Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response
Abstract
1. Introduction
2. Results
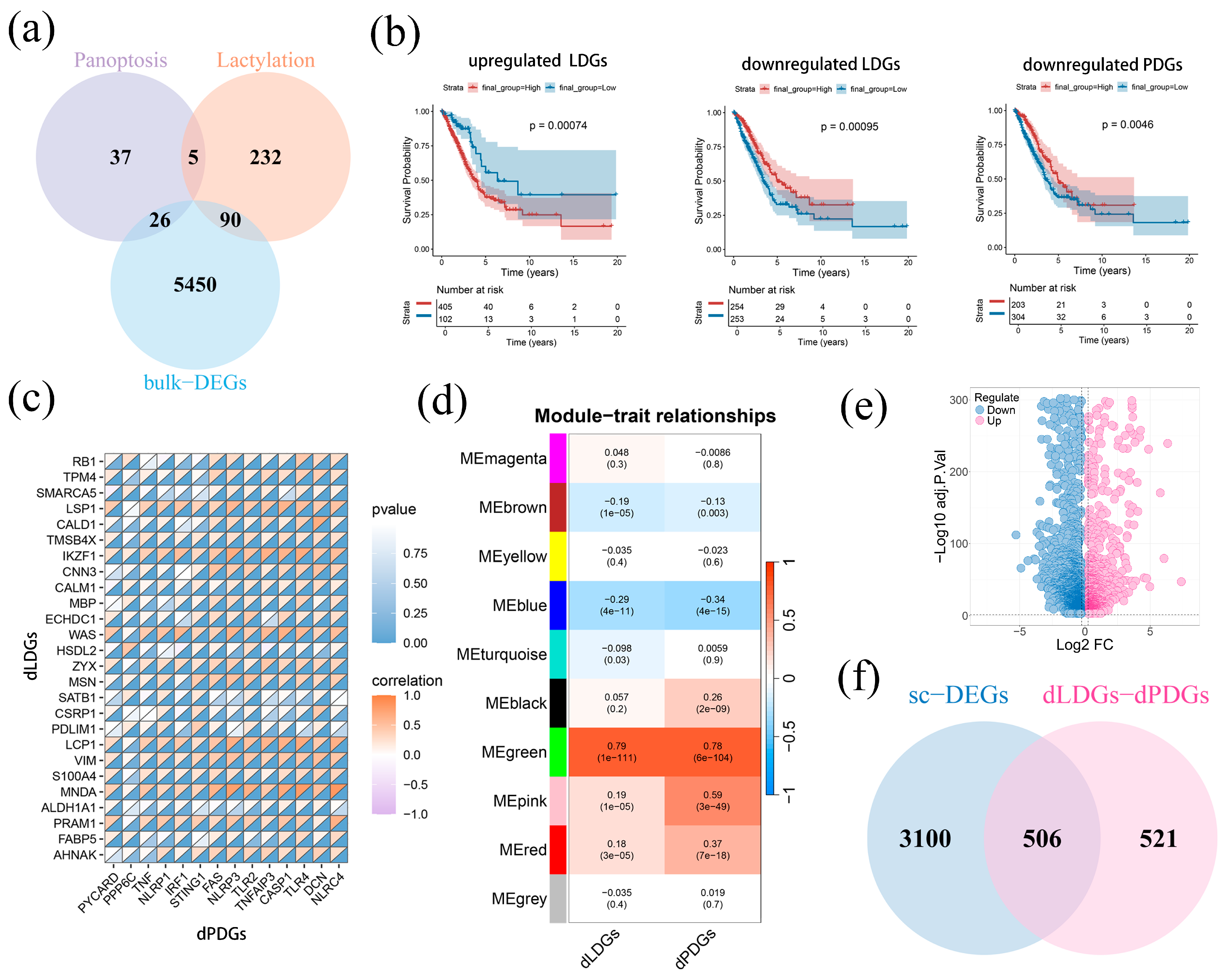
2.1. Identification of Hub Module and Genes Related to PANoptosis and Lactylation
2.2. The Development and Evaluation of LAPRS
2.3. LAPRS Achieved Better Predictive Capability LUAD Patients with Clinical Information
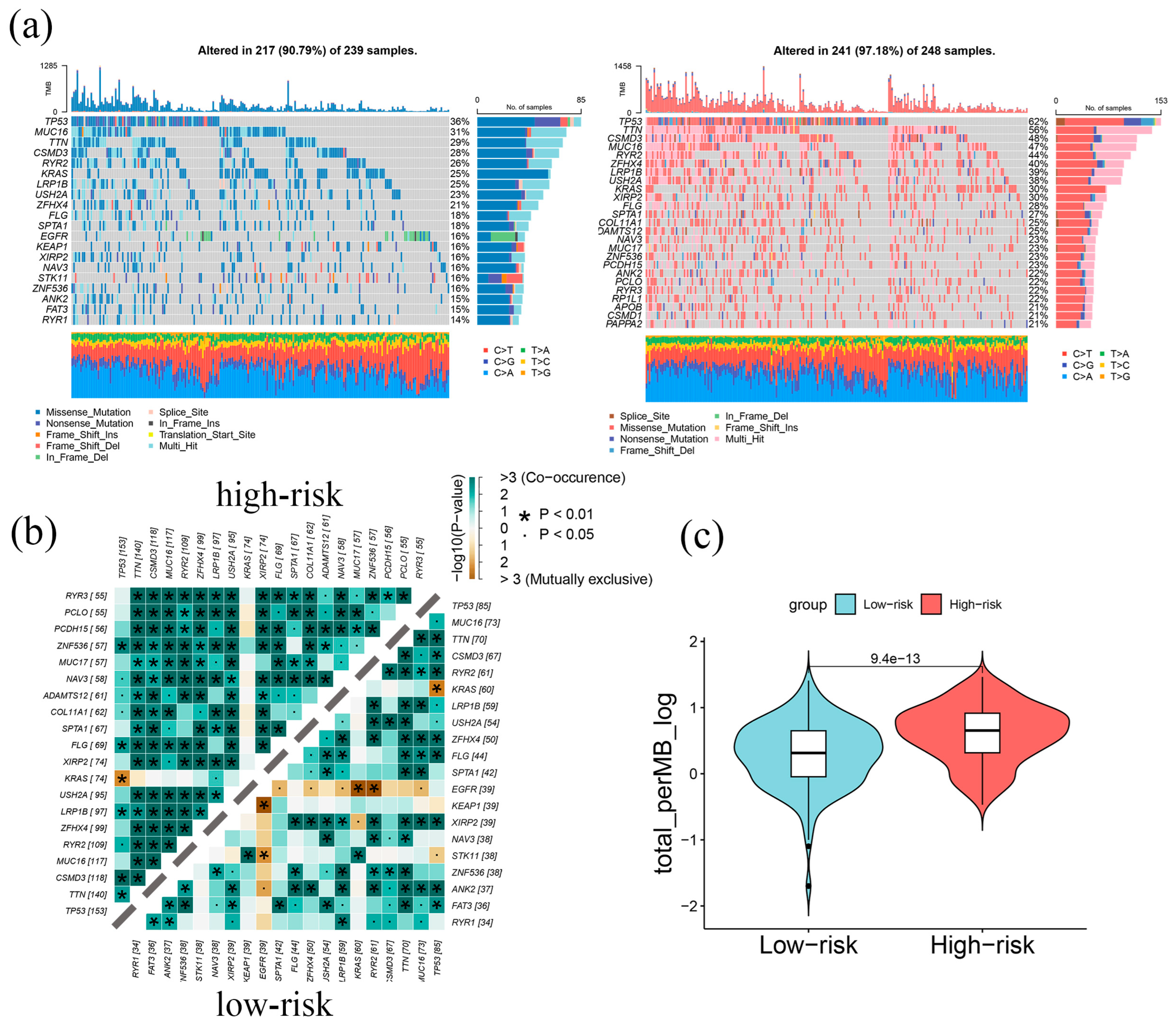
2.4. Genomic Alterations in LAPRS Subgroups
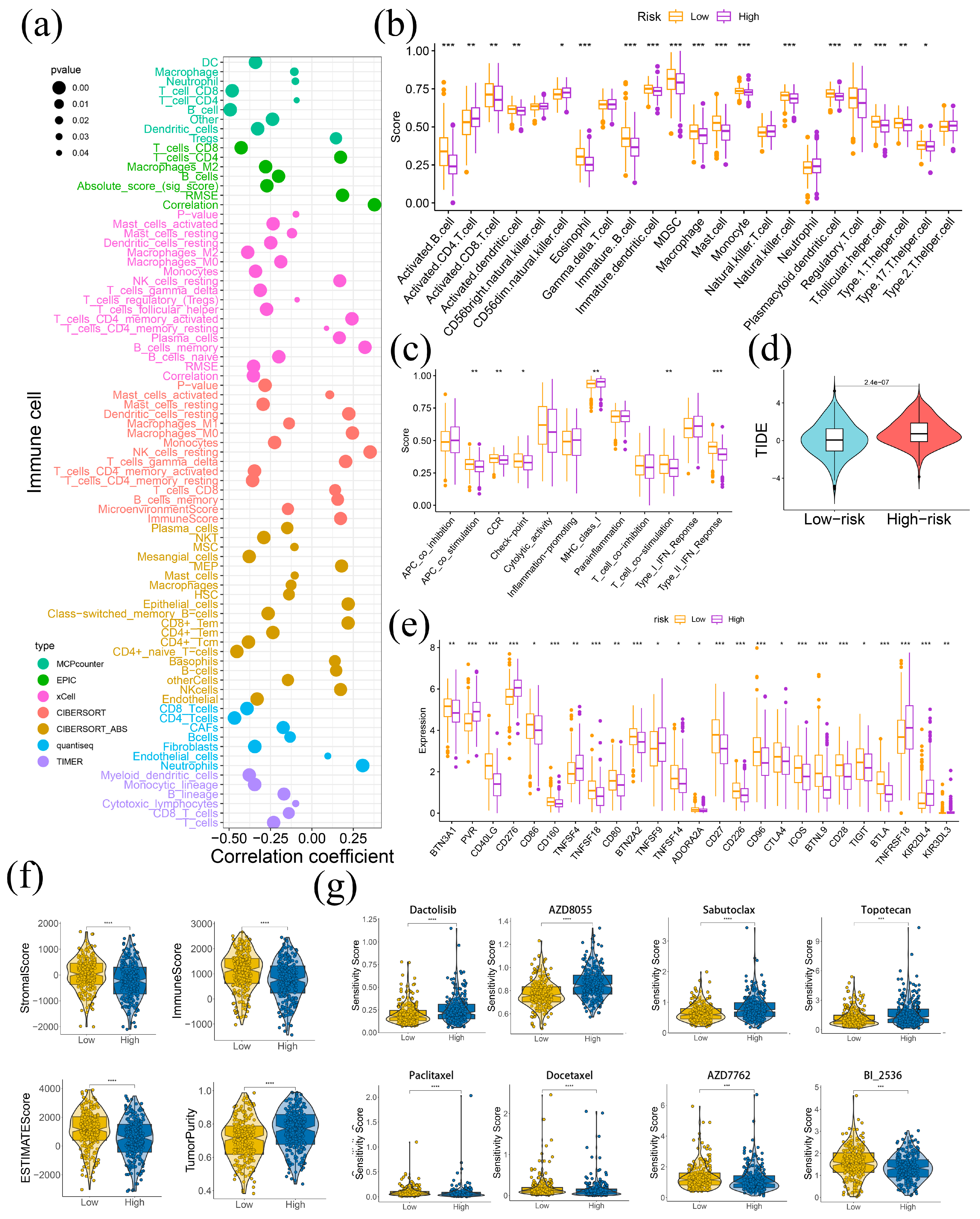
2.5. LAPRS Associated with Tumor Immune Microenvironment
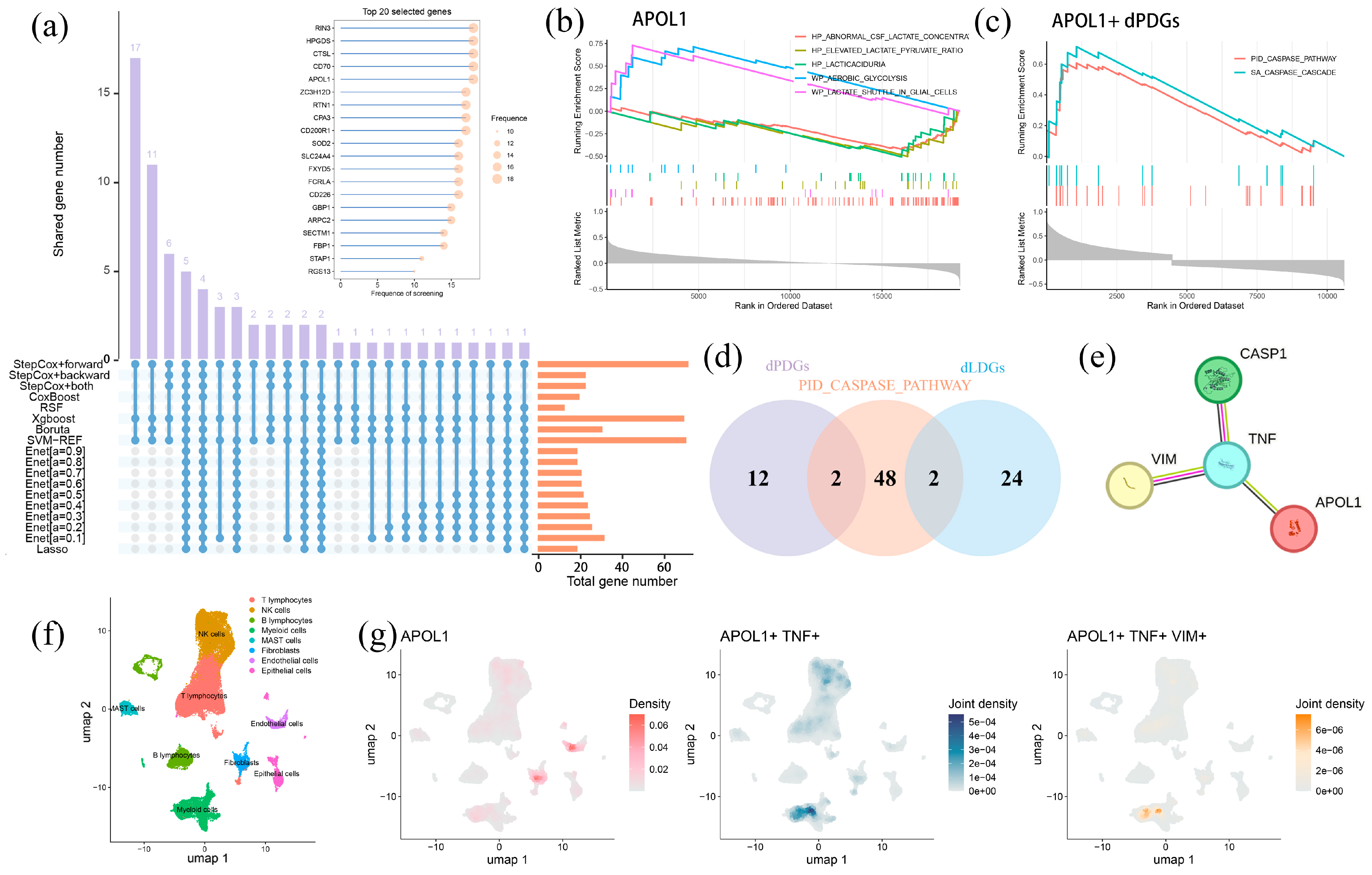
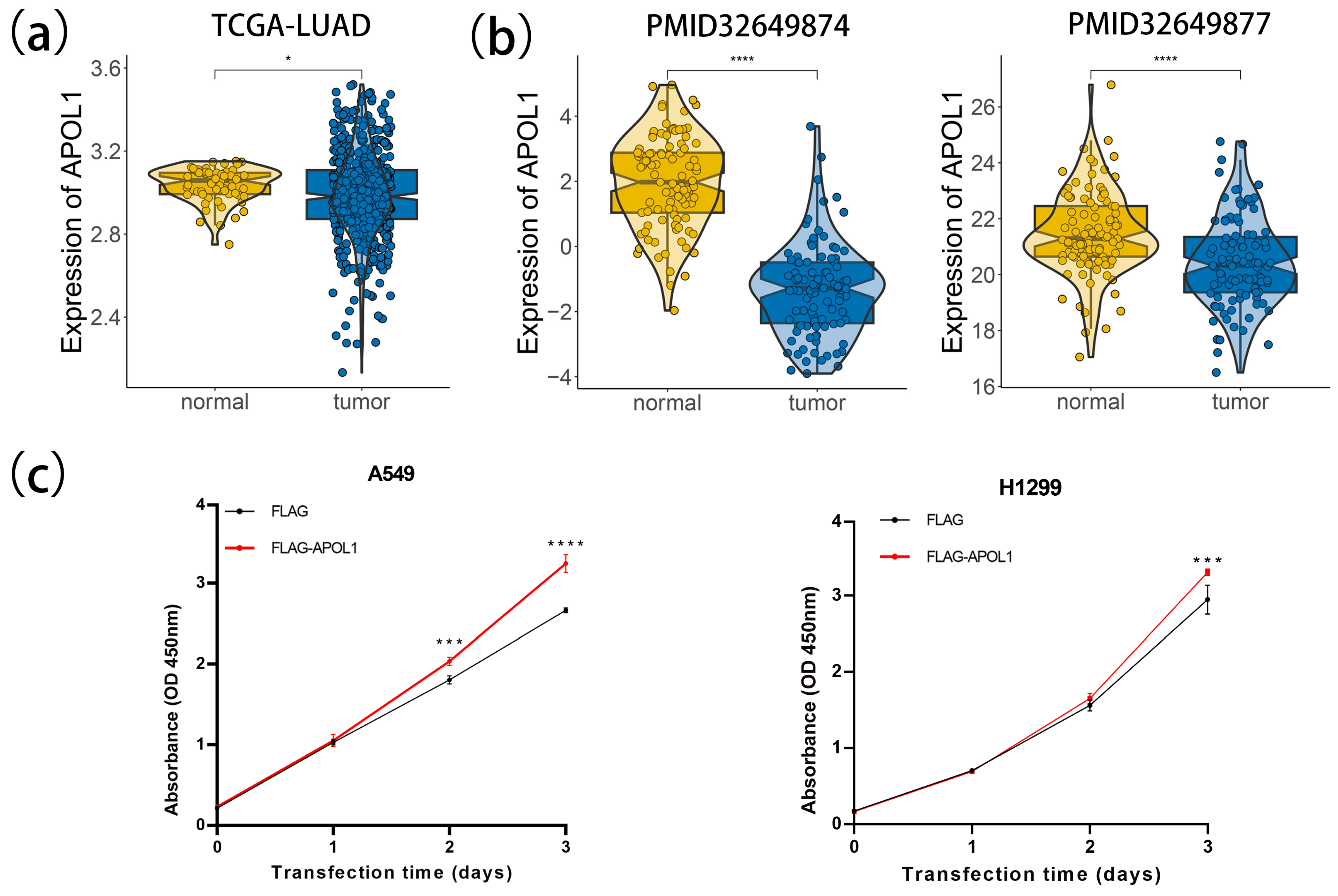
2.6. APOL1-Mediated Tumor Progression Is Potentially Mitigated by Lactylation and PANoptosis
2.7. APOL1 Promotes Cell Proliferation and Viability in LUAD
3. Discussion
4. Material and Methods
4.1. Data Collection and Statistical Analysis
4.2. Weighted Gene Co-Expression Network Analysis
4.3. Single-Cell RNA Sequencing Analysis
4.4. Development and Validation of Prognostic LAPRS
4.5. Construction and Validation of Predictive Nomogram
4.6. Analysis of Genomic Variation Between LAPRS Risk Group
4.7. Enrichment Analysis
4.8. Analysis of Immune Characteristics, Drug Sensitivity, and Immune Checkpoint
4.9. Hub Prognostic Genes Screening
4.10. Cell Culture and Transfection
4.11. Western Blotting
4.12. Cell Counting Kit 8 (CCK8) Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. The Lancet Oncology. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Succony, L.; Rassl, D.M.; Barker, A.P.; McCaughan, F.M.; Rintoul, R.C. Adenocarcinoma spectrum lesions of the lung: Detection, pathology and treatment strategies. Cancer Treat. Rev. 2021, 99, 102237. [Google Scholar] [CrossRef]
- Kolb, T.; Müller, S.; Möller, P.; Barth, T.F.E.; Marienfeld, R. Molecular heterogeneity in histomorphologic subtypes of lung adeno carcinoma represents a challenge for treatment decision. Neoplasia 2024, 49, 100955. [Google Scholar] [CrossRef]
- Johansson, Å.; Andreassen, O.A.; Brunak, S.; Franks, P.W.; Hedman, H.; Loos, R.J.F.; Meder, B.; Melén, E.; Wheelock, C.E.; Jacobsson, B. Precision medicine in complex diseases—Molecular subgrouping for improved prediction and treatment stratification. J. Intern. Med. 2023, 294, 378–396. [Google Scholar] [CrossRef]
- Peng, S.-M.; Ren, J.-J.; Yu, N.; Xu, J.-Y.; Chen, G.-C.; Li, X.; Li, D.-P.; Yang, J.; Li, Z.-N.; Zhang, Y.-S.; et al. The prognostic value of the Naples prognostic score for patients with non-small-cell lung cancer. Sci. Rep. 2022, 12, 5782. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xun, Z.; Zhang, N.; Liu, K.; Chen, X.; Zhao, H. Single-cell RNA sequencing in cancer research: Discovering novel biomarkers and therapeutic targets for immune checkpoint blockade. Cancer Cell Int. 2023, 23, 313. [Google Scholar] [CrossRef]
- Kaur, H.; Dhall, A.; Kumar, R.; Raghava, G.P.S. Identification of Platform-Independent Diagnostic Biomarker Panel for Hepatocellular Carcinoma Using Large-Scale Transcriptomics Data. Front. Genet. 2020, 10, 1306. [Google Scholar] [CrossRef]
- Acharya, D.; Mukhopadhyay, A. A comprehensive review of machine learning techniques for multi-omics data integration: Challenges and applications in precision oncology. Brief. Funct. Genom. 2024, 23, 549–560. [Google Scholar] [CrossRef]
- Iozzo, M.; Pardella, E.; Giannoni, E.; Chiarugi, P. The role of protein lactylation: A kaleidoscopic post-translational modification in cancer. Mol. Cell 2025, 85, 1263–1279. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, Y.; Su, J.; Wang, S.; Su, X.; Ding, X.; Jiang, L.; Fei, X.; Zhang, W. Lactate-induced activation of tumor-associated fibroblasts and IL-8-mediated macrophage recruitment promote lung cancer progression. Redox Biol. 2024, 74, 103209. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Meng, X.; Xie, S.M.; Liu, F.; Chen, H.; Yao, D.; Li, M.; Guo, M.; Shen, H.; et al. HDAC6-G3BP2 promotes lysosomal-TSC2 and suppresses mTORC1 under ETV4 targeting-induced low-lactate stress in non-small cell lung cancer. Oncogene 2023, 42, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Liu, W.; Xia, S.; Zhou, Y.; Tang, M.; Xu, M.; Lin, M.; Li, X.; Wang, Q. Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis. J. Transl. Med. 2023, 21, 547. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhou, C.; Ren, H.; Zhang, Z.; Cheng, F.; Xiong, Z.; Chen, C.; Yang, J.; Gao, J.; Zhang, Y.; et al. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun. Signal. 2020, 18, 167. [Google Scholar] [CrossRef]
- Huang, H.; Chen, K.; Zhu, Y.; Hu, Z.; Wang, Y.; Chen, J.; Li, Y.; Li, D.; Wei, P. A multi-dimensional approach to unravel the intricacies of lactylation related signature for prognostic and therapeutic insight in colorectal cancer. J. Transl. Med. 2024, 22, 211. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, C.; Yang, J.; Chen, M.; Hu, Y. Exploring the prognostic and diagnostic value of lactylation-related genes in sepsis. Sci. Rep. 2024, 14, 23130. [Google Scholar] [CrossRef]
- Zhu, M.; Xiao, Q.; Cai, X.; Chen, Z.; Shi, Q.; Sun, X.; Xie, X.; Sun, M. Predicting lymphoma prognosis using machine learning-based genes associated with lactylation. Transl. Oncol. 2024, 49, 102102. [Google Scholar] [CrossRef]
- Peng, T.; Sun, F.; Yang, J.-C.; Cai, M.-H.; Huai, M.-X.; Pan, J.-X.; Zhang, F.-Y.; Xu, L.-M. Novel lactylation-related signature to predict prognosis for pancreatic adenocarcinoma. World J. Gastroenterol. 2024, 30, 2575–2602. [Google Scholar] [CrossRef]
- Pandian, N.; Kanneganti, T.-D. PANoptosis: A Unique Innate Immune Inflammatory Cell Death Modality. J. Immunol. 2022, 209, 1625–1633. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, A.; Liu, J.; Li, X.; Wang, J.; Zhang, L.; Liu, Y.; Xiong, Y.; Li, G.; He, X. PANoptosis: Bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther. 2024, 31, 970–983. [Google Scholar] [CrossRef]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.-D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.-D. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. ImmunoHorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Z.; Ling, X.; Zhang, W.; Jiang, L. Comprehensive analysis illustrating the role of PANoptosis-related genes in lung cancer based on bioinformatic algorithms and experiments. Front. Pharmacol. 2023, 14, 1115221. [Google Scholar] [CrossRef]
- Bao, C.; Ma, Q.; Ying, X.; Wang, F.; Hou, Y.; Wang, D.; Zhu, L.; Huang, J.; He, C. Histone lactylation in macrophage biology and disease: From plasticity regulation to therapeutic implications. eBioMedicine 2025, 111, 105502. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhao, J.; Fang, X. Dynamics of the immune microenvironment and immune cell PANoptosis in colorectal cancer: Recent advances and insights. Front. Immunol. 2024, 15, 1502257. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, S.; Ye, Q. The lactate metabolism and protein lactylation in epilepsy. Front. Cell. Neurosci. 2025, 18, 1464169. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Lin, S.; Yu, X. Effect of TTN Mutations on Immune Microenvironment and Efficacy of Immunotherapy in Lung Adenocarcinoma Patients. Front. Oncol. 2021, 11, 725292. [Google Scholar] [CrossRef]
- Huang, L.; Lou, N.; Xie, T.; Tang, L.; Han, X.; Shi, Y. Identification of an antigen-presenting cells/T/NK cells-related gene signature to predict prognosis and CTSL to predict immunotherapeutic response for lung adenocarcinoma: An integrated analysis of bulk and single-cell RNA sequencing. Cancer Immunol. Immunother. 2023, 72, 3259–3277. [Google Scholar] [CrossRef]
- Shao, F.; Mao, H.; Luo, T.; Li, Q.; Xu, L.; Xie, Y. HPGDS is a novel prognostic marker associated with lipid metabolism and aggressiveness in lung adenocarcinoma. Front. Oncol. 2022, 12, 894485. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Yang, J.; Zhu, Y.; Li, T.; Liu, X.; Zhang, P.; Cheng, J.; Sun, S.; Wei, C.; et al. CTSL, a prognostic marker of breast cancer, that promotes proliferation, migration, and invasion in cells in triple-negative breast cancer. Front. Oncol. 2023, 13, 1158087. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Deschoolmeester, V.; Zwaenepoel, K.; Rolfo, C.; Silence, K.; Rottey, S.; Lardon, F.; Smits, E.; Pauwels, P. CD70: An emerging target in cancer immunotherapy. Pharmacol. Ther. 2015, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xu, Z.; Xie, J.; Deng, X.; Jiang, L.; Chen, H.; Peng, C.; Li, H.; Zhang, J.; Shen, B. Oncogene APOL1 promotes proliferation and inhibits apoptosis via activating NOTCH1 signaling pathway in pancreatic cancer. Cell Death Dis. 2021, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Le, L.N.-H.; Choi, C.; Han, J.-A.; Kim, E.-B.; Trinh, V.N.; Kim, Y.-J.; Ryu, S. Apolipoprotein L1 is a tumor suppressor in clear cell renal cell carcinoma metastasis. Front. Oncol. 2024, 14, 1371934. [Google Scholar] [CrossRef]
- Hou, J.-M.; Greystoke, A.; Lancashire, L.; Cummings, J.; Ward, T.; Board, R.; Amir, E.; Hughes, S.; Krebs, M.; Hughes, A.; et al. Evaluation of Circulating Tumor Cells and Serological Cell Death Biomarkers in Small Cell Lung Cancer Patients Undergoing Chemotherapy. Am. J. Pathol. 2009, 175, 808–816. [Google Scholar] [CrossRef]
- Vasiukov, G.; Zou, Y.; Senosain, M.-F.; Rahman, J.S.M.; Antic, S.; Young, K.M.; Grogan, E.L.; Kammer, M.N.; Maldonado, F.; Reinhart-King, C.A.; et al. Cancer-associated fibroblasts in early-stage lung adenocarcinoma correlate with tumor aggressiveness. Sci. Rep. 2023, 13, 17604. [Google Scholar] [CrossRef]
- Alexander, S.; Friedl, P. Cancer invasion and resistance: Interconnected processes of disease progression and therapy failure. Trends Mol. Med. 2012, 18, 13–26. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhou, X. Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges. Front. Cell Dev. Biol. 2022, 10, 972020. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Y.; Meng, X.; Li, J.; Liu, X.; Liu, H. PANoptosis: Mechanisms, biology, and role in disease. Immunol. Rev. 2023, 321, 246–262. [Google Scholar] [CrossRef]
- Gong, T.; Wang, Q.-D.; Loughran, P.A.; Li, Y.-H.; Scott, M.J.; Billiar, T.R.; Liu, Y.-T.; Fan, J. Mechanism of lactic acidemia-promoted pulmonary endothelial cells death in sepsis: Role for CIRP-ZBP1-PANoptosis pathway. Mil. Med. Res. 2024, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, S.; Lindenthal, L.; Zeitler, L.; Behnke, K.; Schlösser, D.; Strobl, B.; Scheller, J.; El Kasmi, K.C.; Murray, P.J. Lactate and IL6 define separable paths of inflammatory metabolic adaptation. Sci. Adv. 2021, 7, eabg3505. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ye, Y.; Gu, W.; Xu, X.; Chen, N.; Zhang, L.; Cai, W.; Hu, J.; Wang, T.; Chao, H.; et al. Histone lactylation stimulated upregulation of PSMD14 alleviates neuron PANoptosis through deubiquitinating PKM2 to activate PINK1-mediated mitophagy after traumatic brain injury. Autophagy 2025, 21, 1–19. [Google Scholar] [CrossRef]
- Limou, S.; Dummer, P.D.; Nelson, G.W.; Kopp, J.B.; Winkler, C.A. APOL1 toxin, innate immunity, and kidney injury. Kidney Int. 2015, 88, 28–34. [Google Scholar] [CrossRef]
- Xiao, H.; Qu, Y.; Li, H.; Zhang, Y.; Fei, M.; Liang, C.; Yang, H.; Zhang, X. HIF-2α/LINC02609/APOL1-mediated lipid storage promotes endoplasmic reticulum homeostasis and regulates tumor progression in clear-cell renal cell carcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Zhang, L.; Guo, L.; Wang, X.; Pan, Z.; Jiang, X.; Wu, F.; He, G. Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases. Cell Death Dis. 2023, 14, 851. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168. [Google Scholar] [CrossRef]
- Li, L.-N.; Li, W.-W.; Xiao, L.-S.; Lai, W.-N. Lactylation signature identifies liver fibrosis phenotypes and traces fibrotic progression to hepatocellular carcinoma. Front. Immunol. 2024, 15, 1433393. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Shi, Y.; Ji, C.; Kong, Q.; Sun, K.; Sun, X. Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury. CNS Neurosci. Ther. 2024, 30, e70028. [Google Scholar] [CrossRef]
- Usman, S.; Jamal, A.; Bushaala, A.; Waseem, N.H.; Al-Dehlawi, H.; Yeudall, W.A.; Teh, M.-T.; Tummala, H.; Waseem, A. Transcriptome Analysis Reveals Vimentin-Induced Disruption of Cell–Cell Associations Augments Breast Cancer Cell Migration. Cells 2022, 11, 4035. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, G.; Zhang, N.; Zeng, X. MAP3K4 kinase action and dual role in cancer. Discov. Oncol. 2024, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245–261.e17. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zheng, N.; Zhu, X. Comprehensive analysis identifies a lactylation-related signature for predicting prognosis and guiding therapies in colon adenocarcinoma. Gene 2025, 939, 149191. [Google Scholar] [CrossRef]
- Yang, P.; Huang, G.; Li, Y.; Yu, L.; Yin, Z.; Li, Q. Identification of PANoptosis-related biomarkers and analysis of prognostic values in head and neck squamous cell carcinoma. Sci. Rep. 2024, 14, 9824. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Huang, Y.; Yu, X.; Xuan, J.; Liu, W. Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response. Int. J. Mol. Sci. 2025, 26, 5999. https://doi.org/10.3390/ijms26135999
Xu Z, Huang Y, Yu X, Xuan J, Liu W. Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response. International Journal of Molecular Sciences. 2025; 26(13):5999. https://doi.org/10.3390/ijms26135999
Chicago/Turabian StyleXu, Zhenhao, Yisha Huang, Xiuling Yu, Jiajia Xuan, and Wanting Liu. 2025. "Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response" International Journal of Molecular Sciences 26, no. 13: 5999. https://doi.org/10.3390/ijms26135999
APA StyleXu, Z., Huang, Y., Yu, X., Xuan, J., & Liu, W. (2025). Multi-Omics Integration of Lactylation- and PANoptosis-Based Signatures in Lung Adenocarcinoma: Prognostic Stratification and Immune Response. International Journal of Molecular Sciences, 26(13), 5999. https://doi.org/10.3390/ijms26135999




