Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues
Abstract
1. Introduction
2. Immunohistochemical Differences Between Ectopic Endometriotic Lesions and Eutopic Endometrium
3. Immunohistochemical Differences Across Menstrual Phases
4. Immunohistochemical Findings in Deep Infiltrating Versus Peritoneal Endometriotic Lesions
5. Immunohistochemical Characterization and Comparison Between Ectopic and Non-Endometriosis Tissues
6. Immunohistochemical Evaluation of Pain-Related Markers in Endometriosis
7. Conclusions
Funding
Conflicts of Interest
References
- Cano-Herrera, G.; Salmun Nehmad, S.; Ruiz de Chávez Gascón, J.; Méndez Vionet, A.; van Tienhoven, X.A.; Osorio Martínez, M.F.; Muleiro Alvarez, M.; Vasco Rivero, M.X.; López Torres, M.F.; Barroso Valverde, M.J.; et al. Endometriosis: A Comprehensive Analysis of the Pathophysiology, Treatment, and Nutritional Aspects, and Its Repercussions on the Quality of Life of Patients. Biomedicines 2024, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sheng, S.; Pan, Z.; Zhao, L.; Yang, C.; Li, C.; Wang, F. Immune and endocrine regulation in endometriosis: What we know. J. Endometr. Uterine Disord. 2023, 4, 100049. [Google Scholar] [CrossRef]
- Ochoa Bernal, M.A.; Fazleabas, A.T. The Known, the Unknown and the Future of the Pathophysiology of Endometriosis. Int. J. Mol. Sci. 2024, 25, 5815. [Google Scholar] [CrossRef]
- Zutautas, K.B.; Sisnett, D.J.; Miller, J.E.; Lingegowda, H.; Childs, T.; Bougie, O.; Lessey, B.A.; Tayade, C. The dysregulation of leukemia inhibitory factor and its implications for endometriosis pathophysiology. Front. Immunol. 2023, 14, 1089098. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Pouly, J.L.; Wattiez, A.; Okamura, K.; Mage, G. Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil. Steril. 2004, 82, 1309–1315. [Google Scholar] [CrossRef]
- Vergetaki, A.; Jeschke, U.; Vrekoussis, T.; Taliouri, E.; Sabatini, L.; Papakonstanti, E.A.; Makrigiannakis, A. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLoS ONE 2013, 8, e62313. [Google Scholar] [CrossRef]
- Béliard, A.; Donnez, J.; Nisolle, M.; Foidart, J.M. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil. Steril. 1997, 67, 266–272. [Google Scholar] [CrossRef]
- Sancakli Usta, C.; Turan, G.; Bulbul, C.B.; Usta, A.; Adali, E. Differential expression of Oct-4, CD44, and E-cadherin in eutopic and ectopic endometrium in ovarian endometriomas and their correlations with clinicopathological variables. Reprod. Biol. Endocrinol. 2020, 18, 116. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Jeong, J.W.; Fazleabas, A.T.; Tayade, C.; Young, S.L.; Lessey, B.A. Protein Inhibitor of Activated STAT3 (PIAS3) Is Down-Regulated in Eutopic Endometrium of Women with Endometriosis. Biol. Reprod. 2016, 95, 1–7. [Google Scholar] [CrossRef]
- Alotaibi, F.T.; Peng, B.; Klausen, C.; Lee, A.F.; Abdelkareem, A.O.; Orr, N.L.; Noga, H.; Bedaiwy, M.A.; Yong, P.J. Plasminogen activator inhibitor-1 (PAI-1) expression in endometriosis. PLoS ONE 2019, 14, e0219064. [Google Scholar] [CrossRef]
- Biyik, I.; Kalkan, U.; Simsek, S. The deep infiltrating endometriosis tissue has lower T-cadherin, E-cadherin, progesterone receptor and oestrogen receptor than endometrioma tissue. Taiwan J. Obstet. Gynecol. 2021, 60, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Sommar, A.; Bahat, P.Y.; Özaydin, I.Y.; Bixo, M.; Bäckström, T.; Oral, E.; Turkmen, S. Influence of endometrial nerve fibers and hormones on pain in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 310, 113950. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Dolmans, M.M.; Donnez, J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil. Steril. 2002, 77, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Barrueto, F.F.; Gogusev, J.; Im, D.D.; Morin, P.J. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod. Biol. Endocrinol. 2008, 6, 59. [Google Scholar] [CrossRef]
- Chu, L.H.; Liao, C.C.; Liew, P.L.; Chen, C.W.; Su, P.H.; Wen, K.C.; Lai, H.C.; Huang, R.L.; Chen, L.Y. Epigenomic Analysis Reveals the KCNK9 Potassium Channel as a Potential Therapeutic Target for Adenomyosis. Int. J. Mol. Sci. 2022, 23, 5973. [Google Scholar] [CrossRef]
- Bohonyi, N.; Pohóczky, K.; Szalontai, B.; Perkecz, A.; Kovács, K.; Kajtár, B.; Orbán, L.; Varga, T.; Szegedi, S.; Bódis, J.; et al. Local upregulation of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 ion channels in rectosigmoid deep infiltrating endometriosis. Mol. Pain 2017, 13, e36. [Google Scholar] [CrossRef]
- Mikhaleva, L.M.; Solomatina, A.A.; Milovanov, A.P.; Beeraka, N.M.; Khovanskaya, T.N.; Chabieva, L.B.; Mikhalev, S.A.; Gracheva, N.A.; Chigray, L.V.; Beylerli, O.; et al. Histomorphological and Functional Features of the Eutopic Endometrium in Patients with Ovarian Endometriosis After Surgery-a Clinical Study. Reprod. Sci. 2021, 28, 2350–2358. [Google Scholar] [CrossRef]
- Coroleucă, C.A.; Coroleucă, C.B.; Coroleucă, R.; Brătilă, P.C.; Nodiți, A.R.; Roșca, I.; Brîndușe, L.A.; Brătilă, E.; Boț, M. Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. Int. J. Mol. Sci. 2025, 26, 2983. [Google Scholar] [CrossRef]
- Lenz, J.; Chvatal, R.; Fiala, L.; Konecna, P.; Lenz, D. Comparative immunohistochemical study of deep infiltrating endometriosis, lymph node endometriosis and atypical ovarian endometriosis including description of a perineural invasion. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2021, 165, 69–79. [Google Scholar] [CrossRef]
- Béliard, A.; Noël, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.K.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Matasariu, D.R.; Bausic, A.I.G.; Mandici, C.E.; Bujor, I.E.; Cristofor, A.E.; Bratila, E.; Lozneanu, L.; Boiculese, L.V.; Grigore, M.; Ursache, A. Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis. Biomedicines 2023, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Trapero, C.; Vidal, A.; Fernández-Montolí, M.E.; Coroleu, B.; Tresserra, F.; Barri, P.; De Aranda, I.G.; Sévigny, J.; Ponce, J.; Matias-Guiu, X.; et al. Impaired Expression of Ectonucleotidases in Ectopic and Eutopic Endometrial Tissue Is in Favor of ATP Accumulation in the Tissue Microenvironment in Endometriosis. Int. J. Mol. Sci. 2019, 20, 5532. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2001, 75, 785–790. [Google Scholar] [CrossRef]
- Adamyan, L.; Pivazyan, L.; Zarova, E.; Avetisyan, J.; Laevskaya, A.; Sarkisova, A.; Stepanian, A. Metabolomic biomarkers of endometriosis: A systematic review. J. Endometr. Uterine Disord. 2024, 7, 100077. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noël, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef]
- Matasariu, D.R.; Lozneanu, L.; Dumitraşcu, I.; Grigore, M.; Cristofor, A.E.; Mandici, C.E.; Bujor, I.E.; Ursache, A.; Brăila, A.D.; Bauşic, A.; et al. Hormonal, apoptotic, proliferative and inflammatory markers’ expression in Desogestrel-treated women with ovarian endometriosis. Rom. J. Morphol. Embryol. 2022, 63, 137. [Google Scholar] [CrossRef]
- ÜLGER ÖZBEK, D.; KARAKUŞ, S.; BAKIR, S. Levels of oxidative stress and apoptosis-related biomarkers in endometriosis. Cukurova Med. J. 2023, 48, 480–488. [Google Scholar] [CrossRef]
- Azam, I.N.A.; Wahab, N.A.; Mokhtar, M.H.; Shafiee, M.N.; Mokhtar, N.M. Roles of microRNAs in Regulating Apoptosis in the Pathogenesis of Endometriosis. Life 2022, 12, 1321. [Google Scholar] [CrossRef]
- Brătilă, E.; Brătilă, C.P.; Comandaşu, D.E.; Bauşic, V.; Vlădescu, C.T.; Mehedinţu, C.; Berceanu, C.; Cîrstoiu, M.M.; Mitroi, G.; Stănculescu, R. The assessment of immunohistochemical profile of endometriosis implants, a practical method to appreciate the aggressiveness and recurrence risk of endometriosis. Rom. J. Morphol. Embryol. 2015, 56, 1301–1307. [Google Scholar] [PubMed]
- Hayashi, N.; Kawamorita, N.; Ishizuka, Y.; Kimura, S.; Satake, Y.; Ito, A. Ectopic endometriosis in the pelvic cavity evokes bladder hypersensitivity via transient receptor potential ankyrin 1 hyperexpression in rats. Int. Urogynecol. J. 2023, 34, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bamps, D.; Vriens, J.; De Hoon, J.; Voets, T. TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Verhoef, L.; Aguirre-Hernandez, R.; Nazeran, T.M.; Tessier-Cloutier, B.; Praetorius, T.; Orr, N.L.; Noga, H.; Lum, A.; Khattra, J.; et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum. Reprod. 2019, 34, 69–78. [Google Scholar] [CrossRef]
- Yoo, J.N.; Shin, H.; Kim, T.H.; Choi, W.S.; Ferguson, S.D.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Ha, U.H.; Jeong, J.W. CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PLoS ONE 2014, 9, e100481. [Google Scholar] [CrossRef]
- Sarsenova, M.; Lawarde, A.; Pathare, A.D.S.; Saare, M.; Modhukur, V.; Soplepmann, P.; Terasmaa, A.; Käämbre, T.; Gemzell-Danielsson, K.; Lalitkumar, P.G.L.; et al. Endometriotic lesions exhibit distinct metabolic signature compared to paired eutopic endometrium at the single-cell level. Commun. Biol. 2024, 7, 1026. [Google Scholar] [CrossRef]
- Adamyan, L.; Aznaurova, Y.; Stepanian, A.; Nikitin, D.; Garazha, A.; Suntsova, M.; Sorokin, M.; Buzdin, A. Gene Expression Signature of Endometrial Samples from Women with and without Endometriosis. J. Minim. Invasive Gynecol. 2021, 28, 1774–1785. [Google Scholar] [CrossRef]
- Usta, A.; Turan, G.; Adali, E. The Expression of Cyclophilin A in Ovarian Endometrioma: Its Correlation with Recurrence and Vascularity. Tohoku J. Exp. Med. 2017, 243, 141–150. [Google Scholar] [CrossRef]
- Gołąbek-Grenda, A.; Olejnik, A. In Vitro modeling of endometriosis and endometriotic microenvironment—Challenges and recent advances. Cell Signal 2022, 97, 110375. [Google Scholar] [CrossRef]
- Mai, H.; Liao, Y.; Luo, S.; Wei, K.; Yang, F.; Shi, H. Histone deacetylase HDAC2 silencing prevents endometriosis by activating the HNF4A/ARID1A axis. J. Cell. Mol. Med. 2021, 25, 9972–9982. [Google Scholar] [CrossRef]
- McKinnon, B.; Bersinger, N.A.; Wotzkow, C.; Mueller, M.D. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertil. Steril. 2012, 97, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zutautas, K.B.; Yolmo, P.; Xu, M.; Childs, T.; Koti, M.; Tayade, C. Tertiary lymphoid structures in endometriosis. F&S Sci. 2024, 5, 335–341. [Google Scholar] [CrossRef]
- Chen, X.; Zuo, W.W.; Zhang, G.D.; Xu, C.J. C5aR1+ Macrophages are Involved in the Progression of Endometriosis. Reprod. Dev. Med. 2024, 9, 22–29. [Google Scholar] [CrossRef]
- Istrate-Ofiţeru, A.M.; Berbecaru, E.I.A.; Zorilă, G.L.; Roşu, G.C.; Dîră, L.M.; Comănescu, C.M.; Drăguşin, R.C.; Ruican, D.; Nagy, R.D.; Iliescu, D.G.; et al. Specific Local Predictors That Reflect the Tropism of Endometriosis-A Multiple Immunohistochemistry Technique. Int. J. Mol. Sci. 2022, 23, 5614. [Google Scholar] [CrossRef]
- Alhallak, I.; Quick, C.M.; Graham, G.L.; Simmen, R.C.M. A Pilot Study on the Co-existence of Diabetes and Endometriosis in Reproductive-Age Women: Potential for Endometriosis Progression. Reprod. Sci. 2023, 30, 2429–2438. [Google Scholar] [CrossRef]
- Spasova, V.; Karamisheva, V.; Hammoudeh, Z.; Antonova, O.; Todorov, R.; Koleva, L.; Kolev, A.; Staneva, R.; Toncheva, D.; Hadjidekova, S. MiRNA Expression Profiles in an Ectopic Endometrium of Patients at Different Stage of Endometriosis. Proc. Bulg. Acad. Sci. 2023, 76, 1601–1608. [Google Scholar] [CrossRef]
- Zeng, X.; Yue, Z.; Gao, Y.; Jiang, G.; Zeng, F.; Shao, Y.; Huang, J.; Yin, M.; Li, Y. NR4A1 is Involved in Fibrogenesis in Ovarian Endometriosis. Cell. Physiol. Biochem. 2018, 46, 1078–1090. [Google Scholar] [CrossRef]
- Allam, S.; Paris, E.; Lazcano, I.; Bitterman, P.; Basu, S.; O’Donnell, J.; Barua, A. Detection of Cannabinoid Receptor Expression by Endometriotic Lesions in Women with Endometriosis as an Alternative to Opioid-Based Pain Medication. J. Immunol. Res. 2022, 2022, 4323259. [Google Scholar] [CrossRef]
- Kajitani, T.; Maruyama, T.; Asada, H.; Uchida, H.; Oda, H.; Uchida, S.; Miyazaki, K.; Arase, T.; Ono, M.; Yoshimura, Y. Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr. J. 2013, 60, 1155–1164. [Google Scholar] [CrossRef]
- Peloggia, A.; Andres, M.P.; Abrão, M.S. Expression of ezrin protein and phosphorylated ezrin in pelvic endometriotic lesions. Clinics 2022, 77, 100074. [Google Scholar] [CrossRef]
- Pluchino, N.; Mamillapalli, R.; Wenger, J.M.; Ramyead, L.; Drakopoulos, P.; Tille, J.C.; Taylor, H.S. Estrogen receptor-α immunoreactivity predicts symptom severity and pain recurrence in deep endometriosis. Fertil. Steril. 2020, 113, 1224–1231.e1. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhao, C.; Laganà, A.S.; Liu, X.; Guo, S.W. Identification of lesional attributes of dysmenorrhea severity and the serum antimüllerian hormone levels in women with ovarian endometriomas. Fertil. Steril. 2022, 118, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, S.W. Dysmenorrhea: Risk factors in women with endometriosis. Women’s Health 2008, 4, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Legge, F.; Lauriola, L.; Gessi, M.; Castelli, P.; Barbieri, F.; Minelli, L.; Scambia, G. Analysis of cyclooxygenase-2 (COX-2) expression in different sites of endometriosis and correlation with clinico-pathological parameters. Hum. Reprod. 2004, 19, 393–397. [Google Scholar] [CrossRef]
- Peng, B.; Alotaibi, F.T.; Sediqi, S.; Bedaiwy, M.A.; Yong, P.J. Role of interleukin-1β in nerve growth factor expression, neurogenesis and deep dyspareunia in endometriosis. Hum. Reprod. 2020, 35, 901–912. [Google Scholar] [CrossRef]
- Honda, H.; Nishimichi, N.; Kaneko, M.; Yamashita, M.; Akimoto, Y.; Tanimoto, H.; Teramoto, M.; Teramoto, H.; Yokosaki, Y. Genome-Wide Gene Expression Profiling Reveals the Direct Effect of Dienogest on Ovarian Endometriotic Stromal Cells. Reprod. Sci. 2023, 30, 2457–2467. [Google Scholar] [CrossRef]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
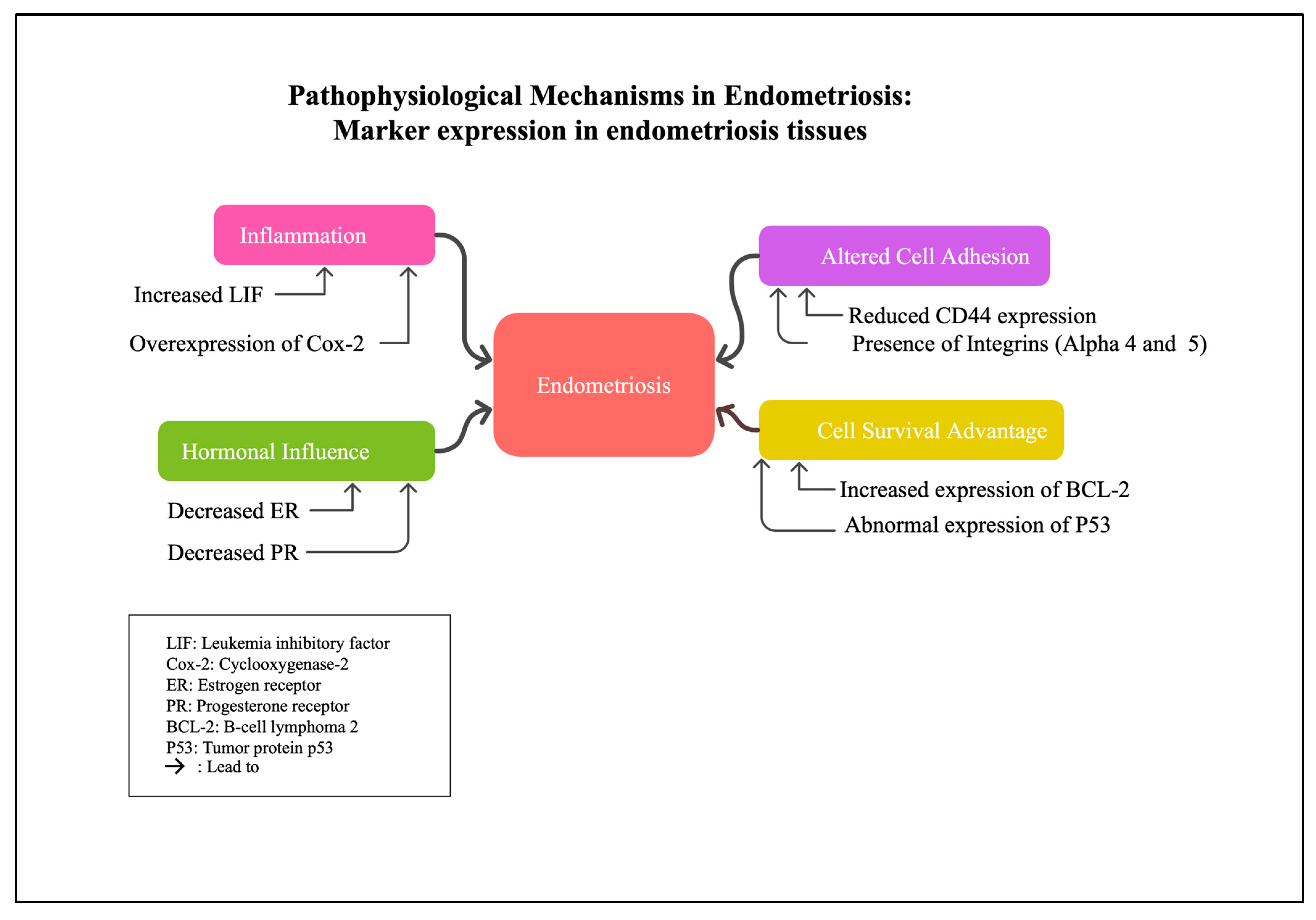
| IHC Marker | Expression Pattern in Ectopic Tissue | Expression Pattern in Eutopic Endometrium | Associated Clinical Features/Functional Relevance |
|---|---|---|---|
| LIF | Elevated, especially during secretory phase | Lower levels | Involved in inflammatory milieu; may contribute to implantation failure and inflammation |
| COX-2 | Overexpressed, particularly during secretory phase | Lower | Promotes inflammation and prostaglandin synthesis, involved in pain and lesion development |
| CRH | Higher levels | Lower or absent | Stress response, inflammation modulation |
| Integrins (Alpha-5 chain) | Predominantly expressed | Lower or absent | Altered adhesion properties in endometriosis lesions |
| Integrins (Alpha-4 chain) | Present in all endometriotic glands | Absent in proliferative phase endometrial glands | Indicates altered cellular adhesion in lesions |
| Oct-4 | Elevated in ectopic tissues | Lower or absent | Marker of pluripotency, linked to cellular plasticity |
| CD44 & E-cadherin | Reduced | Normal levels | Altered cell adhesion, increased migratory potential |
| p53 (apoptotic regulator) | Reduced expression | Higher in eutopic endometrium | Suggests decreased apoptosis in ectopic tissues |
| BCL-2 | Increased | Lower | Anti-apoptotic, promotes cell survival in ectopic tissues |
| PIAS3 | Lower levels | Higher | Dysregulation of STAT3 signaling pathway |
| Estrogen receptor (ER) | Lower amounts, no cyclic modulation | Higher and cyclically regulated | Hormonal responsiveness, influences proliferation |
| PGP9.5 (nerve fiber marker) | Positive in ectopic tissues | Absent in controls | Nerve involvement, pain perception |
| Heme oxygenase (HO) | Increased, varies by lesion type | Lower or absent | Oxidative stress marker |
| AXL, SHC1, ACTN4 | Overexpressed | Lower or absent | Signaling pathways, cytoskeletal dynamics |
| TRPA1, TRPV1 | Elevated in deep infiltrating endometriosis | Lower or absent | Pain perception, sensory transduction |
| IHC Marker | Expression Pattern During Menstrual Cycle | Notes/Functional Relevance |
|---|---|---|
| ER | Slight decrease during mid/late secretory phases | Hormonal sensitivity, proliferation regulation |
| PR | Significant decrease during secretory phases | Hormonal regulation, differentiation |
| Ki-67 (Proliferation marker) | Highest during proliferative phase, declines thereafter | Cell proliferation status |
| MR1A | Present across all phases; mainly in glands | Circadian regulation, possibly linked to tissue response |
| MR1B | Present in glands throughout cycle | Similar to MR1A, involved in circadian signaling |
| Integrin alpha-4 | Present in all phases | Adhesion during cycle |
| Integrin alpha-5 | Increase during proliferative phase, decrease during secretory phases | Cell adhesion and migration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, F.T. Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues. Int. J. Mol. Sci. 2025, 26, 5998. https://doi.org/10.3390/ijms26135998
Alotaibi FT. Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues. International Journal of Molecular Sciences. 2025; 26(13):5998. https://doi.org/10.3390/ijms26135998
Chicago/Turabian StyleAlotaibi, Fahad T. 2025. "Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues" International Journal of Molecular Sciences 26, no. 13: 5998. https://doi.org/10.3390/ijms26135998
APA StyleAlotaibi, F. T. (2025). Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues. International Journal of Molecular Sciences, 26(13), 5998. https://doi.org/10.3390/ijms26135998






