Upregulated BAP31 Links to Poor Prognosis and Tumor Immune Microenvironment in Breast Cancer
Abstract
1. Introduction
2. Results
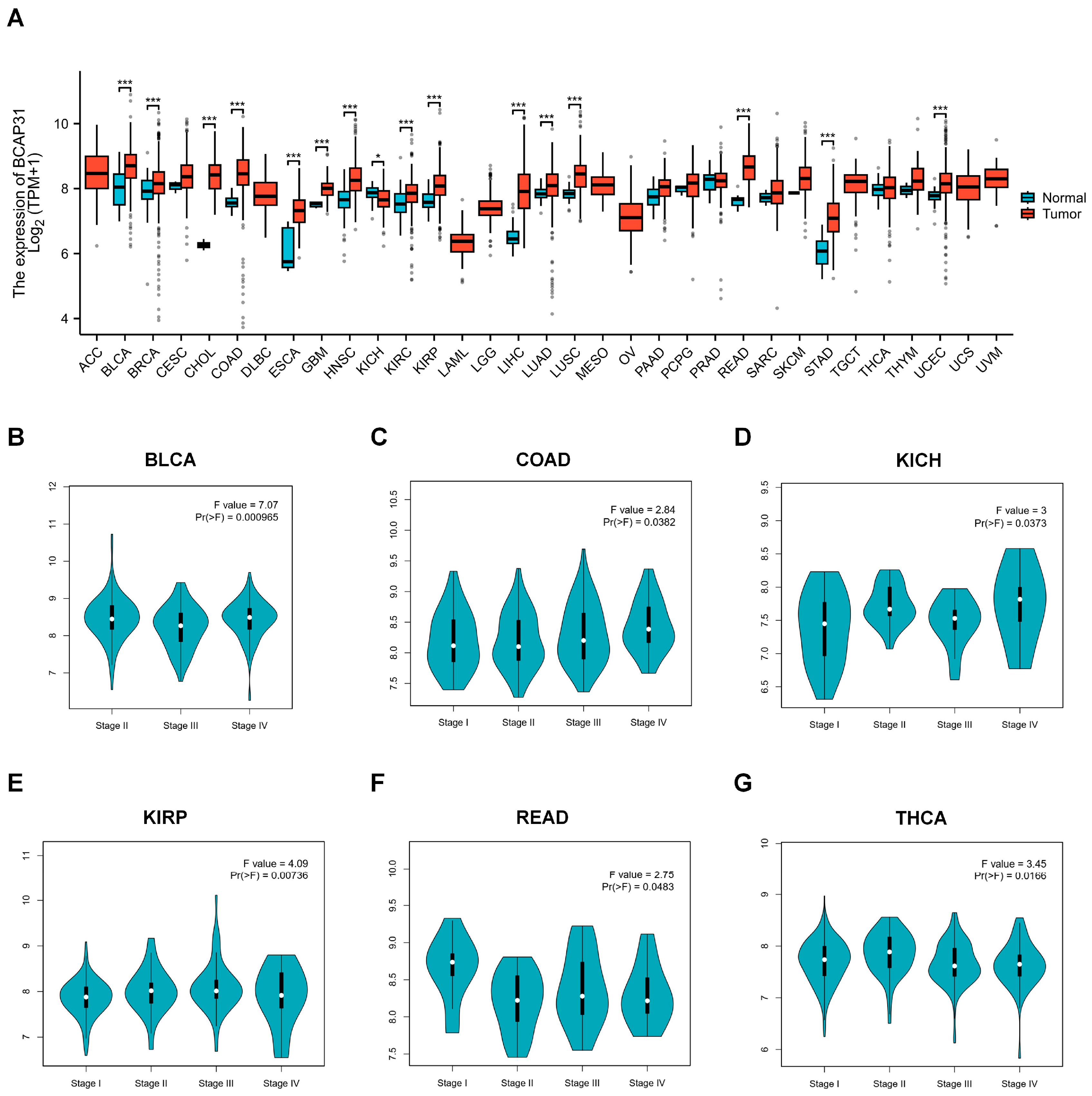
2.1. BAP31 Is Upregulated in a Variety of Human Cancers
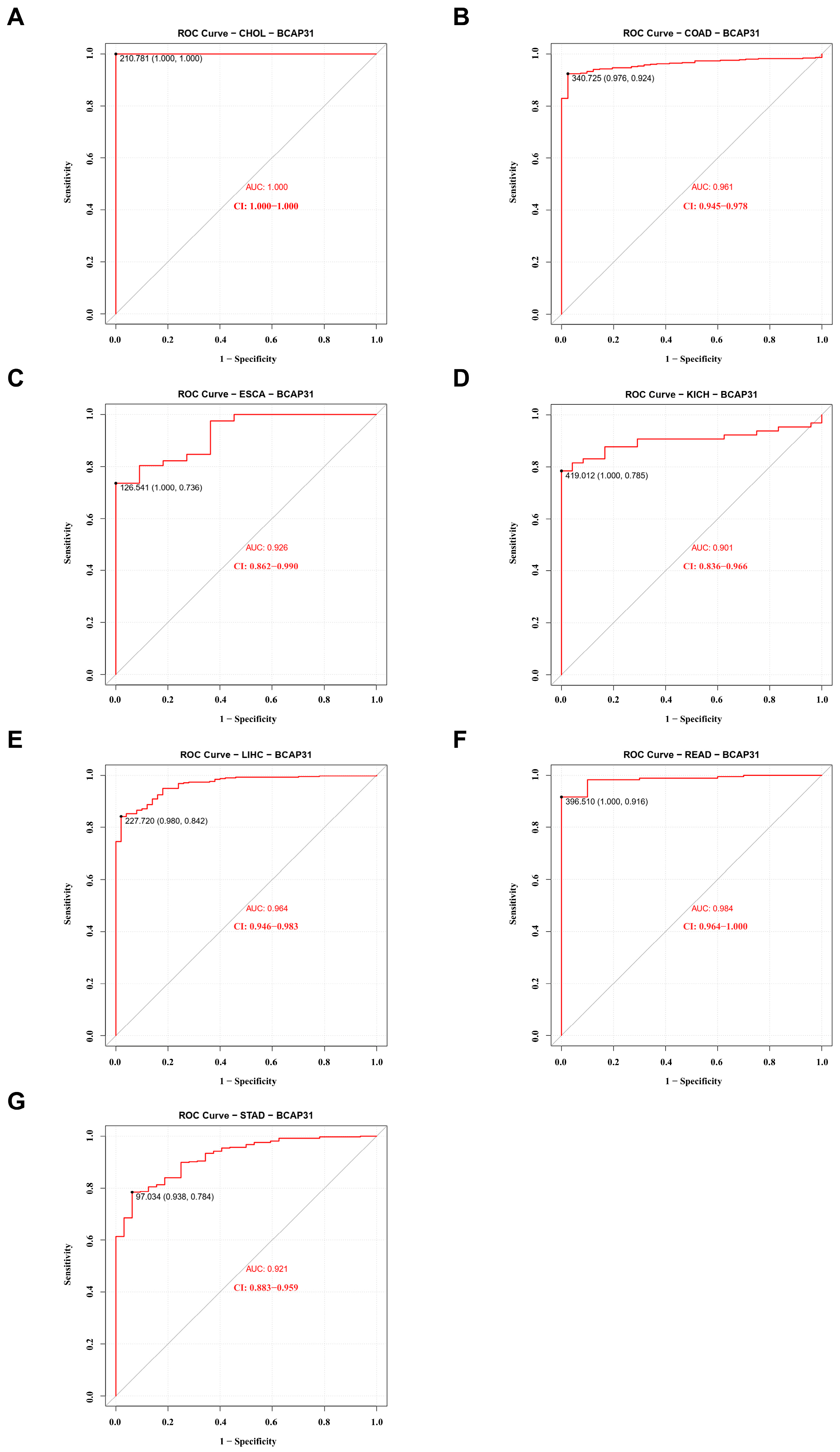
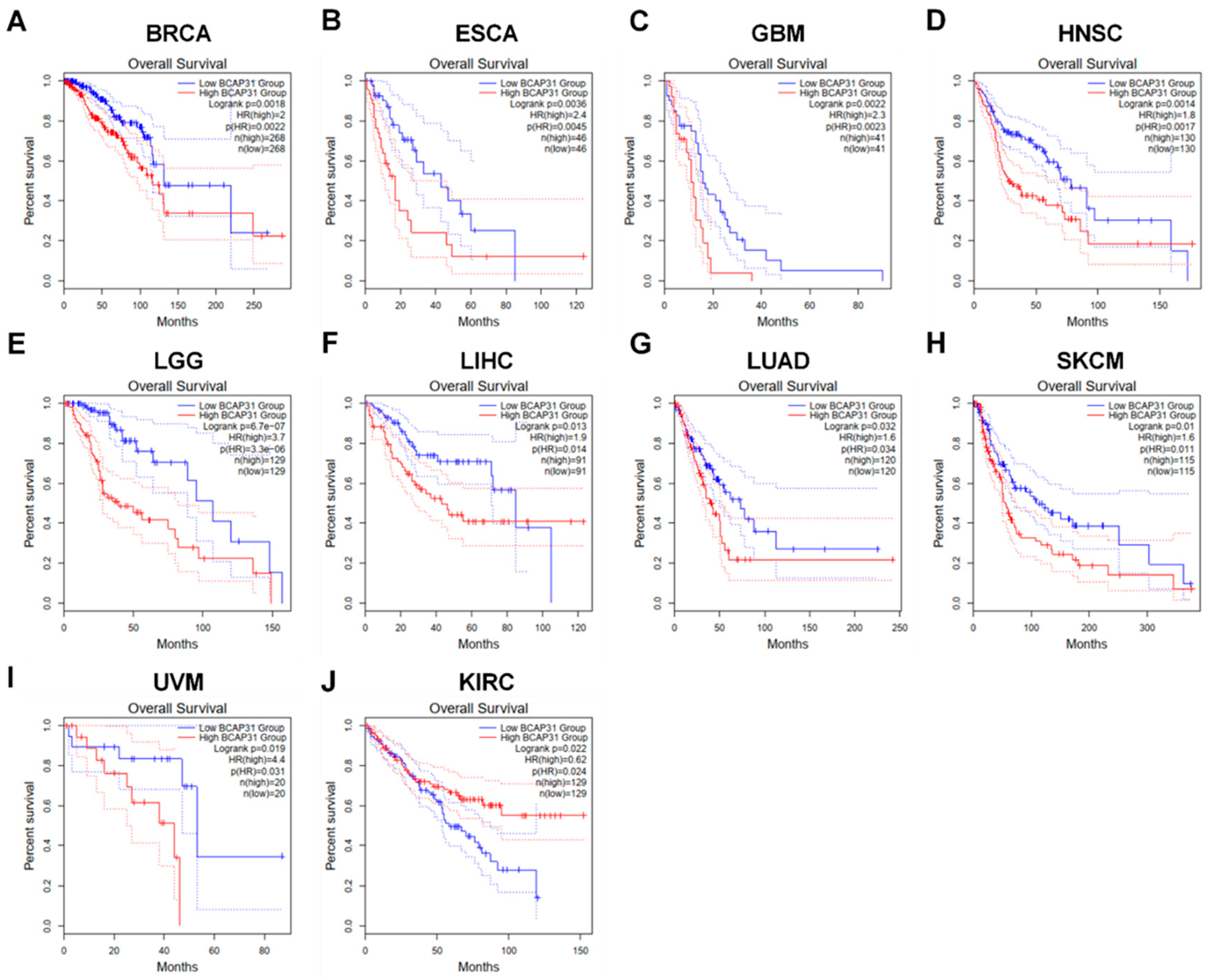
2.2. BAP31 Expression Was Associated with Diagnostic and Prognostic Value in Pan-Cancer
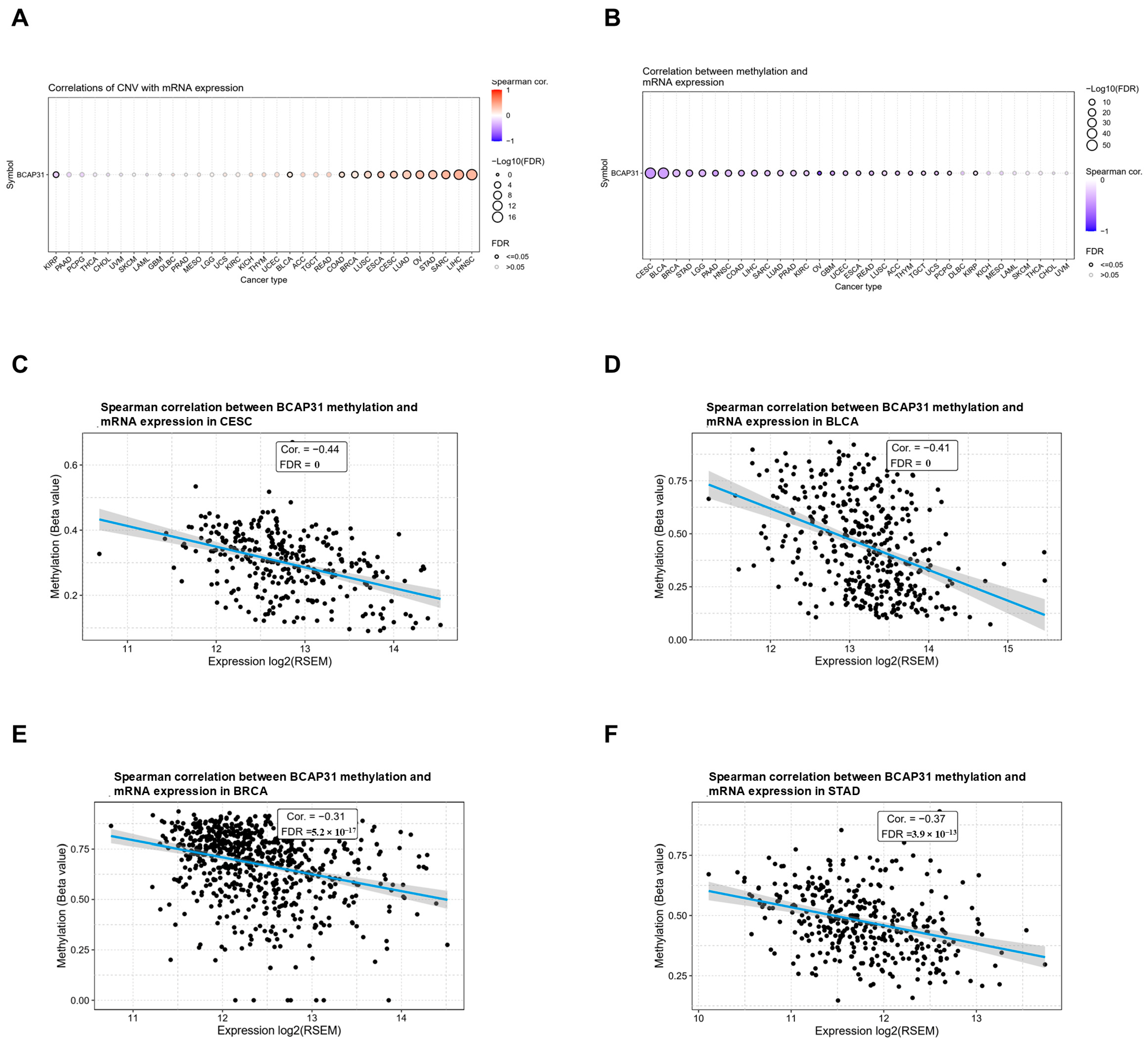
2.3. Abnormal Expression of BAP31 Was Associated with CNV, Methylation in Pan-Cancer
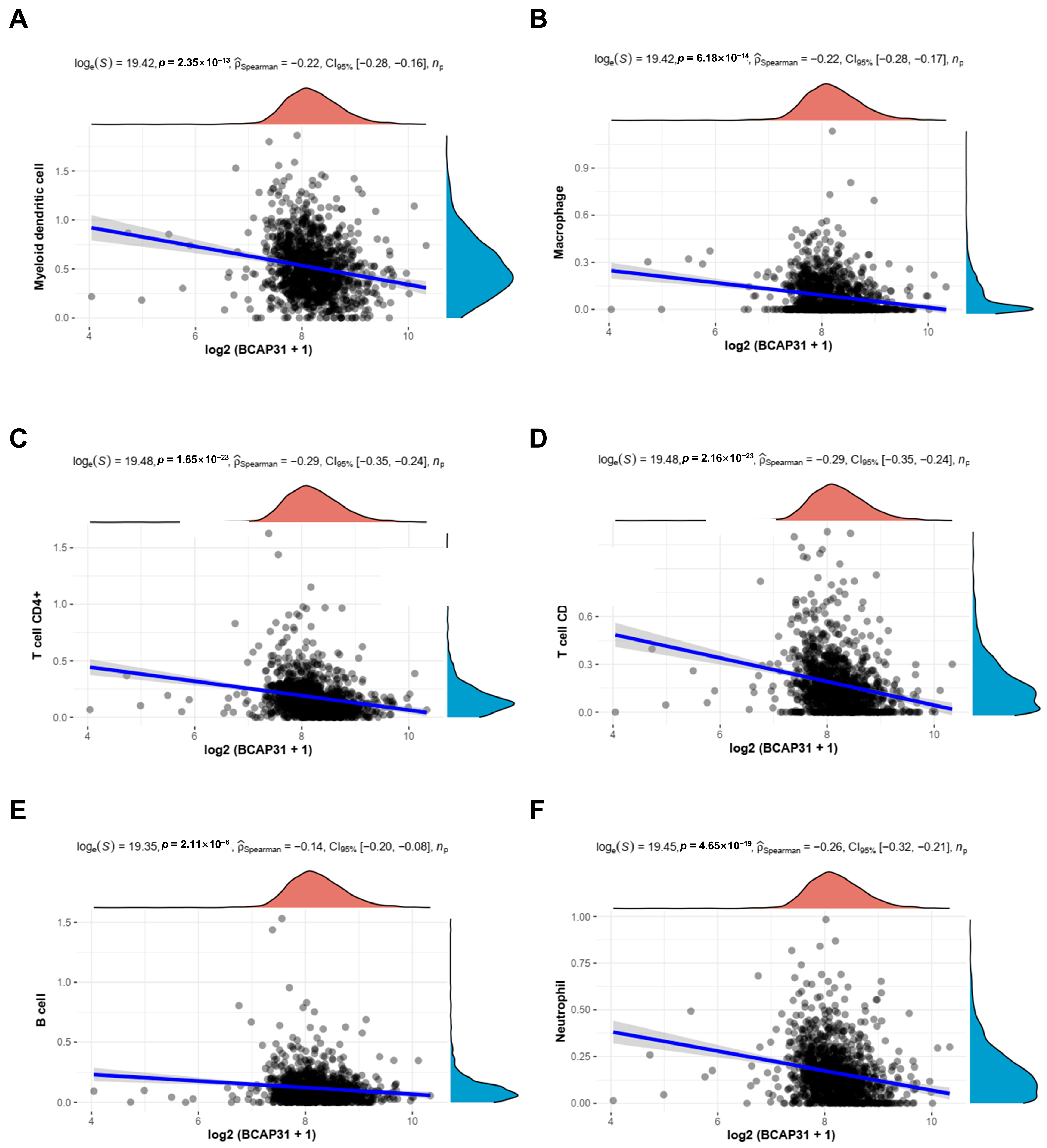
2.4. Correlation Between BAP31 Expression and Immune Cell Infiltration in Breast Cancer
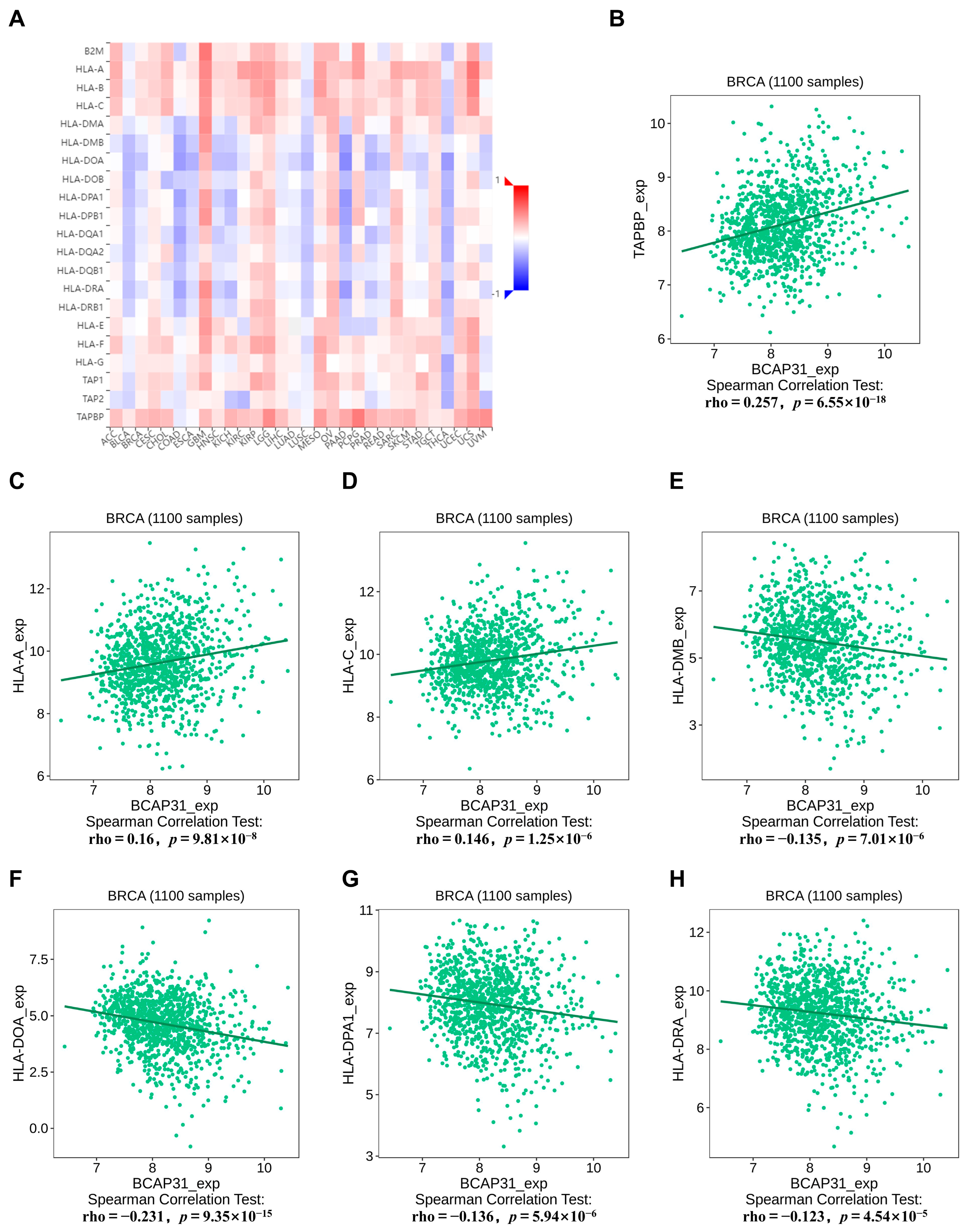
2.5. Correlation Between BAP31 Expression and MHC Molecules in Breast Cancer
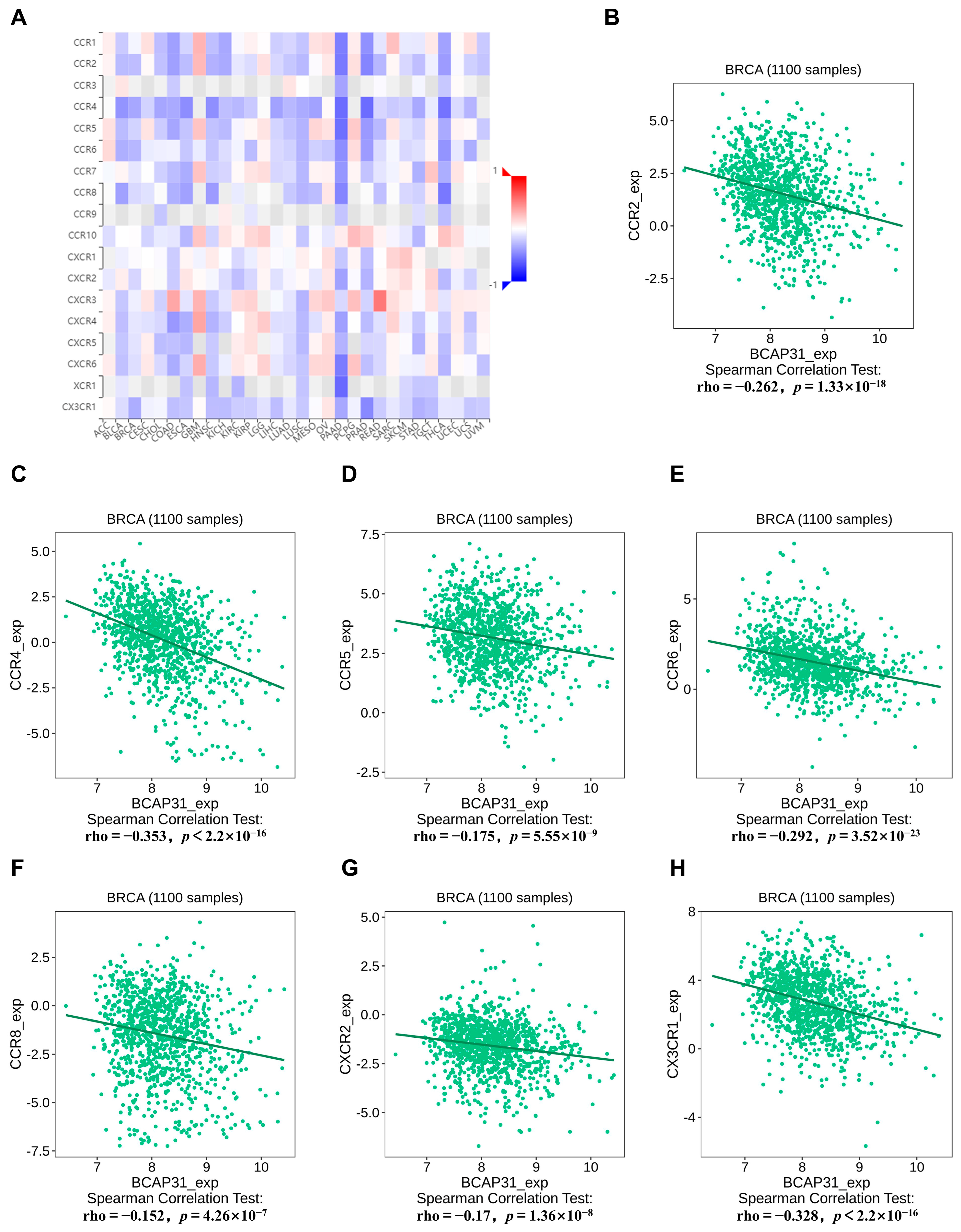
2.6. Correlation Between BAP31 Expression and Chemokine Receptors in Breast Cancer
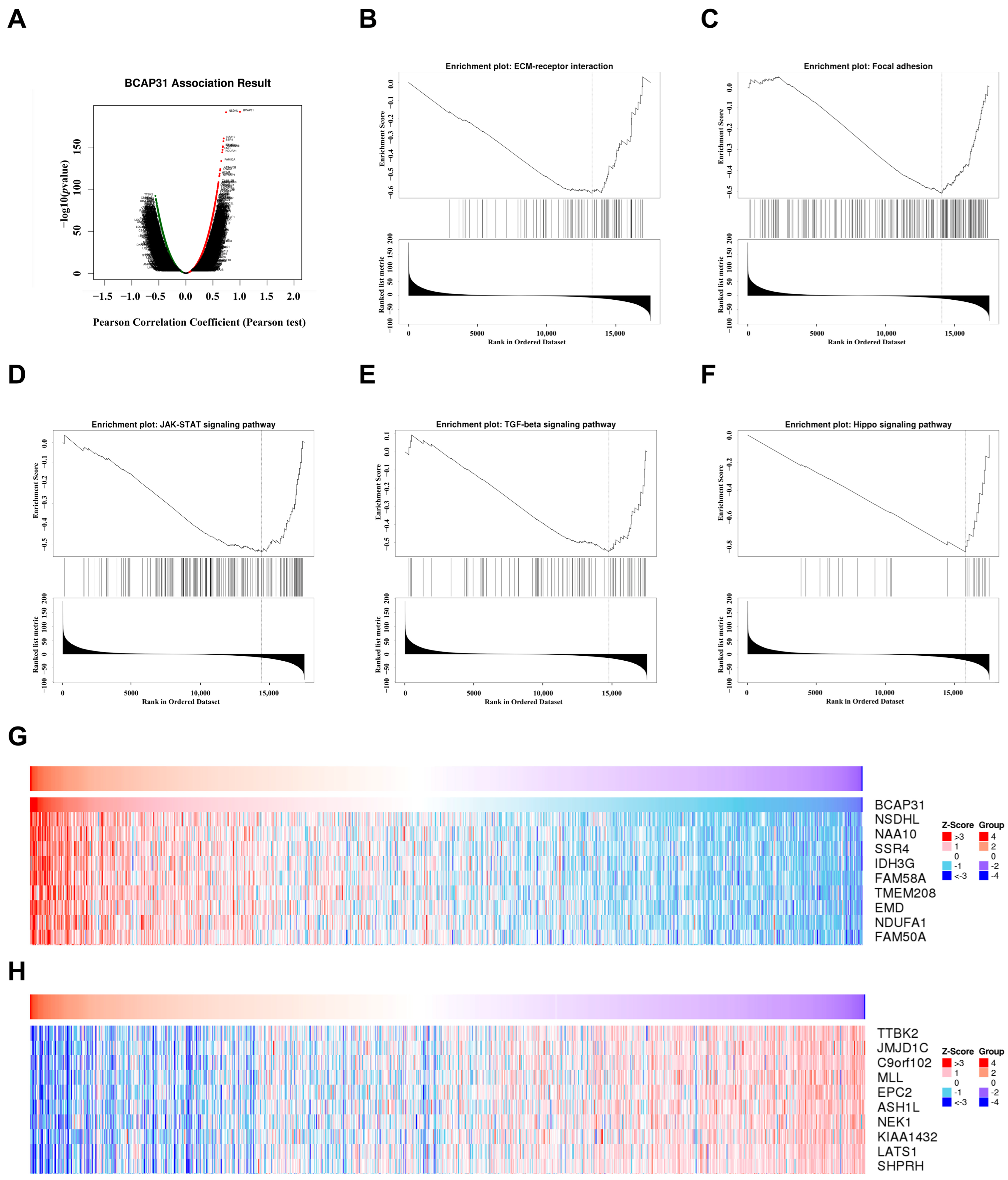
2.7. Functional Analysis of BAP31 in Breast Cancer
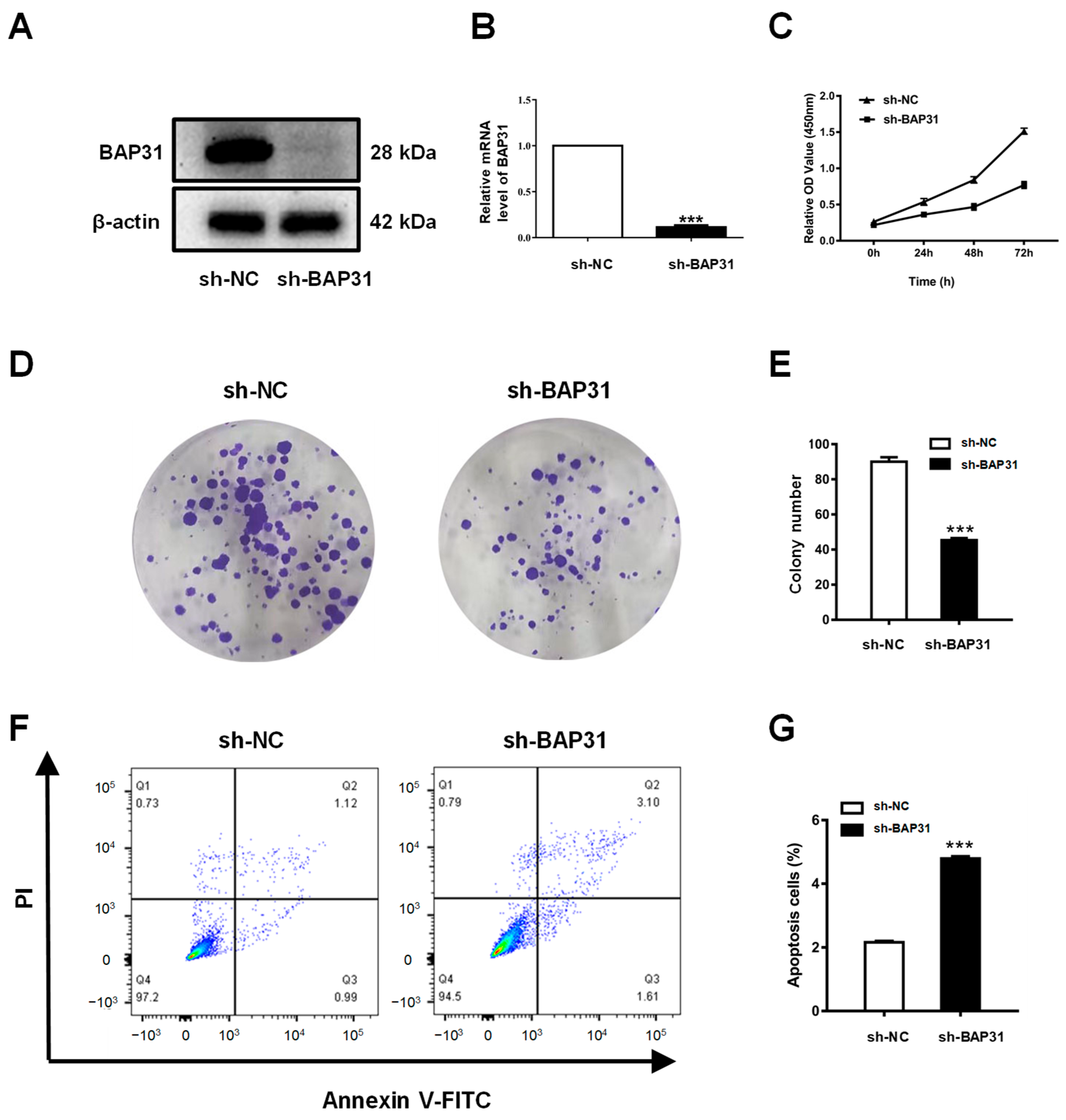
2.8. Knockdown of BAP31 Inhibits Proliferation and Induces Apoptosis of Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. BAP31 Expression Analysis
4.2. Correlation Between BAP31 Expression and CNV, Methylation
4.3. Correlation Analysis of BAP31 Expression Levels with Diagnosis and Prognosis in Human Cancers
4.4. Correlation Analysis Between BAP31 and Tumor Microenvironment
4.5. Function and Enrichment Analysis
4.6. Cell Culture
4.7. Construction of Stable Cell Lines
4.8. RNA Isolation and qPCR
4.9. Western Blot Analysis
4.10. CCK-8 and Colony Formation Assays
4.11. Cell Apoptosis Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trapani, D.; Ginsburg, O.; Fadelu, T.; Lin, N.U.; Hassett, M.; Ilbawi, A.M.; Anderson, B.O.; Curigliano, G. Global challenges and policy solutions in breast cancer control. Cancer Treat. Rev. 2022, 104, 102339. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.Z.; Piyawajanusorn, C.; Gonçalves, A.; Ghislat, G.; Ballester, P.J. Interpretable Machine Learning Models to Pre-dict the Resistance of Breast Cancer Patients to Doxorubicin from Their microRNA Profiles. Adv. Sci. 2022, 9, 2201501. [Google Scholar] [CrossRef] [PubMed]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, S.; Du, Y.; Xu, F.; Sun, W.; Xu, Z.; Wang, X.; Qian, P.; Zhang, Q.; Feng, J.; et al. RelB upregulates PD-L1 and exacerbates prostate cancer immune evasion. J. Exp. Clin. Cancer Res. 2022, 41, 66. [Google Scholar] [CrossRef]
- Chen, M.; Hu, S.; Li, Y.; Jiang, T.T.; Jin, H.; Feng, L. Targeting nuclear acid-mediated immunity in cancer immune checkpoint inhibitor therapies. Signal Transduct. Target. Ther. 2020, 5, 270. [Google Scholar] [CrossRef]
- Kim, K.M.; Adachi, T.; Nielsen, P.J.; Terashima, M.; Lamers, M.C.; Kohler, G.; Reth, M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994, 13, 3793–3800. [Google Scholar] [CrossRef]
- Adachi, T.; Schamel, W.W.; Kim, K.M.; Watanabe, T.; Becker, B.; Nielsen, P.J.; Reth, M. The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J. 1996, 15, 1534–1541. [Google Scholar] [CrossRef]
- Ducret, A.; Nguyen, M.; Breckenridge, D.G.; Shore, G.C. The resident endoplasmic reticulum protein, BAP31, associates with γ-actin and myosin B heavy chain. Eur. J. Biochem. 2003, 270, 342–349. [Google Scholar] [CrossRef]
- Annaert, W.G.; Becker, B.; Kistner, U.; Reth, M.; Jahn, R. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J. Cell. Biol. 1997, 139, 1397–1410. [Google Scholar] [CrossRef]
- Szczesna-Skorupa, E.; Kemper, B. BAP31 Is Involved in the Retention of Cytochrome P450 2C2 in the Endoplasmic Reticulum. J. Biol. Chem. 2006, 281, 4142–4148. [Google Scholar] [CrossRef]
- Ladasky, J.J.; Boyle, S.; Seth, M.; Li, H.; Pentcheva, T.; Abe, F.; Steinberg, S.J.; Edidin, M. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J. Immunol. 2006, 177, 6172–6181. [Google Scholar] [CrossRef]
- Zen, K.; Utech, M.; Liu, Y.; Soto, I.; Nusrat, A.; Parkos, C.A. Association of BAP31 with CD11b/CD18. J. Biol. Chem. 2004, 279, 44924–44930. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Heath-Engel, H.; Zhang, D.; Nguyen, N.; Thomas, D.Y.; Hanrahan, J.W.; Shore, G.C. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 2008, 133, 1080–1092. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Yang, S.; Sun, Y.; Liu, Y.; Shi, J.; Hu, C.; Pan, J.; Liu, T.; Jin, B.; et al. BAP31 Promotes Tumor Cell Proliferation by Stabilizing SERPINE2 in Hepatocellular Carcinoma. Front. Cell. Dev. Biol. 2020, 8, 607906. [Google Scholar] [CrossRef]
- Dang, E.; Yang, S.; Song, C.; Jiang, D.; Li, Z.; Fan, W.; Sun, Y.; Tao, L.; Wang, J.; Liu, T.; et al. BAP31, a newly defined cancer/testis antigen, regulates proliferation, migration, and invasion to promote cervical cancer progression. Cell Death Dis. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, H.; Jiang, H.; Namusamba, M.; Wang, C.; Lan, T.; Wang, T.; Wang, B. A BAP31 intrabody induces gastric cancer cell death by inhibiting p27(kip1) proteasome degradation. Int. J. Cancer 2019, 144, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Namusamba, M.; Wu, Y.; Yang, J.; Zhang, Q.; Wang, C.; Wang, T.; Wang, B. BAP31 Promotes Angiogenesis via Galectin-3 Upregulation in Neuroblastoma. Int. J. Mol. Sci. 2024, 25, 2946. [Google Scholar] [CrossRef]
- Yuan, Q.; Niu, K.; Sun, L.; Zhao, B.; Wang, X.; Wang, B. BAP31 affects macrophage polarization through regulating helper T cells activation. J. Mol. Histol. 2022, 53, 843–855. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, L.; Yuan, Q.; Hao, Z.; An, F.; Zhang, W.; Zhu, X.; Wang, B. BAP31 Knockout in Macrophages Affects CD4+T Cell Activation through Upregulation of MHC Class II Molecule. Int. J. Mol. Sci. 2023, 24, 13476. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Wang, J.; Wang, B. An Intrabody against B-Cell Receptor-Associated Protein 31 (BAP31) Suppresses the Glycosylation of the Epithelial Cell-Adhesion Molecule (EpCAM) via Affecting the Formation of the Sec61-Translocon-Associated Protein (TRAP) Complex. Int. J. Mol. Sci. 2023, 24, 14787. [Google Scholar] [CrossRef]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat. Commun. 2016, 7, 10346. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Pineiro-Hermida, S.; Bosso, G.; Sanchez-Vazquez, R.; Martinez, P.; Blasco, M.A. Telomerase deficiency and dysfunctional telomeres in the lung tumor microenvironment impair tumor progression in NSCLC mouse models and patient-derived xenografts. Cell Death Differ. 2023, 30, 1585–1600. [Google Scholar] [CrossRef]
- Lu, C.; Klement, J.D.; Smith, A.D.; Yang, D.; Waller, J.L.; Browning, D.D.; Munn, D.H.; Liu, K. p50 suppresses cytotoxic T lymphocyte effector function to regulate tumor immune escape and response to immunotherapy. J. Immunother. Cancer 2020, 8, e001365. [Google Scholar] [CrossRef]
- Lu Espinoza-Sánchez, N.A.; Götte, M. Role of cell surface proteoglycans in cancer immunotherapy. Semin. Cancer Biol. 2020, 62, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Quistgaard, E.M. BAP31: Physiological functions and roles in disease. Biochimie 2021, 186, 105–129. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Jiang, D.; Wang, J.; Dang, E.; Li, Z.; Zhou, J.; Lu, Y.; Shi, J.; Tao, L.; et al. MiR-362 suppresses cervical cancer progression via directly targeting BAP31 and activating TGFβ/Smad pathway. Cancer Med. 2021, 10, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Dong, J.; Cheng, Z.; Li, Q.; Feng, D.; Ling, B. B-cell receptor-associated protein 31 promotes migration and invasion in ovarian cancer cells. Exp. Ther. Med. 2021, 22, 858. [Google Scholar] [CrossRef]
- Xu, K.; Han, B.; Bai, Y.; Ma, X.; Ji, Z.; Xiong, Y.; Miao, S.; Zhang, Y.; Zhou, L. MiR-451a suppressing BAP31 can inhibit proliferation and increase apoptosis through inducing ER stress in colorectal cancer. Cell Death Dis. 2019, 10, 152. [Google Scholar] [CrossRef]
- Ma, C.; Jin, R.; Chen, K.; Hao, T.; Li, B.; Zhao, D.; Jiang, H. Low expression of B-Cell-Associated protein 31 is associated with unfavorable prognosis in human colorectal cancer. Pathol. Res. Pract. 2018, 214, 661–666. [Google Scholar] [CrossRef]
- Liu, T.; Yu, J.; Ge, C.; Zhao, F.; Miao, C.; Jin, W.; Su, Y.; Geng, Q.; Chen, T.; Xie, H.; et al. B-Cell Receptor-Associated Protein 31 Promotes Metastasis via AKT/β-Catenin/Snail Pathway in Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 656151. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hao, Z.; Tang, Z.; Li, C.; Cheng, L.; Wang, T.; Zhu, X.; He, Y.; Huang, Y.; Wang, B. BAP31 Regulates Wnt Signaling to Modulate Cell Migration in Lung Cancer. Front. Oncol. 2022, 12, 859195. [Google Scholar] [CrossRef] [PubMed]
- Veroniaina, H.; Wu, Z.; Qi, X. Innate tumor-targeted nanozyme overcoming tumor hypoxia for cancer theranostic use. J. Adv. Res. 2021, 33, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Xia, H.; Yan, Y.; Zhu, X.; Sun, F.; Sun, L.; Li, S.; Li, D.; Wang, J.; et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Med. 2023, 15, 14. [Google Scholar] [CrossRef]
- He, S.; Zheng, L.; Qi, C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol. Cancer 2025, 24, 5. [Google Scholar] [CrossRef]
- Taggart, D.; Andreou, T.; Scott, K.J.; Williams, J.; Rippaus, N.; Brownlie, R.J.; Ilett, E.J.; Salmond, R.J.; Melcher, A.; Lorger, M. Anti–PD-1/anti–CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E1540–E1549. [Google Scholar] [CrossRef]
- Lindo, L.; Wilkinson, L.H.; Hay, K.A. Befriending the Hostile Tumor Microenvironment in CAR T-Cell Therapy. Front. Immunol. 2021, 11, 618387. [Google Scholar] [CrossRef]
- Deng, J.; Galipeau, J. B cells for cancer immunotherapy. OncoImmunology 2014, 3, e955702. [Google Scholar] [CrossRef]
- Sammut, S.; Galson, J.D.; Minter, R.; Sun, B.; Chin, S.; De Mattos-Arruda, L.; Finch, D.K.; Schätzle, S.; Dias, J.; Rueda, O.M.; et al. Predictability of B cell clonal persistence and immunosurveillance in breast cancer. Nat. Immunol. 2024, 25, 916–924. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Carretero, F.J.; Del Campo, A.B.; Flores-Martín, J.F.; Mendez, R.; García-Lopez, C.; Cozar, J.M.; Adams, V.; Ward, S.; Cabrera, T.; Ruiz-Cabello, F.; et al. Frequent HLA class I alterations in human prostate cancer: Molecular mechanisms and clinical relevance. Cancer Immunol. Immunother. 2016, 65, 47–59. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shklovskaya, E.; Lee, J.H.; Pedersen, B.; Stewart, A.; Ming, Z.; Irvine, M.; Shivalingam, B.; Saw, R.P.M.; Menzies, A.M.; et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2023, 14, 1516. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Zhao, B.; Zhu, X.; Zhang, W.; Wang, B. Upregulated BAP31 Links to Poor Prognosis and Tumor Immune Microenvironment in Breast Cancer. Int. J. Mol. Sci. 2025, 26, 5975. https://doi.org/10.3390/ijms26135975
Hao Z, Zhao B, Zhu X, Zhang W, Wang B. Upregulated BAP31 Links to Poor Prognosis and Tumor Immune Microenvironment in Breast Cancer. International Journal of Molecular Sciences. 2025; 26(13):5975. https://doi.org/10.3390/ijms26135975
Chicago/Turabian StyleHao, Zhenzhen, Bo Zhao, Xiaoshuang Zhu, Wanting Zhang, and Bing Wang. 2025. "Upregulated BAP31 Links to Poor Prognosis and Tumor Immune Microenvironment in Breast Cancer" International Journal of Molecular Sciences 26, no. 13: 5975. https://doi.org/10.3390/ijms26135975
APA StyleHao, Z., Zhao, B., Zhu, X., Zhang, W., & Wang, B. (2025). Upregulated BAP31 Links to Poor Prognosis and Tumor Immune Microenvironment in Breast Cancer. International Journal of Molecular Sciences, 26(13), 5975. https://doi.org/10.3390/ijms26135975





