Exploring the Therapeutic Potential of 177Lu-PSMA-617 in a Mouse Model of Prostate Cancer Bone Metastases
Abstract
1. Introduction
2. Results
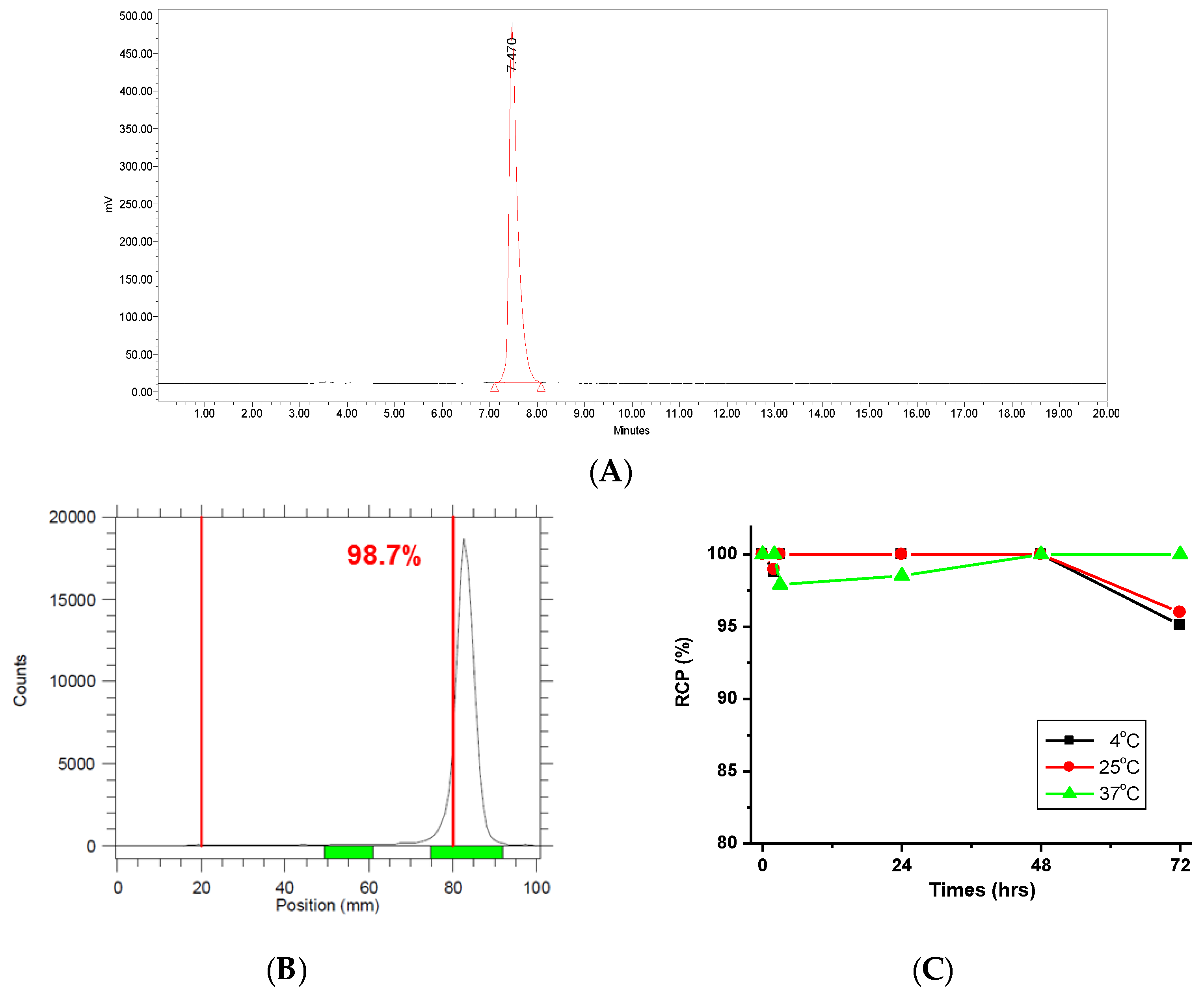
2.1. Characterization of 177Lu-PSMA-617
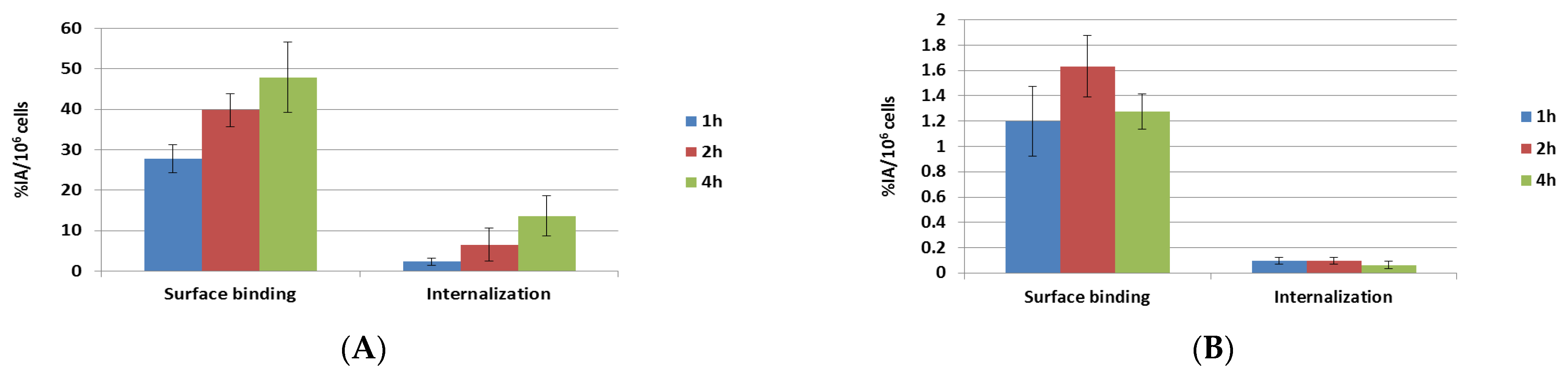
2.2. Surface Binding and Internalization of 177Lu-PSMA-617
2.3. Binding Affinity of natLu-PSMA-617
2.4. Biodistribution of 177Lu-PSMA-617 in Mice with LNCaP Prostate Cancer
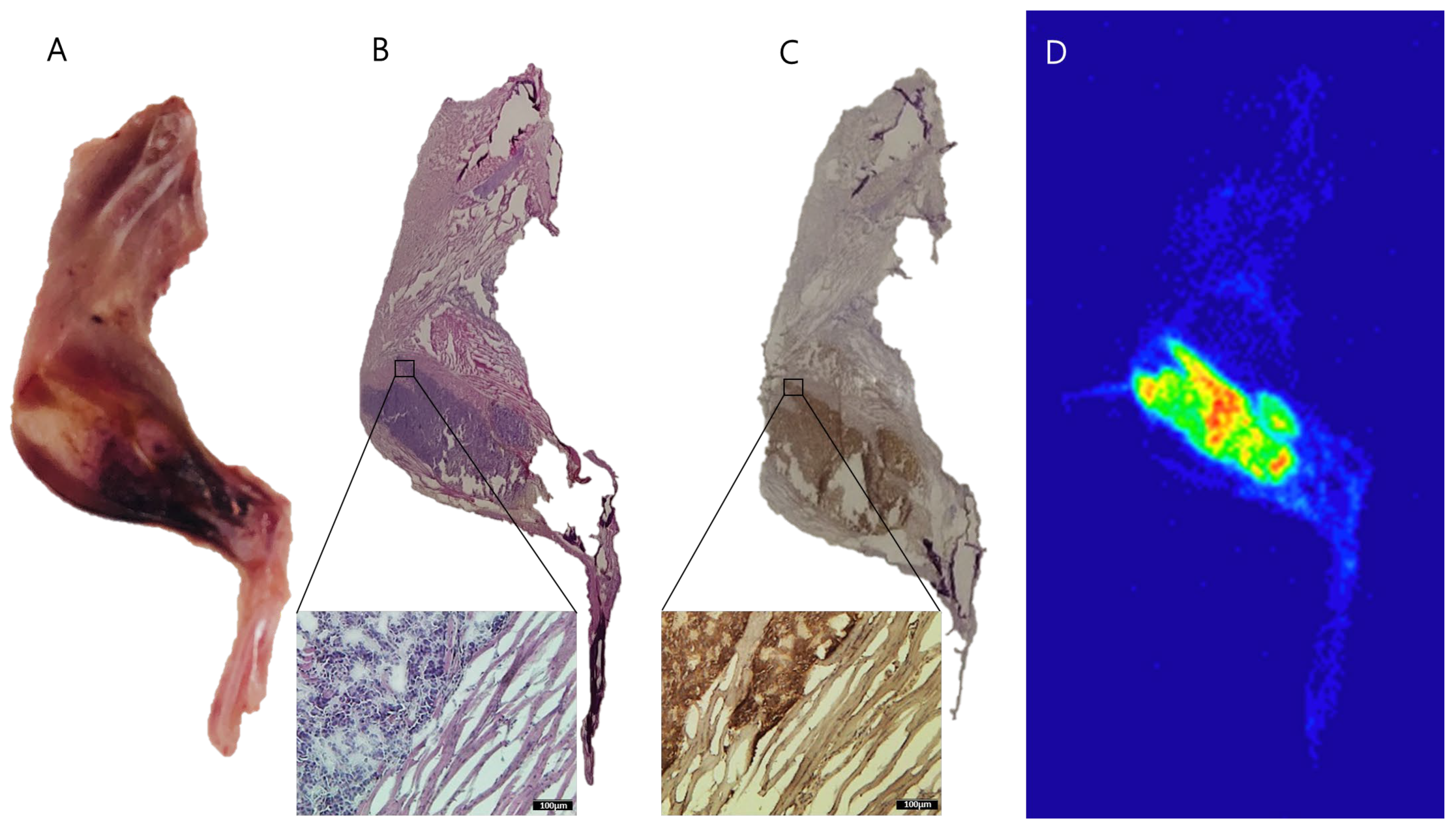
2.5. Autoradiography of 177Lu-PSMA-617 in Bone Metastasis of Prostate Cancer
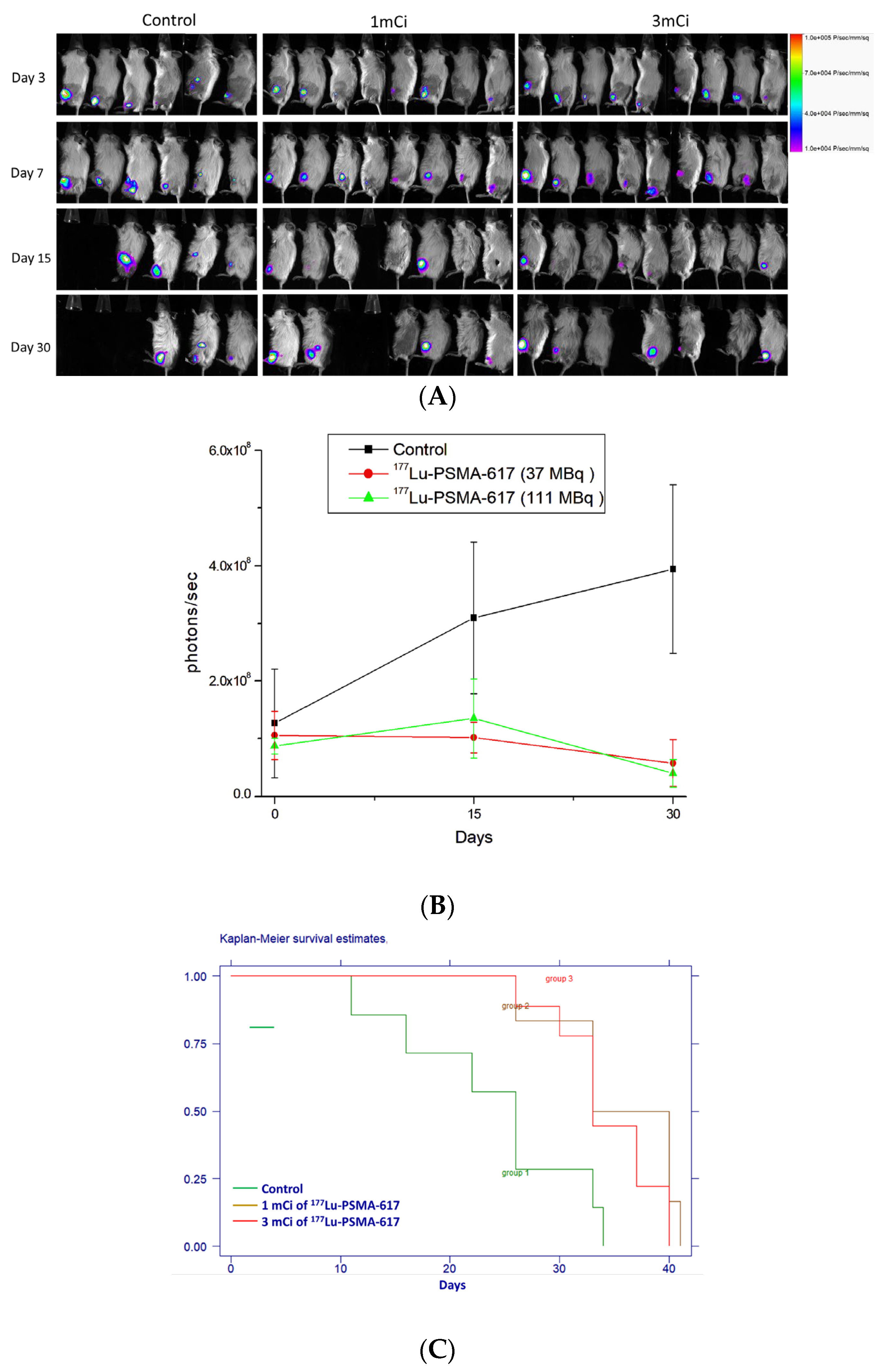
2.6. Therapeutic Efficacy of 177Lu-PSMA-617
2.7. Absorbed Radiation Dose Calculations
3. Discussion
4. Materials and Methods
4.1. Preparation of 177Lu-PSMA-617
4.2. Surface Binding and Internalization of 177Lu-PSMA-617
4.3. Binding Affinity of natLu-PSMA-617
4.4. Bone Metastasis of Prostate Cancer
4.5. Biodistribution of 177Lu-PSMA-617
4.6. Autoradiography of 177Lu-PSMA-617 in Bone Metastasis of Prostate Cancer
4.7. Immunohistochemical Analysis
4.8. Therapeutic Efficacy of 177Lu-PSMA-617
4.9. Absorbed Radiation Dose Calculations
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Smith, A.E.; Muralidharan, A.; Smith, M.T. Prostate cancer induced bone pain: Pathobiology, current treatments and pain responses from recent clinical trials. Discov. Oncol. 2022, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hyväkkä, A.; Kääriäinen, O.S.; Utriainen, T.; Löyttyniemi, E.; Mattila, K.; Reinikainen, P.; Sormunen, J.; Jääskeläinen, M.; Auvinen, P.; Minn, H.; et al. Radium-223 dichloride treatment in metastatic castration-resistant prostate cancer in Finland: A real-world evidence multicenter study. Cancer Med. 2023, 12, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Xie, W.; Ajmera, A.; Araneta, A.; Jamieson, C.; Folefac, E.; Hussain, A.; Kyriakopoulos, C.E.; Olson, A.; Parikh, M.; et al. A Phase I Study of Combination Olaparib and Radium-223 in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) with Bone Metastases (COMRADE). Mol. Cancer Ther. 2023, 22, 511–518. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Gomella, L.G.; Petrylak, D.P. When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials. Eur. Urol. Oncol. 2020, 3, 594–611. [Google Scholar] [CrossRef]
- Parker, C.; Sartor, O. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 365, 767. [Google Scholar] [CrossRef]
- Kręcisz, P.; Czarnecka, K.; Królicki, L.; Mikiciuk-Olasik, E.; Szymański, P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjug. Chem. 2021, 32, 25–42. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy—The Bad Berka Experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef]
- Dash, A.; Chakraborty, S.; Pillai, M.R.; Knapp, F.F., Jr. Peptide receptor radionuclide therapy: An overview. Cancer Biother. Radiopharm. 2015, 30, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Bluemel, C.; Weineisen, M.; Schottelius, M.; Wester, H.J.; Czernin, J.; Eberlein, U.; Beykan, S.; Lapa, C.; Riedmiller, H.; et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Soon, Y.Y.; Marschner, I.C.; Schou, M.; Hofman, M.S.; Emmett, L.; Davis, I.D.; Stockler, M.R.; Martin, A.J. Lu-177 PSMA vs Comparator Treatments and Survival in Metastatic Castration-Resistant Prostate Cancer. JAMA Netw. Open 2024, 7, e2433863. [Google Scholar] [CrossRef]
- Flippot, R.; Telli, T.; Velev, M.; Flechon, A.; Turpin, L.; Bergman, A.M.; Turco, F.; Fendler, W.P.; Giraudet, A.L.; Montravers, F.; et al. Activity of lutetium-177 PSMA (Lu-PSMA) and determinants of outcomes in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with cabazitaxel: The PACAP study. J. Clin. Oncol. 2023, 41, 180. [Google Scholar] [CrossRef]
- Chi, K.N.; Yip, S.M.; Bauman, G.; Probst, S.; Emmenegger, U.; Kollmannsberger, C.K.; Martineau, P.; Niazi, T.; Pouliot, F.; Rendon, R.; et al. 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: A Review of the Evidence and Implications for Canadian Clinical Practice. Curr. Oncol. 2024, 31, 1400–1415. [Google Scholar] [CrossRef]
- Peng, C.L.; Shih, Y.H.; Chiang, P.F.; Chen, C.T.; Chang, M.C. Multifunctional Cyanine-Based Theranostic Probe for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2021, 22, 12214. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Pillai, M.R.; Knapp, F.F., Jr. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (Pluvicto(TM)): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Castellano, D.; Herrmann, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef]
- Guleria, M.; Amirdhanayagam, J.; Sarma, H.D.; Rallapeta, R.P.; Krishnamohan, V.S.; Nimmagadda, A.; Ravi, P.; Patri, S.; Kalawat, T.; Das, T. Preparation of 177Lu-PSMA-617 in Hospital Radiopharmacy: Convenient Formulation of a Clinical Dose Using a Single-Vial Freeze-Dried PSMA-617 Kit Developed In-House. BioMed Res. Int. 2021, 2021, 1555712. [Google Scholar] [CrossRef]
- Cui, C.; Hanyu, M.; Hatori, A.; Zhang, Y.; Xie, L.; Ohya, T.; Fukada, M.; Suzuki, H.; Nagatsu, K.; Jiang, C.; et al. Synthesis and evaluation of [64Cu]PSMA-617 targeted for prostate-specific membrane antigen in prostate cancer. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 40–52. [Google Scholar] [PubMed]
- Zhao, Y.; Culman, J.; Cascorbi, I.; Nithack, N.; Marx, M.; Zuhayra, M.; Lützen, U. PSMA-617 inhibits proliferation and potentiates the 177Lu-PSMA-617-induced death of human prostate cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Gourni, E.; Canovas, C.; Goncalves, V.; Denat, F.; Meyer, P.; Maecke, H. (R)-NODAGA-PSMA: A versatile precursor for radiometal labeling and nuclear imaging of PSMA-positive tumors. PLoS ONE 2015, 10, e0145755. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Eder, M.; Afshar-Oromieh, A.; Benešová, M.; Mier, W.; Kopka, K.; Haberkorn, U. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.; Schäfer, M.; Benešová, M.; Bauder-Wüst, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical Evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef]
- Abou, D.S.; Ulmert, D.; Doucet, M.; Hobbs, R.F.; Riddle, R.C.; Thorek, D.L. Whole-Body and Microenvironmental Localization of Radium-223 in Naïve and Mouse Models of Prostate Cancer Metastasis. J. Natl. Cancer Inst. 2016, 108, djv380. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Humm, J.L.; Sartor, O.; Parker, C.; Bruland, O.S.; Macklis, R. Radium-223 in the treatment of osteoblastic metastases: A critical clinical review. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 898–906. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. An Off. J. Am. Assoc. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef]
- Fendler, W.P.; Stuparu, A.D.; Evans-Axelsson, S.; Lückerath, K.; Wei, L.; Kim, W.; Poddar, S.; Said, J.; Radu, C.G.; Eiber, M.; et al. Establishing 177Lu-PSMA-617 Radioligand Therapy in a Syngeneic Model of Murine Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1786–1792. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Kumar, V.; Lisok, A.; Chen, J.; Minn, I.; Brummet, M.; Boinapally, S.; Cole, M.; Ngen, E.; Wharram, B.; et al. 177Lu-labeled low-molecular-weight agents for PSMA-targeted radiopharmaceutical therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2545–2557. [Google Scholar] [CrossRef]
- Kabasakal, L.; AbuQbeitah, M.; Aygün, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Mizokami, A.; Keller, E.T.; Taichman, R.; Zhang, J.; Namiki, M. The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Cancer Res. 2005, 65, 8818–8825. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Tarnowski, C.P.; Zhang, J.; Dai, J.; Rohn, E.; Patel, A.H.; Morris, M.D.; Keller, E.T. Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate 2001, 47, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Peng, C.L.; Chiang, P.F.; Lin, W.J.; Luo, T.Y.; Shieh, M.J. Therapeutic and scintigraphic applications of polymeric micelles: Combination of chemotherapy and radiotherapy in hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 7443–7454. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chang, Y.-J.; Lee, T.-W.; Ting, G.; Chang, K.-P. Dosimetric evaluation of nanotargeted 188Re-liposome with the MIRDOSE3 and OLINDA/EXM programs. Ann. Nucl. Med. 2012, 26, 419–425. [Google Scholar] [CrossRef]


| Organ | Estimated Dose (mSv/MBq) |
|---|---|
| Adrenals | 8.15E−04 |
| Brain | 3.54E−05 |
| Breasts | 7.86E−03 |
| LLI Wall | 2.01E−02 |
| Small Intestine | 8.52E−04 |
| Stomach Wall | 1.98E−02 |
| ULI Wall | 8.45E−04 |
| Kidneys | 2.19E−04 |
| Liver | 5.81E−04 |
| Lungs | 1.47E−03 |
| Muscle | 6.33E−05 |
| Pancreas | 8.27E−04 |
| Red Marrow | 1.47E−02 |
| Osteogenic Cells | 4.98E−03 |
| Skin | 1.54E−03 |
| Spleen | 6.92E−05 |
| Testes | 2.16E−02 |
| Thymus | 8.01E−04 |
| Thyroid | 8.08E−03 |
| Urinary Bladder Wall | 8.32E−03 |
| Uterus | 8.48E−04 |
| Effective Dose | 1.27E−01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-L.; Chen, C.-T.; Tang, I.-C. Exploring the Therapeutic Potential of 177Lu-PSMA-617 in a Mouse Model of Prostate Cancer Bone Metastases. Int. J. Mol. Sci. 2025, 26, 5970. https://doi.org/10.3390/ijms26135970
Peng C-L, Chen C-T, Tang I-C. Exploring the Therapeutic Potential of 177Lu-PSMA-617 in a Mouse Model of Prostate Cancer Bone Metastases. International Journal of Molecular Sciences. 2025; 26(13):5970. https://doi.org/10.3390/ijms26135970
Chicago/Turabian StylePeng, Cheng-Liang, Chun-Tang Chen, and I-Chung Tang. 2025. "Exploring the Therapeutic Potential of 177Lu-PSMA-617 in a Mouse Model of Prostate Cancer Bone Metastases" International Journal of Molecular Sciences 26, no. 13: 5970. https://doi.org/10.3390/ijms26135970
APA StylePeng, C.-L., Chen, C.-T., & Tang, I.-C. (2025). Exploring the Therapeutic Potential of 177Lu-PSMA-617 in a Mouse Model of Prostate Cancer Bone Metastases. International Journal of Molecular Sciences, 26(13), 5970. https://doi.org/10.3390/ijms26135970






