MicroRNA-Mediated Regulation of Vascular Endothelium: From Pro-Inflammation to Atherosclerosis
Abstract
1. Introduction
Non-Coding RNA Classification
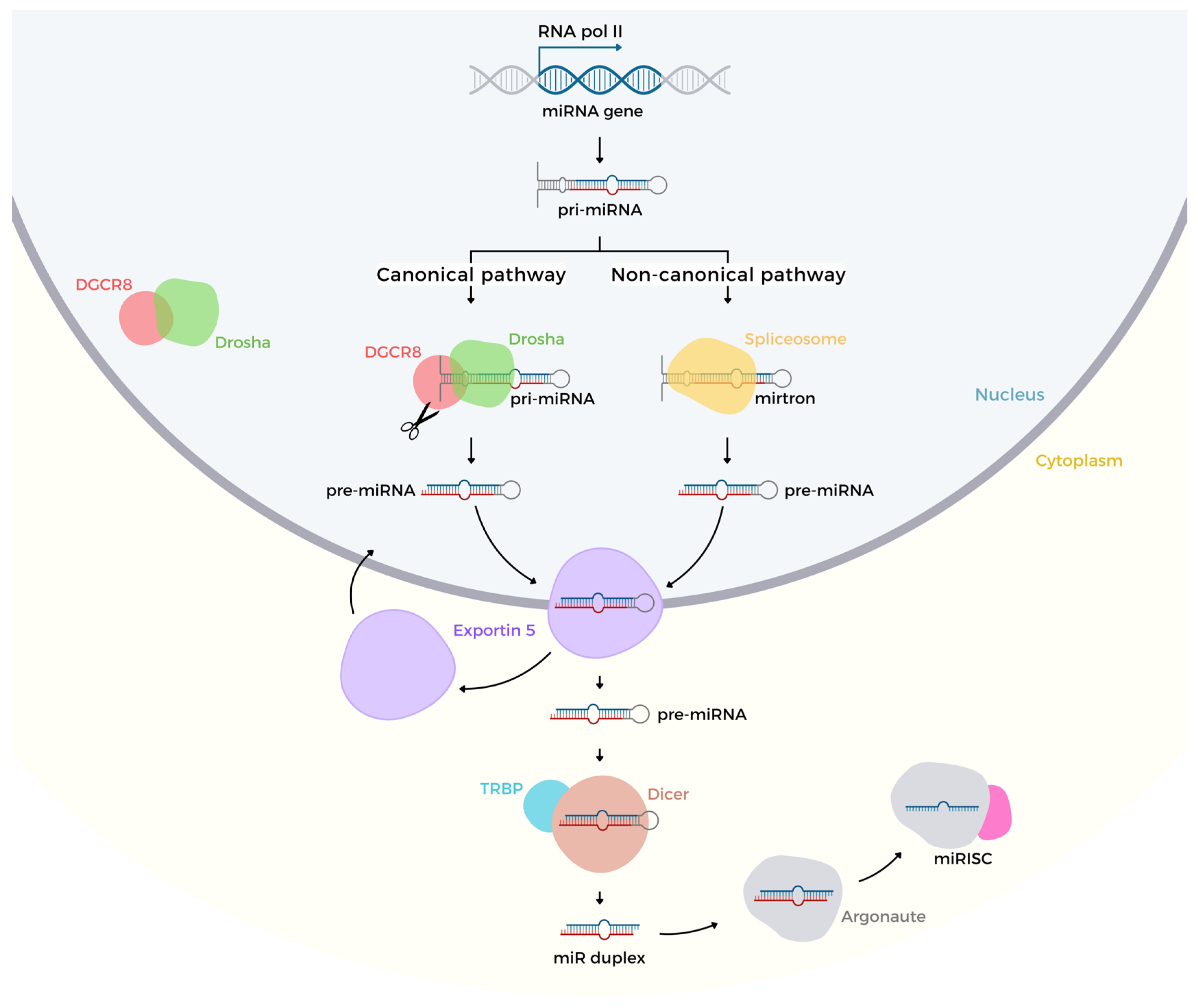
2. Biogenesis and Regulation of Gene Expression
MicroRNA Genes Regulation
3. miRNAs Regulation in Non-Infectious Pro-Inflammation
3.1. Sterile Inflammation DAMPs-Induced
3.2. Inflammasome Activation
3.3. miRNA in Pyroptosis Mechanism
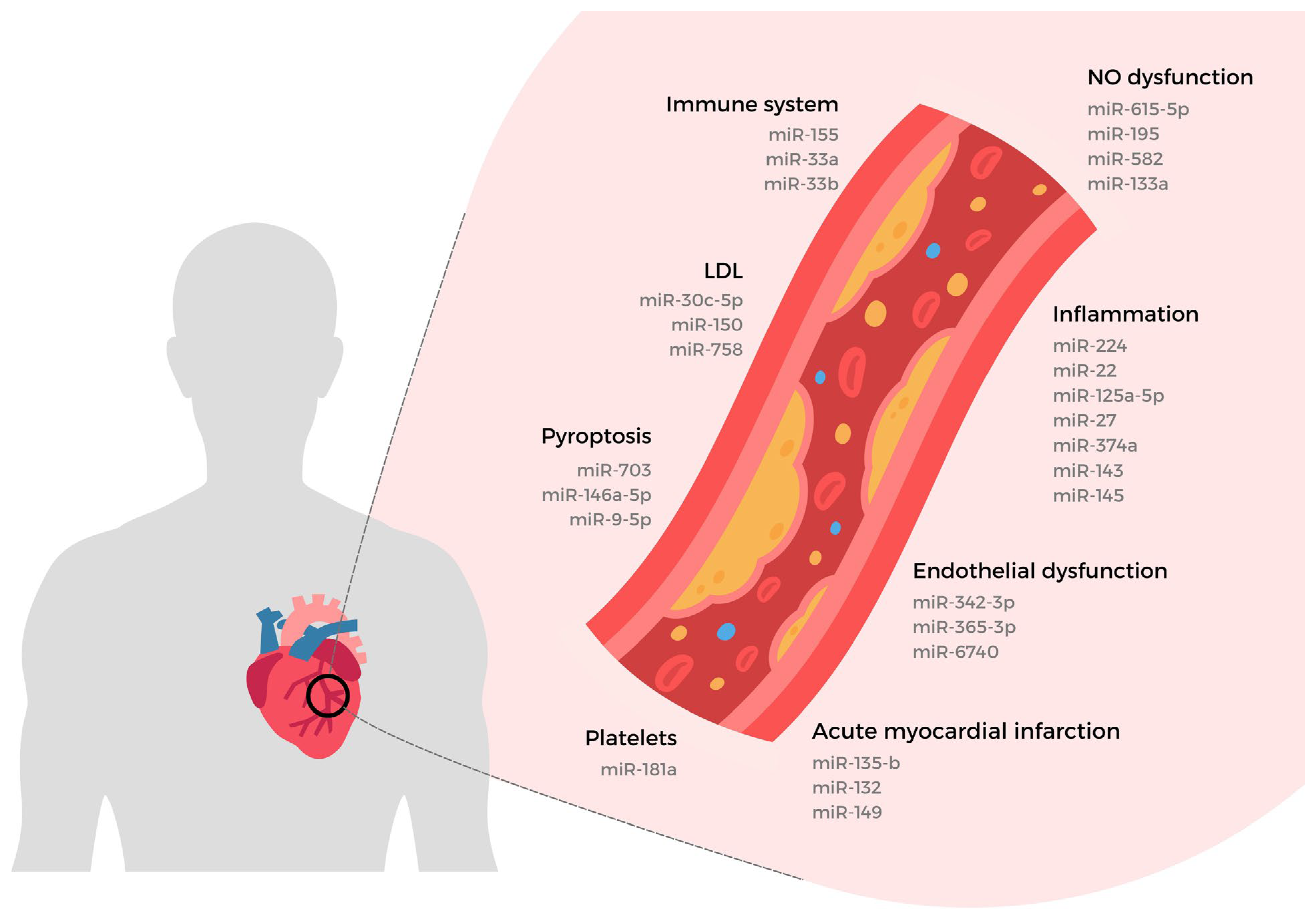
4. miRNAs and Vascular Endothelial Dysfunction
4.1. The Vascular Endothelial Cell in Activation State
4.2. Vascular Endothelial Dysfunction Evaluation
4.3. Nitric Oxide Biosynthesis in Vascular Endothelial Cell
4.4. miRNAs Interacting with Endothelial Nitric Oxide Synthase
4.5. Vascular Endothelial Dysfunction Biomarkers
4.6. Vascular Endothelial Dysfunction by Pathomorphological Manifestations
5. miRNAs and Atherosclerosis
5.1. Pre-Clinical miRNA Study in Atherosclerosis Protocols
5.2. miRNA Targeting Lipid Metabolism
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 Mediates the Nuclear Export of Pre-MicroRNAs and Short Hairpin RNAs. Genes. Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Chateauvieux, S.; Gaigneaux, A.; Dicato, M.; Diederich, M. Long and Short Non-Coding RNAs as Regulators of Hematopoietic Differentiation. Int. J. Mol. Sci. 2013, 14, 14744–14770. [Google Scholar] [CrossRef]
- Ibrahim, F.; Nakaya, T.; Mourelatos, Z. RNA Dysregulation in Diseases of Motor Neurons. Annu. Rev. Pathol. 2012, 7, 323–352. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, D.; Xue, H.; Taylor, D.W.; Patnode, H.; Mishima, Y.; Cheloufi, S.; Ma, E.; Mane, S.; Hannon, G.J.; Lawson, N.D.; et al. A Novel MiRNA Processing Pathway Independent of Dicer Requires Argonaute2 Catalytic Activity. Science 2010, 328, 1694–1698. [Google Scholar] [CrossRef]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of MiRNA-Mediated Gene Regulation from Common Downregulation to MRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of MRNA Translation and Stability by MicroRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Kwak, P.B.; Iwasaki, S.; Tomari, Y. The MicroRNA Pathway and Cancer. Cancer Sci. 2010, 101, 2309–2315. [Google Scholar] [CrossRef]
- Eulalio, A.; Huntzinger, E.; Izaurralde, E. Getting to the Root of MiRNA-Mediated Gene Silencing. Cell 2008, 132, 9–14. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in Endothelial Cell Homeostasis and Vascular Disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef]
- Varani, G. Twenty Years of RNA: The Discovery of MicroRNAs. RNA 2015, 21, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a Molecular Understanding of MicroRNA-Mediated Gene Silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yanez, A.; Novina, C.D. MicroRNA-Repressed MRNAs Contain 40S but Not 60S Components. Proc. Natl. Acad. Sci. USA 2008, 105, 5343–5348. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of MiRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. MicroRNA Sponges: Progress and Possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA Signatures in Tissues and Plasma Predict Development and Prognosis of Computed Tomography Detected Lung Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef]
- Di Stefano, V.; Zaccagnini, G.; Capogrossi, M.C.; Martelli, F. MicroRNAs as Peripheral Blood Biomarkers of Cardiovascular Disease. Vasc. Pharmacol. 2011, 55, 111–118. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in Body Fluids--the Mix of Hormones and Biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef]
- Yamakuchi, M. MicroRNAs in Vascular Biology. Int. J. Vasc. Med. 2012, 2012, 794898. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Holmstrøm, K. Combined MicroRNA in Situ Hybridization and Immunohistochemical Detection of Protein Markers. Methods Mol. Biol. 2013, 986, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Vemuganti, R.; Silva, V.R.; Mehta, S.L.; Hazell, A.S. Acute Liver Failure-Induced Hepatic Encephalopathy Is Associated with Changes in MicroRNA Expression Profiles in Cerebral Cortex of the Rat. Metab. Brain Dis. 2014, 29, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Tuschl, T. Mechanisms of Gene Silencing by Double-Stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- De Felippes, F.F.; Marchais, A.; Sarazin, A.; Oberlin, S.; Voinnet, O. A Single MiR390 Targeting Event Is Sufficient for Triggering TAS3-TasiRNA Biogenesis in Arabidopsis. Nucleic Acids Res. 2017, 45, 5339–5354. [Google Scholar] [CrossRef]
- Frenk, S.; Lister-Shimauchi, E.H.; Ahmed, S. Telomeric Small RNAs in the Genus Caenorhabditis. RNA 2019, 25, 1061–1077. [Google Scholar] [CrossRef]
- Silva, V.R.; Secolin, R.; Vemuganti, R.; Lopes-Cendes, I.; Hazell, A.S. Acute Liver Failure Is Associated with Altered Cerebral Expression Profiles of Long Non-Coding RNAs. Neurosci. Lett. 2017, 656, 58–64. [Google Scholar] [CrossRef]
- Ding, D.; Liu, J.; Dong, K.; Melnick, A.F.; Latham, K.E.; Chen, C. Mitochondrial Membrane-Based Initial Separation of MIWI and MILI Functions during Pachytene PiRNA Biogenesis. Nucleic Acids Res. 2019, 47, 2594–2608. [Google Scholar] [CrossRef]
- Huescas, C.G.Y.; Pereira, R.I.; Prichula, J.; Azevedo, P.A.; Frazzon, J.; Frazzon, A.P.G. Frequency of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) in Non-Clinical Enterococcus Faecalis and Enterococcus Faecium Strains. Braz. J. Biol. 2019, 79, 460–465. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhang, W.-Q.; Huang, M.-Y. Transcription Start Site-Associated Small RNAs in the PTEN Gene. Proc. Natl. Acad. Sci. USA 2017, 114, E10510–E10511. [Google Scholar] [CrossRef]
- Ma, X.; Han, N.; Shao, C.; Meng, Y. Transcriptome-Wide Discovery of PASRs (Promoter-Associated Small RNAs) and TASRs (Terminus-Associated Small RNAs) in Arabidopsis Thaliana. PLoS ONE 2017, 12, e0169212. [Google Scholar] [CrossRef]
- Abel, Y.; Rederstorff, M. SnoRNAs and the Emerging Class of SdRNAs: Multifaceted Players in Oncogenesis. Biochimie 2019, 164, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ratner, H.K.; Escalera-Maurer, A.; Le Rhun, A.; Jaggavarapu, S.; Wozniak, J.E.; Crispell, E.K.; Charpentier, E.; Weiss, D.S. Catalytically Active Cas9 Mediates Transcriptional Interference to Facilitate Bacterial Virulence. Mol. Cell 2019, 75, 498–510.e5. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, W.; Xue, R.; Dong, B.; Liang, Z.; Chen, C.; Li, J.; Wang, Y.; Zhao, J.; Huang, H.; et al. Transcribed Ultraconserved Regions, Uc.323, Ameliorates Cardiac Hypertrophy by Regulating the Transcription of CPT1b (Carnitine Palmitoyl Transferase 1b). Hypertension 2020, 75, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.M.; Dowell, R.D. Survey of Cryptic Unstable Transcripts in Yeast. BMC Genom. 2016, 17, 305. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, W.; Gagneur, J.; Perocchi, F.; Clauder-Münster, S.; Camblong, J.; Guffanti, E.; Stutz, F.; Huber, W.; Steinmetz, L.M. Bidirectional Promoters Generate Pervasive Transcription in Yeast. Nature 2009, 457, 1033–1037. [Google Scholar] [CrossRef]
- Marión, R.M.; Montero, J.J.; López de Silanes, I.; Graña-Castro, O.; Martínez, P.; Schoeftner, S.; Palacios-Fábrega, J.A.; Blasco, M.A. TERRA Regulate the Transcriptional Landscape of Pluripotent Cells through TRF1-Dependent Recruitment of PRC2. Elife 2019, 8, e44656. [Google Scholar] [CrossRef]
- Preker, P.; Almvig, K.; Christensen, M.S.; Valen, E.; Mapendano, C.K.; Sandelin, A.; Jensen, T.H. PROMoter UPstream Transcripts Share Characteristics with MRNAs and Are Produced Upstream of All Three Major Types of Mammalian Promoters. Nucleic Acids Res. 2011, 39, 7179–7193. [Google Scholar] [CrossRef]
- Wapinski, O.; Chang, H.Y. Long Noncoding RNAs and Human Disease. Trends Cell Biol. 2011, 21, 354–361. [Google Scholar] [CrossRef]
- Bernstein, E.; Allis, C.D. RNA Meets Chromatin. Genes. Dev. 2005, 19, 1635–1655. [Google Scholar] [CrossRef]
- Whitehead, J.; Pandey, G.K.; Kanduri, C. Regulation of the Mammalian Epigenome by Long Noncoding RNAs. Biochim. Biophys. Acta 2009, 1790, 936–947. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Fareh, M.; Yeom, K.H.; Haagsma, A.C.; Chauhan, S.; Heo, I.; Joo, C. TRBP Ensures Efficient Dicer Processing of Precursor MicroRNA in RNA-Crowded Environments. Nat. Commun. 2016, 7, 13694. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shishkin, A.A.; Zhu, X.; Kadri, S.; Maza, I.; Guttman, M.; Hanna, J.H.; Regev, A.; Garber, M. Evolutionary Analysis across Mammals Reveals Distinct Classes of Long Non-Coding RNAs. Genome Biol. 2016, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Terai, G.; Iwakiri, J.; Kameda, T.; Hamada, M.; Asai, K. Comprehensive Prediction of LncRNA-RNA Interactions in Human Transcriptome. BMC Genomics 2016, 17 (Suppl. S1), 12. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Xing, Y.H.; Bai, Z.; Liu, C.X.; Hu, S.B.; Ruan, M.; Chen, L.L. Research Progress of Long Noncoding RNA in China. IUBMB Life 2016, 68, 887–893. [Google Scholar] [CrossRef]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and Abundance Analysis of Human LncRNAs at Single-Cell and Single-Molecule Resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Tan, G.S.; Lamprinaki, S.; De Planell-Saguer, M.; Nelson, P.T.; Mourelatos, Z. An MRNA M7G Cap Binding-like Motif within Human Ago2 Represses Translation. Cell 2007, 129, 1141–1151. [Google Scholar] [CrossRef]
- Chendrimada, T.P.; Finn, K.J.; Ji, X.; Baillat, D.; Gregory, R.I.; Liebhaber, S.A.; Pasquinelli, A.E.; Shiekhattar, R. MicroRNA Silencing through RISC Recruitment of EIF6. Nature 2007, 447, 823–828. [Google Scholar] [CrossRef]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 MicroRNA-Mediated MRNA Deadenylation and Translational Repression in a Mammalian Cell-Free System. Genes. Dev. 2007, 21, 1857–1862. [Google Scholar] [CrossRef]
- Petersen, C.P.; Bordeleau, M.E.; Pelletier, J.; Sharp, P.A. Short RNAs Repress Translation after Initiation in Mammalian Cells. Mol. Cell 2006, 21, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Maroney, P.A.; Yu, Y.; Fisher, J.; Nilsen, T.W. Evidence That MicroRNAs Are Associated with Translating Messenger RNAs in Human Cells. Nat. Struct. Mol. Biol. 2006, 13, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Nottrott, S.; Simard, M.J.; Richter, J.D. Human Let-7a MiRNA Blocks Protein Production on Actively Translating Polyribosomes. Nat. Struct. Mol. Biol. 2006, 13, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.I.; Izaurralde, E. Decay of MRNAs Targeted by RISC Requires XRN1, the Ski Complex, and the Exosome. RNA 2005, 11, 459–469. [Google Scholar] [CrossRef]
- Behm-Ansmant, I. MRNA Degradation by MiRNAs and GW182 Requires Both CCR4:NOT Deadenylase and DCP1:DCP2 Decapping Complexes. Genes. Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef]
- Khraiwesh, B.; Arif, M.A.; Seumel, G.I.; Ossowski, S.; Weigel, D.; Reski, R.; Frank, W. Transcriptional Control of Gene Expression by MicroRNAs. Cell 2010, 140, 111–122. [Google Scholar] [CrossRef]
- Kim, D.H.; Saetrom, P.; Snove, O.; Rossi, J.J. MicroRNA-Directed Transcriptional Gene Silencing in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16230–16235. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, M.; Geng, G.; Liu, B.; Huang, Z.; Luo, H.; Zhou, J.; Guo, X.; Cai, W.; Zhang, H. A Novel HIV-1-Encoded MicroRNA Enhances Its Viral Replication by Targeting the TATA Box Region. Retrovirology 2014, 11, 23. [Google Scholar] [CrossRef]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a Binds the 5′UTR of Ribosomal Protein MRNAs and Enhances Their Translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Smekalova, E.M.; Kotelevtsev, Y.V.; Leboeuf, D.; Shcherbinina, E.Y.; Fefilova, A.S.; Zatsepin, T.S.; Koteliansky, V. LncRNA in the Liver: Prospects for Fundamental Research and Therapy by RNA Interference. Biochimie 2016, 131, 159–172. [Google Scholar] [CrossRef]
- Bonasio, R.; Shiekhattar, R. Regulation of Transcription by Long Noncoding RNAs. Annu. Rev. Genet. 2014, 48, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The Noncoding RNA Revolution-Trashing Old Rules to Forge New Ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Schmitz, K.M.; Li, J.; Grummt, I.; Santoro, R. Intergenic Transcripts Regulate the Epigenetic State of RRNA Genes. Mol. Cell 2006, 22, 351–361. [Google Scholar] [CrossRef]
- Schmitz, K.M.; Mayer, C.; Postepska, A.; Grummt, I. Interaction of Noncoding RNA with the RDNA Promoter Mediates Recruitment of DNMT3b and Silencing of RRNA Genes. Genes. Dev. 2010, 24, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Carmo, H.R.P.; Bonilha, I.; Barreto, J.; Tognolini, M.; Zanotti, I.; Sposito, A.C. High-Density Lipoproteins at the Interface between the NLRP3 Inflammasome and Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 1290. [Google Scholar] [CrossRef]
- Land, W.G. The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases Part II: DAMPs as Diagnostics, Prognostics and Therapeutics in Clinical Medicine. Sultan Qaboos Univ. Med. J. 2015, 15, e157–e170. [Google Scholar] [CrossRef]
- Golino, M.; Harding, D.; Del Buono, M.G.; Fanti, S.; Mohiddin, S.; Toldo, S.; Smyth, J.; Sanna, T.; Marelli-Berg, F.; Abbate, A. Innate and Adaptive Immunity in Acute Myocarditis. Int. J. Cardiol. 2024, 404, 131901. [Google Scholar] [CrossRef]
- Lin, L.; Knowlton, A.A. Innate Immunity and Cardiomyocytes in Ischemic Heart Disease. Life Sci. 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zheng, L.; Hu, Y.W.; Wang, Q. Pyroptosis and Its Relationship to Atherosclerosis. Clin. Chim. Acta 2018, 476, 28–37. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, G.; Yin, Y.; Han, S.; Zheng, D.; Zhou, S.; Zhao, Y.; Chen, Y.; Jin, Y. Low Shear Stress Regulates Vascular Endothelial Cell Pyroptosis through MiR-181b-5p/STAT-3 Axis. J. Cell Physiol. 2021, 236, 318–327. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qiu, J.; Song, Y.; Liang, T.; Liu, S.; Ren, C.; Song, X.; Cui, L.; Sun, Y. Pyroptosis and Degenerative Diseases of the Elderly. Cell Death Dis. 2023, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Rühl, S.; Broz, P. The Gasdermin-D Pore: Executor of Pyroptotic Cell Death. Oncotarget 2016, 7, 57481–57482. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Frelin, C.; Imbert, V.; Bottero, V.; Gonthier, N.; Samraj, A.K.; Schulze-Osthoff, K.; Auberger, P.; Courtois, G.; Peyron, J.F. Inhibition of the NF-KappaB Survival Pathway via Caspase-Dependent Cleavage of the IKK Complex Scaffold Protein and NF-KappaB Essential Modulator NEMO. Cell Death Differ. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Julio, A.; Guedes-Silva, T.C.; Berni, M.; Bisch, P.M.; Araujo, H. A Rhodnius prolixus Catalytically Inactive Calpain Protease Patterns the Insect Embryonic Dorsal-Ventral Axis. Curr. Res. Insect Sci. 2024, 6, 100094. [Google Scholar] [CrossRef]
- Shumway, S.D.; Maki, M.; Miyamoto, S. The PEST Domain of IkappaBalpha Is Necessary and Sufficient for in Vitro Degradation by Mu-Calpain. J. Biol. Chem. 1999, 274, 30874–39881. [Google Scholar] [CrossRef]
- Skommer, J.; Rana, I.; Marques, F.Z.; Zhu, W.; Du, Z.; Charchar, F.J. Small Molecules, Big Effects: The Role of MicroRNAs in Regulation of Cardiomyocyte Death. Cell Death Dis. 2014, 5, e1325. [Google Scholar] [CrossRef]
- dos Santos, C.C.; Lopes-Pacheco, M.; English, K.; Rolandsson Enes, S.; Krasnodembskaya, A.; Rocco, P.R.M. The MSC-EV-MicroRNAome: A Perspective on Therapeutic Mechanisms of Action in Sepsis and ARDS. Cells 2024, 13, 122. [Google Scholar] [CrossRef]
- Vande Walle, L.; Lamkanfi, M. Drugging the NLRP3 Inflammasome: From Signalling Mechanisms to Therapeutic Targets. Nat. Rev. Drug Discov. 2024, 23, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, X.; Chen, X.; Yang, S.; Chen, H. Inhibition of MiR-223 Attenuates the NLRP3 Inflammasome Activation, Fibrosis, and Apoptosis in Diabetic Cardiomyopathy. Life Sci. 2020, 256, 117980. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhong, X.; Li, J.; Liu, H.; Ma, X.; He, R.; Zhao, Y. MicroRNA-30c-5p Inhibits NLRP3 Inflammasome-Mediated Endothelial Cell Pyroptosis through FOXO3 down-Regulation in Atherosclerosis. Biochem. Biophys. Res. Commun. 2018, 503, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, L.; Guo, R.; Lu, N.; Shi, Y.; Wang, X. Long Noncoding RNA MALAT1 Promotes High Glucose-Induced Human Endothelial Cells Pyroptosis by Affecting NLRP3 Expression through Competitively Binding MiR-22. Biochem. Biophys. Res. Commun. 2019, 509, 359–366. [Google Scholar] [CrossRef]
- Zhaolin, Z.; Jiaojiao, C.; Peng, W.; Yami, L.; Tingting, Z.; Jun, T.; Shiyuan, W.; Jinyan, X.; Dangheng, W.; Zhisheng, J.; et al. OxLDL Induces Vascular Endothelial Cell Pyroptosis through MiR-125a-5p/TET2 Pathway. J. Cell Physiol. 2019, 234, 7475–7491. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, J.; Wang, H.; Zhang, Z.; Yin, M.; Zhu, Y.; Li, L.; Chen, C.; Wei, M.; Hu, M.; et al. MiR-30d Attenuates Pulmonary Arterial Hypertension via Targeting MTDH and PDE5A and Modulates the Beneficial Effect of Sildenafil. Adv. Sci. 2024, 11, e2407712. [Google Scholar] [CrossRef]
- Li, X.; Du, N.; Zhang, Q.; Li, Q.; Chen, X.; Liu, X.; Hu, Y.; Qin, W.; Shen, N.; Xu, C.; et al. MicroRNA-30d Regulates Cardiomyocyte Pyroptosis by Directly Targeting Foxo3a in Diabetic Cardiomyopathy. Cell Death Dis. 2014, 5, e1479. [Google Scholar] [CrossRef]
- Jeyabal, P.; Thandavarayan, R.A.; Joladarashi, D.; Suresh Babu, S.; Krishnamurthy, S.; Bhimaraj, A.; Youker, K.A.; Kishore, R.; Krishnamurthy, P. MicroRNA-9 Inhibits Hyperglycemia-Induced Pyroptosis in Human Ventricular Cardiomyocytes by Targeting ELAVL1. Biochem. Biophys. Res. Commun. 2016, 471, 423–429. [Google Scholar] [CrossRef]
- Wang, H.; Wang, E.Z.R.; Feng, B.; Chakrabarti, S. CircRNA_012164/MicroRNA-9-5p Axis Mediates Cardiac Fibrosis in Diabetic Cardiomyopathy. PLoS ONE 2024, 19, e0302772. [Google Scholar] [CrossRef]
- Li, A.; Yu, Y.; Ding, X.; Qin, Y.; Jiang, Y.; Wang, X.; Liu, G.; Chen, X.; Yue, E.; Sun, X.; et al. MiR-135b Protects Cardiomyocytes from Infarction through Restraining the NLRP3/Caspase-1/IL-1β Pathway. Int. J. Cardiol. 2020, 307, 137–145. [Google Scholar] [CrossRef]
- Wei, X.; Peng, H.; Deng, M.; Feng, Z.; Peng, C.; Yang, D. MiR-703 Protects against Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury via Inhibiting the NLRP3/Caspase-1-Mediated Pyroptosis. J. Bioenerg. Biomembr. 2020, 52, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.M.; Ji, J.L.; Gan, W.; Zhang, A.; Shi, H.J.; Wang, H.; Lv, L.; Li, Z.; Tang, T.; et al. Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy. JACC Basic. Transl. Sci. 2020, 5, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, H.; Ma, C.; Dong, F.; Hu, Y.; Li, H. MiR-149 Aggravates Pyroptosis in Myocardial Ischemia-Reperfusion Damage via Silencing FoxO3. Med. Sci. Monit. 2019, 25, 8733–8743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, K.S.; Liu, L.; Li, S.L. MicroRNA-132 Promotes Oxidative Stress-induced Pyroptosis by Targeting Sirtuin 1 in Myocardial Ischaemia-reperfusion Injury. Int. J. Mol. Med. 2020, 45, 1942–1950. [Google Scholar] [CrossRef]
- Ghaffari, M.; Razi, S.; Zalpoor, H.; Nabi-Afjadi, M.; Mohebichamkhorami, F.; Zali, H. Association of MicroRNA-146a with Type 1 and 2 Diabetes and Their Related Complications. J. Diabetes Res. 2023, 2023, 2587104. [Google Scholar] [CrossRef]
- Roshanravan, N.; Alamdari, N.M.; Jafarabadi, M.A.; Mohammadi, A.; Shabestari, B.R.; Nasirzadeh, N.; Asghari, S.; Mansoori, B.; Akbarzadeh, M.; Ghavami, A.; et al. Effects of Oral Butyrate and Inulin Supplementation on Inflammation-Induced Pyroptosis Pathway in Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Cytokine 2020, 131, 155101. [Google Scholar] [CrossRef]
- Dawes, M.G.; Bartlett, G.; Coats, A.J.; Juszczak, E. Comparing the Effects of White Coat Hypertension and Sustained Hypertension on Mortality in a UK Primary Care Setting. Ann. Fam. Med. 2008, 6, 390–396. [Google Scholar] [CrossRef]
- Chen, W.; Druhan, L.J.; Chen, C.A.; Hemann, C.; Chen, Y.R.; Berka, V.; Tsai, A.L.; Zweier, J.L. Peroxynitrite Induces Destruction of the Tetrahydrobiopterin and Heme in Endothelial Nitric Oxide Synthase: Transition from Reversible to Irreversible Enzyme Inhibition. Biochemistry 2010, 49, 3129–3137. [Google Scholar] [CrossRef]
- Chennupati, R.; Solga, I.; Wischmann, P.; Dahlmann, P.; Celik, F.G.; Pacht, D.; Şahin, A.; Yogathasan, V.; Hosen, M.R.; Gerdes, N.; et al. Chronic Anemia Is Associated with Systemic Endothelial Dysfunction. Front. Cardiovasc. Med. 2023, 10, 1099069. [Google Scholar] [CrossRef]
- Wagner, K.T.; Lu, R.X.Z.; Landau, S.; Shawky, S.A.; Zhao, Y.; Bodenstein, D.F.; Jiménez Vargas, L.F.; Jiang, R.; Okhovatian, S.; Wang, Y.; et al. Endothelial Extracellular Vesicles Enhance Vascular Self-Assembly in Engineered Human Cardiac Tissues. Biofabrication 2024, 16, 045037. [Google Scholar] [CrossRef]
- Romaine, S.P.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Schoenenberger, A.; Urbanek, N.; Erne, P. The Prevalence of Endothelial Dysfunction in Patients with and without Coronary Artery Disease. Clin. Cardiol. 2010, 33, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Shruthi, N.R.; Banerjee, A.; Jothimani, G.; Duttaroy, A.K.; Pathak, S. Endothelial Dysfunction, Platelet Hyperactivity, Hypertension, and the Metabolic Syndrome: Molecular Insights and Combating Strategies. Front. Nutr. 2023, 10, 1221438. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Matsuzawa, Y.; Yoshii, T.; Akiyama, E.; Konishi, M.; Nakahashi, H.; Minamimoto, Y.; Kimura, Y.; Okada, K.; Maejima, N.; et al. Impact of Low-Density Lipoprotein Cholesterol Levels at Acute Coronary Syndrome Admission on Long-Term Clinical Outcomes. J. Atheroscler. Thromb. 2024, 31, 444–460. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Li, J.; Aoki, T.; Guddeti, R.R.; Kwon, T.G.; Cilluffo, R.; Widmer, R.J.; Gulati, R.; Lennon, R.J.; Lerman, L.O.; et al. Predictive Value of Endothelial Function by Noninvasive Peripheral Arterial Tonometry for Coronary Artery Disease. Coron. Artery Dis. 2015, 26, 231–238. [Google Scholar] [CrossRef]
- Camarda, N.D.; Lu, Q.; Meola, D.M.; Man, J.J.; Song, Z.; Travers, R.J.; Lopez, K.E.; Powers, S.N.; Papanastasiou, M.; DeRuff, K.C.; et al. Identifying Mitigating Strategies for Endothelial Cell Dysfunction and Hypertension in Response to VEGF Receptor Inhibitors. Clin. Sci. 2024, 138, 1131–1150. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular Nitric Oxide: Beyond ENOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Icli, B.; Wu, W.; Ozdemir, D.; Li, H.; Cheng, H.S.; Haemmig, S.; Liu, X.; Giatsidis, G.; Avci, S.N.; Lee, N.; et al. MicroRNA-615-5p Regulates Angiogenesis and Tissue Repair by Targeting AKT/ENOS (Protein Kinase B/Endothelial Nitric Oxide Synthase) Signaling in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1458–1474. [Google Scholar] [CrossRef]
- Li, J.-B.; Wang, H.-Y.; Yao, Y.; Sun, Q.-F.; Liu, Z.-H.; Liu, S.-Q.; Zhuang, J.-L.; Wang, Y.-P.; Liu, H.-Y. Overexpression of MicroRNA-138 Alleviates Human Coronary Artery Endothelial Cell Injury and Inflammatory Response by Inhibiting the PI3K/Akt/ENOS Pathway. J. Cell Mol. Med. 2017, 21, 1482–1491. [Google Scholar] [CrossRef]
- Pilz, R.B.; Casteel, D.E. Regulation of Gene Expression by Cyclic GMP. Circ. Res. 2003, 93, 1034–1046. [Google Scholar] [CrossRef]
- Lehners, M.; Dobrowinski, H.; Feil, S.; Feil, R. CGMP Signaling and Vascular Smooth Muscle Cell Plasticity. J. Cardiovasc. Dev. Dis. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, S.; Xia, C. MicroRNAs Regulate Nitric Oxide Release from Endothelial Cells by Targeting NOS3. J. Thromb. Thrombolysis 2018, 46, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, L.; Li, Y.; Chen, W.; Hu, N.; Wang, H.; Ou, H. Roles of MiRNA-24 in Regulating Endothelial Nitric Oxide Synthase Expression and Vascular Endothelial Cell Proliferation. Mol. Cell Biochem. 2015, 405, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of Oxidative Stress in the Dysfunction of the Placental Endothelial Nitric Oxide Synthase in Preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Guo, T.; Sun, X.Y.; Ma, H.; Zhu, M.L.; Zhao, F.R.; Xu, P.; Chen, Y.; Wan, G.R.; et al. Inhibition of Aberrant MicroRNA-133a Expression in Endothelial Cells by Statin Prevents Endothelial Dysfunction by Targeting GTP Cyclohydrolase 1 in Vivo. Circulation 2016, 134, 1752–1765. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal CircHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the MiR-29a/IGF-1 Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7954657. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Khalyfa, A.A.; Gozal, D. Circulating MicroRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. Chest 2016, 149, 786–800. [Google Scholar] [CrossRef]
- Han, Q.A.; Yan, C.; Wang, L.; Li, G.; Xu, Y.; Xia, X. Urolithin A Attenuates Ox-LDL-Induced Endothelial Dysfunction Partly by Modulating MicroRNA-27 and ERK/PPAR-γ Pathway. Mol. Nutr. Food Res. 2016, 60, 1933–1943. [Google Scholar] [CrossRef]
- Ebert, M.L.A.; Schmidt, V.F.; Pfaff, L.; von Thaden, A.; Kimm, M.A.; Wildgruber, M. Animal Models of Neointimal Hyperplasia and Restenosis: Species-Specific Differences and Implications for Translational Research. JACC Basic. Transl. Sci. 2021, 6, 900–917. [Google Scholar] [CrossRef]
- Ahanchi, S.S.; Tsihlis, N.D.; Kibbe, M.R. The Role of Nitric Oxide in the Pathophysiology of Intimal Hyperplasia. J. Vasc. Surg. 2007, 45 (Suppl. A), A64–A73. [Google Scholar] [CrossRef]
- Vanchin, B.; Offringa, E.; Friedrich, J.; Brinker, M.G.L.; Kiers, B.; Pereira, A.C.; Harmsen, M.C.; Moonen, J.R.A.; Krenning, G. MicroRNA-374b Induces Endothelial-to-Mesenchymal Transition and Early Lesion Formation through the Inhibition of MAPK7 Signaling. J. Pathol. 2019, 247, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Quintavalle, M.; Miragoli, M.; Chen, J.; Condorelli, G.; Elia, L. TGFbeta Triggers MiR-143/145 Transfer From Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilisation. Circ. Res. 2015, 116, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, Z.; Chen, G.; Shang, P.; You, G.; Zhao, J.; Liu, S.; Han, D.; Zhou, H. Matrix Stiffening Induces Endothelial Dysfunction via the TRPV4/MicroRNA-6740/Endothelin-1 Mechanotransduction Pathway. Acta Biomater. 2019, 100, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Shah, P.K. Inflammation, Infection and Atherosclerosis. Trends Cardiovasc. Med. 2019, 29, 468–472. [Google Scholar] [CrossRef]
- von Scheidt, M.; Zhao, Y.; Kurt, Z.; Pan, C.; Zeng, L.; Yang, X.; Schunkert, H.; Lusis, A.J. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017, 25, 248–261. [Google Scholar] [CrossRef]
- Huntley, R.P.; Sitnikov, D.; Orlic-Milacic, M.; Balakrishnan, R.; D’Eustachio, P.; Gillespie, M.E.; Howe, D.; Kalea, A.Z.; Maegdefessel, L.; Osumi-Sutherland, D.; et al. Guidelines for the Functional Annotation of MicroRNAs Using the Gene Ontology. RNA 2016, 22, 667–676. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Mellis, D.; Caporali, A. MicroRNA-Based Therapeutics in Cardiovascular Disease: Screening and Delivery to the Target. Biochem. Soc. Trans. 2018, 46, 11–21. [Google Scholar] [CrossRef]
- Lv, B.; He, S.; Li, P.; Jiang, S.; Li, D.; Lin, J.; Feinberg, M.W. MicroRNA-181 in Cardiovascular Disease: Emerging Biomarkers and Therapeutic Targets. FASEB J. 2024, 38, e23635. [Google Scholar] [CrossRef]
- Niimi, M.; Chen, Y.; Zhao, H.; Tang, X.; Matsuhisa, F.; Zhou, H.; Yan, H.; Chen, L.; Kitajima, S.; Sato, A.; et al. Enhanced Atherosclerosis in Apolipoprotein E Knockout Rabbits: Role of ApoB48-Rich Remnant Lipoproteins. Front. Cardiovasc. Med. 2024, 11, 1424064. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit Models for the Study of Human Atherosclerosis: From Pathophysiological Mechanisms to Translational Medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, R.; Zhang, X.; Wu, Y.; Li, X.; Zhang, S.; Hou, W.; Ding, Y.; Tian, J.; Sun, L.; et al. Comprehensive Analysis of CircRNA Expression Pattern and CircRNA-MiRNA-MRNA Network in the Pathogenesis of Atherosclerosis in Rabbits. Aging 2018, 10, 2266–2283. [Google Scholar] [CrossRef] [PubMed]
- Desjarlais, M.; Dussault, S.; Dhahri, W.; Mathieu, R.; Rivard, A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 900–908. [Google Scholar] [CrossRef]
- Wang, L.; Xia, J.W.; Ke, Z.P.; Zhang, B.H. Blockade of NEAT1 Represses Inflammation Response and Lipid Uptake via Modulating MiR-342-3p in Human Macrophages THP-1 Cells. J. Cell Physiol. 2019, 234, 5319–5326. [Google Scholar] [CrossRef]
- Näär, A.M. MiR-33: A Metabolic Conundrum. Trends Endocrinol. Metab. 2018, 29, 667–668. [Google Scholar] [CrossRef]
- Price, N.L.; Miguel, V.; Ding, W.; Singh, A.K.; Malik, S.; Rotllan, N.; Moshnikova, A.; Toczek, J.; Zeiss, C.; Sadeghi, M.M.; et al. Genetic Deficiency or Pharmacological Inhibition of MiR-33 Protects from Kidney Fibrosis. JCI Insight 2019, 4, e131102. [Google Scholar] [CrossRef]
- Goedeke, L.; Salerno, A.; Ramírez, C.M.; Guo, L.; Allen, R.M.; Yin, X.; Langley, S.R.; Esau, C.; Wanschel, A.; Fisher, E.A.; et al. Long-Term Therapeutic Silencing of MiR-33 Increases Circulating Triglyceride Levels and Hepatic Lipid Accumulation in Mice. EMBO Mol. Med. 2014, 6, 1133–1141. [Google Scholar] [CrossRef]
- Montaño-Samaniego, M.; Sánchez-Cedillo, J.; Lucas-González, A.; Bravo-Estupiñan, D.M.; Alarcón-Hernández, E.; Rivera-Gutiérrez, S.; Balderas-López, J.A.; Ibáñez-Hernández, M. Targeted Expression to Liver of an AntimiR-33 Sponge as a Gene Therapy Strategy against Hypercholesterolemia: In Vitro Study. Curr. Issues Mol. Biol. 2023, 45, 7043–7057. [Google Scholar] [CrossRef]
| ncRNA; Nucleotides Numbers; Classification | Putative Functions | Reference |
|---|---|---|
| miRNA (18–23, small nc RNA) | Translational repression; Transcriptional activation | Nielsen & Holmstrøm (2013) [21] Vemuganti et al. (2014) [22] |
| tiRNA (14–30, small nc RNA) | Transcriptional initiation | Vemuganti et al. (2014) [22] |
| siRNA (19–25, small nc RNA) | mRNA degradation | Meister & Tuschl (2004) [23] |
| tasiRNA (20–24, small nc RNA) | Gene silencing in plants | De Felippes (2017) [24] |
| tel-sRNA (23–28, small nc RNA) | Epigenetic regulation of telomerase | Frenk et al. (2019) [25] |
| rasiRNA (24, small nc RNA) | DNA-methylation | Silva et al. (2017) [26] |
| piRNA (24–32, small nc RNA) | Transposon mobilisation | Ding et al. (2019) [27] |
| CRISPR (24–48, small nc RNA) | Prokaryotic immune control | Huescas et al. (2019) [28] |
| TSS-miRNAs (20–90, medium-size ncRNA) | Transcriptional regulation | Liu et al. (2017) [29] |
| PASR (20–200, medium-size ncRNA) | chromatin modifications | Ma et al. (2017) [30] |
| snoRNA (60–300, medium-size ncRNA) | Maturation of other ncRNAs | Abel & Rederstorff (2019) [31] |
| scaRNA (83–330, medium-size ncRNA) | Guiding spliceosomal RNAs | Ratner et al. (2019) [32] |
| Long ncRNAs: lncRNA (>200, long ncRNAs) | Transcriptional regulation | Silva et al. (2017) [26] |
| T-UCR (200–779, long ncRNAs) | Antisense inhibition of mRNAs and ncRNAs | Sun et al. (2020) [33] |
| CUT (200–800, long ncRNAs) | Chromatin regulation | Vera & Dowell (2016) [34] |
| SUT (200–800, long ncRNAs) | Transposon silencing | Xu et al. (2009) [35] |
| TERRA (100–9000, long ncRNAs) | Regulation of telomere length | Marión et al. (2019) [36] |
| PROMPT (500–2500, long ncRNAs) | Promoter control | Preker et al. (2011) [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.R.; Azar, A.; Goncalves, E.R.; Nascimento, T.C.d.M.; Buchaim, R.L.; Buchaim, D.V.; de Oliveira, F.A.A.; Nassar, C.C.; de Camargo, T.M.; Caboclo, R.F.; et al. MicroRNA-Mediated Regulation of Vascular Endothelium: From Pro-Inflammation to Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 5919. https://doi.org/10.3390/ijms26135919
Silva VR, Azar A, Goncalves ER, Nascimento TCdM, Buchaim RL, Buchaim DV, de Oliveira FAA, Nassar CC, de Camargo TM, Caboclo RF, et al. MicroRNA-Mediated Regulation of Vascular Endothelium: From Pro-Inflammation to Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(13):5919. https://doi.org/10.3390/ijms26135919
Chicago/Turabian StyleSilva, Vinícius Rodrigues, Ashraf Azar, Edmilson Ricardo Goncalves, Thatiane Cristina de Moura Nascimento, Rogerio Leone Buchaim, Daniela Vieira Buchaim, Fernando Antonio Antunes de Oliveira, Carolina Costa Nassar, Tais Mendes de Camargo, Ricardo Farinasso Caboclo, and et al. 2025. "MicroRNA-Mediated Regulation of Vascular Endothelium: From Pro-Inflammation to Atherosclerosis" International Journal of Molecular Sciences 26, no. 13: 5919. https://doi.org/10.3390/ijms26135919
APA StyleSilva, V. R., Azar, A., Goncalves, E. R., Nascimento, T. C. d. M., Buchaim, R. L., Buchaim, D. V., de Oliveira, F. A. A., Nassar, C. C., de Camargo, T. M., Caboclo, R. F., & da Cunha, M. R. (2025). MicroRNA-Mediated Regulation of Vascular Endothelium: From Pro-Inflammation to Atherosclerosis. International Journal of Molecular Sciences, 26(13), 5919. https://doi.org/10.3390/ijms26135919








