Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models
Abstract
1. Introduction
2. Results
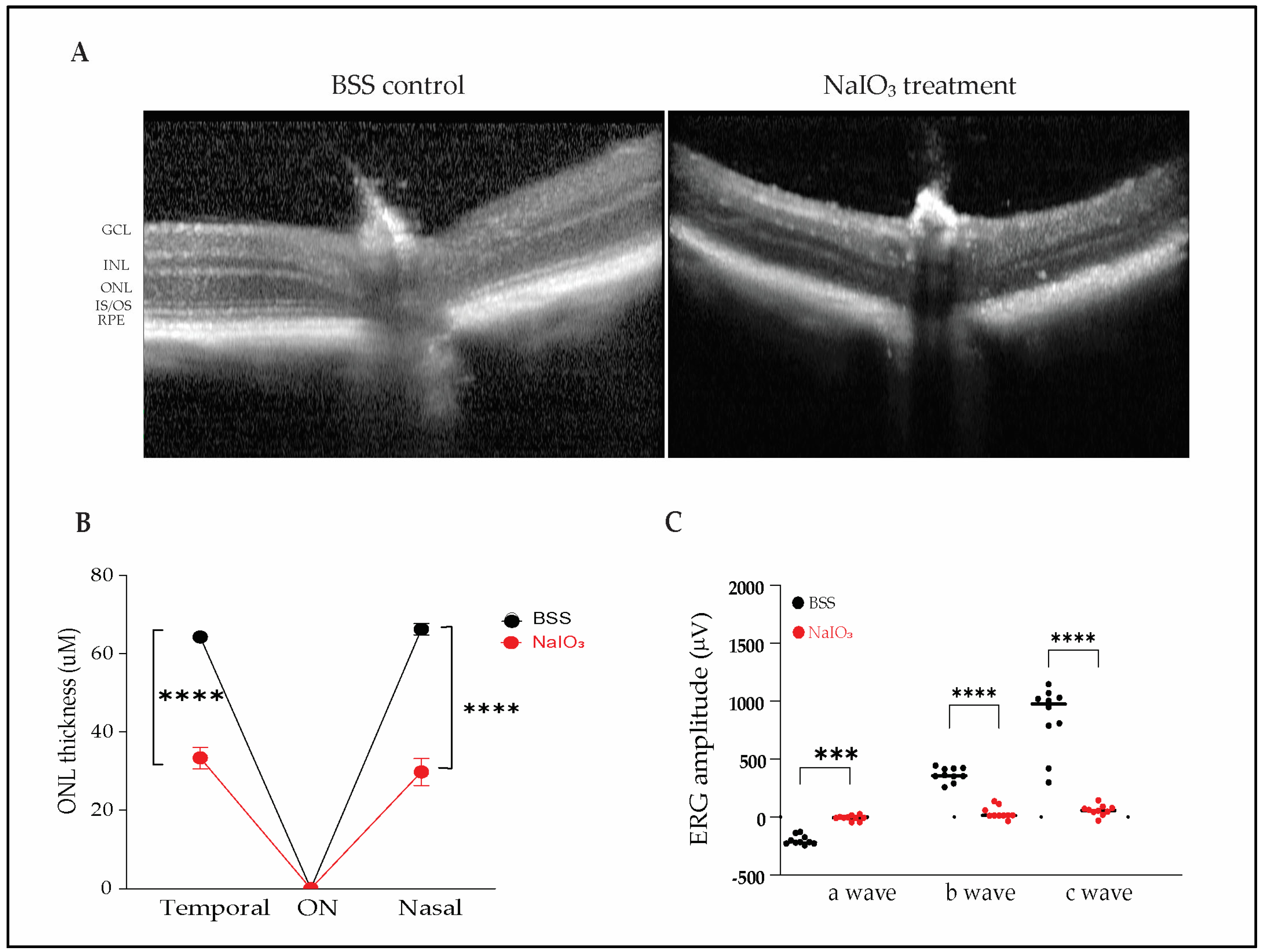
2.1. Sodium Iodate Induces Severe Structural and Functional Retinal Degeneration in Mice
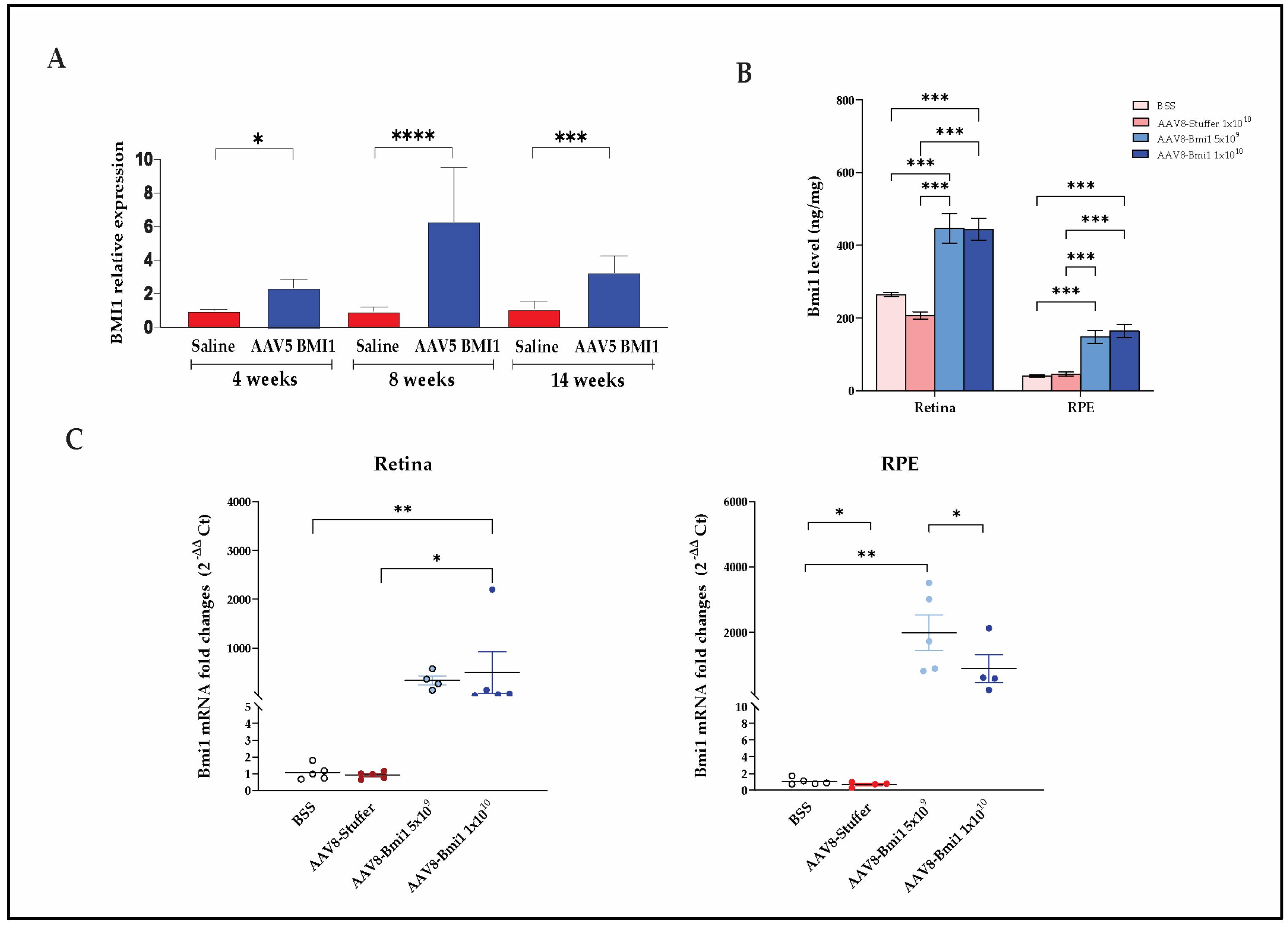
2.2. Durable, Dose-Dependent Increases in Retinal BMI1 Expression Following AAV-Mediated Delivery
2.2.1. AAV5.BMI1 Subretinal Delivery Increases Retinal BMI1 mRNA Expression over Time
2.2.2. AAV8.BMI1 Suprachoroidal Delivery Achieves Robust, Dose-Dependent BMI1 Expression
2.3. BMI1 Expression Preserves Photoreceptors and Retinal Structure
2.4. AAV8.BMI1 Treatment Preserves Retinal Function Following Sodium Iodate Exposure
3. Discussion
4. Materials and Methods
4.1. AAV Vectors
4.2. Animals
4.3. Subretinal Injection
4.4. Suprachoroidal Injection
4.5. Electroretinography
4.6. Optical Coherence Tomography
4.7. RNA Extraction and qRT-PCR
4.8. Meso Scale Discovery (MSD) Assay for BMI1 Quantification
4.9. Immunofluorescence and Immunohistochemistry
4.10. Quantification of Outer Nuclear Layer Thickness
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef]
- Khanani, A.M.; Hershberger, V.S.; Pieramici, D.J.; Khurana, R.N.; Brunstein, F.; Ma, L.; Maass, K.F.; Honigberg, L.A.; Tom, I.; Chen, H.; et al. Phase 1 Study of the Anti-HtrA1 Antibody-binding Fragment FHTR2163 in Geographic Atrophy Secondary to Age-related Macular Degeneration. Am. J. Ophthalmol. 2021, 232, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chatoo, W.; Abdouh, M.; David, J.; Champagne, M.P.; Ferreira, J.; Rodier, F.; Bernier, G. The polycomb group gene Bmi1 regulates antioxidant defenses in neurons by repressing p53 pro-oxidant activity. J. Neurosci. 2009, 29, 529–542. [Google Scholar] [CrossRef]
- Lessard, J.; Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003, 423, 255–260. [Google Scholar] [CrossRef]
- Liu, J.; Cao, L.; Chen, J.; Song, S.; Lee, I.H.; Quijano, C.; Liu, H.; Keyvanfar, K.; Chen, H.; Cao, L.Y.; et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009, 459, 387–392. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Iwashita, T.; Park, I.K.; Clarke, M.F.; Morrison, S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003, 425, 962–967. [Google Scholar] [CrossRef]
- Bardales, S.; Lu, Z.; Whitlock, A.; Ramkumar, H. Potency Assay for AAV vector Encoding BMI1 Protein for the Treatment of Dry Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1134. [Google Scholar]
- Dhirachaikulpanich, D.; Li, X.; Porter, L.F.; Paraoan, L. Integrated Microarray and RNAseq Transcriptomic Analysis of Retinal Pigment Epithelium/Choroid in Age-Related Macular Degeneration. Front. Cell Dev. Biol. 2020, 8, 808. [Google Scholar] [CrossRef]
- Lu, Z.; Morales, M.G.; Liu, S.; Ramkumar, H.L. The Endogenous Expression of BMI1 in Adult Human Eyes. Cells 2024, 13, 1672. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Whitlock, A.; Ramkumar, R.; Ramkumar, H. Pharmacokinetics and Safety of Suprachoroidal Delivery of AAV.BMI1 in C57/B6 Mice. Investig. Ophthalmol. Vis. Sci. 2024, 65, 201. [Google Scholar]
- Chen, G.; Zhang, Y.; Yu, S.; Sun, W.; Miao, D. Bmi1 Overexpression in Mesenchymal Stem Cells Exerts Antiaging and Antiosteoporosis Effects by Inactivating p16/p19 Signaling and Inhibiting Oxidative Stress. Stem Cells 2019, 37, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Ni, W.; Zhang, Y.; Sun, S.; Miao, D.; Chai, R.; Li, H. Bmi1 regulates auditory hair cell survival by maintaining redox balance. Cell Death Dis. 2015, 6, e1605. [Google Scholar] [CrossRef]
- Dibenedetto, S.; Niklison-Chirou, M.; Cabrera, C.P.; Ellis, M.; Robson, L.G.; Knopp, P.; Tedesco, F.S.; Ragazzi, M.; Di Foggia, V.; Barnes, M.R.; et al. Enhanced Energetic State and Protection from Oxidative Stress in Human Myoblasts Overexpressing BMI1. Stem Cell Rep. 2017, 9, 528–542. [Google Scholar] [CrossRef]
- Nakamura, S.; Oshima, M.; Yuan, J.; Saraya, A.; Miyagi, S.; Konuma, T.; Yamazaki, S.; Osawa, M.; Nakauchi, H.; Koseki, H.; et al. Bmi1 confers resistance to oxidative stress on hematopoietic stem cells. PLoS ONE 2012, 7, e36209. [Google Scholar] [CrossRef]
- Wang, R.; Xue, X.; Wang, Y.; Zhao, H.; Zhang, Y.; Wang, H.; Miao, D. BMI1 Deficiency Results in Female Infertility by Activating p16/p19 Signaling and Increasing Oxidative Stress. Int. J. Biol. Sci. 2019, 15, 870–881. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.Z.; Ma, M.; Shao, G.F. MiR-15 suppressed the progression of bladder cancer by targeting BMI1 oncogene via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8813–8822. [Google Scholar] [CrossRef]
- Chatoo, W.; Abdouh, M.; Duparc, R.H.; Bernier, G. Bmi1 distinguishes immature retinal progenitor/stem cells from the main progenitor cell population and is required for normal retinal development. Stem Cells 2010, 28, 1412–1423. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Barabino, A.; Plamondon, V.; Abdouh, M.; Chatoo, W.; Flamier, A.; Hanna, R.; Zhou, S.; Motoyama, N.; Hebert, M.; Lavoie, J.; et al. Loss of Bmi1 causes anomalies in retinal development and degeneration of cone photoreceptors. Development 2016, 143, 1571–1584. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Gong, X.; Gong, L.; Zhang, L.; Tang, X.; Wang, L.; Liu, F.; Fu, J.L.; Xiang, J.W.; Xiao, Y.; et al. Sodium Iodate-Induced Mouse Model of Age-Related Macular Degeneration Displayed Altered Expression Patterns of Sumoylation Enzymes E1, E2 and E3. Curr. Mol. Med. 2018, 18, 550–555. [Google Scholar] [CrossRef]
- Kim, S.Y.; Zhao, Y.; Kim, H.L.; Oh, Y.; Xu, Q. Sodium iodate-induced retina degeneration observed in non-separate sclerochoroid/retina pigment epithelium/retina whole mounts. Ann. Eye Sci. 2022, 7, 3. [Google Scholar] [CrossRef]
- Geathers, J.S.; Grillo, S.L.; Karakoleva, E.; Campbell, G.P.; Du, Y.; Chen, H.; Barber, A.J.; Zhao, Y.; Sundstrom, J.M. Sodium Iodate: Rapid and Clinically Relevant Model of AMD. Front. Biosci. 2024, 29, 380. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dubey, R.; Jung, K.; Hernandez, A.G.; Kleinman, M.E. Deciphering age-related transcriptomic changes in the mouse retinal pigment epithelium. Aging 2025, 17, 657–684. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dubey, R.; Prajapati, S.C.; Jung, K.; Mohan, K.; Liu, X.; Roney, J.; Tian, W.; Abney, J.; Giarmarco, M.M.; et al. Histone deficiency and hypoacetylation in the aging retinal pigment epithelium. Aging Cell 2024, 23, e14108. [Google Scholar] [CrossRef]
- Maurya, M.; Bora, K.; Blomfield, A.K.; Pavlovich, M.C.; Huang, S.; Liu, C.H.; Chen, J. Oxidative stress in retinal pigment epithelium degeneration: From pathogenesis to therapeutic targets in dry age-related macular degeneration. Neural Regen. Res. 2023, 18, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra176. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Pang, J.J.; Dai, X.; Boye, S.E.; Barone, I.; Boye, S.L.; Mao, S.; Everhart, D.; Dinculescu, A.; Liu, L.; Umino, Y.; et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol. Ther. 2011, 19, 234–242. [Google Scholar] [CrossRef]
- Lim, Y.; Campochiaro, P.A.; Green, J.J. Suprachoroidal Delivery of Viral and Nonviral Vectors for Treatment of Retinal and Choroidal Vascular Diseases. Am. J. Ophthalmol. 2024; in press. [Google Scholar] [CrossRef]
- Habot-Wilner, Z.; Noronha, G.; Wykoff, C.C. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: A targeted approach. Acta Ophthalmol. 2019, 97, 460–472. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef]
- He, X.; Fu, Y.; Ma, L.; Yao, Y.; Ge, S.; Yang, Z.; Fan, X. AAV for Gene Therapy in Ocular Diseases: Progress and Prospects. Research 2023, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Khan, H.; Khanani, Z.A.; Thomas, M.J.; Khan, H.; Ahmed, A.; Gahn, G.M.; Khanani, A.M. Review of Gene Therapy Clinical Trials for Retinal Diseases. Int. Ophthalmol. Clin. 2024, 64, 141–151. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Liu, S.; Morales, M.G.; Whitlock, A.; Ramkumar, R.; Ramkumar, H.L. Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models. Int. J. Mol. Sci. 2025, 26, 5907. https://doi.org/10.3390/ijms26125907
Lu Z, Liu S, Morales MG, Whitlock A, Ramkumar R, Ramkumar HL. Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models. International Journal of Molecular Sciences. 2025; 26(12):5907. https://doi.org/10.3390/ijms26125907
Chicago/Turabian StyleLu, Zhongyang, Shufeng Liu, Maria G. Morales, Andy Whitlock, Ram Ramkumar, and Hema L. Ramkumar. 2025. "Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models" International Journal of Molecular Sciences 26, no. 12: 5907. https://doi.org/10.3390/ijms26125907
APA StyleLu, Z., Liu, S., Morales, M. G., Whitlock, A., Ramkumar, R., & Ramkumar, H. L. (2025). Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models. International Journal of Molecular Sciences, 26(12), 5907. https://doi.org/10.3390/ijms26125907




