.; McMorrow, T.; Campbell, E.; Slattery, C.; Ryan, M.P. TNF-α and IL-1β–mediated regulation of MMP-9 and TIMP-1 in renal proximal tubular cells. Kidney Int. 2004, 66, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kassiri, Z. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and Its Therapeutic Implications in Cardiovascular Pathology. Front. Physiol. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Quiroz, I.; Guzmán-Martín, C.A.; Peña-Peña, M.; Juárez-Villa, J.D.; Soto-Abraham, M.V.; Vázquez-Toledo, M.A.; Jiménez-Ortega, R.F.; Moguel-González, B.; Osorio-Alonso, H.; Sánchez-Muñoz, F.; et al. Plasma miR-150-5p in Renal Transplant Recipients with Acute Antibody-Mediated Rejection. J. Clin. Med. 2024, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, R.; Legaz, I.; Jimenez-Coll, V.; El kaaoui El band, J.; Martínez-Banaclocha, H.; Galián, J.A.; Parrado, A.; Mrowiec, A.; Botella, C.; Moya-Quiles, M.R.; et al. MicroRNA Expression Changes in Kidney Transplant: Diagnostic Efficacy of miR-150-5p as Potential Rejection Biomarker, Pilot Study. J. Clin. Med. 2021, 10, 2748. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 27) | NR (n = 12) | ABMR (n = 15) | p Value | |
|---|---|---|---|---|
| Female | 14 (51.9) | 8 (66.7) | 6 (40) | 0.16 |
| Age | 42 (32–51) | 51 (37–59) | 34 (31–42) | 0.03 |
| BMI (kg/m2) | 26.10 (21.90–29.30) | 25.75 (21.85–29.85) | 26.1 (21.95–28.2) | 0.94 |
| Etiology | 0.12 | |||
| Unknown | 16 (59.3) | 4 (41.6) | 11 (73.3) | |
| MGN | 9 (33.3) | 5 (41.7) | 4 (26.7) | |
| Diabetes | 2 (7.4) | 2 (16.7) | 0 | |
| Time post-transplantation (months) | 80.5 (16–112) | 37.8 (10.2–102) | 85 (37.5–112) | 0.22 |
| Deceased donor | 13 (48.2) | 7 (58.3) | 6 (40) | 0.29 |
| Allosensitization | 17 (63) | 6 (50) | 11 (73.3) | 0.2 |
| PRA I | 1 (0–5) | 1 (0–2) | 3 (0–10) | 0.34 |
| PRA II | 2 (1–6) | 2 (0.5–6) | 2 (1–44) | 0.57 |
| DSAs pre-transplantation | 9(33.3) | 2 (16.7) | 7 (46.7) | 0.15 |
| DSAs post-transplantation | 20 (74.1) | 6 (50) | 15 (100) | 0.003 |
| Total (n = 27) | NR (n = 12) | ABMR (n = 15) | p-Value | |
|---|---|---|---|---|
| g > 0 | 22 (81.5) | 7 (58.3) | 15 (100) | 0.01 |
| ptc > 0 | 16 (59.3) | 1 (8.3) | 15 (100) | <0.001 |
| mm > 0 | 22 (81.5) | 7 (58.3) | 15 (100) | 0.01 |
| i > 0 | 9 (33.3) | 0 | 9 (60) | 0.001 |
| t > 0 | 9 (33.3) | 1 (8.3) | 8 (53.3) | 0.02 |
| v > 0 | 3 (11.1) | 0 | 3 (20) | 0.16 |
| cg > 0 | 6 (22.2) | 0 | 6 (40) | 0.02 |
| ci > 0 | 22 (81.5) | 9 (75) | 13 (86.7) | 0.39 |
| ct > 0 | 22 (81.5) | 9 (75) | 13 (86.7) | 0.39 |
| cv > 0 | 11 (40.7) | 3 (25) | 8 (53.3) | 0.14 |
| i-IFTA > 0 | 14 (52.9) | 5 (41.7) | 9 (60) | 0.29 |
| C4d > 0 | 9 (33.3) | 5 (41.7) | 4 (26.7) | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Toledo, M.A.; Sánchez-Muñoz, F.; Zepeda-Quiroz, I.; Guzmán-Martín, C.A.; Osorio-Alonso, H.; Daniel, J.-V.; Soto-Abraham, M.V.; Moguel-González, B.; Chacón-Salinas, R.; Flores-Gama, C.; et al. Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles. Int. J. Mol. Sci. 2025, 26, 6011. https://doi.org/10.3390/ijms26136011
Vázquez-Toledo MA, Sánchez-Muñoz F, Zepeda-Quiroz I, Guzmán-Martín CA, Osorio-Alonso H, Daniel J-V, Soto-Abraham MV, Moguel-González B, Chacón-Salinas R, Flores-Gama C, et al. Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles. International Journal of Molecular Sciences. 2025; 26(13):6011. https://doi.org/10.3390/ijms26136011
Chicago/Turabian StyleVázquez-Toledo, Miguel A., Fausto Sánchez-Muñoz, Iván Zepeda-Quiroz, Carlos A. Guzmán-Martín, Horacio Osorio-Alonso, Juárez-Villa Daniel, Ma. Virgilia Soto-Abraham, Bernardo Moguel-González, Rommel Chacón-Salinas, César Flores-Gama, and et al. 2025. "Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles" International Journal of Molecular Sciences 26, no. 13: 6011. https://doi.org/10.3390/ijms26136011
APA StyleVázquez-Toledo, M. A., Sánchez-Muñoz, F., Zepeda-Quiroz, I., Guzmán-Martín, C. A., Osorio-Alonso, H., Daniel, J.-V., Soto-Abraham, M. V., Moguel-González, B., Chacón-Salinas, R., Flores-Gama, C., & Springall, R. (2025). Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles. International Journal of Molecular Sciences, 26(13), 6011. https://doi.org/10.3390/ijms26136011








