Solar Panel Corrosion: A Review
Abstract
1. Introduction
2. Corrosion Mechanisms and Degradation Models
2.1. Ohm’s Law Model
2.2. Pan Model
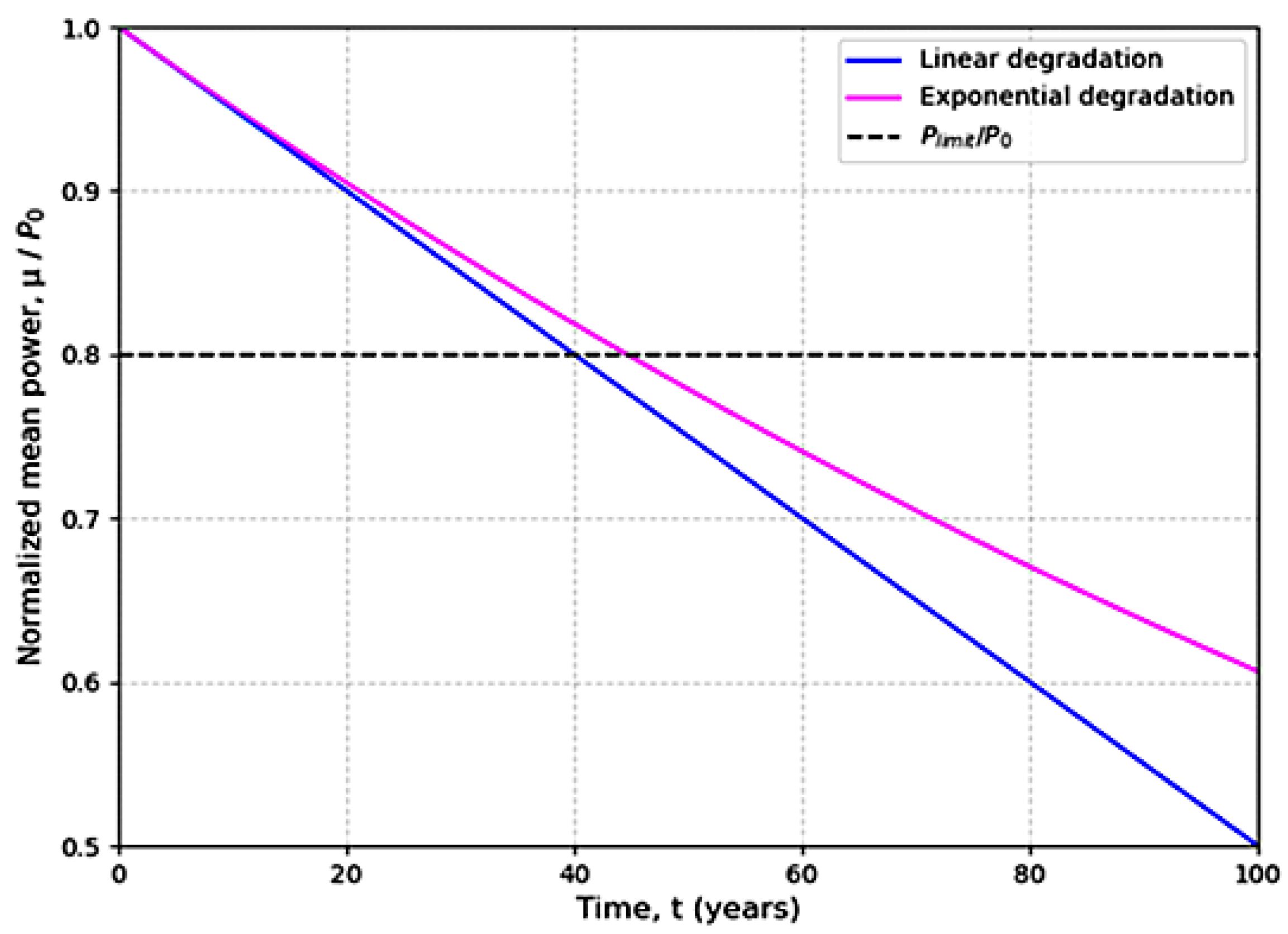
2.3. The Model of Exponential Development
- The module power (P) is a guide for performance evaluation.
- The degradation of solar cell modules is evaluated based on the initial power (P0).
2.4. Model Degradation by UV Stress
2.5. Model Degradation by Temperature Stress
- The rate constant K applies to a change in a property or performance. For example, if a material degrades due to color change or loss of mechanical properties, the rate constant of two degradation processes will likely differ.
- The entire temperature-dependent process leading to changes in performance (either in the presence or absence of light) follows Arrhenius dependence.
- The rate constant varies linearly with irradiance in the range of irradiances considered. This may not be the case for all polymers or at high irradiances, such as several times the maximum daytime irradiance near the equator. In such cases, a power law may apply to irradiance [30].
- The activation energy Ea of the entire temperature-dependent process (in the presence or absence of light) is constant in the considered temperature range.
- Weathering energy activation under accelerated conditions is only known for some materials. When a specific material’s activation energy is unavailable, it must be estimated from published activation energies for similar materials or determined from in-house experiments. For instance, the activation energy for the photochemical yellowing of EVA has not been published but can be estimated based on the Ea of photochemical yellowing established for various aromatic polymers [31].
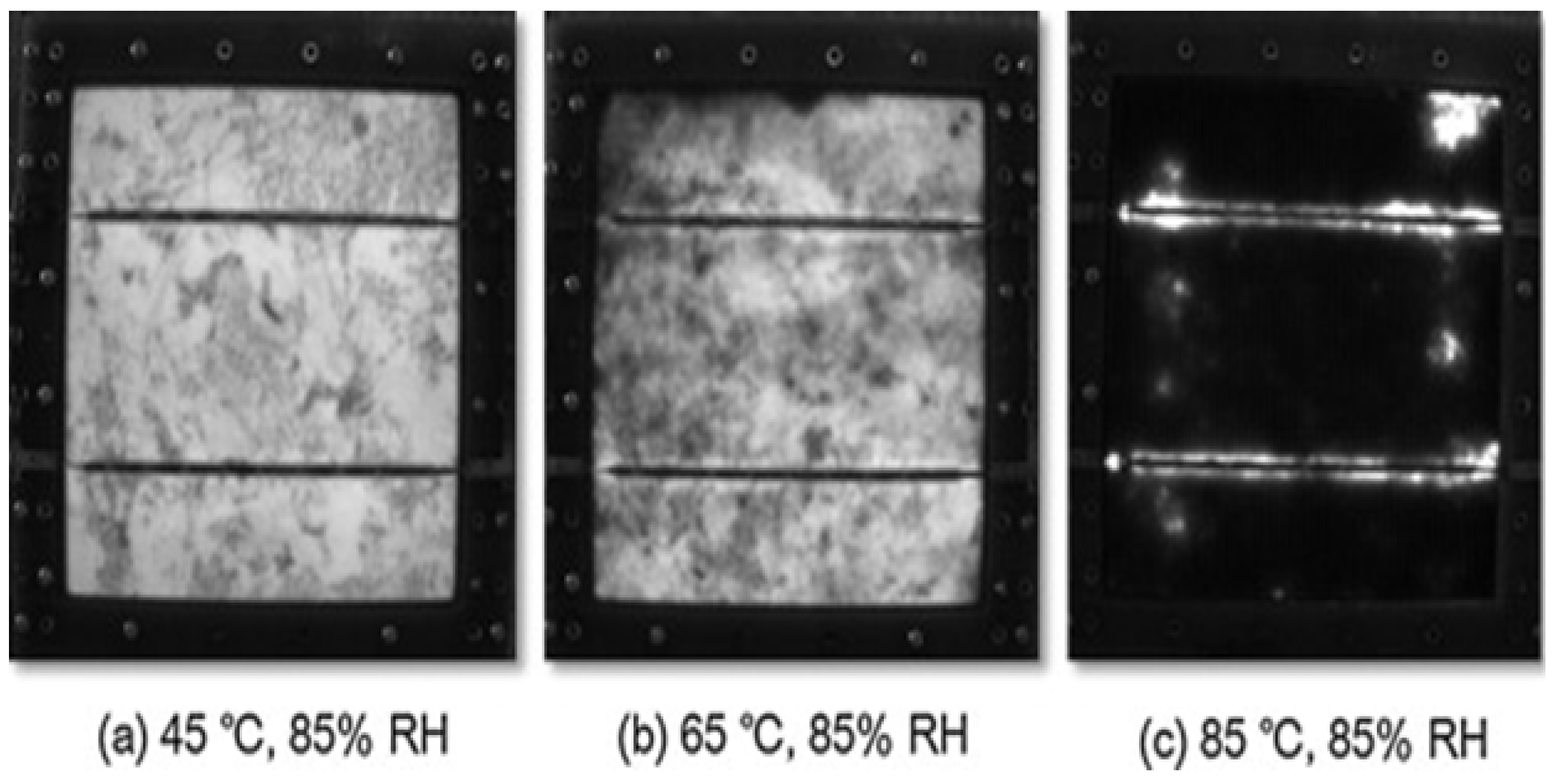
2.6. Model Degradation Due to Temperature and Humidity Stress
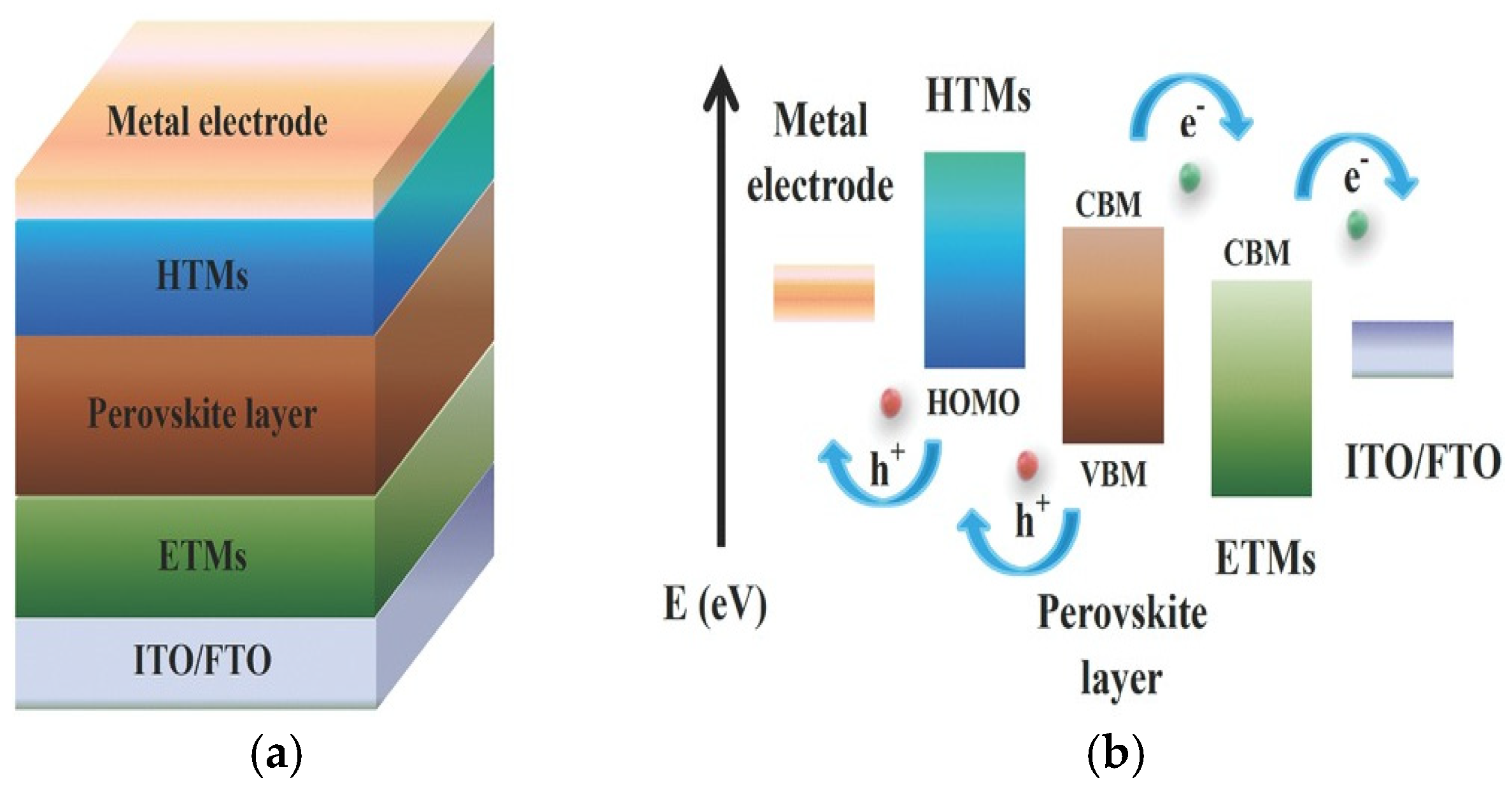
2.7. Degradation Mechanisms of Perovskite Solar Cells
2.7.1. Moisture Sensitivity of PSCs
2.7.2. Thermal Instability of PSCs
2.7.3. Light-Induced Degradation of PSCs
2.7.4. Ion Migration of PSCs
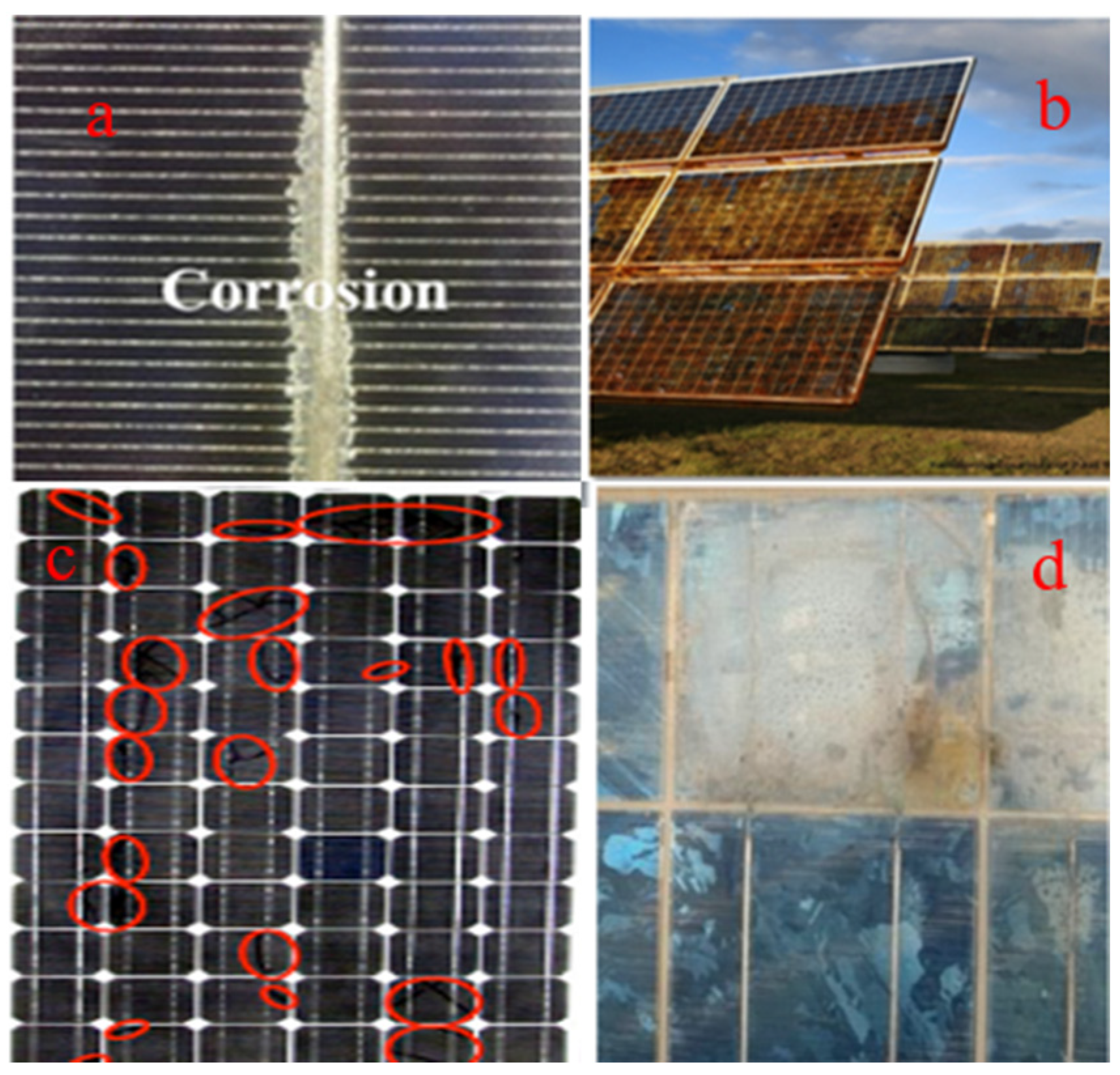
3. Effects of Corrosion on Solar Panel Performance
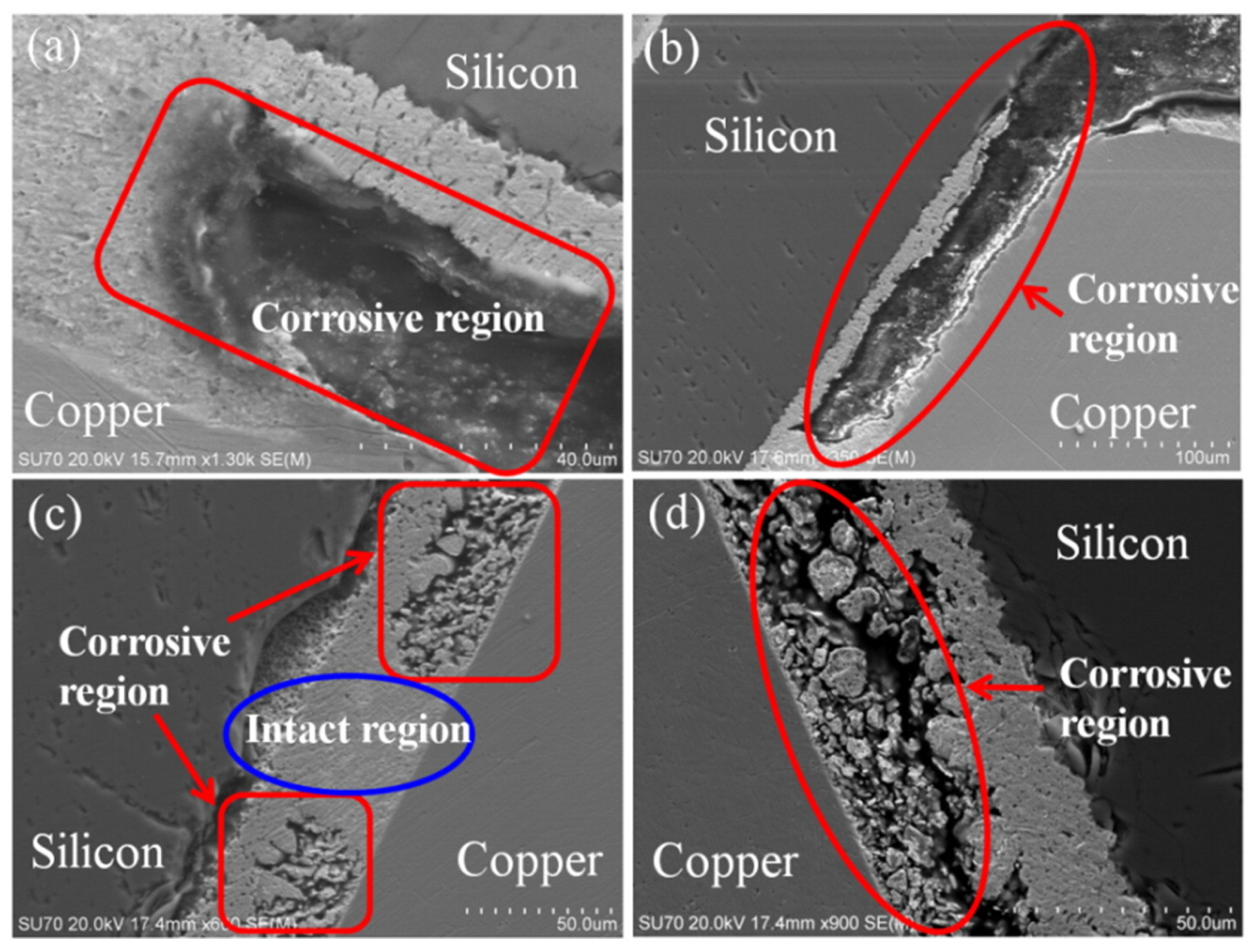
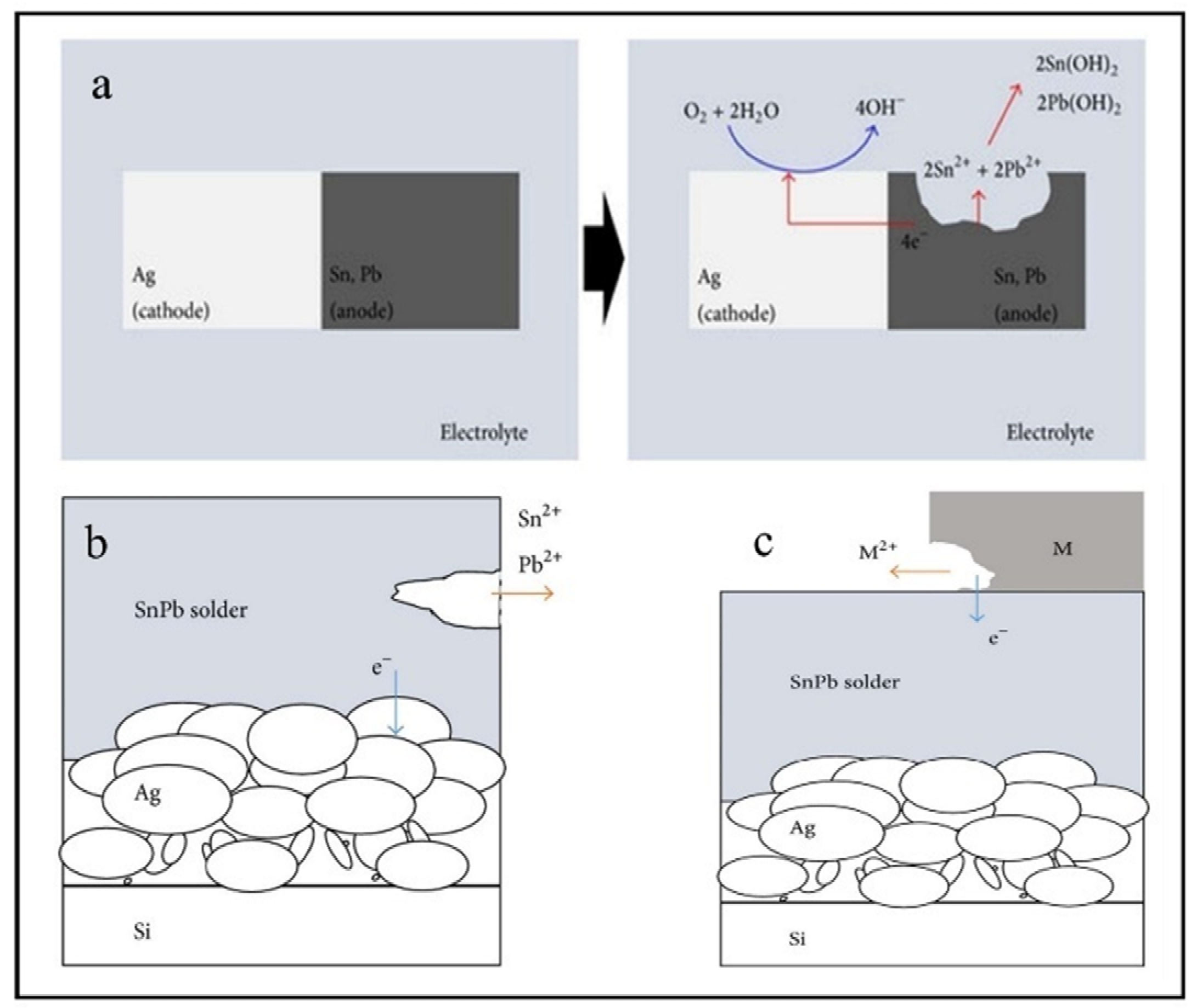
4. Corrosion Mechanism of Solder Interconnection
4.1. The Cathodic Sub-Processes
4.1.1. Hydrogen Evolution Reaction (HER)
4.1.2. Oxygen Reduction Reaction (ORR)
4.2. The Anodic Sub-Processes
5. Perspectives in Mitigation Related to Corrosion Management
5.1. Material Selection and Coatings
5.2. Design Improvements
5.3. Environmental Considerations
5.4. Monitoring and Maintenance
6. Critical Analysis
7. Typical Electrochemical and Surface Characterization Techniques
7.1. Electroluminescence Imaging Analysis (EL)
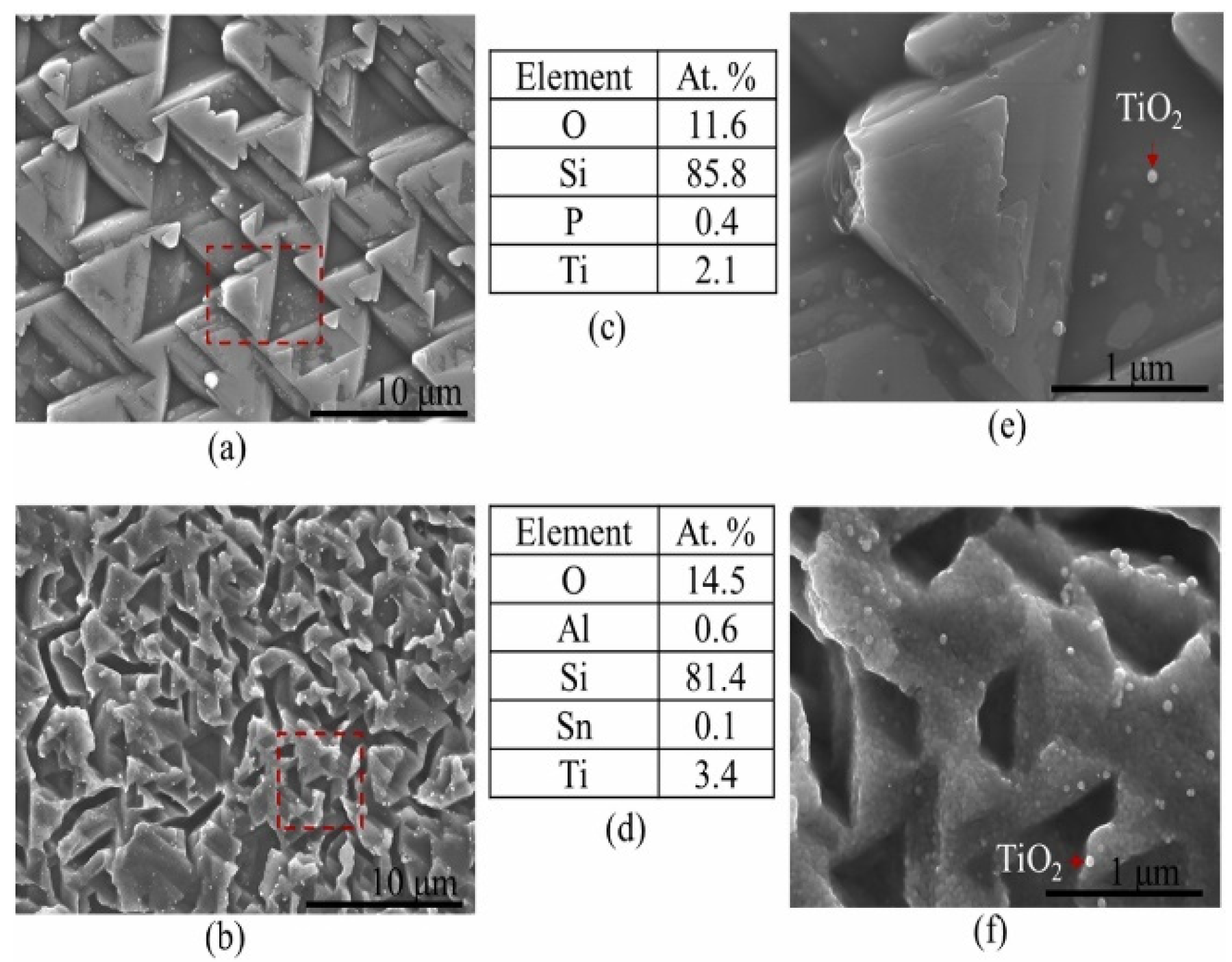
7.2. Scanning Electron Microscopy (SEM), Energy Dispersive Spectroscopy (EDS) Analysis
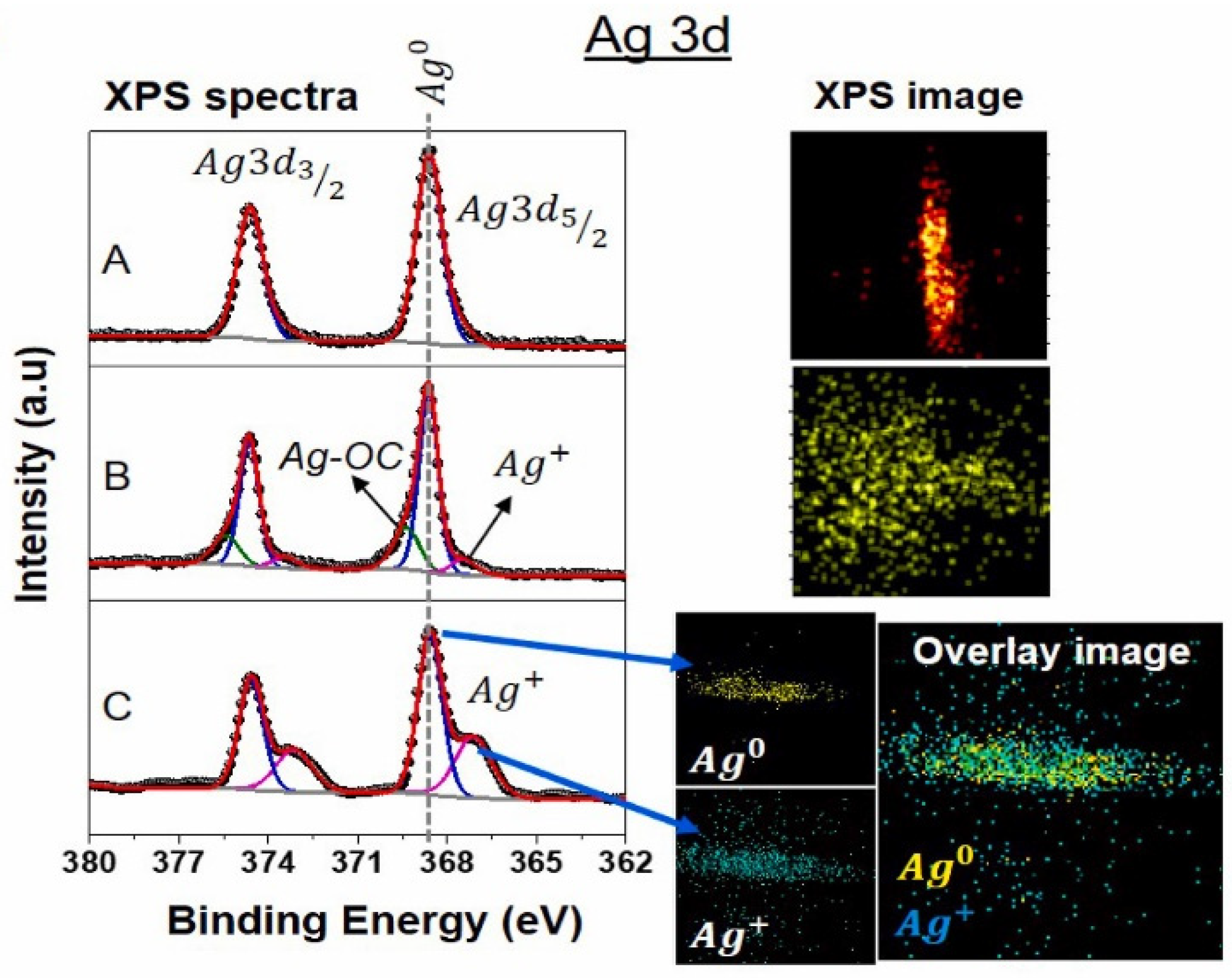
7.3. X-Ray Photoelectron Spectroscopy Analysis (XPS)
8. Organic Solar Cells (OSC)
8.1. Exposure to Light and Photo-Oxidation
8.2. Influence of Oxygen and Humidity
8.3. Thermal Degradation
8.4. Mechanical Degradation
8.5. Corrosion in Organic Solar Cells
8.5.1. Electrode Corrosion
8.5.2. Galvanic Corrosion
8.5.3. Light-Induced Chemical Reactions
9. Strategies to Mitigate Degradation and Corrosion
9.1. Use of High-Barrier Encapsulants
9.2. The Search for New Semiconductor Materials
9.3. Electrode Modification
9.4. Device Engineering
10. Applications of DFT in the Design of Materials for Organic Solar Cells
10.1. Prediction of Energy Band and Molecular Energy Levels
10.2. Simulation of Donor–Acceptor Interactions
- π-π stacking interactions, which enhance charge delocalization and conductivity.
- Interfacial energy levels affect how efficiently electrons and holes are extracted at the electrodes.
- Molecular reorganization energies predict how easily charges move within the material.
10.3. Evaluation of Light Absorption
10.4. Charge Transport in Organic Solar Cells
11. Discussion
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moschella, A.; Salemi, A.; Lo Faro, A.; Sanfilippo, G.; Detommaso, M.; Privitera, A. Historic Buildings in Mediterranean Area and Solar Thermal Technologies: Architectural Integration vs Preservation Criteria. Energy Procedia 2013, 42, 416–425. [Google Scholar] [CrossRef]
- Alves dos Santos, S.A.; João, J.P.; Carlos, C.A.; Marques Lameirinhas, R.A. The Impact of Aging of Solar Cells on the Performance of Photovoltaic Panels. Energy Convers. Manag. X 2021, 10, 100082. [Google Scholar] [CrossRef]
- Ravi Kumar, K.; Krishna Chaitanya, N.V.V.; Sendhil Kumar, N. Solar Thermal Energy Technologies and Its Applications for Process Heating and Power Generation—A Review. J. Clean. Prod. 2021, 282, 125296. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, X.; Wu, J.; Shu, C.; Gao, F.L.; Li, C.; Lu, X.H.; Zhang, Y.; Wang, Q.; Li, X.; et al. An Intelligent, Solar-Responsive, and Thermally Conductive Phase-Change System Toward Solar-Thermal-Electrical Conversion Featuring Daytime Blooming for Solar Energy Harvesting and Nighttime Closing for Thermal Preservation. Adv. Funct. Mater. 2024, 34, 2406236. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials Corrosion for Thermal Energy Storage Systems in Concentrated Solar Power Plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar]
- Hou, Y.; Du, X.; Scheiner, S.; Mcmeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I.; et al. A Generic Interface to Reduce the Efficiency-Stability-Cost Gap of Perovskite Solar Cells. Science 2017, 358, 1192–1197. [Google Scholar]
- Bender, R.; Féron, D.; Mills, D.; Ritter, S.; Bäßler, R.; Bettge, D.; De Graeve, I.; Dugstad, A.; Grassini, S.; Hack, T.; et al. Corrosion Challenges towards a Sustainable Society. Mater. Corros. 2022, 73, 1730–1751. [Google Scholar] [CrossRef]
- Wang, H.; Oguz, E.; Jeong, B.; Zhou, P. Life Cycle and Economic Assessment of a Solar Panel Array Applied to a Short Route Ferry. J. Clean. Prod. 2019, 219, 471–484. [Google Scholar] [CrossRef]
- Rouhandeh, M.; Ahmadi, A.; Mirhosseini, M.; Alirezaei, R. Economic Energy Supply Using Renewable Sources Such as Solar and Wind in Hard-to-Reach Areas of Iran with Two Different Geographical Locations. Energy Strategy Rev. 2024, 55, 101494. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jonai, S.; Hara, K.; Komaki, H.; Shimizu-Kamikawa, Y.; Shibata, H.; Niki, S.; Kawakami, Y.; Masuda, A. Potential-Induced Degradation of Cu(In,Ga)Se2 Photovoltaic Modules. Jpn. J. Appl. Phys. 2015, 54. [Google Scholar] [CrossRef]
- Klemchuk, P.; Ezrin, M.; Lavigne, G.; Holley, W.; Galica, J.; Agro, S. Investigation of the Degradation and Stabilization of EVA-Based Encapsulant in Field-Aged Solar Energy Modules. Polym. Degrad. Stab. 1997, 55, 347–365. [Google Scholar] [CrossRef]
- Oreski, G.; Ottersböck, B.; Omazic, A. Degradation Processes and Mechanisms of Encapsulants. In Durability and Reliability of Polymers and Other Materials in Photovoltaic Modules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152. ISBN 9780128115459. [Google Scholar]
- Radavičius, T.; Van Der Heide, A.; Palitzsch, W.; Rommens, T.; Denafas, J.; Tvaronavičienė, M. Circular Solar Industry Supply Chain through Product Technological Design Changes Circular Solar Industry Supply Chain through Product Technological Design Changes. Insights Into Reg. Dev. 2021, 3, 10–30. [Google Scholar] [CrossRef]
- Dada, M.; Popoola, P. Recent Advances in Solar Photovoltaic Materials and Systems for Energy Storage Applications: A Review. Beni-Suef Univ. J. Basic. Appl. Sci. 2023, 12, 66. [Google Scholar] [CrossRef]
- Hossain, R.; Ahmed, A.J.; Islam, S.M.K.N.; Saha, N.; Debnath, P.; Kouzani, A.Z.; Mahmud, M.A.P. New Design of Solar Photovoltaic and Thermal Hybrid System for Performance Improvement of Solar Photovoltaic. Int. J. Photoenergy 2020, 2020, 8825489. [Google Scholar] [CrossRef]
- Govindasamy, D.; Kumar, A. Experimental Analysis of Solar Panel Efficiency Improvement with Composite Phase Change Materials. Renew. Energy 2023, 212, 175–184. [Google Scholar] [CrossRef]
- de Oliveira, M.C.C.; Diniz Cardoso, A.S.A.; Viana, M.M.; Lins, V.d.F.C. The Causes and Effects of Degradation of Encapsulant Ethylene Vinyl Acetate Copolymer (EVA) in Crystalline Silicon Photovoltaic Modules: A Review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar]
- Jiang, S.; Wang, K.; Zhang, H.; Ding, Y.; Yu, Q. Encapsulation of PV Modules Using Ethylene Vinyl Acetate Copolymer as the Encapsulant. Macromol. React. Eng. 2015, 9, 522–529. [Google Scholar] [CrossRef]
- Rabelo, M.; Yousuf, H.; Cha, Y.; Dao, V.A.; Kim, Y.; Phong Pham, D.; Yi, J. Crystalline Silicon Photovoltaic Module Degradation: Galvanic Corrosion and Its Solution. Eng. Fail. Anal. 2022, 138, 106329. [Google Scholar] [CrossRef]
- Mon, G.R.; Orehotsky, J.; Ross, R.G.; Whitla, G. Predicting Electrochemical Breakdown in Terrestrial Photovoltaic Modules. In Proceedings of the 17th IEEE Photovoltaic Specialists Conference, Orlando, FL, USA, 1 May 1984. [Google Scholar]
- Kempe, M.; Hacke, P.; Morse, J.; Li, J.; Shen, Y.C.; Han, K. Electrochemical Mechanisms of Leakage-Current in Photovoltaic Modules. Prog. Photovolt. Res. Appl. 2023, 31, 700–715. [Google Scholar] [CrossRef]
- Kaaya, I.; Ascencio-Vásquez, J.; Weiss, K.A.; Topič, M. Assessment of Uncertainties and Variations in PV Modules Degradation Rates and Lifetime Predictions Using Physical Models. Sol. Energy 2021, 218, 354–367. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Q.; Dai, Z.; Yan, W. Module Defect Detection and Diagnosis for Intelligent Maintenance of Solar Photovoltaic Plants: Techniques, Systems and Perspectives. Energy 2024, 297, 131222. [Google Scholar]
- Hao, Y.; Yang, H.; Han, H.; Nan, C.; Huang, X.; Wang, H. Potential-Induced Electrochemical Corrosion in Crystalline Silicon Solar Cells. Solar RRL 2024, 8, 2300980. [Google Scholar] [CrossRef]
- Vazquez, M.; Rey-Stolle, I. Photovoltaic Module Reliability Model Based on Field Degradation Studies. Prog. Photovolt. Res. Appl. 2008, 16, 419–433. [Google Scholar] [CrossRef]
- Al Mahdi, H.; Leahy, P.G.; Alghoul, M.; Morrison, A.P. A Review of Photovoltaic Module Failure and Degradation Mechanisms: Causes and Detection Techniques. Solar 2024, 4, 43–82. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Chorover, J.; Goyne, K.W.; Brantley, S.L. Protection of Mesopore-Adsorbed Organic Matter from Enzymatic Degradation. Environ. Sci. Technol. 2004, 38, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Kherici, Z.; Kahoul, N.; Cheghib, H.; Younes, M.; Chekal Affari, B. Main Degradation Mechanisms of Silicon Solar Cells in Algerian Desert Climates. Sol. Energy 2021, 224, 279–284. [Google Scholar] [CrossRef]
- Mavlonov, A.; Hishikawa, Y.; Kawano, Y.; Negami, T.; Hayakawa, A.; Tsujimura, S.; Okumura, T.; Minemoto, T. Thermal Stability Test on Flexible Perovskite Solar Cell Modules to Estimate Activation Energy of Degradation on Temperature. Sol. Energy Mater. Sol. Cells 2024, 277, 113148. [Google Scholar] [CrossRef]
- Zaghdoudi, M.; Kömmling, A.; Jaunich, M.; Wolff, D. Erroneous or Arrhenius: A Degradation Rate-Based Model for EPDM during Homogeneous Ageing. Polymers 2020, 12, 2152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, B.X.; Wang, X.; Qin, S. Optimal Plan for Wiener Constant-Stress Accelerated Degradation Model. Appl. Math. Model. 2020, 84, 191–201. [Google Scholar] [CrossRef]
- Park, N.C.; Oh, W.W.; Kim, D.H. Effect of Temperature and Humidity on the Degradation Rate of Multicrystalline Silicon Photovoltaic Module. Int. J. Photoenergy 2013, 2013, 925280. [Google Scholar] [CrossRef]
- Damo, U.M.; Ozoegwu, C.G.; Ogbonnaya, C.; Maduabuchi, C. Effects of Light, Heat and Relative Humidity on the Accelerated Testing of Photovoltaic Degradation Using Arrhenius Model. Sol. Energy 2023, 250, 335–346. [Google Scholar] [CrossRef]
- Escobar, L.A.; Meeker, W.Q. A Review of Accelerated Test Models. Stat. Sci. 2006, 21, 552–577. [Google Scholar]
- Kurtz, M.M. Neurocognition as a Predictor of Response to Evidence-Based Psychosocial Interventions in Schizophrenia: What Is the State of the Evidence? Clin. Psychol. Rev. 2011, 31, 663–672. [Google Scholar]
- Kim, S.Y.; Kumachang, C.C.F.; Doumon, N.Y. Characterization Tools to Probe Degradation Mechanisms in Organic and Perovskite Solar Cells. Solar RRL 2023, 7, 2300155. [Google Scholar]
- Tong, X.; Lin, F.; Wu, J.; Wang, Z.M. High Performance Perovskite Solar Cells. Adv. Sci. 2015, 3, 1500201. [Google Scholar]
- Mon, G.R.; Ross, R.G., Jr. Electrochemical degradation of amorphous-silicon photovoltaic modules. In Proceedings of the Photovoltaic Specialists Conference 1985, Las Vegas, NV, USA, 21–25 October 1985; pp. 1142–1149. [Google Scholar]
- Mon Wen, G.L.; Meyer, J.; Ross, R., Jr. Electrochemical and galvanic corrosion efffcts in thin-film photovoitaic modules. In Proceedings of the Conference Record of the Twentieth IEEE Photovoltaic Specialists Conference, Washington, DC, USA, 26 September 1996. [Google Scholar]
- Realini, A.; Burà, E.; Cereghetti, N.; Chianese, D.; Rezzonico, S.; Sample, T.; Ossenbrink, H. Study of a 20-year old pv plant (MTBF project). In Proceedings of the 17th European Photovoltaic Solar Energy Conference and Exhibition, Munich, Germany, 22–26 October 2001. [Google Scholar]
- Reis, A.M.; Coleman, N.T.; Marshall, M.W.; Lehman, P.A.; Chamberlin, C.E. Comparison of PV module performance before and after 11-years of field exposure. In Proceedings of the Conference Record of the Twenty-Ninth IEEE Photovoltaic Specialists Conference, New Orleans, Louisiana, 19–24 May 2002; IEEE: Piscataway, NJ, USA, 2002. ISBN 0780374711. [Google Scholar] [CrossRef]
- Osterwald, C.R.; Mcmahon, T.J.; Del Cueto, J.A. Electrochemical Corrosion of SnO2: F Transparent Conducting Layers in Thin-Film Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2003, 79, 21–33. [Google Scholar]
- Dunlop, E.D. Lifetime performance of crystalline silicon PV modules. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003. [Google Scholar]
- Carlson, D.E.; Romero, R.; Willing, F.; Meakin, D.; Gonzalez, L.; Murphy, R.; Moutinho, H.R.; Al-Jassim, M. Corrosion Effects in Thin-Film Photovoltaic Modules. Prog. Photovolt. Res. Appl. 2003, 11, 377–386. [Google Scholar] [CrossRef]
- Kempe, M.D. Modeling of Rates of Moisture Ingress into Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2006, 90, 2720–2738. [Google Scholar] [CrossRef]
- Dunlop, E.D.; Halton, D. The Performance of Crystalline Silicon Photovoltaic Solar Modules after 22 Years of Continuous Outdoor Exposure. Prog. Photovolt. Res. Appl. 2006, 14, 53–64. [Google Scholar]
- Jorgensen, G.J.; Terwilliger, K.M.; DelCueto, J.A.; Glick, S.H.; Kempe, M.D.; Pankow, J.W.; Pern, F.J.; McMahon, T.J. Moisture Transport, Adhesion, and Corrosion Protection of PV Module Packaging Materials. Sol. Energy Mater. Sol. Cells 2006, 90, 2739–2775. [Google Scholar] [CrossRef]
- Granata, J.E.; Boyson, W.E.; Kratochvil, J.A.; Quintana, M.A. Long-term performance and reliability assessment of 8 PV arrays at Sandia National Laboratories. In Proceedings of the 34th IEEE Photovoltaic Specialists Conference, Philadelphia, PA, USA, 7–12 June 2009; IEEE: Piscataway, NJ, USA, 2010. ISBN 9781424429509. [Google Scholar] [CrossRef]
- Kempe, M.D. Ultraviolet Light Test and Evaluation Methods for Encapsulants of Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2010, 94, 246–253. [Google Scholar] [CrossRef]
- Sánchez-Friera, P.; Piliougine, M.; Peláez, J.; Carretero, J.; De Cardona, M.S. Analysis of Degradation Mechanisms of Crystalline Silicon PV Modules after 12 Years of Operation in Southern Europe. Prog. Photovolt. Res. Appl. 2011, 19, 658–666. [Google Scholar] [CrossRef]
- Poulek, V.; Strebkov, D.S.; Persic, I.S.; Libra, M. Towards 50 Years Lifetime of PV Panels Laminated with Silicone Gel Technology. Sol. Energy 2012, 86, 3103–3108. [Google Scholar] [CrossRef]
- Berghold, J.; Roericht, M.; Hanusch, M.; Koch, S.; Böttcher, A.; Wendlandt, S.; Grunow, P.; Stegemann, B. Electrochemical Corrosion within Solar Panels. In Proceedings of the 27th European Photovoltaic Solar Energy Conference and Exhibition Conference, Frankfurt, Germany, 24–28 September 2012; pp. 3511–3518. [Google Scholar] [CrossRef]
- Ndiaye, A.; Kébé, C.M.F.; Ndiaye, P.A.; Charki, A.; Kobi, A.; Sambou, V. A Novel Method for Investigating Photovoltaic Module Degradation. Energy Procedia 2013, 36, 1222–1231. [Google Scholar]
- Laronde, R.; Charki, A.; Bigaud, D. Fetime Estimation of a Photovoltaic Module Subjected to Corrosion Due to Damp Heat Testing. J. Sol. Energy Eng. Trans. ASME 2013, 135, 021010. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, N.C.; Kim, D.H. The Effect of Moisture on the Degradation Mechanism of Multi-Crystalline Silicon Photovoltaic Module. Microelectron. Reliab. 2013, 53, 1823–1827. [Google Scholar]
- Sharma, V.; Kumar, A.; Sastry, O.S.; Chandel, S.S. Performance Assessment of Different Solar Photovoltaic Technologies under Similar Outdoor Conditions. Energy 2013, 58, 511–518. [Google Scholar] [CrossRef]
- Park, N.; Han, C.; Kim, D. Effect of Moisture Condensation on Long-Term Reliability of Crystalline Silicon Photovoltaic Modules. Microelectron. Reliab. 2013, 53, 1922–1926. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.; Kim, D.; Park, N. Study on Mitigation Method of Solder Corrosion for Crystalline Silicon Photovoltaic Modules. Int. J. Photoenergy 2014, 2014, 809075. [Google Scholar] [CrossRef]
- Ndiaye, A.; Kébé, C.M.F.; Charki, A.; Ndiaye, P.A.; Sambou, V.; Kobi, A. Degradation Evaluation of Crystalline-Silicon Photovoltaic Modules after a Few Operation Years in a Tropical Environment. Sol. Energy 2014, 103, 70–77. [Google Scholar] [CrossRef]
- Masuda, A.; Uchiyama, N.; Hara, Y. Degradation by Acetic Acid for Crystalline Si Photovoltaic Modules. Jpn. J. Appl. Phys. 2015, 54, 04DR04. [Google Scholar]
- Sweelem, E.A.; El Shenawy, E.T.; Nafeh, A.A. Effect of different dust accumulation on the performance of PV module in Egypt. Int. J. Adv. Inf. Sci. Technol. (IJAIST) 2015, 44, 44. [Google Scholar]
- Chandel, S.S.; Nagaraju Naik, M.; Sharma, V.; Chandel, R. Degradation Analysis of 28 Year Field Exposed Mono-c-Si Photovoltaic Modules of a Direct Coupled Solar Water Pumping System in Western Himalayan Region of India. Renew. Energy 2015, 78, 193–202. [Google Scholar] [CrossRef]
- Oh, W.; Kim, S.; Bae, S.; Park, N.; Chan, S.I.; Kang, Y.; Lee, H.S.; Kim, D. Migration of Sn and Pb from Solder Ribbon onto Ag Fingers in Field-Aged Silicon Photovoltaic Modules. Int. J. Photoenergy 2015, 2015, 257343. [Google Scholar] [CrossRef]
- Ndiaye, A.; Kébé, C.M.F.; Charki, A.; Sambou, V.; Ndiaye, P.A. Photovoltaic Platform for Investigating PV Module Degradation. Energy Procedia 2015, 74, 1370–1380. [Google Scholar] [CrossRef]
- Shrestha, S.M.; Mallineni, J.K.; Yedidi, K.R.; Knisely, B.; Tatapudi, S.; Kuitche, J.; Tamizhmani, G. Determination of Dominant Failure Modes Using FMECA on the Field Deployed C-Si Modules under Hot-Dry Desert Climate. IEEE J. Photovolt. 2015, 5, 174–182. [Google Scholar] [CrossRef]
- Bandou, F.; Arab, A.; Belkaid, M.; Rosca, V.; Guichoux, M.; Eerenstein, W.; Roosmalen, J.; Logerais, P. Reliability of Back-Contact MWT Modules under Hot and Humid Conditions. Br. J. Appl. Sci. Technol. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Mohammed, S.; Boumediene, B.; Miloud, B. Assessment of PV Modules Degradation Based on Performances and Visual Inspection in Algerian Sahara. Int. J. Renew. Energy Res. 2016, 6, 106–116. [Google Scholar]
- Quansah, D.A.; Adaramola, M.S.; Takyi, G.; Edwin, I.A. Reliability and Degradation of Solar PV Modules-Case Study of 19-Year Old Polycrystalline Modules in Ghana. Technologies 2017, 5, 22. [Google Scholar] [CrossRef]
- Fezzani, A.; Mahammed, I.H.; Said, D.; Zaghba, L.; Bouchakour, A.; Benbitour, M.; Oudjana, S.H. Degradation and Performance Evaluation of PV Module in Desert Climate Conditions with Estimate Uncertainty in Measuring. Serbian J. Electr. Eng. 2017, 14, 277–299. [Google Scholar] [CrossRef]
- Bouraiou, A.; Hamouda, M.; Chaker, A.; Lachtar, S.; Neçaibia, A.; Boutasseta, N.; Mostefaoui, M. Experimental Evaluation of the Performance and Degradation of Single Crystalline Silicon Photovoltaic Modules in the Saharan Environment. Energy 2017, 132, 22–30. [Google Scholar] [CrossRef]
- Xiong, H.; Gan, C.; Yang, X.; Hu, Z.; Niu, H.; Li, J.; Si, J.; Xing, P.; Luo, X. Corrosion Behavior of Crystalline Silicon Solar Cells. Microelectron. Reliab. 2017, 70, 49–58. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Sample, T. Characterisation of C-Si Modules After 20 Years of Outdoor Exposure on an Electric Vehicle; European Commission Joint Research Centre Institute: Brussels, Belgium, 2017. [Google Scholar]
- Ndiaye, A.; Kébé, C.M.F.; Bilal, B.O.; Charki, A.; Sambou, V.; Ndiaye, P.A. Investigation of Degradation in Crystalline Silicon Photovoltaic Modules After 10 Years Exposition in Senegal by Infrared (IR) and Electroluminescence (EL). In Proceedings of the Lecture Notes of the Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering, LNICST, London, UK, 23–24 August 2018; Springer: Berlin/Heidelberg, Germany, 2018; Volume 249, pp. 23–40. [Google Scholar]
- Singh Chaudhary, A.; Chaturvedi, D.K. Analyzing Defects of Solar Panels under Natural Atmospheric Conditions with Thermal Image Processing. Int. J. Image Graph. Signal Process. 2018, 10, 10–21. [Google Scholar] [CrossRef]
- Virtuani, A.; Caccivio, M.; Annigoni, E.; Friesen, G.; Chianese, D.; Ballif, C.; Sample, T. 35 Years of Photovoltaics: Analysis of the TISO-10-KW Solar Plant, Lessons Learnt in Safety and Performance—Part 1. Prog. Photovolt. Res. Appl. 2019, 27, 328–339. [Google Scholar] [CrossRef]
- Han, H.; Dong, X.; Lai, H.; Yan, H.; Zhang, K.; Liu, J.; Verlinden, P.J.; Liang, Z.; Shen, H. Analysis of the Degradation of Monocrystalline Silicon Photovoltaic Modules after Long-Term Exposure for 18 Years in a Hot-Humid Climate in China. IEEE J. Photovolt. 2018, 8, 806–812. [Google Scholar] [CrossRef]
- Abdou, N.; Faye, I.; Kobor, D.; Blieske, U.; Ayituv, M. Comparative Experimental Study of Performance Degradation of Amorphous Silicon and Crystalline Silicon in Outdoor Exposed in Cologne. Afr. J. Environ. Sci. Tech. 2018, 12, 461–468. [Google Scholar] [CrossRef]
- Li, J.; Shen, Y.C.; Hacke, P.; Kempe, M. Electrochemical Mechanisms of Leakage-Current-Enhanced Delamination and Corrosion in Si Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2018, 188, 273–279. [Google Scholar] [CrossRef]
- Ogbomo, O.O.; Amalu, E.H.; Ekere, N.N.; Olagbegi, P.O. Effect of Operating Temperature on Degradation of Solder Joints in Crystalline Silicon Photovoltaic Modules for Improved Reliability in Hot Climates. Sol. Energy 2018, 170, 682–693. [Google Scholar] [CrossRef]
- Hamaoka, R.; Iwami, K.; Itayama, T.; Nagasaki, H.; Takemoto, S.; Yamamoto, C.; Hara, Y.; Masuda, A.; Umeda, N. Detection of Acetic Acid Produced in Photovoltaic Modules Based on Tin Film Corrosion during Damp Heat Test. Jpn. J. Appl. Phys. 2018, 57, 08RG16. [Google Scholar] [CrossRef]
- Luo, W.; Khoo, Y.S.; Hacke, P.; Jordan, D.; Zhao, L.; Ramakrishna, S.; Aberle, A.G.; Reindl, T. Analysis of the Long-Term Performance Degradation of Crystalline Silicon Photovoltaic Modules in Tropical Climates. IEEE J. Photovolt. 2019, 9, 266–271. [Google Scholar] [CrossRef]
- Tanesab, J.A.; Amheka, A.; Sinaga, R.; Mauta, J.J.; Hattu, E. A Preliminary Study of Common Defects of Photovoltaic Modules in West Timor, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 6 August 2020; Institute of Physics Publishing: Bristol, UK, 2020; Volume 542. [Google Scholar]
- Kilikeviciene, K.; Matijosius, J.; Kilikevicius, A.; Jurevicius, M.; Makarskas, V.; Caban, J.; Marczuk, A. Research of the Energy Losses of Photovoltaic (PV) Modules after Hail Simulation Using a Newly-Created Testbed. Energies 2019, 12, 4537. [Google Scholar] [CrossRef]
- Tanahashi, T.; Sakamoton, N.; Shibata, H.; Masuda, A. Corrosion-Induced AC Impedance Elevation in Front Electrodes of Crystalline Silicon Photovoltaic Cells Within Field-Aged Photovoltaic Modules. IEEE J. Photovolt. 2019, 9, 741–751. [Google Scholar] [CrossRef]
- Benabdelkrim, B.; Benatiallah, A.; Ghaitaoui, T. Study of Degradation of Amorphous PV Module Performance under Different Climatic Conditions. Alger. J. Renew. Energy Sustain. Dev. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Bouaichi, A.; El Amrani, A.; Ouhadou, M.; Lfakir, A.; Messaoudi, C. In-Situ Performance and Degradation of Three Different Photovoltaic Module Technologies Installed in Arid Climate of Morocco. Energy 2020, 190, 116368. [Google Scholar] [CrossRef]
- Pierce, B.G.; Karimi, A.M.; Liu, J.; French, R.H.; Braid, J.L. Identifying Degradation Modes of Photovoltaic Modules Using Unsupervised Machine Learning on Electroluminescense Images. In Proceedings of the Conference Record of the IEEE Photovoltaic Specialists Conference, Calgary, ON, Canada, 14 June 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 1850–1855. [Google Scholar]
- Atsu, D. Assessing the Long-Term Performance Degradation of PV Modules in the Sub-Saharan Environment; International Solar Energy Society (ISES): Freiburg im Breisgau, Germany, 2021; pp. 1–10. [Google Scholar]
- Mota, F.; Neto Torres, J.P.; Ferreira Fernandes, C.A.; Marques Lameirinhas, R.A. Influence of an Aluminium Concentrator Corrosion on the Output Characteristic of a Photovoltaic System. Sci. Rep. 2020, 10, 21865. [Google Scholar] [CrossRef]
- Oreski, G.; Omazic, A.; Eder, G.C.; Voronko, Y.; Neumaier, L.; Mühleisen, W.; Hirschl, C.; Ujvari, G.; Ebner, R.; Edler, M. Properties and Degradation Behaviour of Polyolefin Encapsulants for Photovoltaic Modules. Prog. Photovolt. Res. Appl. 2020, 28, 1277–1288. [Google Scholar] [CrossRef]
- Chen, J.; Pan, G.; Ouyang, J.; Ma, J.; Fu, L.; Zhang, L. Study on Impacts of Dust Accumulation and Rainfall on PV Power Reduction in East China. Energy 2020, 194, 116915. [Google Scholar] [CrossRef]
- da Fonseca, J.E.F.; de Oliveira, F.S.; Massen Prieb, C.W.; Krenzinger, A. Degradation Analysis of a Photovoltaic Generator after Operating for 15 Years in Southern Brazil. Sol. Energy 2020, 196, 196–206. [Google Scholar] [CrossRef]
- Hossion, M.A. Visual and Electrical Degradation Data of Five Years Aged Rooftop Photovoltaic Modules. Data Brief. 2020, 31, 105762. [Google Scholar] [CrossRef]
- Bdour, M.; Dalala, Z.; Al-Addous, M.; Radaideh, A.; Al-Sadi, A. A Comprehensive Evaluation on Types of Microcracks and Possible Eects on Power Degradation in Photovoltaic Solar Panels. Sustainability 2020, 12, 6416. [Google Scholar] [CrossRef]
- Chandel, T.A.; Mallick, M.A.; Yasin, M.Y. Oxidation: A Dominant Source for Reduced Efficiency of Silicon Solar Photovoltaic Modules. Mater. Today: Proc. 2020, 27, 1092–1098. [Google Scholar] [CrossRef]
- Semba, T. Corrosion Mechanism Analysis of the Front-Side Metallization of a Crystalline Silicon PV Module by a High-Temperature and High-Humidity Test. Jpn. J. Appl. Phys. 2020, 59, 054001. [Google Scholar] [CrossRef]
- Sangpongsanont, Y.; Chenvidhya, D.; Chuangchote, S.; Kirtikara, K. Corrosion Growth of Solar Cells in Modules after 15 Years of Operation. Sol. Energy 2020, 205, 409–431. [Google Scholar] [CrossRef]
- Sinha, A.; Moffitt, S.L.; Hurst, K.; Kempe, M.; Han, K.; Shen, Y.C.; Miller, D.C.; Hacke, P.; Schelhas, L.T. Understanding Interfacial Chemistry of Positive Bias High-Voltage Degradation in Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2021, 223, 110959. [Google Scholar] [CrossRef]
- Baldus-Jeursen, C.; Côté, A.; Deer, T.; Poissant, Y. Analysis of Photovoltaic Module Performance and Life Cycle Degradation for a 23 Year-Old Array in Quebec, Canada. Renew. Energy 2021, 174, 547–556. [Google Scholar] [CrossRef]
- Dahbi, H.; Aoun, N.; Sellam, M. Performance Analysis and Investigation of a 6 MW Grid-Connected Ground-Based PV Plant Installed in Hot Desert Climate Conditions. Int. J. Energy Environ. Eng. 2021, 12, 577–587. [Google Scholar] [CrossRef]
- Tang, J.; Ju, C.; Lv, R.; Zeng, X.; Chen, J.; Fu, D.; Jaubert, J.N.; Xu, T. The Performance of Double Glass Photovoltaic Modules under Composite Test Conditions. Energy Procedia 2017, 130, 87–93. [Google Scholar]
- Kyranaki, N.; Smith, A.; Yendall, K.; Hutt, D.A.; Whalley, D.C.; Gottschalg, R.; Betts, T.R. Damp-Heat Induced Degradation in Photovoltaic Modules Manufactured with Passivated Emitter and Rear Contact Solar Cells. Prog. Photovolt. Res. Appl. 2022, 30, 1061–1071. [Google Scholar] [CrossRef]
- Oh, W.; Choi, H.; Kim, D. Analysis of the Impact of Power Loss Due to Snail Trails in a 95-KWp Photovoltaic Power System. Microelectron. Reliab. 2021, 126, 114230. [Google Scholar] [CrossRef]
- Luo, W.; Clement, C.E.; Khoo, Y.S.; Wang, Y.; Khaing, A.M.; Reindl, T.; Kumar, A.; Pravettoni, M. Photovoltaic Module Failures after 10 Years of Operation in the Tropics. Renew. Energy 2021, 177, 327–335. [Google Scholar] [CrossRef]
- Beaucarne, G.; Dupont, A.; Puthenmadom, D.; Shephard, N.; Sample, T. Material Study of Photovoltaic Modules with Silicone Encapsulation after Long-Term Outdoor Exposure. Sol. Energy Mater. Sol. Cells 2021, 230, 111298. [Google Scholar] [CrossRef]
- Zhuang, H.; Huang, G.; Jiang, Y.; Bai, X.; Sun, G.; Li, J. Study of Novel High-Density Panels Based on Negative Space Welding Technology with Cells Cut by Different Techniques. In Proceedings of the E3S Web of Conferences, Les Ulis, France, 19 May 2021; EDP Sciences: Les Ulis, France, 2021; Volume 260. [Google Scholar]
- Lillo-Sánchez, L.; López-Lara, G.; Vera-Medina, J.; Pérez-Aparicio, E.; Lillo-Bravo, I. Degradation Analysis of Photovoltaic Modules after Operating for 22 Years. A Case Study with Comparisons. Sol. Energy 2021, 222, 84–94. [Google Scholar] [CrossRef]
- Adar, M.; Najih, Y.; Chebak, A.; Mabrouki, M.; Bennouna, A. Performance Degradation Assessment of the Three Silicon PV Technologies. Prog. Photovolt. Res. Appl. 2022, 30, 1149–1165. [Google Scholar] [CrossRef]
- Fezzani, A.; Hadj-Mahammed, I.; Kouzou, A.; Zaghba, L.; Drid, S.; Khennane, M.; Kennel, R.; Abdelrahem, M. Energy Efficiency of Multi-Technology PV Modules under Real Outdoor Conditions—An Experimental Assessment in Ghardaïa, Algeria. Sustainability 2022, 14, 1771. [Google Scholar] [CrossRef]
- Fairbrother, A.; Gnocchi, L.; Ballif, C.; Virtuani, A. Corrosion Testing of Solar Cells: Wear-out Degradation Behavior. Sol. Energy Mater. Sol. Cells 2022, 248, 111974. [Google Scholar] [CrossRef]
- Ameur, A.; Berrada, A.; Bouaichi, A.; Loudiyi, K. Long-Term Performance and Degradation Analysis of Different PV Modules under Temperate Climate. Renew. Energy 2022, 188, 37–51. [Google Scholar] [CrossRef]
- Daher, D.H.; Aghaei, M.; Quansah, D.A.; Adaramola, M.S.; Parvin, P.; Ménézo, C. Multi-Pronged Degradation Analysis of a Photovoltaic Power Plant after 9.5 Years of Operation under Hot Desert Climatic Conditions. Prog. Photovolt. Res. Appl. 2023, 31, 888–907. [Google Scholar] [CrossRef]
- Iqbal, N.; Colvin, D.J.; Schneller, E.J.; Sakthivel, T.S.; Ristau, R.; Huey, B.D.; Yu, B.X.J.; Jaubert, J.N.; Curran, A.J.; Wang, M.; et al. Characterization of Front Contact Degradation in Monocrystalline and Multicrystalline Silicon Photovoltaic Modules Following Damp Heat Exposure. Sol. Energy Mater. Sol. Cells 2022, 235, 111468. [Google Scholar] [CrossRef]
- Bansal, N.; Jaiswal, S.P.; Singh, G. Prolonged Degradation and Reliability Assessment of Installed Modules Operational for 10 Years in 5 MW PV Plant in Hot Semi-Arid Climate. Energy Sustain. Dev. 2022, 68, 373–389. [Google Scholar] [CrossRef]
- Singh, S.K.; Chander, N. Mid-Life Degradation Evaluation of Polycrystalline Si Solar Photovoltaic Modules in a 100 KWp Grid-Tied System in East-Central India. Renew. Energy 2022, 199, 351–367. [Google Scholar] [CrossRef]
- Del Pero, C.; Aste, N.; Leonforte, F.; Sfolcini, F. Long-Term Reliability of Photovoltaic c-Si Modules—A Detailed Assessment Based on the First Italian BIPV Project. Sol. Energy 2023, 264, 112074. [Google Scholar] [CrossRef]
- Hacke, P.; Owen-Bellini, M.; Kempe, M.D.; Sulas-Kern, D.B.; Miller, D.C.; Jankovec, M.; Mitterhofer, S.; Topič, M.; Spataru, S.; Gambogi, W.; et al. Acceleration Factors for Combined-Accelerated Stress Testing of Photovoltaic Modules. Solar RRL 2023, 7, 2300068. [Google Scholar] [CrossRef]
- Zaghba, L.; Khennane, M.; Mekhilef, S.; Fezzani, A.; Borni, A. Long-Term Outdoor Performance of Grid-Connected Photovoltaic Power Plant in a Desert Climate. Energy Sustain. Dev. 2023, 74, 430–453. [Google Scholar] [CrossRef]
- Ebhota, W.S.; Tabakov, P.Y. Influence of Photovoltaic Cell Technologies and Elevated Temperature on Photovoltaic System Performance. Ain Shams Eng. J. 2023, 14, 101984. [Google Scholar] [CrossRef]
- Segbefia, O.K. Temperature Profiles of Field-Aged Photovoltaic Modules Affected by Optical Degradation. Heliyon 2023, 9, e19566. [Google Scholar] [CrossRef]
- Gyamfi, S.; Aboagye, B.; Peprah, F.; Obeng, M. Degradation Analysis of Polycrystalline Silicon Modules from Different Manufacturers under the Same Climatic Conditions. Energy Convers. Manag. X 2023, 20, 100403. [Google Scholar] [CrossRef]
- Aboagye, B.; Gyamfi, S.; Ofosu, E.A.; Djordjevic, S. Characterisation of Visual Defects on Installed Solar Photovoltaic (PV) Modules in Different Climatic Zones in Ghana. Sci. Afr. 2023, 20, e01682. [Google Scholar] [CrossRef]
- Segbefia, O.K.; Akhtar, N.; Sætre, T.O. Moisture Induced Degradation in Field-Aged Multicrystalline Silicon Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2023, 258, 112407. [Google Scholar] [CrossRef]
- Mannino, G.; Tina, G.M.; Cacciato, M.; Todaro, L.; Bizzarri, F.; Canino, A. A Photovoltaic Degradation Evaluation Method Applied to Bifacial Modules. Sol. Energy 2023, 251, 39–50. [Google Scholar] [CrossRef]
- Mishra, P.R.; Rathore, S.; Varma, K.S.V.; Yadav, S.K. Long-Term Performance and Degradation Analysis of a 5 MW Solar PV Plant in the Andaman & Nicobar Islands. Energy Sustain. Dev. 2024, 79, 101413. [Google Scholar] [CrossRef]
- Awoyinka, T.D.; David, T.W.; Somefun, T.E.; Somefun, C.T.; Orovwode, H.E. I-V Response Test of 60–150 W Mono-Crystalline Solar Panel. Front. Energy Res. 2024, 12, 1375854. [Google Scholar] [CrossRef]
- Pimpalkar, R.; Sahu, A.; Yadao, A.; Patil, R.B. Failure Modes and Effects Analysis of Polycrystalline Photovoltaic Modules Exposed to the Composite Climate of India. J. Inst. Eng. Ser. C 2024, 105, 339–355. [Google Scholar] [CrossRef]
- Galleguillos Madrid, F.M.; Soliz, A.; Cáceres, L.; Bergendahl, M.; Leiva-Guajardo, S.; Portillo, C.; Olivares, D.; Toro, N.; Jimenez-Arevalo, V.; Páez, M. Green Corrosion Inhibitors for Metal and Alloys Protection in Contact with Aqueous Saline. Materials 2024, 17, 3996. [Google Scholar] [CrossRef]
- Bozzini, B.; Amati, M.; Dobrovolska, T.; Gregoratti, L.; Krastev, I.; Sgura, I.; Taurino, A.; Kiskinova, M. Depth-Dependent Scanning Photoelectron Microspectroscopy Unravels the Mechanism of Dynamic Pattern Formation in Alloy Electrodeposition. J. Phys. Chem. C 2018, 122, 15996–16007. [Google Scholar] [CrossRef]
- Escobar Galindo, R.; Gago, R.; Duday, D.; Palacio, C. Towards Nanometric Resolution in Multilayer Depth Profiling: A Comparative Study of RBS, SIMS, XPS and GDOES. Anal. Bioanal. Chem. 2010, 396, 2725–2740. [Google Scholar]
- Tang, Q.; Shen, H.; Yao, H.; Gao, K.; Jiang, Y.; Zheng, C.; Yang, W.; Li, Y.; Liu, Y.; Zhang, L. Potential of Quasi-Inverted Pyramid with Both Efficient Light Trapping and Sufficient Wettability for Ultrathin c-Si/PEDOT:PSS Hybrid Solar Cells. Sol. Energy Mater. Sol. Cells 2017, 169, 226–235. [Google Scholar] [CrossRef]
- Cattin, L.; Bernède, J.C.; Lare, Y.; Dabos-Seignon, S.; Stephant, N.; Morsli, M.; Zamora, P.P.; Diaz, F.R.; Del Valle, M.A. Improved Performance of Organic Solar Cells by Growth Optimization of MoO3/CuI Double-Anode Buffer. Phys. Status Solidi Appl. Mater. Sci. 2013, 210, 802–808. [Google Scholar] [CrossRef]
- Nojiri, K.; Shahiduzzaman, M.; Yamamoto, K.; Kuwabara, T.; Takahashi, K.; Taima, T. Interpenetrating Heterojunction Photovoltaic Cells Based on C60 Nano-Crystallized Thin Films. Org. Electron. 2016, 38, 107–114. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Y.; Ran, G.; Liu, F.; Zhang, W.; Zhu, X. 19.7% Efficiency Binary Organic Solar Cells Achieved by Selective Core Fluorination of Nonfullerene Electron Acceptors. Joule 2024, 8, 835–851. [Google Scholar] [CrossRef]
- Gupta, S.K.; Banerjee, S.; Singh, A.; Pali, L.S.; Garg, A. Modeling of degradation in normal and inverted OSC devices. Sol. Energy Mater. Sol. Cells 2019, 191, 329–338. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Hone, F.G.; Mola, G.T.; Tegegne, N.A. The Roles of Acceptors in the Thermal-Degradation of P3HT Based Organic Solar Cells. Phys. B Condens. Matter 2023, 653, 414666. [Google Scholar] [CrossRef]
- Bastos, J.P.; Voroshazi, E.; Fron, E.; Brammertz, G.; Vangerven, T.; Van Der Auweraer, M.; Poortmans, J.; Cheyns, D. Oxygen-Induced Degradation in C60-Based Organic Solar Cells: Relation between Film Properties and Device Performance. ACS Appl. Mater. Interfaces 2016, 8, 9798–9805. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhu, R.; Chen, X.; Wang, T.; Pu, X.; Chen, H.; Cao, Q.; Li, X. Enhanced Corrosion Resistance of Ag Electrode Through Ionized 2-Mercaptobenzothiazole in Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2024, 37, 386–395. [Google Scholar] [CrossRef]
- Sato, H.; Binti Azmi, W.S.; Onaru, Y.; Harafuji, K. Degradation by Ultra-Violet Light and Its Mechanism in Organic Solar Cells. Org. Electron. 2016, 37, 386–395. [Google Scholar] [CrossRef]
- Zamora, P.P.; Díaz, F.R.; del Valle, M.A.; Cattin, L.; Louarn, G.; Bernède, J.C. Effect of CuI Anode Buffer Layer on the Growth of Polymers Thin Films and on the Performances of Organic Solar Cells. Nat. Resour. 2013, 4, 123–133. [Google Scholar] [CrossRef]
- Hou, X.; Clarke, A.J.; Azzouzi, M.; Yan, J.; Eisner, F.; Shi, X.; Wyatt, M.F.; Dennis, T.J.S.; Li, Z.; Nelson, J. Relationship between Molecular Properties and Degradation Mechanisms of Organic Solar Cells Based on Bis-Adducts of Phenyl-C61 Butyric Acid Methyl Ester. J. Mater. Chem. C Mater. 2022, 10, 7875–7885. [Google Scholar] [CrossRef]
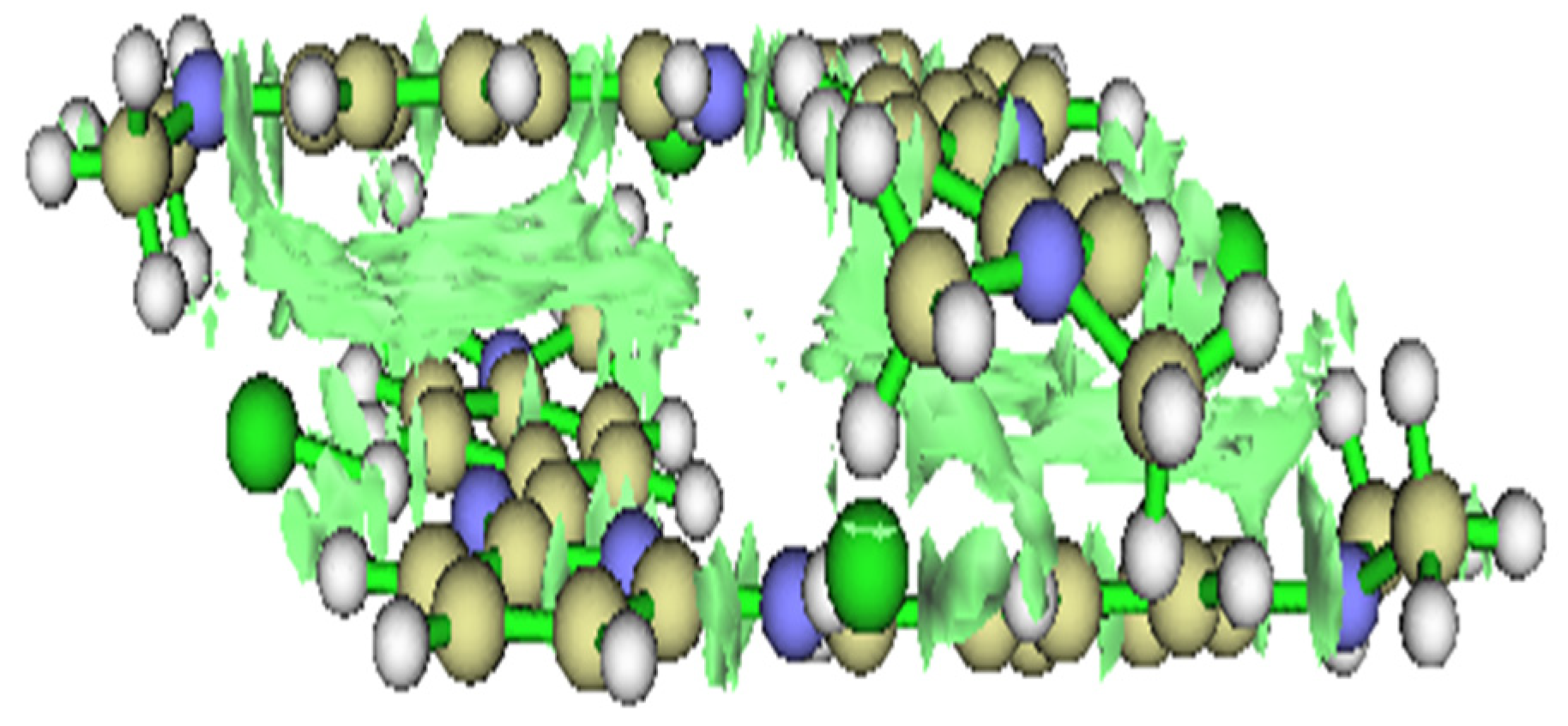
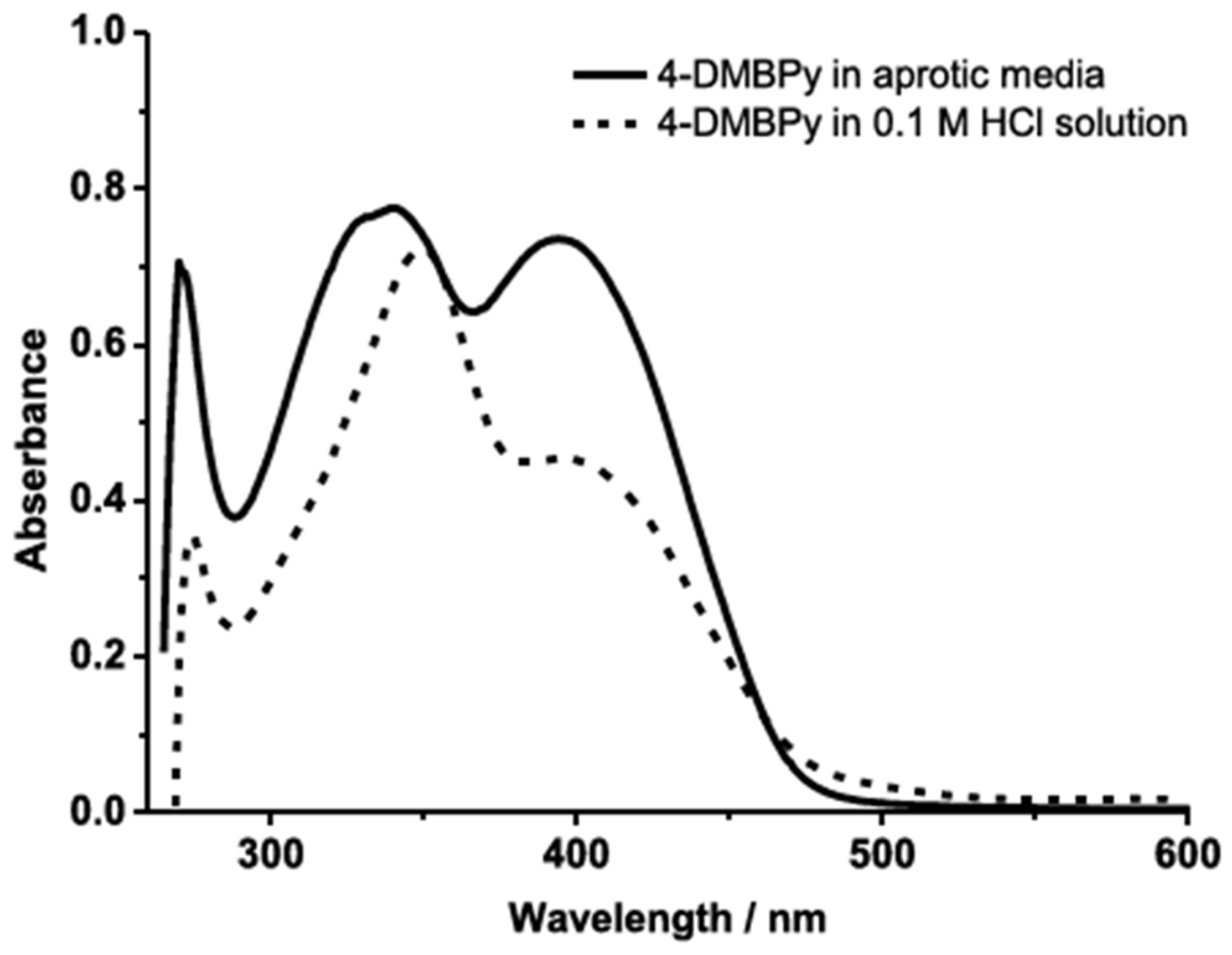
- Soliz, A.; Zamora, P.P.; Muena, J.P.; Bieger, K.; Haribabu, J.; Landaeta, E.; Arulraj, A.; Mangalaraja, R.V. Experimental and DFT Analysis of the Concentration Dependency of Corrosion Inhibition by a Pyridine Schiff Base for Mild Steel in HCl Solution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135283. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Li, J.; Sun, S.; Wu, Z.; Xu, B. A Method for Accurately Assessing Field Performance Degradation of PV Modules in Different Geographical Regions. Sustain. Energy Technol. Assess. 2021, 48, 101638. [Google Scholar] [CrossRef]
- Jordan, D.C.; Kurtz, S.R. Photovoltaic Degradation Rates—An Analytical Review. Prog. Photovolt. Res. Appl. 2013, 21, 12–29. [Google Scholar] [CrossRef]
- Aboagye, B.; Gyamfi, S.; Ofosu, E.A.; Djordjevic, S. Degradation Analysis of Installed Solar Photovoltaic (PV) Modules under Outdoor Conditions in Ghana. Energy Rep. 2021, 7, 6921–6931. [Google Scholar] [CrossRef]
- Bouaichi, A.; Logerais, P.O.; El Amrani, A.; Ennaoui, A.; Messaoudi, C. Comprehensive Analysis of Aging Mechanisms and Design Solutions for Desert-Resilient Photovoltaic Modules. Sol. Energy Mater. Sol. Cells 2024, 267, 112707. [Google Scholar] [CrossRef]
- Ascencio-Vásquez, J.; Kaaya, I.; Brecl, K.; Weiss, K.A.; Topič, M. Global Climate Data Processing and Mapping of Degradation Mechanisms and Degradation Rates of PV Modules. Energies 2019, 12, 4749. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, M.; Yadav, K. Degradation and Reliability Analysis of Photovoltaic Modules after Operating for 12 Years: A Case Study with Comparisons. Renew. Energy 2022, 196, 1170–1186. [Google Scholar] [CrossRef]
- McMahon, T.J. Accelerated Testing and Failure of Thin-Film PV Modules. Prog. Photovolt. Res. Appl. 2004, 12, 235–248. [Google Scholar] [CrossRef]
- Omazic, A.; Oreski, G.; Halwachs, M.; Eder, G.C.; Hirschl, C.; Neumaier, L.; Pinter, G.; Erceg, M. Relation between Degradation of Polymeric Components in Crystalline Silicon PV Module and Climatic Conditions: A Literature Review. Sol. Energy Mater. Sol. Cells 2019, 192, 123–133. [Google Scholar] [CrossRef]

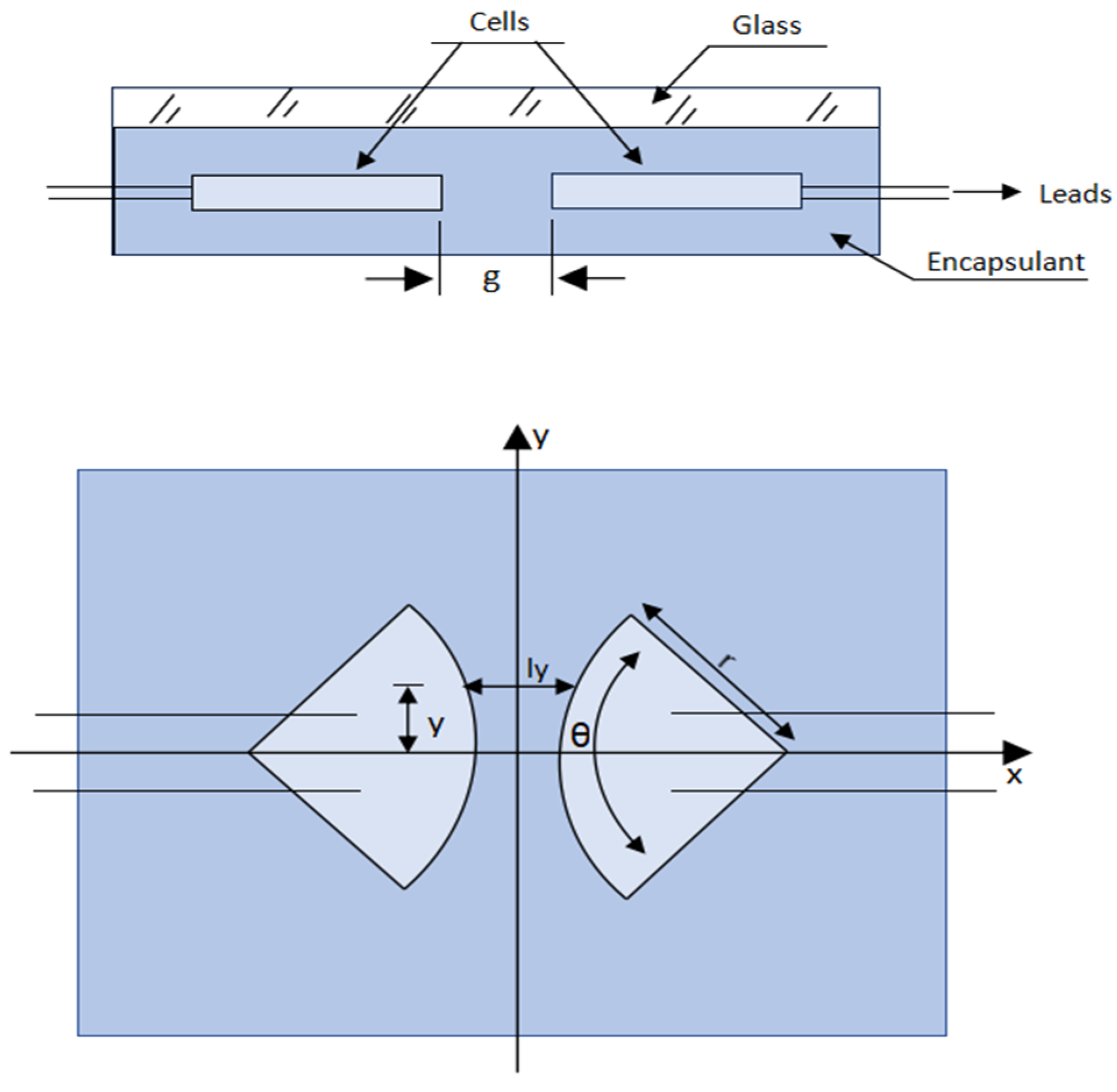


| Ref. | Technology | Degradation Modes | Temp °C | HR (%) | Irradiation (kWh/m2/Year) | Location | Exposure Period | Characterization Techniques |
|---|---|---|---|---|---|---|---|---|
| [20] | c-Si | Electrochemical and galvanic | 85 | 2.5/70/98 | - | USA | 1944 h | I-V and P-V measurements Visual measurements: SEM, EDAX, ESCA, Auger Surface analysis |
| [38] | a-Si | Electrochemical and galvanic | 85 | 85 | - | USA | 1200 h | SEM, EDX, SIMS. LSV, I vs. t, power output vs. Q |
| [39] | TF/a-Si | Electrochemical, galvanic, and pitting | 85 | 85 | - | USA | 1910 h | LSV (Ecorr = 500 mV and Icorr = 1.5 mA) |
| [40] | sc-Si | - | −10 to 35 | <90 | 1393.6 | Italy | 19 years | Visual inspection IR I-V and P-V measurements Insulation tests |
| [41] | sc-Si | - | 7 to 17 | 75 to 80 | 1493 (Arcata) | USA | 11 years | I-V and P-V measurements |
| [42] | TF/a-Si | Electrochemical | 60/72/85 | 85 | - | USA | 170 to 1340 h | Visual inspection BHAST |
| [43] | c-Si | Electrochemical, pitting, and photocorrosion | −10 to 35 | <90 | 1393.6 | Italy | 19 years | I-V and P-V measurements |
| [44] | TF/a-Si/a-SiGe | Electrochemical | 85 | 85 | - | USA | 125 h | AFM, DRX |
| [45] | c-Si | Electrochemical, oxidative degradation, hydrolytic reaction, and delamination | 85 | 100 | - | USA | - | Diffusivity and solubility measurements WVTR |
| [46] | sc-Si/mc-Si | Electrochemical, photocorrosion, and delamination | 40/90 | 93/95 | 1393.6 | Italy | 20 to 22 years | I-V and P-V measurements Visual inspection Electrical insulation test Bypass diode check Laser scan measurements |
| [47] | TF | Electrochemical, galvanic, and delamination | 60/85/95 | 60/85/100 | - | USA | 19 to 967 h | Xenon arc lamp radiation test Damp-heat test Adhesion measurements Scotch tape adhesion test XPS |
| [25] | c-Si | Electrochemical and galvanic | - | - | - | Spain | - | - |
| [48] | a-Si/c-Si/mc-Si | Electrochemical, photocorrosion, and delamination | 25 | - | 2054.5 | USA | 1 to 7 years | Visual inspection I-V and P-V measurements IR |
| [49] | c-Si | Electrochemical, photocorrosion, and delamination | 60 | 60 | - | USA | 6.25 months | Lap shear tests UV exposure tests Accelerated stress chamber tests Transmittance measurements Water absorption measurements |
| [50] | c-Si | Electrochemical, photocorrosion, and delamination | 18 | 63 | 1890 | Spain | 12 years | Visual inspection I-V and P-V measurements IR Spectrophotometry |
| [51] | c-Si | Electrochemical and photocorrosion | 85 | - | - | Rusia | 1000 | UV radiation exposure test Spectral transmittance measurement Visual inspection I-V and P-V measurements |
| [52] | - | Electrochemical, general corrosion, and delamination | 85 | 85 | - | Germany | 1000 | Visual investigations EL Wet leakage testing Peel strength measurement Bulk resistivity measurements |
| [53] | sc-Si | - | 16 to 38 | 75 to 95 | 2114 | Senegal | 1 year | I-V and P-V measurements |
| [54] | c-Si | General corrosion | ~7.5 | ~86.5 | - | France | 2700 | - |
| [55] | mc-Si | Galvanic corrosion | 45/65/85 | 85 | - | - | 4000 | SEM, EDX, AES, EL |
| [56] | a-Si, mc-Si HIT | General corrosion | 11 to 34 | 42 to 62 | 1621 | India | 1 year | I-V and P-V measurements |
| [57] | c-Si | Electrochemical | 45/65/85 | 85 | - | USA | 1 year | I-V and P-V measurements Moisture weight gain measurements Solubility determination WVTR |
| [58] | c-Si | Galvanic corrosion | 85 | 85 | - | Republic of Korea | 3000 | SEM, EDX, EL |
| [59] | sc-Si/mc-Si | Electrochemical | 16 to 38 | 75 to 95 | 2114 | Senegal | 16 to 48 months | I-V and P-V measurements |
| [60] | c-Si | Chemical corrosion | 85 | 85 | - | Japan | 4000 | EL, EPMA, FTIR, SCM |
| [61] | a-Si | Electrochemical | 25 | - | - | Egypt | - | I-V and P-V measurements |
| [62] | sc-Si | Electrochemical and galvanic corrosion | 20 to 25 | 80 | 1517.6 | India | 28 years | Visual inspection IR I-V and P-V measurements |
| [63] | Si | Electrochemical | 13.6 | 64 | 1515.4 | Republic of Korea | 6 years | FE-SEM, EDS, FIB, |
| [64] | sc-Si | - | 16 to 38 | 75 to 95 | 2114 | Senegal | 1 year | I-V and P-V measurements |
| [65] | c-Si | Electrochemical and galvanic corrosion | 6 to 28 | 19 to 45 | 2014.8 | USA | 6–16 years | Visual inspection IR I-V and P-V measurements |
| [66] | c-Si | Electrochemical | 85 | 85 | - | The Netherlands | 2000 h | Visual inspections I-V and P-V measurements |
| [67] | sc-Si | Electrochemical | 7 to 42 | 19 | 2209.7 | Algeria | - | Visual inspection I-V and P-V measurements |
| [68] | mc-Si | 24.4 to 27.8 | 65 to 83.5 | 1725 (Kumasi) | Ghana | 19 years | Visual inspection IR I-V and P-V measurements | |
| [69] | sc-Si | Electrochemical and photocorrosion | 47.4 | 16 to 52 | 2066.5 | Algeria | 12 years | Visual inspection I-V and P-V measurements Analytical calculations of degradation rates Insulation resistance measurements Cost analysis |
| [70] | sc-Si | Electrochemical | 9 to 44 | 10 to 40 | 2209.7 | Algeria | >12 years | Visual inspection I-V and P-V measurements |
| [71] | mc-Si | Grid corrosion, galvanic | 25 | 45/85 | - | China | 240 h to 2 months | EL I-V characteristic measurement SEM EDS |
| [72] | c-Si | - | 10 to 35 | 90 | 1393.6 | Italy | 20 years | Visual inspection Electrical performance measurements EL IR |
| [73] | sc-Si/mc-Si | General corrosion | 20 to 28 | ~70 | 2114 | Senegal | 10 years | Visual inspection EL IR |
| [74] | c-Si/TH | Electrochemical, photocorrosion, and pitting | 14 to 34 | - | 1764.9 | India | - | Visual inspection Thermal image |
| [75] | c-Si | - | −0.4 to 19.7 | 70 to 80 | 1393.2 | Switzerland | 35 years | I-V and P-V measurements Visual inspection Electrical insulation (INS) test EL IR |
| [76] | sc-Si | Electrochemical, galvanic, photocorrosion, and general corrosion | 14.2 to 28.3 | 67 to 80 | 1470.3 | China | 18 years | I-V and P-V measurements EL SEM EDX XPS |
| [77] | a-Si/sc-Si | Electrochemical | 2.5 to 19 | 58 to 92 | 1072.5 | Germany | 5 years | I-V and P-V measurements |
| [78] | c-Si | Electrochemical and galvanic | 72/85 | 85/95 | - | USA | 292 to 1000 h | SEM EDS Pourbaix diagrams |
| [79] | c-Si | Electrochemical and photocorrosion | 25 to 120 | - | - | UK | - | Finite element method (FEM) Dynamic mechanical analysis (DMA) |
| [80] | c-Si | Chemical and galvanic corrosion | 85 | 85 | - | Japan | 7203 | DH test Optical measurements. I-V and P-V measurements EL Ion chromatography |
| [81] | c-Si | Electrochemical and photo-corrosion | 26 to 28 | 82 to 86 | 1637 | Singapore | 7 years | I-V and P-V measurements |
| [82] | sc-Si | Electrochemical and pitting | 23 to 27 | 70–90 | 2003.2 | Indonesia | 6/8/12 years | Visual inspection |
| [83] | c-Si | - | 25 | - | - | Lithuania | - | Visual inspection EL |
| [84] | mc-Si | Electrochemical | 25 | - | - | Japan | - | Visual inspection I-V and P-V measurements EL Impedance spectroscopy Statistical evaluation |
| [85] | a-Si | - | 9 to 44 | 10 to 40 | 2209.7 | Algeria | 14 months | Visual inspection I-V and P-V measurements |
| [86] | c-Si/a-Si | - | 12.2 to 31 | 28.4 to 31.5 | 2099.6 | Morocco | 6 years | I-V and P-V measurements |
| [87] | c-Si | - | 85 | 85 | - | USA | 4200 h | EL Feature extraction algorithms Hierarchical clustering Principal component analysis (PCA) |
| [88] | sc-Si | - | 24 to 28 | 76.5 to 88.3 | 1733 | Ghana | 13 years | Visual inspection I-V and P-V measurements IR |
| [89] | c-Si | Atmospheric corrosion and pitting corrosion | - | - | - | Portugal | - | I-V and P-V measurements |
| [90] | sc-Si | - | 30 to 85 | 50 to 85 | - | Austria | 1000 to 3000 h | I-V and P-V measurements EL Visual inspection FTIR Differential scanning calorimetry (DSC) Thermal expansion behavior characterization Thermally extractable components analysis |
| [91] | sc-Si | - | 6 to 30 | 73 to 80 | 1289.6 | China | 2 years | SEM XRS |
| [92] | sc-Si | - | 19.6 | 76.5 | 1675.5 | Brazil | 15 years | EL I-V and P-V measurements Visual inspection Thermography Electrical insulation test |
| [93] | sc-Si/mc-Si/TF | - | 19.2 to 28.9 | 45 to 81 | 1645 | Bangladesh | 5 years | Visual inspection I-V and P-V measurements |
| [94] | c-Si | - | 7.3 to 28.6 | - | 2141 (Amman) | Jordania | 0 to 4 years | EL I-V and P-V measurements |
| [95] | mc-Si | - | 15 to 31 | - | 1731 (Lucknow) | India | 2 to 5 years | Portable monocular metallurgical microscope Photoluminescence (PL) |
| [96] | sc-Si | - | 95 | 95 | - | Japan | 2664 h | FE-SEM EDX FIB ICP-OES |
| [97] | mc-Si | - | ~29 | ~78 | 1843 to 2005 | Thailand | 15 years | Visual inspection I-V and P-V measurements EL Electrical insulation test |
| [98] | c-Si | - | 75 to 85 | 85 | - | USA | 3 months | XPS Elemental depth profiling Optical microscopy |
| [99] | sc-Si | - | −10 to 21 | 67 to 80 | 1140 (Quebec) | Canada | 23 years | Visual inspection Solar simulator I-V Curves Auger electron spectroscopy |
| [100] | mc-Si | - | 12.4 to 38.6 | 10 to 40 | 2209.7 | Algeria | 1 year | I-V and P-V measurements |
| [101] | c-Si | - | 85 | 85 | - | China | 1000 h | I-V and P-V measurements Dynamic load + shearing sequence test Distortion experiment (module distortion + DH2000 h) Long-term PID test |
| [102] | c-Si | - | 85 | 85 | - | UK | 5350 h | Visual inspection EL I-V and P-V measurements Spectral response (SR) measurements |
| [103] | mc-Si | - | - | - | - | Republic of Korea | 5 years | Visual inspection EL I-V and P-V measurements |
| [104] | mc-Si/sc-Si/CIS | - | 26 to 28 | 82 to 86 | 1637 | Singapore | 10 years | Visual inspection I-V and P-V measurements EL |
| [105] | c-Si | - | 10 to 35 | 90 | 1393.6 | Italy | 37 to 39 years | XRF FTIR DSC DLTMA |
| [106] | c-Si | - | 85 | 85 | - | China | 1000 h | I-V and P-V measurements Visual inspection |
| [107] | sc-Si | - | 10.9 to 28.2 | 44 to 74 | 1845 (Sevilla) | Spain | 22 years | Visual inspection IR EL I-V and P-V measurements |
| [108] | sc-Si/mc-si/a-Si | - | 8 to 31 | 31 to 57 | 1949 (Beni Mellal) | Morocco | 4 years | I-V and P-V measurements |
| [109] | c-Si/HIT/CIS | - | 16.3 to 38.2 | 16 to 52 | 2081 (Ghardaïa) | Algeria | 1 year | I-V and P-V measurements |
| [110] | c-Si/HIT | - | 20 to 60 | - | - | Switzerland | 3000 h | Visual inspection SEM EDX Raman spectroscopy |
| [111] | mc-Si/sc-Si/a-Si | - | −2 to 28 | 57 to 78 | 1890 (Meknes Tafilalet) | Morocco | 6 years | I-V and P-V measurements Linear regression analysis |
| [112] | mc-si | - | 26.7 to 38 | - | 2200 | Djibouti | 9.5 years | Visual inspection IR UVFL |
| [19] | mc-Si | Galvanic corrosion | 60 | 55.75 and 85 | - | 20 years | Thermodynamic and kinetic analyses Finite element analysis | |
| [113] | mc-Si/m-Si | Metallization corrosion and encapsulant discoloration | 85 | 85 | - | Thailand | 30 years | I-V measurements Electroluminescence SEM EDS XPS |
| [114] | c-Si | Metallization | 25 | - | 1000 W/m2 | India | 10 years | I-V measurements Visual inspection Electrical characterization |
| [115] | poly-Si | Metallization corrosion | 38 | - | 2400 | India | 10 years | I-V measurements Visual inspection |
| [116] | c-Si | - | 10 | - | - | Italy | 20 years | I-V and P-V measurements PV roofs |
| [117] | a-Si | Electrochemical corrosion | 90 | 85 | USA | 6 to 7 years | ||
| [118] | a-Si/μc-Si | General corrosion | 45 | - | - | Africa | 5.5 year | Visual inspection P-V measurements Installation costs |
| [119] | c-Si | General corrosion | −10 to 50 | - | 1995.9 | USA | - | Easy installation Low maintenance cost Reduction in CO2 emissions |
| [120] | mc-Si | Metal grid oxidation and corrosion | 10 to 12 | - | - | Norway | 20 years | I-V measurements Visual inspection IR EL UV-F |
| [121] | poly-Si | Electrochemical corrosion | 25 | - | 1000 W/m2 | Ghana | 20 to 25 years | I-V and P-V measurements Visual inspection |
| [120] | mc-Si | Metal grid oxidation and corrosion | 10 to 12 | - | - | Norway | 20 years | I-V measurements Visual inspection IR EL UV-F |
| [121] | poly-Si | Electrochemical corrosion | 25 | - | 1000 W/m2 | Ghana | 20 to 25 years | I-V and P-V measurements Visual inspection |
| [122] | poly-Si/m-Si/a-Si | General corrosion | - | 75 | - | Ghana | 5 years | I-V measurements Visual inspection |
| [123] | mc-Si | Electrochemical corrosion | 25 | 960 to 1040 W/m2 | Norway | 20 years | I-V measurements EL UV-F IR-T | |
| [124] | c-Si | - | 25 | - | 800–1200 W/m2 | China | 10 years | |
| [125] | m-si | General corrosion | −45 to 85 | - | 1 kW/m2 | Andaman/Nicobar Islands | 10 years | Visual inspection |
| [126] | mc-Si | - | 25 | 80 to 87 | 1000 W/m2 | Nigeria | I-V and P-V measurements Electrical parameters | |
| [127] | c-Si/mc-Si | - | - | - | - | India | 12 to 18 years | I-V measurements FMEA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, Z.; Zamora, P.P.; Soliz, A.; Soler, D.; Reyes Cruz, V.E.; Cobos-Murcia, J.A.; Galleguillos Madrid, F.M. Solar Panel Corrosion: A Review. Int. J. Mol. Sci. 2025, 26, 5960. https://doi.org/10.3390/ijms26135960
Rana Z, Zamora PP, Soliz A, Soler D, Reyes Cruz VE, Cobos-Murcia JA, Galleguillos Madrid FM. Solar Panel Corrosion: A Review. International Journal of Molecular Sciences. 2025; 26(13):5960. https://doi.org/10.3390/ijms26135960
Chicago/Turabian StyleRana, Zuraiz, Pedro P. Zamora, Alvaro Soliz, Denet Soler, Víctor E. Reyes Cruz, José A. Cobos-Murcia, and Felipe M. Galleguillos Madrid. 2025. "Solar Panel Corrosion: A Review" International Journal of Molecular Sciences 26, no. 13: 5960. https://doi.org/10.3390/ijms26135960
APA StyleRana, Z., Zamora, P. P., Soliz, A., Soler, D., Reyes Cruz, V. E., Cobos-Murcia, J. A., & Galleguillos Madrid, F. M. (2025). Solar Panel Corrosion: A Review. International Journal of Molecular Sciences, 26(13), 5960. https://doi.org/10.3390/ijms26135960







