Investigation of In Vitro and In Silico Anti-Inflammatory Potential of Carthamus caeruleus L. Root Juice
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of C. caeruleus Root Juice
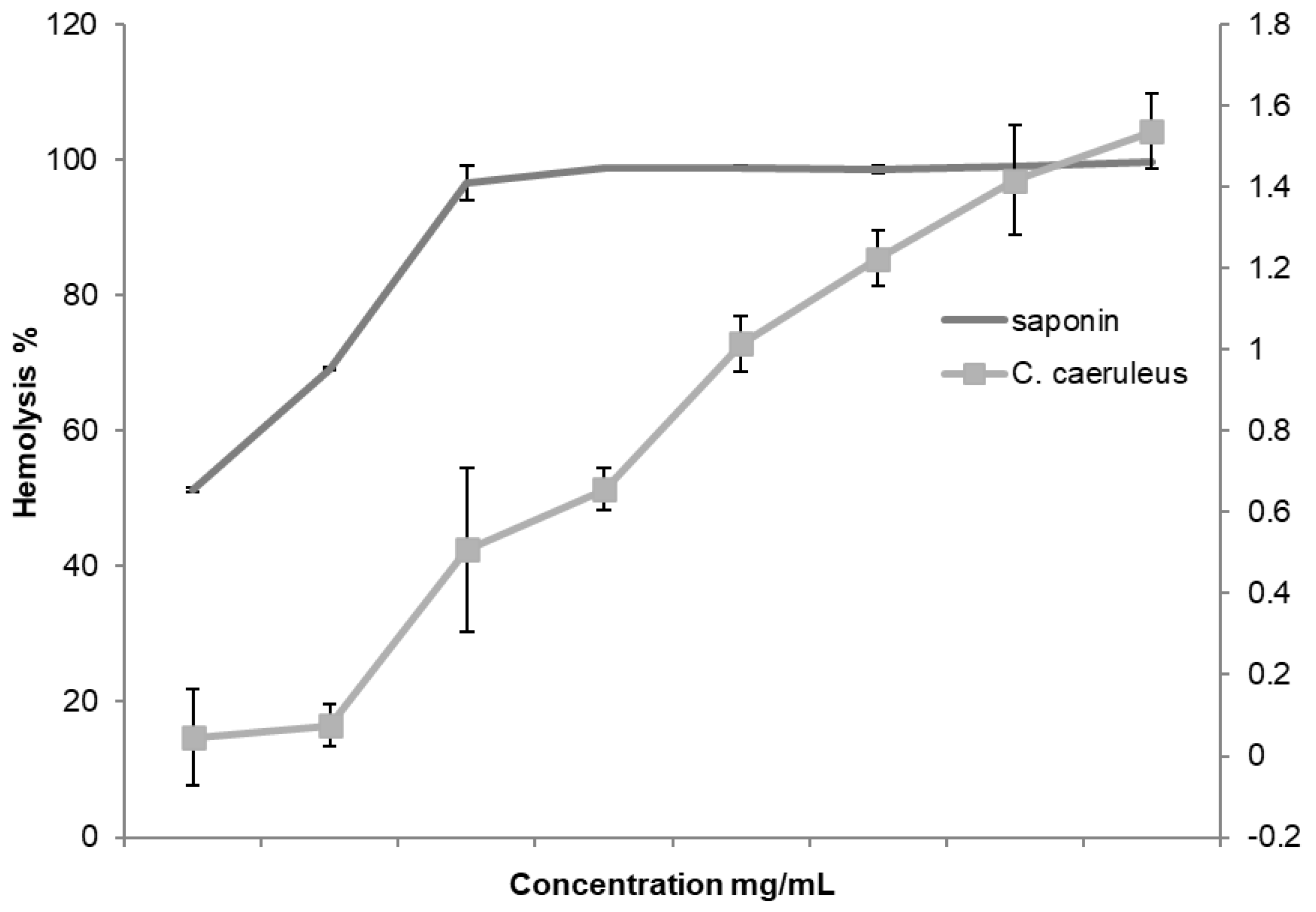
2.2. Hemolytic Activity Assessment
2.3. Anti-Inflammatory Activity In Vitro
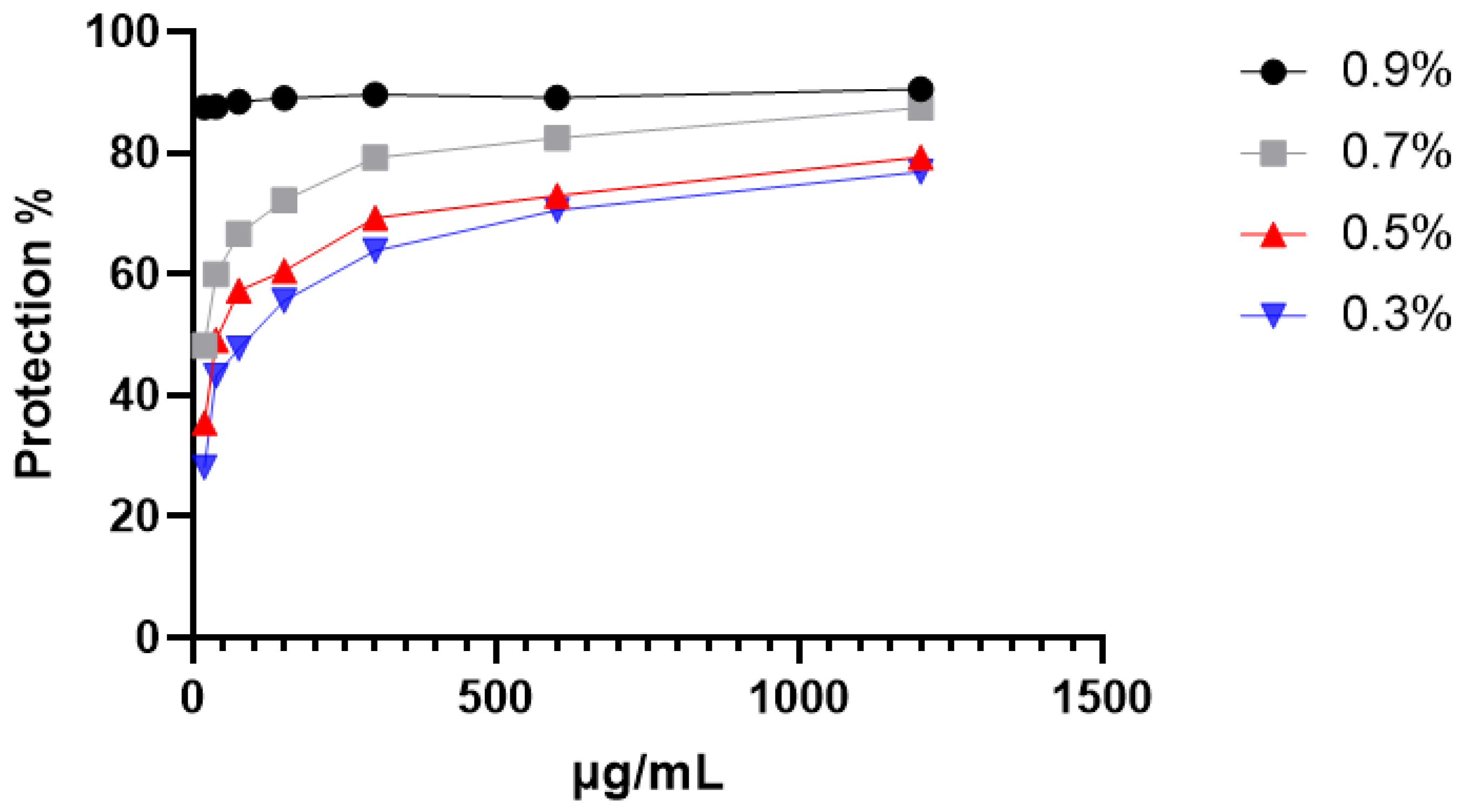
2.3.1. Protection Against Hypotonic-Induced Hemolysis
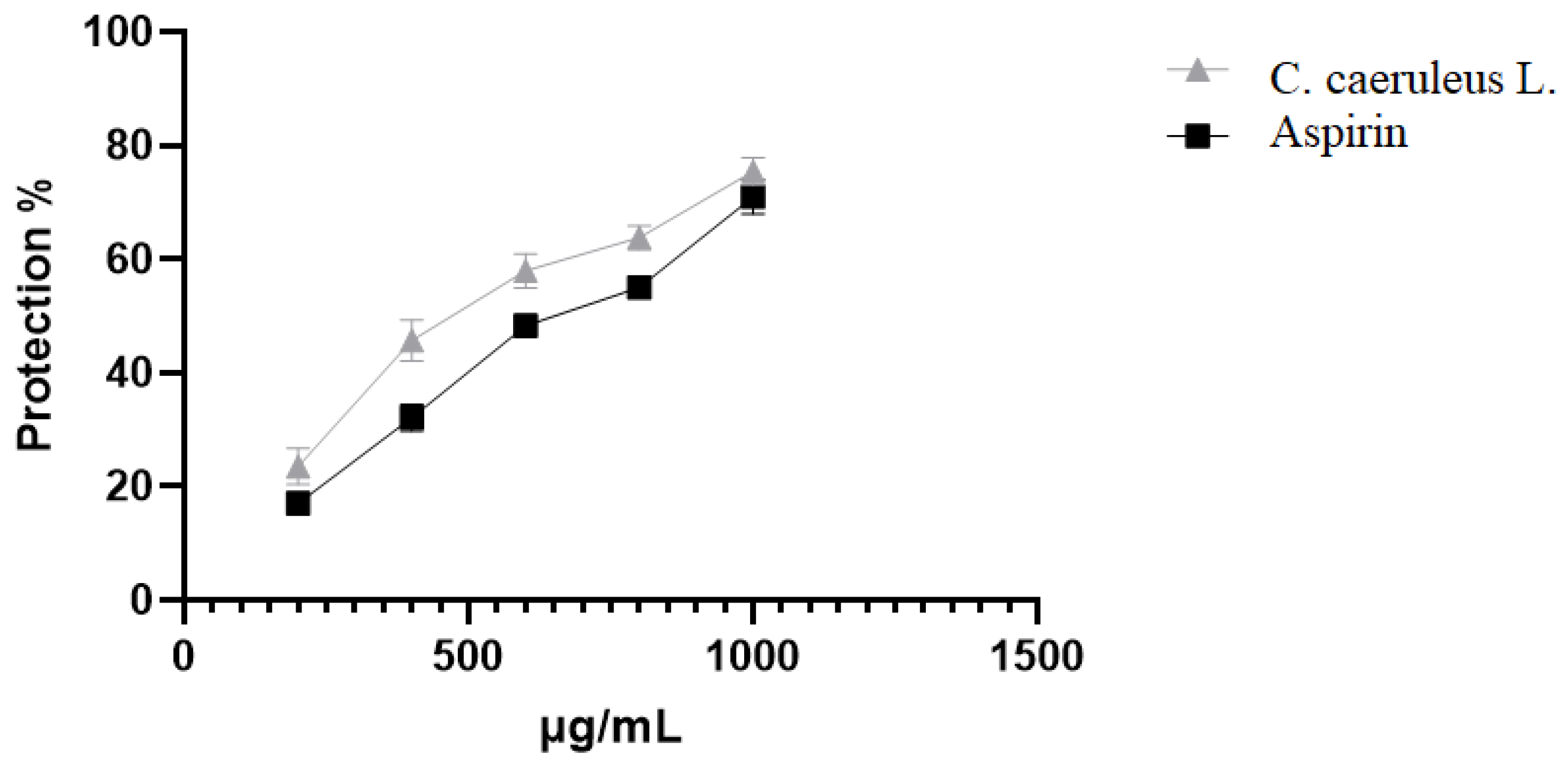
2.3.2. Protection Against Heat-Induced Hemolysis
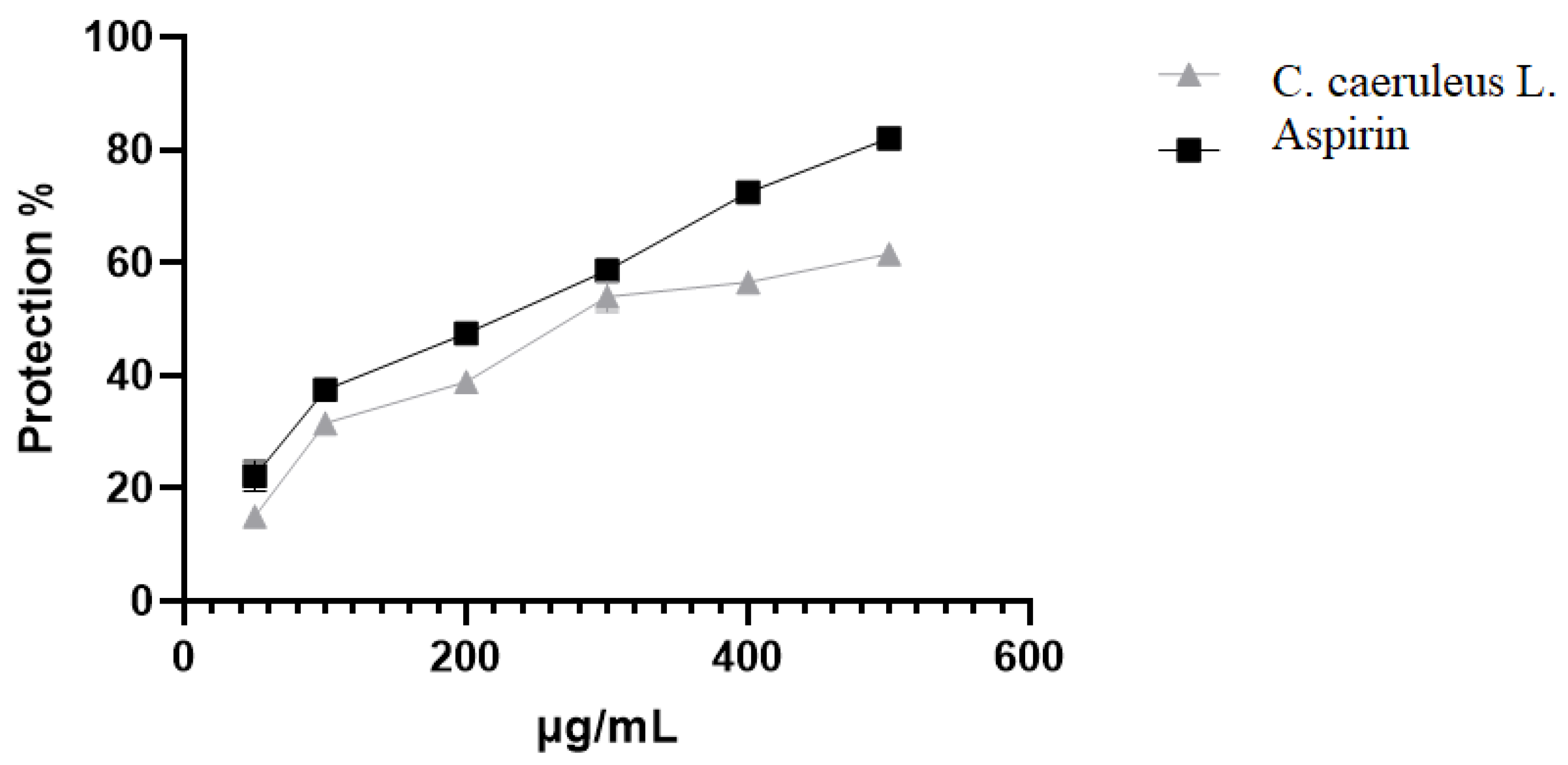
2.3.3. Protection Against Oxidative-Induced Hemolysis
2.3.4. Protection Against Albumin Denaturation
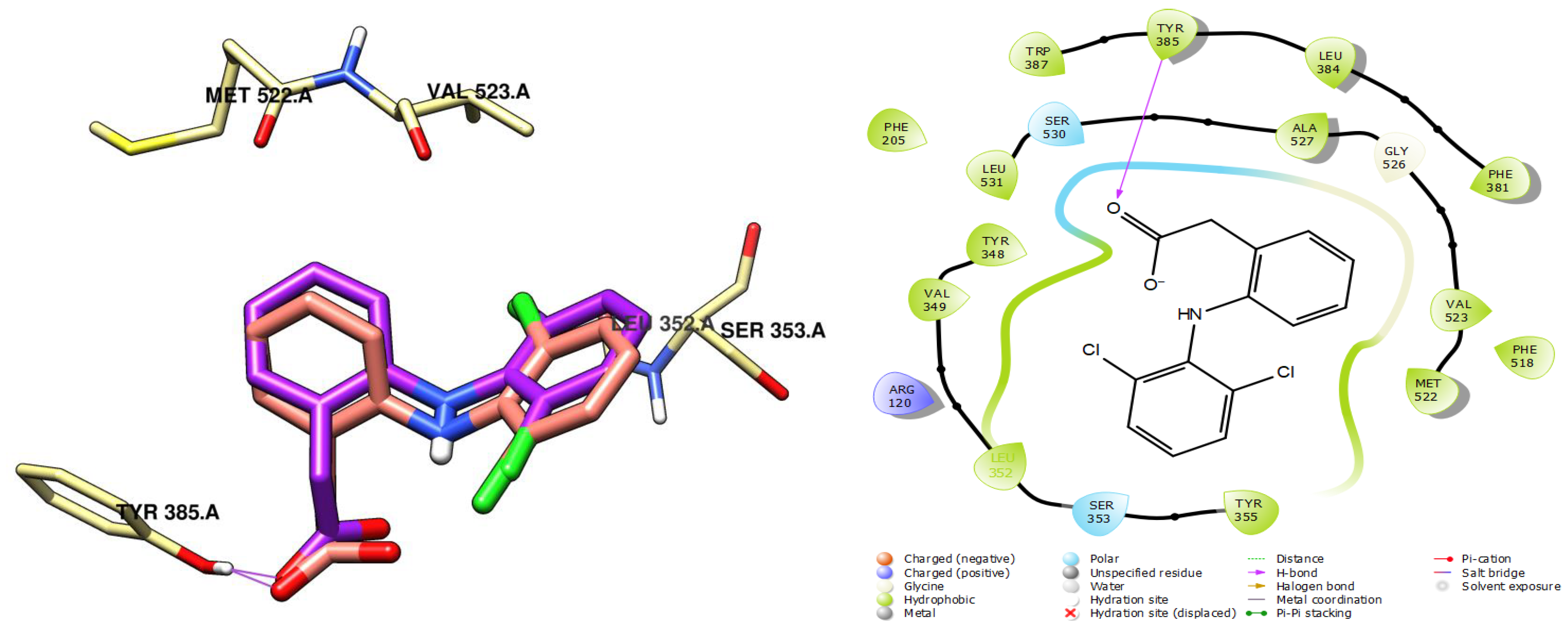
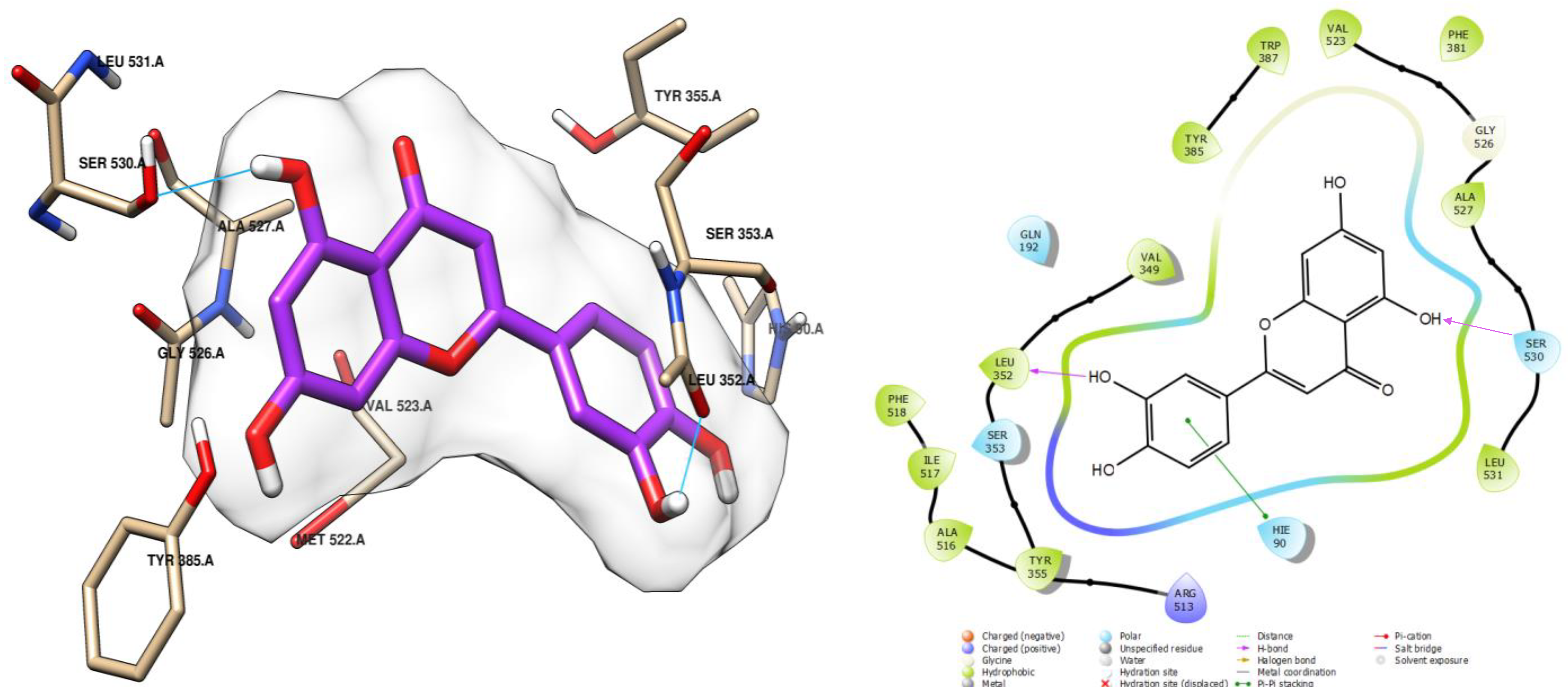
2.4. Molecular Docking of CRJ Compounds with COX-2
3. Discussion
4. Material and Methods
4.1. Plant Material and Extraction
4.2. Determination of Total Polyphenol Content (TPC)
4.3. Determination of Total Flavonoid Content (TFC)
4.4. Determination of Total Tannin Content (TTC)
4.5. Phytochemical Screening of Juice by RP-HPLC-DAD
4.6. Erythrocyte Suspension Preparation
4.7. Hemolytic Effect of C. caeruleus Root Juice
4.8. In Vitro Anti-Inflammatory Assays
4.8.1. Hypotonic Solution-Induced Hemolysis
4.8.2. Heat-Induced Hemolysis
4.8.3. Oxidant-Induced Hemolysis
4.8.4. Inhibition of Protein Denaturation
4.9. Molecular Docking Study
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | absorbance |
| DAD | diode array detector |
| DPPH | 2,2-diphenyl-1-picrylhydrazil |
| GAE | gallic acid equivalents |
| H2O2 | hydrogen peroxide |
| HClO | hypochlorous acid |
| HPLC | high-performance liquid chromatography |
| QE | quercetin equivalents |
| RT | retention time |
| SEM | standard error of mean |
| TAE | tannic acid equivalents |
| TBARS | thiobarbituric acid reactive substance |
References
- GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, D.O.; Mathers, C.D.; Adam, T.; Ortegon, M.; Strong, K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007, 370, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Lucero-Prisno, D.E., III; Shomuyiwa, D.O.; Kouwenhoven, M.B.N.; Dorji, T.; Odey, G.O.; Miranda, A.V.; Wong, M.C. Top 10 public health challenges to track in 2023: Shifting focus beyond a global pandemic. Public Health Chall. 2023, 2, e86. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Fakhri, S.; Mohammadi-Noori, E.; Farzaei, M.H. Naringenin improves ovalbumin-induced allergic asthma in rats through antioxidant and anti-inflammatory effects. Evid.-Based Complement. Altern. Med. 2022, 2022, 9110798. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Chou, C.T. The anti-inflammatory effect of an extract of Tripterygium wilfordii Hook F on adjuvant-induced paw oedema in rats and inflammatory mediators release. Phytother. Res. Int. J. Devoted Med. Sci. Res. Plants Plant Prod. 1997, 11, 152–154. [Google Scholar]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Gorniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Gobe, G. Preclinical Rodent Models of Arthritis and Acute Inflammation Indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans regia Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 1695701. [Google Scholar] [CrossRef]
- Toubane, A.; Rezzoug, S.A.; Besombes, C.; Daoud, K. Optimization of Accelerated Solvent Extraction of Carthamus Caeruleus L. Evaluation of antioxidant and anti-inflammatory activity of extracts. Ind. Crops Prod. 2017, 97, 620–631. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of hesperidin. Indian J. Biochem. Biophys. 2019, 56, 287–300. [Google Scholar]
- Meddour, R.; Meddour-Sahar, O. Medicinal plants and their traditional uses in Kabylia (Tizi Ouzou, Algeria). Arab. J. Med. Aromat. Plants 2015, 1, 137–151. [Google Scholar]
- Meddour, R.; Sahar, O.; Abdoune, N.; Dermouche, M. Quantitative ethnobotanical investigation of medicinal plants used by local population in the rural municipalities of Haizer and El Asnam, province of Bouira, Northern Algeria. Mediterr. Bot. 2022, 43, 13. [Google Scholar] [CrossRef]
- Benhamou, A.; Fazouane, F. Ethnobotanical Study, Phytochemical Characterization and Healing Effect of Carthamus caeruleus L. rhizomes. Int. J. Med. Arom. Plants 2013, 3, 61–68. [Google Scholar]
- Belounis, Y.; Moualek, I.; Sebbane, H.; Dekir, A.; Bendif, H.; Garzoli, S.; Houali, K. Phytochemical Characterization and Antibacterial Activity of Carthamus Caeruleus L. Aqueous Extracts: In Vitro and In Silico Molecular Docking Studies. Chem. Biodivers. 2025, 22, e202402662. [Google Scholar] [CrossRef]
- Belounis, Y.; Moualek, I.; Sebbane, H.; Ait Issad, H.; Saci, S.; Saoudi, B.; Cruz, C. Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation. Processes 2025, 13, 878. [Google Scholar] [CrossRef]
- Boumerfeg, S.; Baghiani, A.; Belkhiri, F.; Arrar, L. Evaluation of inhibitive action on oxidative damage in human erythrocytes and antioxidant activities of Carthamus caeruleus. In Proceedings of the 2nd African Congress on Biology & Health, University Ferhat Abbas Sétif 1, Sétif, Algeria, 10–12 November 2012; Volume 11, p. 102. [Google Scholar]
- Baghiani, A.; Boumerfeg, S.; Belkhiri, F.; Khennouf, S.; Charef, N.; Harzallah, D.; Arrar, L.; Attia, A.W. Antioxidant and radical scavenging properties of Carthamus caeruleus L. extracts grown wild in Algeria flora. Comun. Sci. 2010, 1, 128–136. [Google Scholar]
- Ouda, A.N.; Fatiha, M.; Sadia, M.; Zohra, S.F.; Noureddine, D. In vivo anti-inflammatory activity of aqueous extract of Carthamus caeruleus L. rhizome against carrageenan-induced inflammation in mice. Jordan J. Biol. Sci. 2021, 14, 529–535. [Google Scholar]
- Moussa, H.; Dahmoune, F.; Hentabli, M.; Remini, H.; Mouni, L. Optimization of ultrasound-assisted extraction of phenolic-saponin content from Carthamus caeruleus L. rhizome and predictive model based on support vector regression optimized by dragonfly algorithm. Chemom. Intell. Lab. Syst. 2022, 222, 104493. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Protective effects of ferulic acid on high glucose-induced protein glycation, lipid peroxidation, and membrane ion pump activity in human erythrocytes. PLoS ONE 2015, 10, e0129495. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Morabito, R. Antioxidant activity of quercetin in an H2O2-induced oxidative stress model in red blood cells: Functional role of Band 3 protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef]
- An, F.; Wang, S.; Yuan, D.; Gong, Y.; Wang, S. Attenuation of oxidative stress of erythrocytes by plant-derived flavonoids, orientin, and luteolin. Evid.-Based Complement. Altern. Med. 2016, 2016, 3401269. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin, and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative analysis and in vitro anti-inflammatory effects of gallic acid, ellagic acid, and quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104. [Google Scholar]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; Islam, S. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A comparative study of hesperetin, hesperidin, and hesperidin glucoside: In vitro antioxidant, anti-inflammatory, and antibacterial activities. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Sinjari, B.; Falasca, K.; Reale, M.; Caputi, S.; Jagarlapodii, S.; Murmura, G. Assessment of the vanillin anti-inflammatory and regenerative potentials in inflamed primary human gingival fibroblasts. Mediat. Inflamm. 2021, 2021, 5562340. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Ganeshpurkar, A.; Verma, M.; Golani, P.; Lodhi, D.S.; Jain, N. Evaluation of in-vitro anti-inflammatory activity of gallic acid. Int. J. Res. Rev. 2021, 8, 323–327. [Google Scholar] [CrossRef]
- Henneh, I.T.; Ameyaw, E.O.; Biney, R.P.; Armah, F.A.; Obese, E.; Konjah, D.; Eric, T.O. Ziziphus abyssinica hydro-ethanolic root bark extract attenuates acute inflammation, possibly through membrane stabilization and inhibition of protein denaturation and neutrophil degranulation. West Sfr. J. Pharm. 2018, 29, 81–94. [Google Scholar]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-inflammation activity of flavones and their structure–activity relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Ansari, P.; Shofiul, A.J.; Sarker, S.M.; Kallol, K.M.; Zareen, T.; Tasnim, T.; Sanjeeda, S.; Sarmin, B. Potential investigation of anti-inflammatory and anti-oxidative properties of ethanolic extract of Ixora nigricans leaves. Int. J. Pharmacol. Res. 2015, 5, 104. [Google Scholar]
- Vadivu, R.; Lakshmi, K.S. In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore spp Laurina. Bangladesh J. Pharmacol. 2008, 3, 121–124. [Google Scholar] [CrossRef]
- Kumari, C.S.; Yasmin, N.; Hussain, M.R.; Babuselvam, M. In-vitro anti-inflammatory and anti-arthritic property of Rhizophora mucronata leaves. Int. J. Pharma Sci. Res. 2015, 6, 482–485. [Google Scholar]
- Freitas, M.V.; Rita de Cássia, M.N.; da Costa Huss, J.C.; de Souza, T.M.T.; Costa, J.O.; Firmino, C.B.; Penha-Silva, N. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol. Vitr. 2008, 22, 219–224. [Google Scholar] [CrossRef]
- Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Interaction between plant polyphenols and the erythrocyte membrane. Cell. Mol. Biol. Lett. 2011, 17, 77–88. [Google Scholar] [CrossRef]
- Męczarska, K.; Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Oszmiański, J.; Siejak, K.; Bonarska-Kujawa, D. Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. Int. J. Mol. Sci. 2025, 26, 3213. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Suwalsky, M.; Villena, F.; Gallardo, M.J. In vitro protective effects of resveratrol against oxidative damage in human erythrocytes. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Precupas, A.; Sandu, R.; Popa, V.T. Quercetin Influence on Thermal Denaturation of Bovine Serum Albumin. J. Phys. Chem. B 2016, 120, 9362–9375. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N. Composés bioactifs: Etudein vitro des propriétés anti-inflammatoires de l’extrait méthanolique des fruits de rosa canina l. (rosacées). Nutr. Santé 2020, 9, 117–125. [Google Scholar] [CrossRef]
- Flouda, K.; Gammelgaard, B.; Davies, M.J.; Hawkins, C.L. Modulation of hypochlorous acid (HOCl) induced damage to vascular smooth muscle cells by thiocyanate and selenium analogues. Redox Biol. 2021, 41, 101873. [Google Scholar] [CrossRef]
- Saso, L.; Valentini, G.; Casini, M.L.; Grippa, E.; Gatto, M.T.; Leone, M.G.; Silvestrini, B. Inhibition of heat-induced denaturation of albumin by nonsteroidal antiinflammatory drugs (NSAIDs): Pharmacological implications. Arch. Pharmacal Res. 2001, 24, 150–158. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Stallings, W.C. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Haddouchi, F.; Chaouche, T.M.; Halla, N. Phytochemical screening, antioxidant activities and hemolytic power of four Saharan plants from Algeria. Phytothérapie 2016, 1–9. [Google Scholar] [CrossRef]
- Dahmani, M.M.; Laoufi, R.; Selama, O.; Arab, K. Gas chromatography coupled to mass spectrometry characterization, anti-inflammatory effect, wound-healing potential, and hair growth-promoting activity of Algerian Carthamus caeruleus L. (Asteraceae). Indian J. Pharmacol. 2018, 50, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaeahirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Suwalsky, M.; Orellana, P.; Avello, M.; Villena, F. Protective effect of Ugnimolinae Turcz against oxidative damage of human erythrocytes. Food Chem. Toxicol. 2007, 45, 130–135. [Google Scholar] [CrossRef]
- Ullah, H.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 346. [Google Scholar]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Marnett, L.J. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C. LigPrep, Version 3; Schrödinger: New York, NY, USA, 2012.
- Dekir, A.; Berredjem, M.; Rachedi, K.O.; Bahadi, R.; Djouad, S.E.; Bouacida, S.; Boussaker, M. X-ray crystallographic study, molecular docking, molecular dynamics and DFT/ADMET analyses of carboxylsulfamides. J. Mol. Struct. 2023, 1289, 135831. [Google Scholar] [CrossRef]



| Polyphenols (mg GAE/g) | Flavonoids (mg QE/g) | Tannins (mg TAE/g) |
|---|---|---|
| 58.95 ± 1.02 | 16.8 ± 0.27 | 33.19 ± 1.14 |
| Chemical Class | Compound | RT (min) | Amount (mg/g) |
|---|---|---|---|
| Flavonoids | Vitexin | 7.024 | 0.015 |
| Orientin | 8.26 | 0.025 | |
| Rutin | 8.832 | 0.013 | |
| Myricetin | 11.255 | 0.006 | |
| Luteolin | 12.658 | 0.006 | |
| Quercetin | 12.932 | 0.008 | |
| Hesperidin | 15.579 | 0.002 | |
| Phenolic acids | Dihydroxycinnamic acid | 7.256 | 0.011 |
| Isovanillic acid | 7.545 | 0.021 | |
| Ferulic acid | 9.25 | 0.014 | |
| p-Coumaric acid | 9.616 | 0.011 | |
| Rosmarinic acid | 10.196 | 0.015 | |
| Simple phenolics and aldehydes | Hydroxy-quinone | 3.895 | 0.017 |
| Resorcinol | 5.173 | 0.007 | |
| p-Hydroxybenzaldehyde | 7.892 | 0.014 | |
| Vanillin | 8.415 | 0.023 | |
| Other aromatic compounds | Tannic acid | 3.538 | 0.012 |
| Caffeine | 6.37 | 0.007 | |
| 3,4,5-Trimethoxybenzoic acid | 10.908 | 0.011 | |
| Coumarin | 11.784 | 0.007 | |
| m-Anisic acid | 12.169 | 0.004 | |
| 2,3-Dimethyl cinnamic acid | 14.76 | 0.007 |
| Tests | Concentration of Samples (µg/mL) | Maximal Protection (%) | ||
|---|---|---|---|---|
| CRJ | Standard * | |||
| Hypotonicity-induced hemolysis | 0.7% NaCl | 1200 | 90.51 ±1.6 a | 97.31± 0.98 b |
| 0.5% NaCl | 1200 | 87.46 ±1.5 a | ||
| 0.3% NaCl | 1200 | 76.87 ±1.3 a | ||
| Heat-induced hemolysis | 500 | 81.54 ±2.3 a | 89.74 ± 1.4 b | |
| HClO induced hemolysis | 1000 | 75.43 ±2.4 a | 70.97 ±2.02 b | |
| Albumin denaturation | 2000 | 61.5 ± 1.2 a | 82 ± 0.2 b | |
| Compound | Docking Score (kcal/mol) | Compound | Docking Score (kcal/mol) |
|---|---|---|---|
| Ferulic acid | −6.086 | Orientin | −5.674 |
| Isovanillic acid | −6.967 | Hesperidin | −5.057 |
| m-Anisic acid | −7.001 | Vanillin | −5.368 |
| Rosmarinic acid | −6.932 | Dihydroxycinnamic acid | −6.223 |
| Rutin | −6.110 | Hydroxy-quinone | −5.264 |
| Caffeine | −6.450 | Vitexin | −6.225 |
| Quercetin | −7.925 | p-Coumaric acid | −6.797 |
| Luteolin | −8.051 | Resorcinol | −5.125 |
| Myricetin | −8.260 | Co-crystallized ligand (diclofenac) | −8.433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moualek, I.; Bendif, H.; Dekir, A.; Benarab, K.; Belounis, Y.; Elfalleh, W.; Houali, K.; Peron, G. Investigation of In Vitro and In Silico Anti-Inflammatory Potential of Carthamus caeruleus L. Root Juice. Int. J. Mol. Sci. 2025, 26, 5965. https://doi.org/10.3390/ijms26135965
Moualek I, Bendif H, Dekir A, Benarab K, Belounis Y, Elfalleh W, Houali K, Peron G. Investigation of In Vitro and In Silico Anti-Inflammatory Potential of Carthamus caeruleus L. Root Juice. International Journal of Molecular Sciences. 2025; 26(13):5965. https://doi.org/10.3390/ijms26135965
Chicago/Turabian StyleMoualek, Idir, Hamdi Bendif, Ali Dekir, Karima Benarab, Yousra Belounis, Walid Elfalleh, Karim Houali, and Gregorio Peron. 2025. "Investigation of In Vitro and In Silico Anti-Inflammatory Potential of Carthamus caeruleus L. Root Juice" International Journal of Molecular Sciences 26, no. 13: 5965. https://doi.org/10.3390/ijms26135965
APA StyleMoualek, I., Bendif, H., Dekir, A., Benarab, K., Belounis, Y., Elfalleh, W., Houali, K., & Peron, G. (2025). Investigation of In Vitro and In Silico Anti-Inflammatory Potential of Carthamus caeruleus L. Root Juice. International Journal of Molecular Sciences, 26(13), 5965. https://doi.org/10.3390/ijms26135965










