Exhaled Breath Analysis in Lymphangioleiomyomatosis by Real-Time Proton Mass Spectrometry
Abstract
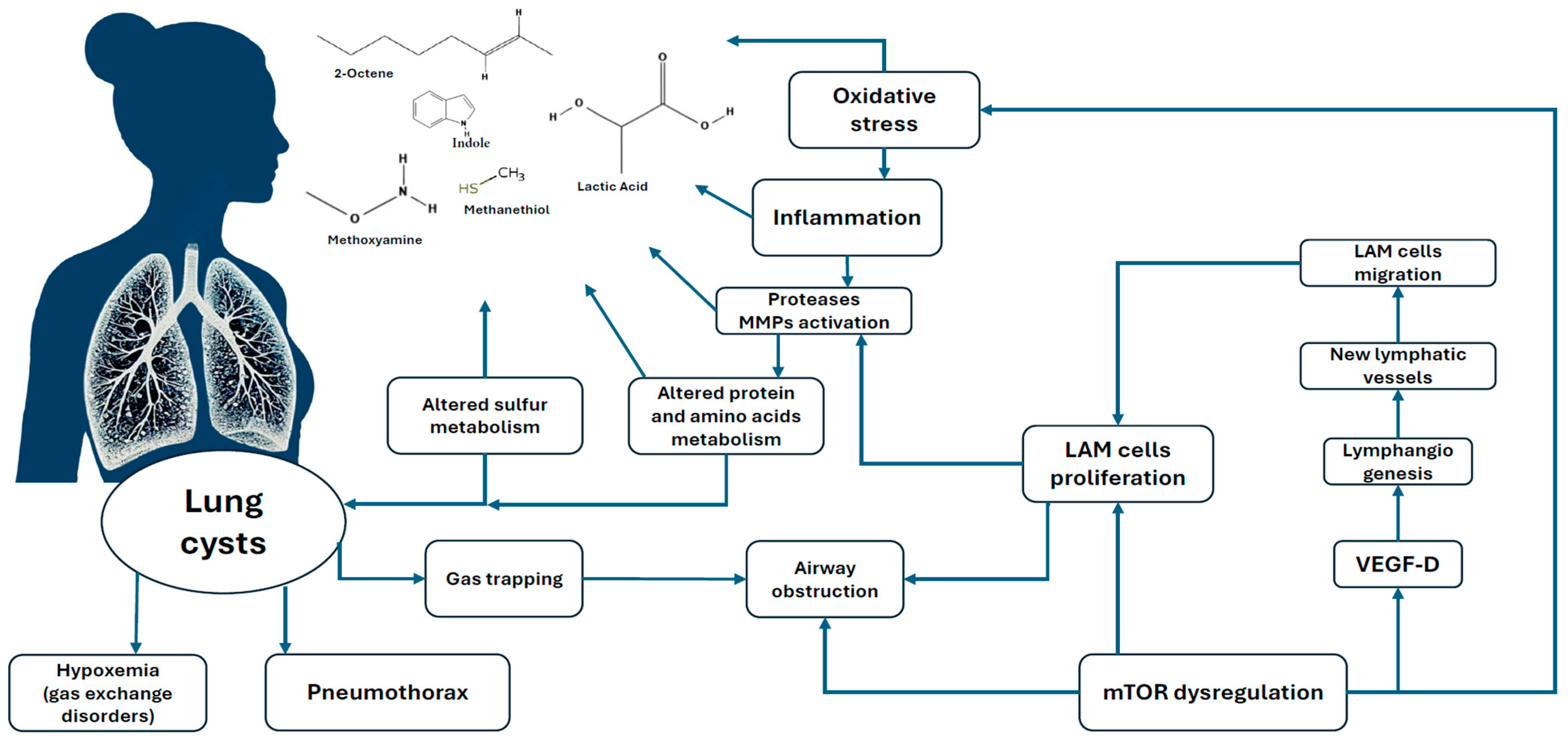
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Identification of VOCs as Predictors of LAM
2.3. Identification of VOCs as Predictors of Respiratory Disorders, Radiological Changes, and Significant Clinical Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Collection of Exhaled Breath and Measurement of VOCs
4.3. Data Processing
4.4. VOCs Annotation
4.5. Statistical Data Analysis
4.5.1. Descriptive Statistics
4.5.2. Identifying VOC Predictors and Their Relationship to Endpoints
4.5.3. Feature Selection Process
4.5.4. Examining the Association Between Selected VOCs and Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTR-TOF-MS | proton-transfer reaction time-of-flight mass spectrometry |
| eVOCs | exhaled volatile organic compounds |
| GC-MC | gas chromatography–mass-spectrometry |
| SIFT-MS | selected ion flow mass spectrometry |
| SESI-MS | secondary ionization electrospray mass spectrometry |
| CO | carbon monoxide |
| ATS | the American Thoracic Society |
| ERS | the European Respiratory Society |
| GLI | the Global Lung Function Initiative |
| AUC | area under the curve |
| BMI | body mass index |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| FEF75 | forced expiratory flow when 75% of FVC has been exhaled |
| mMRC | Modified Medical Research Council |
| LAM | lymphangioleiomyomatosis |
| RV | residual volume |
| SD | standard deviation |
| TLC | total lung capacity |
| VA | alveolar volume |
References
- O’Mahony, A.M.; Lynn, E.; Murphy, D.J.; Fabre, A.; McCarthy, C. Lymphangioleiomyomatosis: A clinical review. Breathe 2020, 16, 200007. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Cordier, J.F.; Lazor, R.; Cottin, V.; Costabel, U.; Harari, S.; Reynaud-Gaubert, M.; Boehler, A.; Brauner, M.; Popper, H.; et al. Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur. Respir. J. 2010, 35, 14–26. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Lee, H.S.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; et al. MILES Trial Group. Serum VEGF-D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response. Lancet Respir. Med. 2013, 1, 445–452. [Google Scholar] [CrossRef]
- Hashoul, D.; Haick, H. Sensors for detecting pulmonary diseases from exhaled breath. Eur. Respir. Rev. 2019, 28, 190011. [Google Scholar] [CrossRef]
- Pleil, J.D.; Hansel, A.; Beauchamp, J. Advances in proton transfer reaction mass spectrometry (PTR-MS): Applications in exhaled breath analysis, food science, and atmospheric chemistry. J. Breath Res. 2019, 13, 039002. [Google Scholar] [CrossRef]
- Shahbazi Khamas, S.; Alizadeh Bahmani, A.H.; Vijverberg, S.J.H.; Brinkman, P.; Maitland-van der Zee, A.H. Exhaled volatile organic compounds associated with risk factors for obstructive pulmonary diseases: A systematic review. ERJ Open Res. 2023, 9, 00143–02023. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, Q.; Pan, Z.; Chen, J.; Sun, M.; Wang, J.; Li, Y.; Ye, Q. Development and validation of a screening model for lung cancer using machine learning. Front. Oncol. 2022, 12, 975563. [Google Scholar]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Suvorova, O.; Schmidt, A.; et al. Identification of exhaled metabolites correlated with respiratory function and clinical features in adult patients with cystic fibrosis. Biomolecules 2024, 14, 1189. [Google Scholar] [CrossRef]
- Montaudon, M.; Desbarats, P.; Berger, P.; de Dietrich, G.; Marthan, R.; Laurent, F. Assessment of bronchial wall thickness and lumen diameter in human adults using multi-detector computed tomography. J. Anat. 2007, 211, 579–588. [Google Scholar] [CrossRef]
- Xie, J.; Liu, H.; He, Q.; Li, C. Relationship Between Lactate-to-Albumin Ratio and 28-Day Mortality in Patients with Exacerbation of Chronic Obstructive Pulmonary Disease Admitted to the Intensive Care Unit. Eur. J. Med. Res. 2024, 29, 258. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Gupta, N.; Johnson, S.R.; Yu, J.J.; McCormack, F.X. Lymphangioleiomyomatosis: Pathogenesis, clinical features, diagnosis, and management. Lancet Respir. Med. 2021, 9, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Kos, R.; Brinkman, P.; Neerincx, A.H.; Paff, T.; Gerritsen, M.G.; Lammers, A.; Kraneveld, A.D.; Heijerman, H.G.M.; Janssens, H.M.; Davies, J.C.; et al. Amsterdam Mucociliary Clearance Disease (AMCD) Research Group and the Amsterdam UMC Breath Research Group. Targeted exhaled breath analysis for detection of Pseudomonas aeruginosa in cystic fibrosis patients. J. Cyst. Fibros. 2022, 21, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.; Yang, L.; Ren, S. The development for emerging biomarkers of lymphangioleiomyomatosis. Orphanet J. Rare Dis. 2024, 19, 445. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, S.J.; Oh, J.H.; Ryu, J.S.; Park, Y.; Hwang, E.S. MPoMA protects against lung epithelial cell injury via p65 degradation. Biomed. Pharmacother. 2024, 175, 116674. [Google Scholar] [CrossRef]
- Philipp, T.M.; Scheller, A.S.; Krafczyk, N.; Klotz, L.O.; Steinbrenner, H. Methanethiol: A scent mark of dysregulated sulfur metabolism in cancer. Antioxidants 2023, 12, 1780. [Google Scholar] [CrossRef]
- Klarquist, J.; Barfuss, A.; Kandala, S.; Reust, M.J.; Braun, R.K.; Hu, J.; Dilling, D.F.; McKee, M.D.; Boissy, R.E.; Love, R.B.; et al. Melanoma-associated antigen expression in lymphangioleiomyomatosis renders tumor cells susceptible to cytotoxic T cells. Am. J. Pathol. 2009, 175, 2463–2472. [Google Scholar] [CrossRef]
- Gupta, N.; Finlay, G.A.; Kotloff, R.M.; Strange, C.; Wilson, K.C.; Young, L.R.; Taveira-DaSilva, A.M.; Johnson, S.R.; Cottin, V.; Sahn, S.A.; et al. Lymphangioleiomyomatosis diagnosis and management: High-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An official American Thoracic Society/Japanese Respiratory Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2017, 196, 1337–1348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.M.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Wanger, J. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- Mustafina, M.; Silantyev, A.; Krasovskiy, S.; Chernyak, A.; Naumenko, Z.; Suvorov, A.; Gognieva, D.; Abdullaev, M.; Bektimirova, A.; Bykova, A.; et al. Exhaled breath analysis in adult patients with cystic fibrosis by real-time proton mass spectrometry. Clin. Chim. Acta 2024, 560, 119733. [Google Scholar] [CrossRef] [PubMed]
- van Mastrigt, E.; Reyes-Reyes, A.; Brand, K.; Bhattacharya, N.; Urbach, H.P.; Stubbs, A.P.; de Jongste, J.C.; Pijnenburg, M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016, 10, 026003. [Google Scholar] [CrossRef]
- van Berkel, J.J.B.N. There’s Something in the Air: Volatile Organic Compounds in Exhaled Breath in Pulmonary Diseases. Doctoral Thesis, Maastricht University, Maastricht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Weber, R.; Perkins, N.; Bruderer, T.; Micic, S.; Moeller, A. Identification of Exhaled Metabolites in Children with Cystic Fibrosis. Metabolites 2022, 12, 980. [Google Scholar] [CrossRef]
- Natal Jorge, R.M.; Tavares, J.M.R.; Barbosa, M.P.; Slade, A.P. (Eds.) Technology and Medical Sciences, 1st ed.; Taylor & Francis: London, UK, 2011. [Google Scholar] [CrossRef]
| LAM | Control | p-Value | |
|---|---|---|---|
| Patients and controls (all women) | 51 | 51 | – |
| Age, years | 48.7 ± 10.9 | 49.0 ± 17.9 | 0.536 |
| BMI kg·m2 | 24.7 ± 5.1 | 25.9 ± 5.7 | 0.347 |
| Smoking status | 0.327 | ||
| Never | 50 (98.0) | 49 (96.1) | |
| Former | 0 | 2 (3.9) | |
| Current | 1 (2.0) | 0 | |
| mMRC, scores | 1.5 ± 1.1 | 0.0 ± 0.1 | <0.001 |
| FVC % predicted | 89.0 ± 18.7 | 100.5 ± 11.6 | 0.008 |
| FEV1 % predicted | 72.9 ± 28.6 | 101.6 ± 11.9 | <0.001 |
| FEV1/FVC % | 63.8 ± 17.1 | 81.8 ± 6.3 | <0.001 |
| FEF75 % predicted | 66.3 ± 44.2 | 141.0 ± 73.4 | <0.001 |
| RV % predicted | 164.6 ± 53.8 | 118.7 ± 22.2 | <0.001 |
| FRC % predicted | 131.6 ± 31.1 | 107.8 ± 20.6 | <0.001 |
| RV/TLC % predicted | 143.1 ± 47.6 | 112.4 ± 21.1 | <0.001 |
| DLCO % predicted | 62.8 ± 24.1 | 97.0 ± 13.5 | <0.001 |
| DLCO/VA % predicted | 67.6 ± 22.6 | 101.6 ± 14.9 | <0.001 |
| CT characteristics of the lungs | |||
| Volume of lung cysts, % * | 22.3 ± 16.5 | NA | |
| B1 dist. average lumen area, mm2 ** | 1.4 ± 0.6 | NA | |
| B10 dist. average lumen area, mm2 | 1.3 ± 0.6 | NA | |
| History of extrapulmonary manifestations | |||
| Pneumothorax | 25 (49.0) | NA | |
| Pulmonary bleeding | 13 (25.5) | NA | |
| Angiomyolipoma | 31 (60.8) | NA | |
| Lymphangioleiomyoma | 25 (49.0) | NA | |
| Chylothorax | 13 (25.5) | NA | |
| Bronchodilators | 27 (52.9) | NA | |
| Oxygen therapy | 6 (11.8) | NA | |
| mTOR inhibitors | 20 (39.2) | NA |
| m/z | Name of the Chemical Substance | Mass Error, ppm | Feature Importances | |
|---|---|---|---|---|
| Forced Expiratory Maneuver | Normal Quiet Breathing | |||
| 44.99 | NA | 0.00677846 | 0.00683706 | |
| 50.00 | NA | 0.00679701 | 0.00683653 | |
| 82.07 | NA | 0.00681889 | 0.00683042 | |
| 90.06 | Lactic Acid | 346.3 * | 0.00679180 | 0.00683309 |
| 99.07 | NA | 0.00679757 | 0.00682748 | |
| 113.13 | NA | 0.00685565 | 0.00691124 | |
| 127.00 | NA | 0.00684542 | 0.00682354 | |
| 150.10 | NA | 0.00683700 | 0.00685841 | |
| 329.82 | NA | 0.00682365 | 0.00682728 | |
| Clinical and Functional Endpoints | m/z | Name of the Chemical Substance | Mass Error, ppm | Feature Importances | |
|---|---|---|---|---|---|
| Forced Expiratory Maneuver | Normal Quiet Breathing | ||||
| Pneumothorax | 103.08 | Isopropyl acetate | −1.80 | 0.00695703 | 0.00701847 |
| 133.10 | 2-Ethoxyethyl acetate | 73.56 | 0.00756860 | 0.00716509 | |
| FEV1/FVC < LLN | 63.02 | Dimethyl sulfide | −110.04 | 0.00696004 | 0.00684350 |
| 129.07 | NA | – | 0.00692103 | 0.00686908 | |
| 141.13 | 2-Nonenal | −13.31 | 0.00687696 | 0.00700937 | |
| FEV1 < LLN | 48.04 | O-methylhydroxylamine | −22.42 | 0.00692541 | 0.00684413 |
| 173.15 | Isomers of Oxononanoic acid | 197.67 | 0.00679017 | 0.00688988 | |
| RV/TLC > LLN | 74.05 | N-methylacetamide | −138.07 | 0.00855004 | 0.0269816 |
| RV > LLN | 42.03 | Acetonitrile | −124.36 | 0.00290517 | 0.00849818 |
| DLCO < LLN | 48.04 | O-methylhydroxylamine | −22.42 | 0.00687678 | 0.00690884 |
| Volume of lung cysts ** | 129.07 | NA | – | 0.00695566 | 0.00699561 |
| B1 and B10 dist. average lumen area *** | 57.06 | Isobutene | −150.66 | 0.00722638 | 0.00729180 |
| 73.06 | Methyl Ethyl Ketone | −20.36 | 0.00720196 | 0.00705437 | |
| 91.06 | 2-Butanethiol | −34.75 | 0.00699667 | 0.00724412 | |
| 181.00 | Oxidized Lipid Fragment | −198.72 | 0.00699667 | 0.00699890 | |
| Clinical and Functional Endpoints | m/z | VOCs Name ** | Forced Expiratory Maneuver | Normal Quiet Breathing | ||||
|---|---|---|---|---|---|---|---|---|
| LAM | Control | LAM | Control | |||||
| LAM diagnosis | 44.99 | NA | 7558.6 ± 1437.1 | 8810.6 ± 2368.3 | p < 0.001 | 7056.6 ± 1291.1 | 8035.1 ± 1770.8 | p < 0.001 |
| 50.00 | NA | 62.5 ± 8.5 | 70.5 ± 14.9 | p < 0.05 | 64.0 ± 9.4 | 73.6 ± 22.3 | p < 0.05 | |
| 82.07 | NA | 95.1 ± 29.3 | 98.9 ± 29.1 | p < 0.05 | 52.8 ± 26.2 | 53.1 ± 27.8 | p < 0.05 | |
| 90.06 | Lactic Acid | 58.4 ± 16.2 | 65.2 ± 20.0 | p < 0.001 | 57.1 ± 17.1 | 60.0 ± 16.1 | p < 0.05 | |
| 99.07 | NA | 76.7 ± 24.1 | 80.0 ± 25.9 | p < 0.05 | 68.7 ± 19.8 | 72.0 ± 17.2 | p < 0.05 | |
| 113.13 | NA | 51.6 ± 9.6 | 54.1 ± 9.0 | p < 0.02 | 51.6 ± 9.0 | 56.7 ± 8.4 | p < 0.03 | |
| 127.00 | NA | 59.4 ± 18.6 | 64.7 ± 24.5 | p < 0.05 | 57.5 ± 18.7 | 60.1 ± 21.4 | p < 0.001 | |
| 150.10 | NA | 28.8 ± 8.7 | 42.0 ± 85.2 | p < 0.05 | 26.3 ± 6.4 | 33.5 ± 40.0 | p < 0.05 | |
| 329.82 | NA | 1598.8 ± 521.9 | 1398.9 ± 562.5 | p < 0.05 | 1626.3 ± 519.1 | 1411.8 ± 545.5 | p < 0.01 | |
| Pneumothorax | 103.08 | Isopropyl acetate | 109.0 ± 68.4 | 107.7 ± 69.5 | p < 0.01 | 113.2 ± 72.5 | 103.2 ± 62.2 | p < 0.001 |
| 133.10 | 2-Ethoxyethyl acetate | 32.4 ± 11.5 | 37.7 ± 9.9 | p < 0.01 | 34.0 ± 7.2 | 35.6 ± 7.7 | p < 0.1 | |
| 63.02 | Dimethyl sulfide | 26.1 ± 6.0 | 87.0 ± 61.6 | p < 0.001 | 27.0 ± 11.4 | 83.6 ± 63.1 | p < 0.001 | |
| FEV1/FVC < LLN | 129.07 | NA | 133.6 ± 31.1 | 135.6 ± 87.0 | p < 0.001 | 137.5 ± 102.4 | 139.7 ± 131.5 | p < 0.05 |
| 141.13 | 2-Nonenal | 2401.3 ± 1690.9 | 2695.1 ± 1420.6 | p < 0.05 | 2902.3 ± 1690.9 | 3145.4 ± 1829.0 | p < 0.015 | |
| 48.04 | O-methylhydroxylamine | 138.1 ± 92.5 | 135.6 ± 87.0 | p < 0.001 | 142.5 ± 102.4 | 139.7 ± 131.5 | p < 0.01 | |
| FEV1 < LLN | 173.15 | Isomers of Oxononanoic acid | 152.5 ± 115.4 | 94.8 ± 31.3 | p < 0.001 | 109.6 ± 27.7 | 90.6 ± 28.8 | p < 0.01 |
| RV/TLC > LLN | 74.05 | N-methylacetamide | 160.6 ± 21.8 | 153.9 ± 47.4 | p < 0.001 | 148.6 ± 39.0 | 145.5 ± 42.4 | p < 0.01 |
| RV > LLN | 42.03 | Acetonitrile | 76.1 ± 26.8 | 98.9 ± 29.1 | p < 0.001 | 73.8 ± 27.7 | 91.0 ± 29.7 | p < 0.05 |
| DLCO < LLN | 48.04 | O-methylhydroxylamine | 138.1 ± 92.5 | 135.6 ± 87.0 | p < 0.05 | 147.5 ± 102.4 | 139.7 ± 131.5 | p < 0.001 |
| Volume of lung cysts *** | 129.07 | NA | 51.7 ± 12.4 | 55.4 ± 10.4 | p < 0.001 | 42.5 ± 8.1 | 50.9 ± 8.1 | p < 0.001 |
| B1 and B10 dist. average lumen area **** | 57.06 | Isobutene | 624.7 ± 389.6 | 610.3 ± 378.8 | p < 0.001 | 601.0 ± 355.5 | 547.0 ± 313.8 | p < 0.001 |
| 73.06 | Methyl Ethyl Ketone | 206.3 ± 66.1 | 215.2 ± 70.1 | p < 0.001 | 196.5 ± 63.4 | 197.0 ± 53.6 | p = 0.1 | |
| 91.06 | 2-Butanethiol | 115.5 ± 35.0 | 127.1 ± 46.8 | p < 0.001 | 108.8 ± 34.1 | 117.3 ± 41.8 | p < 0.001 | |
| 181.00 | Oxidized Lipid Fragment | 31.9 ± 29.2 | 36.9 ± 25.7 | p < 0.05 | 37.3 ± 40.8 | 39.7 ± 38.3 | p < 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafina, M.; Silantyev, A.; Makarova, M.; Suvorov, A.; Chernyak, A.; Naumenko, Z.; Pakhomov, P.; Pershina, E.; Suvorova, O.; Shmidt, A.; et al. Exhaled Breath Analysis in Lymphangioleiomyomatosis by Real-Time Proton Mass Spectrometry. Int. J. Mol. Sci. 2025, 26, 6005. https://doi.org/10.3390/ijms26136005
Mustafina M, Silantyev A, Makarova M, Suvorov A, Chernyak A, Naumenko Z, Pakhomov P, Pershina E, Suvorova O, Shmidt A, et al. Exhaled Breath Analysis in Lymphangioleiomyomatosis by Real-Time Proton Mass Spectrometry. International Journal of Molecular Sciences. 2025; 26(13):6005. https://doi.org/10.3390/ijms26136005
Chicago/Turabian StyleMustafina, Malika, Artemiy Silantyev, Marina Makarova, Aleksandr Suvorov, Alexander Chernyak, Zhanna Naumenko, Pavel Pakhomov, Ekaterina Pershina, Olga Suvorova, Anna Shmidt, and et al. 2025. "Exhaled Breath Analysis in Lymphangioleiomyomatosis by Real-Time Proton Mass Spectrometry" International Journal of Molecular Sciences 26, no. 13: 6005. https://doi.org/10.3390/ijms26136005
APA StyleMustafina, M., Silantyev, A., Makarova, M., Suvorov, A., Chernyak, A., Naumenko, Z., Pakhomov, P., Pershina, E., Suvorova, O., Shmidt, A., Gordeeva, A., Vergun, M., Bahankova, O., Gognieva, D., Bykova, A., Belevskiy, A., Avdeev, S., Betelin, V., & Kopylov, P. (2025). Exhaled Breath Analysis in Lymphangioleiomyomatosis by Real-Time Proton Mass Spectrometry. International Journal of Molecular Sciences, 26(13), 6005. https://doi.org/10.3390/ijms26136005








