When Your Body Tells You to Not Breastfeed—The Connivance of Oxytocin, Prolactin, and Dopamine
Abstract
1. Introduction
2. Why Mothers Stop Breastfeeding Prematurely
3. Maternal Mental Status and Breastfeeding
3.1. Dysphoric Milk Ejection Reflex
3.1.1. Symptomatology of D-MER
3.1.2. Pathophysiology of D-MER
3.2. Breastfeeding Aversion/Agitation
3.2.1. Symptomatology of BAA
3.2.2. Pathophysiology of BAA
4. The Hormones on the Playing Field
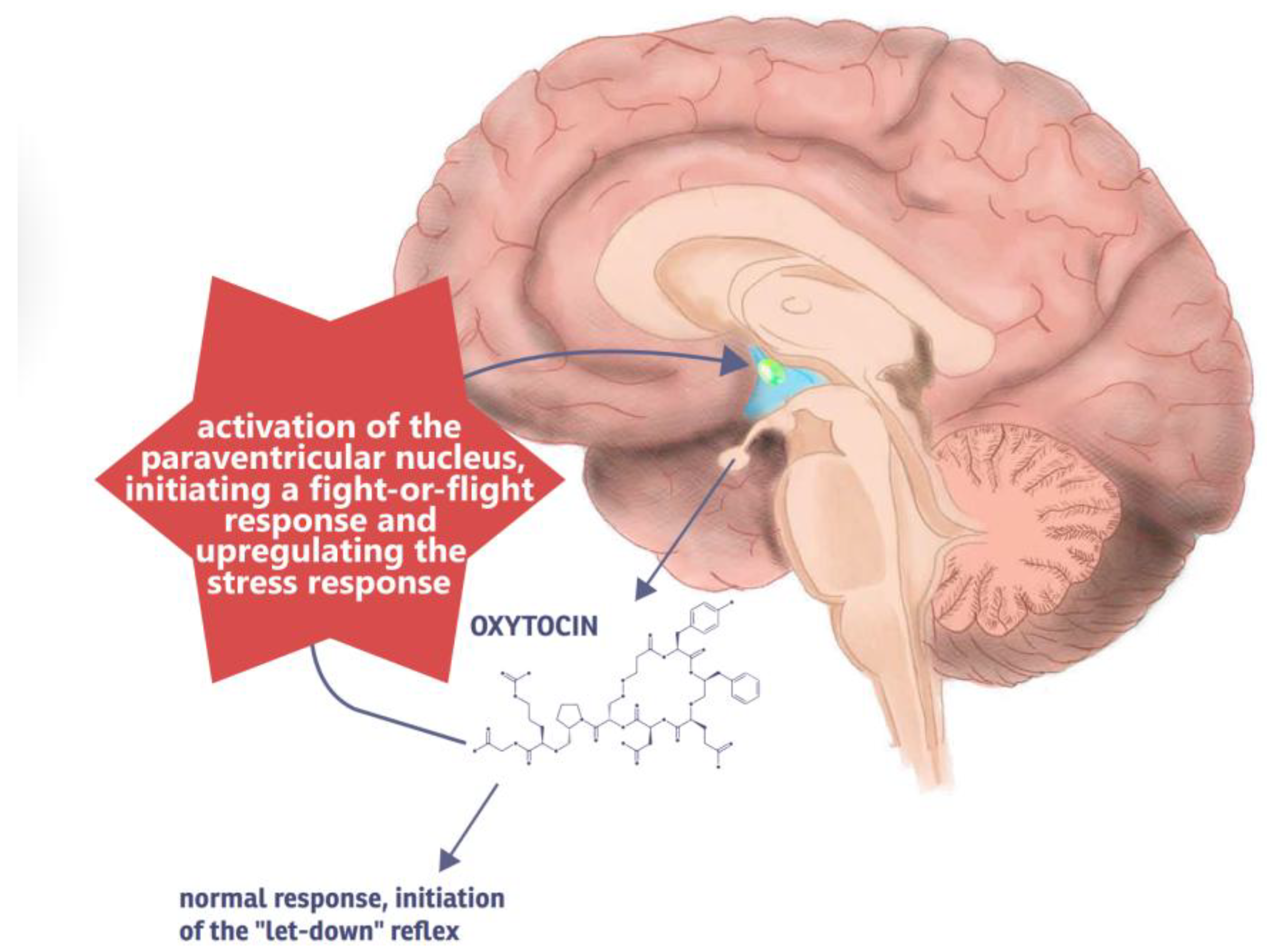
4.1. Oxytocin
4.2. Prolactin
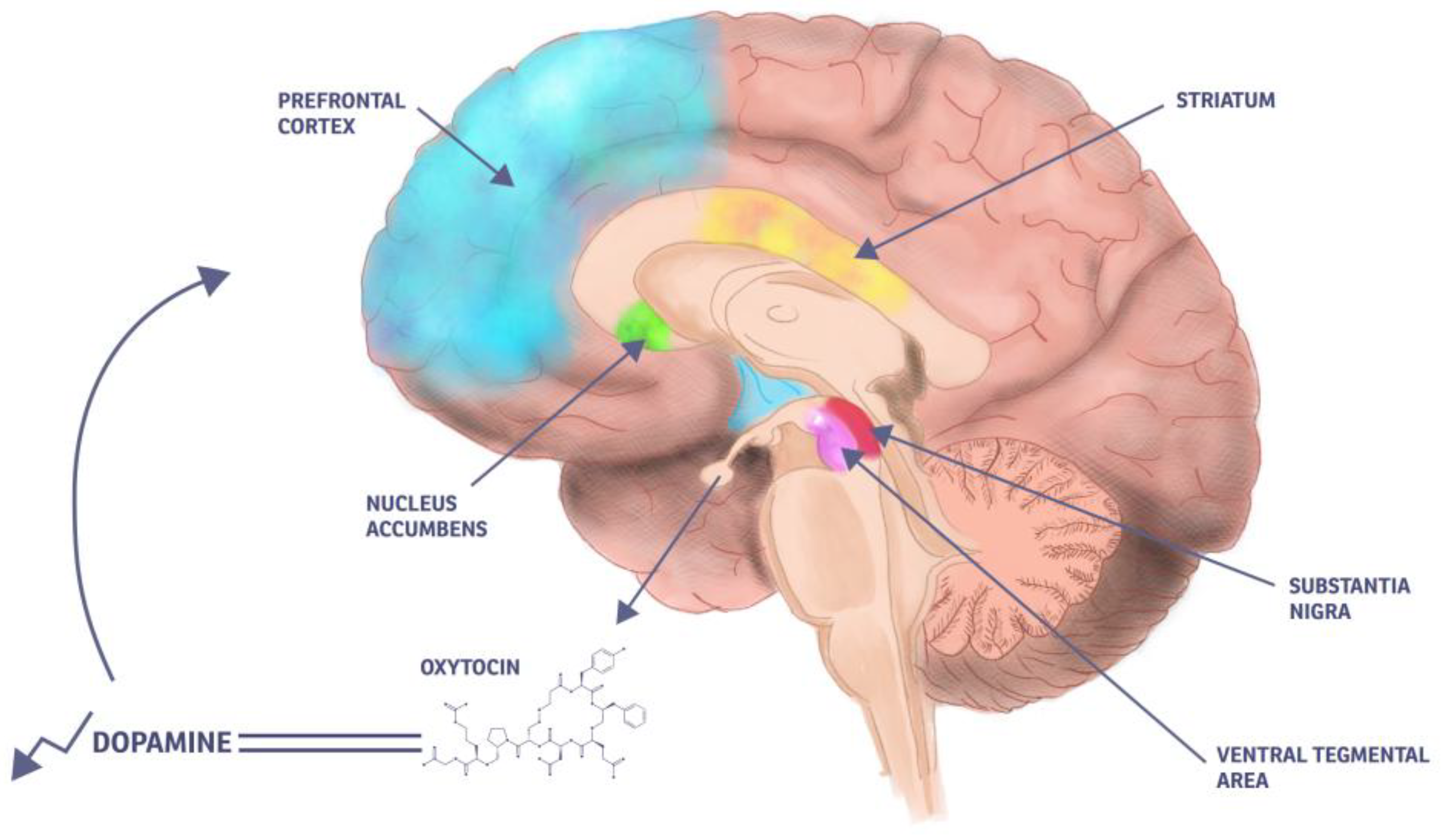
4.3. Dopamine
5. Future Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| D-MER | Dysphoric Milk Ejection Reflex |
| BAA | Breastfeeding Aversion/Agitation |
| PPD | postpartum depression |
| PTSD | post-traumatic stress disorder |
| OT | oxytocin |
| OXTR | oxytocin receptor |
| PRL | prolactin |
| PRL-R | prolactin receptor |
| MPOA | medial preoptic area |
| ACTH | adrenocorticotropic hormone |
| DA | dopamine |
References
- Eglash, A.; Montgomery, A.; Wood, J. Breastfeeding. Disease-A-Month 2008, 54, 343–411. [Google Scholar] [CrossRef]
- Yi, D.; Kim, S. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Modak, A.; Ronghe, V.; Gomase, K.P. The Psychological Benefits of Breastfeeding: Fostering Maternal Well-Being and Child Development. Cureus 2023, 15, e46730. [Google Scholar] [CrossRef]
- Brockway, M.M.; Daniel, A.I.; Reyes, S.M.; Granger, M.; McDermid, J.M.; Chan, D.; Refvik, R.; Sidhu, K.K.; Musse, S.; Patel, P.P.; et al. Human Milk Macronutrients and Child Growth and Body Composition in the First Two Years: A Systematic Review. Adv. Nutr. 2024, 15, 100149. [Google Scholar] [CrossRef]
- Goldman, A.S.; Chheda, S. The Immune System in Human Milk: A Historic Perspective. Ann. Nutr. Metab. 2021, 77, 189–196. [Google Scholar] [CrossRef]
- Sayres, S.; Visentin, L. Breastfeeding: Uncovering Barriers and Offering Solutions. Curr. Opin. Pediatr. 2018, 30, 591–596. [Google Scholar] [CrossRef]
- Westerfield, K.L.; Koenig, K.; Oh, R. Breastfeeding: Common Questions and Answers. Am. Fam. Physician 2018, 98, 368–373. [Google Scholar]
- Billings, H.; Horsman, J.; Soltani, H.; Spencer, R.L. Breastfeeding Experiences of Women with Perinatal Mental Health Problems: A Systematic Review and Thematic Synthesis. BMC Pregnancy Childbirth 2024, 24, 582. [Google Scholar] [CrossRef]
- Xia, M.; Luo, J.; Wang, J.; Liang, Y. Association between Breastfeeding and Postpartum Depression: A Meta-Analysis. J. Affect. Disord. 2022, 308, 512–519. [Google Scholar] [CrossRef]
- Mezzacappa, E.S. Breastfeeding and Maternal Stress Response and Health. Nutr. Rev. 2004, 62, 261–268. [Google Scholar] [CrossRef]
- Bugaeva, P.; Arkusha, I.; Bikaev, R.; Kamenskiy, I.; Pokrovskaya, A.; El-Taravi, Y.; Caso, V.; Avedisova, A.; Chu, D.K.; Genuneit, J.; et al. Association of Breastfeeding with Mental Disorders in Mother and Child: A Systematic Review and Meta-Analysis. BMC Med. 2023, 21, 393. [Google Scholar] [CrossRef]
- Coo, S.; García, M.I.; Prieto, F. Quality of Mother-Infant Interaction, Breastfeeding, and Perinatal Mental Health. Infant Behav. Dev. 2024, 75, 101946. [Google Scholar] [CrossRef]
- Cohen, S.S.; Alexander, D.D.; Krebs, N.F.; Young, B.E.; Cabana, M.D.; Erdmann, P.; Hays, N.P.; Bezold, C.P.; Levin-Sparenberg, E.; Turini, M.; et al. Factors Associated with Breastfeeding Initiation and Continuation: A Meta-Analysis. J. Pediatr. 2018, 203, 190–196.e21. [Google Scholar] [CrossRef]
- Dias, C.C.; Figueiredo, B. Breastfeeding and Depression: A Systematic Review of the Literature. J. Affect. Disord. 2015, 171, 142–154. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Meltzer-Brody, S.; Propper, C.; Pearson, B.; Beiler, P.; Elam, M.; Walker, C.; Mills-Koonce, R.; Grewen, K. The Mood, Mother, and Infant Study: Associations Between Maternal Mood in Pregnancy and Breastfeeding Outcome. Breastfeed. Med. 2019, 14, 551–559. [Google Scholar] [CrossRef]
- Henshaw, E.J. Breastfeeding and Postpartum Depression: A Review of Relationships and Potential Mechanisms. Curr. Psychiatry Rep. 2023, 25, 803–808. [Google Scholar] [CrossRef]
- Frawley, T.; McGuinness, D. Dysphoric Milk Ejection Reflex (D-MER) and Its Implications for Mental Health Nursing. Int. J. Ment. Health Nurs. 2023, 32, 620–626. [Google Scholar] [CrossRef]
- Heise, A.M.; Wiessinger, D. Dysphoric Milk Ejection Reflex: A Case Report. Int. Breastfeed. J. 2011, 6, 6. [Google Scholar] [CrossRef]
- Middleton, C.; Lee, E.; McFadden, A. Negative Emotional Experiences of Breastfeeding and the Milk Ejection Reflex: A Scoping Review. Int. Breastfeed. J. 2025, 20, 13. [Google Scholar] [CrossRef]
- Ureño, T.L.; Berry-Cabán, C.S.; Adams, A.; Buchheit, T.L.; Hopkinson, S.G. Dysphoric Milk Ejection Reflex: A Descriptive Study. Breastfeed. Med. 2019, 14, 666–673. [Google Scholar] [CrossRef]
- Ahmed, M.; Mahmud, A.; Mughal, S.; Shah, H.H. Dysphoric Milk Ejection Reflex—Call for Future Trials. Arch. Gynecol. Obstet. 2024, 310, 627–630. [Google Scholar] [CrossRef]
- Solmonovich, R.L.; Kouba, I.; Bailey, C.; Andria, W.; Demertzis, K.; Blitz, M.J.; Muscat, J. Incidence and Awareness of Dysphoric Milk Ejection Reflex (DMER). J. Perinat. Med. 2025, 53, 258–261. [Google Scholar] [CrossRef]
- Cappenberg, R.; Garcia, J.G.; Liolios, I.; Happle, C.; Zychlinsky Scharff, A. Dysphoric Milk Ejection Reflex: Prevalence, Persistence, and Implications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 308, 127–131. [Google Scholar] [CrossRef]
- Moriyama, Y.; Nakao, Y.; Yamamoto, N.; Oki, T. Dysphoric Milk Ejection Reflex among Japanese Mothers: A Self-Administered Survey. Int. Breastfeed. J. 2024, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, M.; Murray, C.; Simpson, J. Maternal Experiences of Embodied Emotional Sensations during Breast Feeding: An Interpretative Phenomenological Analysis. Midwifery 2016, 36, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Deif, R.; Burch, E.M.; Azar, J.; Yonis, N.; Abou Gabal, M.; El Kramani, N.; DakhlAllah, D. Dysphoric Milk Ejection Reflex: The Psychoneurobiology of the Breastfeeding Experience. Front. Glob. Womens Health 2021, 2, 669826. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Kendall-Tackett, K. The Mystery of D-MER: What Can Hormonal Research Tell Us About Dysphoric Milk-Ejection Reflex? Clin. Lact. 2018, 9, 23–29. [Google Scholar] [CrossRef]
- Ureño, T.L.; Buchheit, T.L.; Hopkinson, S.G.; Berry-Cabán, C.S. Dysphoric Milk Ejection Reflex: A Case Series. Breastfeed. Med. 2018, 13, 85–88. [Google Scholar] [CrossRef]
- Morns, M.A.; Steel, A.E.; McIntyre, E.; Burns, E. Breastfeeding Aversion Response (BAR): A Descriptive Study. J. Midwifery Women’s Health 2023, 68, 430–441. [Google Scholar] [CrossRef]
- Yate, Z. A Qualitative Study on Negative Emotions Triggered by Breastfeeding; Describing the Phenomenon of Breastfeeding/Nursing Aversion and Agitation in Breastfeeding Mothers. Iran. J. Nurs. Midwifery Res. 2017, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Morns, M.A.; Steel, A.E.; Burns, E.; McIntyre, E. Women Who Experience Feelings of Aversion While Breastfeeding: A Meta-Ethnographic Review. Women Birth 2021, 34, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Morns, M.A.; Burns, E.; McIntyre, E.; Steel, A.E. The Prevalence of Breastfeeding Aversion Response in Australia: A National Cross-sectional Survey. Matern. Child Nutr. 2023, 19, e13536. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.; Marshall, J.; Hinsliff, S. Self-conscious Emotions and Breastfeeding Support: A Focused Synthesis of UK Qualitative Research. Matern. Child Nutr. 2022, 18, e13270. [Google Scholar] [CrossRef]
- Mühlrad, H.; Björkegren, E.; Haraldson, P.; Bohm-Starke, N.; Kopp Kallner, H.; Brismar Wendel, S. Interpregnancy Interval and Maternal and Neonatal Morbidity: A Nationwide Cohort Study. Sci. Rep. 2022, 12, 17402. [Google Scholar] [CrossRef]
- Feldman, R.; Bakermans-Kranenburg, M.J. Oxytocin: A Parenting Hormone. Curr. Opin. Psychol. 2017, 15, 13–18. [Google Scholar] [CrossRef]
- Whitley, J.; Wouk, K.; Bauer, A.E.; Grewen, K.; Gottfredson, N.C.; Meltzer-Brody, S.; Propper, C.; Mills-Koonce, R.; Pearson, B.; Stuebe, A. Oxytocin during Breastfeeding and Maternal Mood Symptoms. Psychoneuroendocrinology 2020, 113, 104581. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Florea, T.; Palimariciuc, M.; Cristofor, A.C.; Dobrin, I.; Chiriță, R.; Bîrsan, M.; Dobrin, R.P.; Pădurariu, M. Oxytocin: Narrative Expert Review of Current Perspectives on the Relationship with Other Neurotransmitters and the Impact on the Main Psychiatric Disorders. Medicina 2022, 58, 923. [Google Scholar] [CrossRef]
- Inada, K. Neurobiological Mechanisms Underlying Oxytocin-Mediated Parental Behavior in Rodents. Neurosci. Res. 2024, 207, 1–12. [Google Scholar] [CrossRef]
- Rokicki, J.; Kaufmann, T.; De Lange, A.-M.G.; Van Der Meer, D.; Bahrami, S.; Sartorius, A.M.; Haukvik, U.K.; Steen, N.E.; Schwarz, E.; Stein, D.J.; et al. Oxytocin Receptor Expression Patterns in the Human Brain across Development. Neuropsychopharmacology 2022, 47, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Light, A.E.; Holt-Lunstad, J.; Porter, C.L.; Light, K.C. Early Life Trauma: An Exploratory Study of Effects on OXTR and NR3C1 Gene Expression and Nurturing Self-Efficacy in Mothers of Infants. Int. J. Psychophysiol. 2019, 136, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, M.B.; Rich, M.E.; Reno, E.L.; Lee, H.-J.; Caldwell, H.K. Heightened Aggressive Behavior in Mice with Lifelong versus Postweaning Knockout of the Oxytocin Receptor. Horm. Behav. 2012, 62, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.; Gaffke, L.; Żabińska, M.; Cyske, Z.; Rintz, E.; Wiśniewska, K.; Podlacha, M.; Węgrzyn, G. Roles of the Oxytocin Receptor (OXTR) in Human Diseases. Int. J. Mol. Sci. 2023, 24, 3887. [Google Scholar] [CrossRef]
- Lubin, D.A.; Elliot, J.C.; Black, M.C.; Johns, J.M. An Oxytocin Antagonist Infused into the Central Nucleus of the Amygdala Increases Maternal Aggressive Behavior. Behav. Neurosci. 2003, 117, 195–201. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, R.; Xia, Y.; Zhang, X.; Wang, Z.; Lorimer, G.H.; Ghiladi, R.A.; Bayram, H.; Wang, J. Neuropeptides Affecting Social Behavior in Mammals: Oxytocin. Peptides 2024, 177, 171223. [Google Scholar] [CrossRef]
- Zhao, B.-G.; Chapman, C.; Bicknell, R.J. Functional κ-Opioid Receptors on Oxytocin and Vasopressin Nerve Terminals Isolated from the Rat Neurohypophysis. Brain Res. 1988, 462, 62–66. [Google Scholar] [CrossRef]
- Brown, C.H.; Stern, J.E.; Jackson, K.L.M.; Bull, P.M.; Leng, G.; Russell, J.A. Morphine Withdrawal Increases Intrinsic Excitability of Oxytocin Neurons in Morphine-dependent Rats. Eur. J. Neurosci. 2005, 21, 501–512. [Google Scholar] [CrossRef]
- Douglas, A.J.; Johnstone, L.E.; Neumann, I.; Leng, G.; Russell, J.A. Oxytocin Neurones in the Supraoptic Nucleus (SON) Are Inhibited by Endogenous Opioids in Late Pregnant Rats. Gene Ther. 1994, 1 (Suppl. 1), S84. [Google Scholar]
- Bicknell, R.J.; Leng, G.; Lincoln, D.W.; Russell, J.A. Naloxone Excites Oxytocin Neurones in the Supraoptic Nucleus of Lactating Rats after Chronic Morphine Treatment. J. Physiol. 1988, 396, 297–317. [Google Scholar] [CrossRef]
- Johnstone, L.E.; Brown, C.H.; Meeren, H.K.M.; Vuijst, C.L.; Brooks, P.J.; Leng, G.; Russell, J.A. Local Morphine Withdrawal Increases c -Fos Gene, Fos Protein, and Oxytocin Gene Expression in Hypothalamic Magnocellular Neurosecretory Cells. J. Neurosci. 2000, 20, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Meguro, Y.; Miyano, K.; Hirayama, S.; Yoshida, Y.; Ishibashi, N.; Ogino, T.; Fujii, Y.; Manabe, S.; Eto, M.; Nonaka, M.; et al. Neuropeptide Oxytocin Enhances μ Opioid Receptor Signaling as a Positive Allosteric Modulator. J. Pharmacol. Sci. 2018, 137, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nisbett, K.E.; Vendruscolo, L.F.; Koob, G.F. Μ-Opioid Receptor Antagonism Facilitates the Anxiolytic-like Effect of Oxytocin in Mice. Transl. Psychiatry 2024, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Yoshida, Y.; Hirayama, S.; Takahashi, H.; Ono, H.; Meguro, Y.; Manabe, S.; Komatsu, A.; Nonaka, M.; Mizuguchi, T.; et al. Oxytocin Is a Positive Allosteric Modulator of κ-Opioid Receptors but Not δ-Opioid Receptors in the G Protein Signaling Pathway. Cells 2021, 10, 2651. [Google Scholar] [CrossRef]
- Mizuguchi, T.; Miyano, K.; Yamauchi, R.; Yoshida, Y.; Takahashi, H.; Yamazaki, A.; Ono, H.; Inagaki, M.; Nonaka, M.; Uezono, Y.; et al. The First Structure-Activity Relationship Study of Oxytocin as a Positive Allosteric Modulator for the µ Opioid Receptor. Peptides 2023, 159, 170901. [Google Scholar] [CrossRef]
- Torner, L. Actions of Prolactin in the Brain: From Physiological Adaptations to Stress and Neurogenesis to Psychopathology. Front. Endocrinol. 2016, 7, 25. [Google Scholar] [CrossRef]
- Szewczyk, A.K.; Ulutas, S.; Aktürk, T.; Al-Hassany, L.; Börner, C.; Cernigliaro, F.; Kodounis, M.; Lo Cascio, S.; Mikolajek, D.; Onan, D.; et al. Prolactin and Oxytocin: Potential Targets for Migraine Treatment. J. Headache Pain 2023, 24, 31. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Q.; Yao, D.; Wang, S.; Dang, S.; Ding, D.; Zhu, Z.; Shao, S.; Li, H. Prolactin, a Potential Mediator of Reduced Social Interactive Behavior in Newborn Infants Following Maternal Perinatal Depressive Symptoms. J. Affect. Disord. 2017, 215, 274–280. [Google Scholar] [CrossRef]
- Georgescu, T.; Swart, J.M.; Grattan, D.R.; Brown, R.S.E. The Prolactin Family of Hormones as Regulators of Maternal Mood and Behavior. Front. Glob. Womens Health 2021, 2, 767467. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A Pleiotropic Factor in Health and Disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef]
- PNECAT Group; Tost, M.; Monreal, J.A.; Armario, A.; Barbero, J.D.; Cobo, J.; García-Rizo, C.; Bioque, M.; Usall, J.; Huerta-Ramos, E.; et al. Targeting Hormones for Improving Cognition in Major Mood Disorders and Schizophrenia: Thyroid Hormones and Prolactin. Clin. Drug Investig. 2020, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Pianese, G.; Mureddu, P.; Fanos, V.; Bosco, A. From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression? Nutrients 2024, 16, 2285. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Hu, X.; Roberts, N.; Zhao, Y.; Xu, X.; Zhou, Y.; Tan, X.; Chen, S.; Meng, Y.; Wang, S.; et al. Prolactin Mediates the Relationship between Regional Gray Matter Volume and Postpartum Depression Symptoms. J. Affect. Disord. 2022, 301, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, T.; Khant Aung, Z.; Grattan, D.R.; Brown, R.S.E. Prolactin-Mediated Restraint of Maternal Aggression in Lactation. Proc. Natl. Acad. Sci. USA 2022, 119, e2116972119. [Google Scholar] [CrossRef]
- Speranza, L.; Di Porzio, U.; Viggiano, D.; De Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Rappeneau, V.; Castillo Díaz, F. Convergence of Oxytocin and Dopamine Signalling in Neuronal Circuits: Insights into the Neurobiology of Social Interactions across Species. Neurosci. Biobehav. Rev. 2024, 161, 105675. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K. Interactions of Oxytocin and Dopamine—Effects on Behavior in Health and Disease. Biomedicines 2024, 12, 2440. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef]
- Gogarnoiu, E.S.; Vogt, C.D.; Sanchez, J.; Bonifazi, A.; Saab, E.; Shaik, A.B.; Soler-Cedeño, O.; Bi, G.-H.; Klein, B.; Xi, Z.-X.; et al. Dopamine D3 /D2 Receptor Ligands Based on Cariprazine for the Treatment of Psychostimulant Use Disorders That May Be Dual Diagnosed with Affective Disorders. J. Med. Chem. 2023, 66, 1809–1834. [Google Scholar] [CrossRef]
- Delva, N.C.; Stanwood, G.D. Dysregulation of Brain Dopamine Systems in Major Depressive Disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Grieb, Z.A.; Lonstein, J.S. Oxytocin Interactions with Central Dopamine and Serotonin Systems Regulate Different Components of Motherhood. Phil. Trans. R. Soc. B 2022, 377, 20210062. [Google Scholar] [CrossRef] [PubMed]
- Baskerville, T.A.; Douglas, A.J. Dopamine and Oxytocin Interactions Underlying Behaviors: Potential Contributions to Behavioral Disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef] [PubMed]



| Reasons for Premature Breastfeeding Cessation | |
|---|---|
| perception of inadequate milk supply | late lactogenesis |
| perception of insufficient nutrition | return to work |
| lack of support from family members | unrealistic expectations |
| concerns about the safety of medication taken | maternal obesity |
| uncoordinated suck and swallow reflex | smoking |
| maternal mental health problems | complicated childbirth |
| Aspect | D-MER and BAA/BAR Similarities |
|---|---|
| Associated with breastfeeding | both occur in breastfeeding individuals |
| Emotional/psychological symptoms | both involve distressing emotional experiences |
| Trigger | both are triggered or worsened by nursing or milk release |
| Impact | can lead to early weaning or reduced breastfeeding satisfaction |
| Recognition | under-recognized and often misunderstood by healthcare providers; both require emotional support and sometimes professional care |
| Aspect | D-MER | BAA/BAR |
| Definition | a neurological condition causing brief dysphoria during let-down | psychological aversion or agitation toward nursing |
| Onset timing | occurs just before or during milk ejection (let-down reflex) | can occur anytime during nursing, especially with older infants |
| Duration of episode | lasts 30 s to 2 min | persists as long as nursing continues |
| Primary emotions | sudden wave of sadness, dread, anxiety | anger, irritability, skin-crawling sensation |
| Hormonal involvement | linked to hormonal changes during let-down | not clearly linked to hormonal shifts |
| Typical onset period | often appears in early postpartum period | can develop later, especially during toddler nursing or tandem feeding |
| Resolution | often resolves over time | may persist unless feeding patterns are changed |
| Research status | some emerging scientific understanding | largely anecdotal, with limited formal study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, V., Jr.; Čižmárová, B.; Birková, A. When Your Body Tells You to Not Breastfeed—The Connivance of Oxytocin, Prolactin, and Dopamine. Int. J. Mol. Sci. 2025, 26, 5909. https://doi.org/10.3390/ijms26125909
Kraus V Jr., Čižmárová B, Birková A. When Your Body Tells You to Not Breastfeed—The Connivance of Oxytocin, Prolactin, and Dopamine. International Journal of Molecular Sciences. 2025; 26(12):5909. https://doi.org/10.3390/ijms26125909
Chicago/Turabian StyleKraus, Vladimír, Jr., Beáta Čižmárová, and Anna Birková. 2025. "When Your Body Tells You to Not Breastfeed—The Connivance of Oxytocin, Prolactin, and Dopamine" International Journal of Molecular Sciences 26, no. 12: 5909. https://doi.org/10.3390/ijms26125909
APA StyleKraus, V., Jr., Čižmárová, B., & Birková, A. (2025). When Your Body Tells You to Not Breastfeed—The Connivance of Oxytocin, Prolactin, and Dopamine. International Journal of Molecular Sciences, 26(12), 5909. https://doi.org/10.3390/ijms26125909





