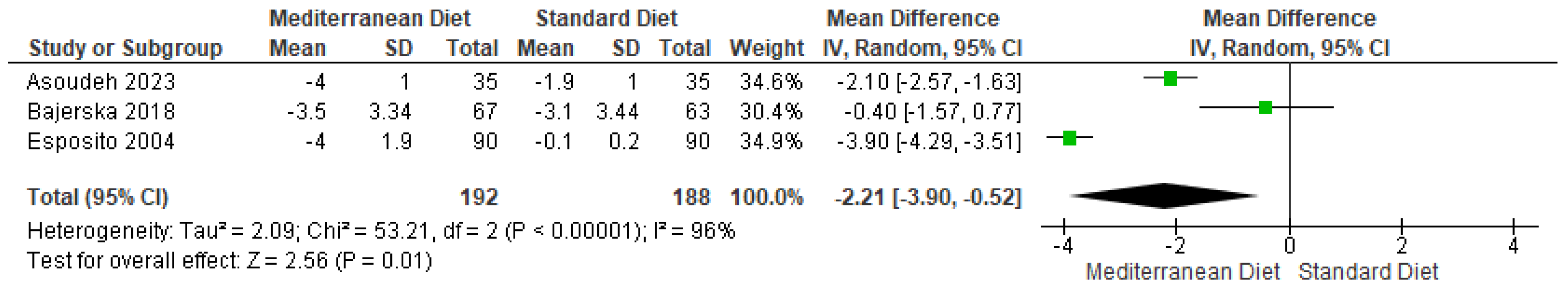Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Prisma Guidelines and PROSPERO Registration
2.2. Literature Search
2.3. Data Extraction
2.4. Types Studies
2.5. Data Analysis and Rating Quality of Evidence
3. Results
3.1. Selection of Studies
3.2. Study Characteristics
3.3. Assessment of Risk of Bias in Individual Studies
3.4. Synthesis of Results
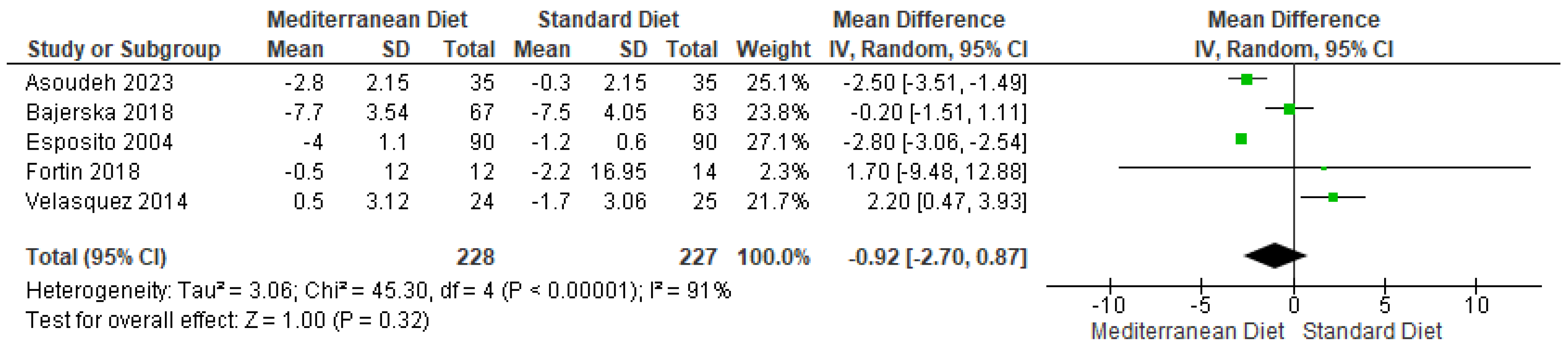
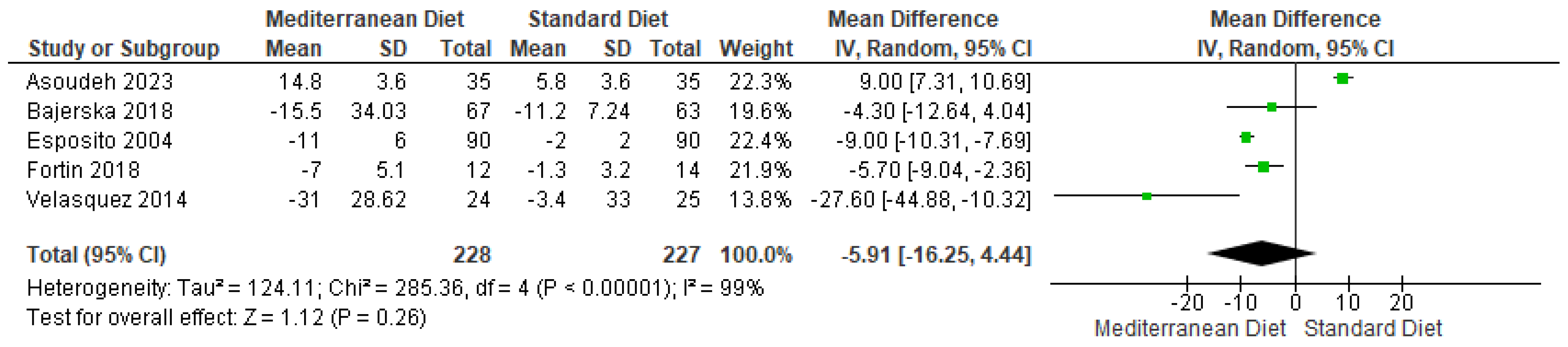
3.4.1. Body Weight
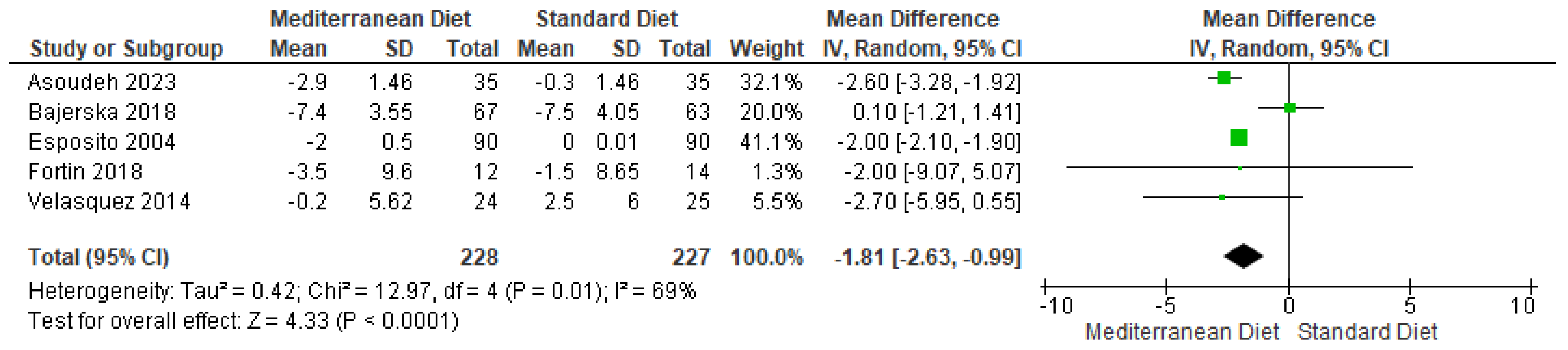
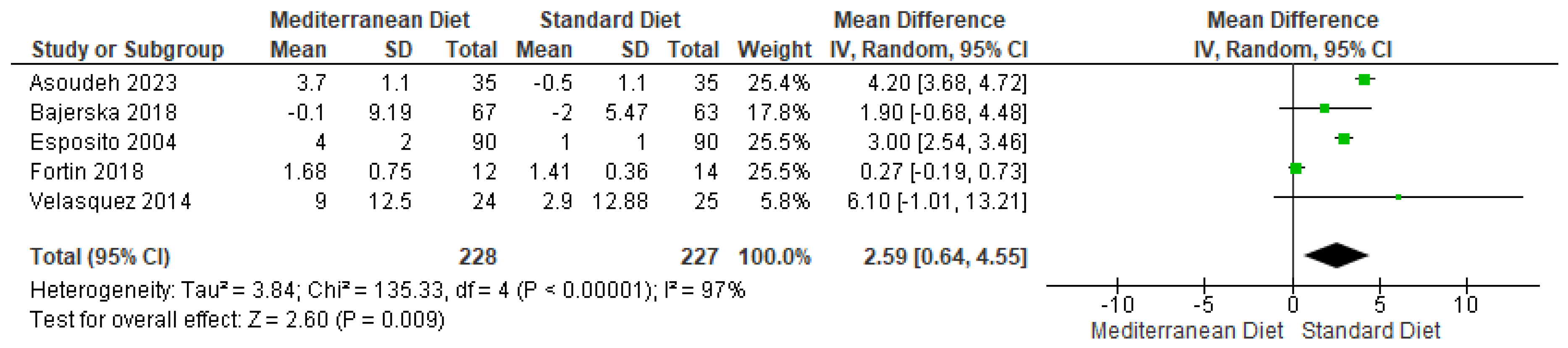
3.4.2. Outcome BMI
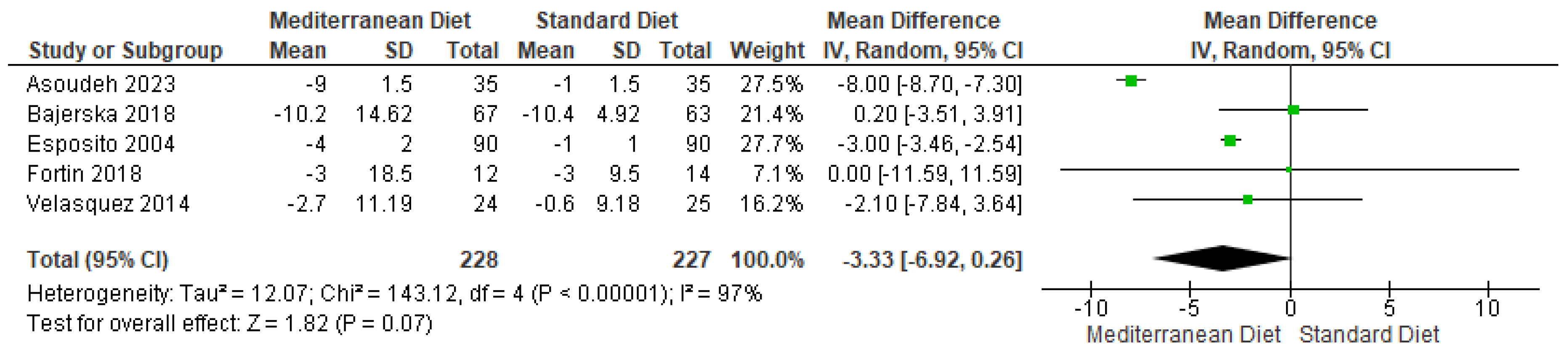
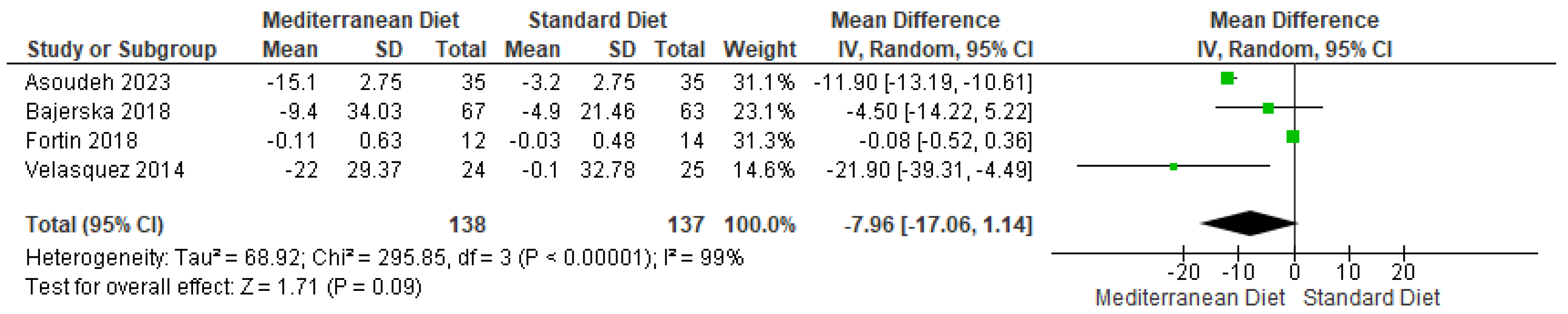
3.4.3. Outcome Waist Circumference
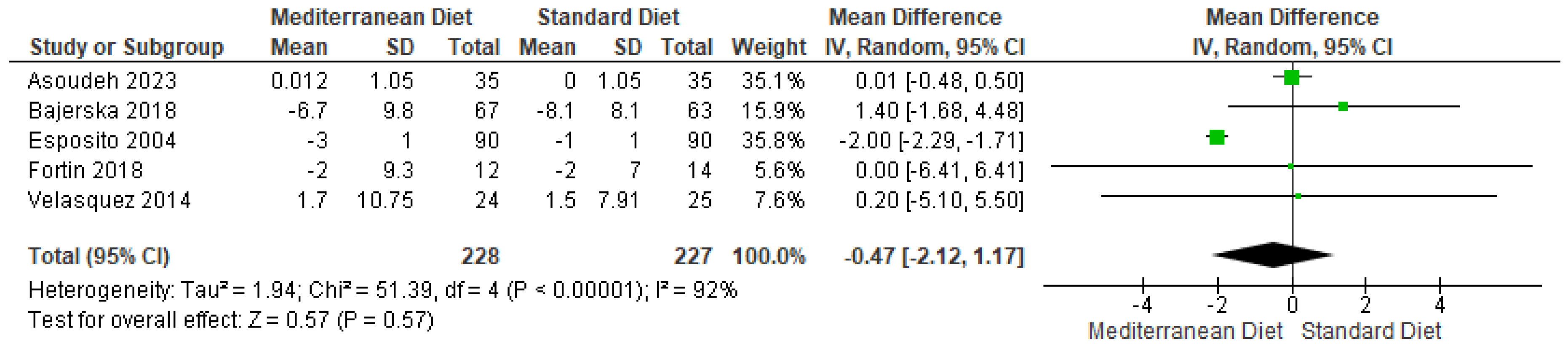
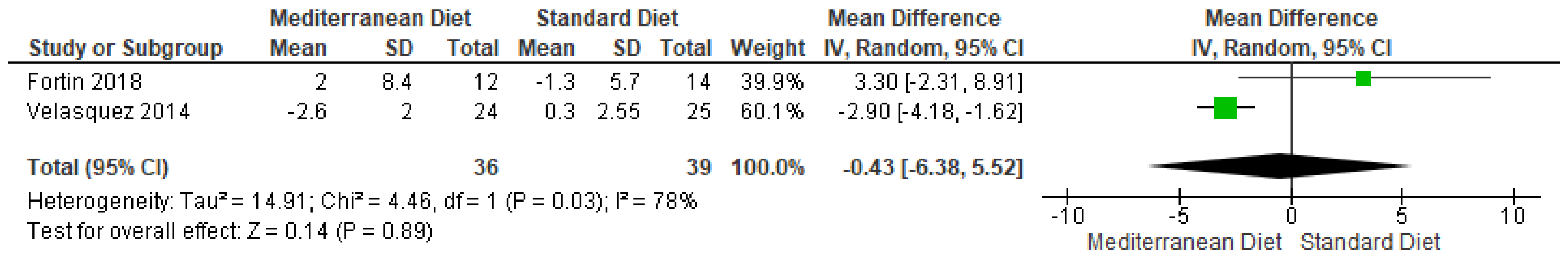
3.4.4. Outcome SBP
3.4.5. Outcome DBP
3.4.6. Outcome Triglycerides
3.4.7. Outcome Glucose
3.4.8. Outcome Total Cholesterol
3.4.9. Outcome HDL
3.4.10. Outcome LDL
3.4.11. Body Fat
3.4.12. HOMA-IR
3.4.13. Insulin
4. Adverse Effects
5. Meta-Regression
6. Sensitivity Analysis
7. Discussion
8. Limitations of the Study
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babio, N.; Toledo, E.; Estruch, R.; Ros, E.; Martínez-González, M.A.; Castañer, O.; Bulló, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Mediterranean diets and metabolic syndrome status in the PREDIMEDrandomized trial. CMAJ 2014, 186, E649–E657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh, F.; Fallah, M.; Aminianfar, A.; Djafarian, K.; Shirzad, N.; Clark, C.C.T.; Larijani, B.; Esmaillzadeh, A. The effect of Mediterranean diet on inflammatory biomarkers and components of metabolic syndrome in adolescent girls. J. Endocrinol. Investig. 2023, 46, 1995–2004, Erratum in J. Endocrinol. Investig. 2024, 47, 257. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Sala-Vila, A.; Chisaguano, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Lamuela-Raventós, R.; et al. Effects of 1-year intervention with a Mediterranean diet on plasma fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PLoS ONE 2014, 9, e85202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Marcucci, R.; Casini, A. Mediterranean versus vegetarian diet for cardiovascular disease prevention (the CARDIVEG study): Study protocol for a randomized controlled trial. Trials 2016, 17, 233, Erratum in Trials 2016, 17, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The Effect of the Traditional Mediterranean-Style Diet on Metabolic Risk Factors: A Meta-Analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández, J.M.; Rosado-Álvarez, D.; Da Silva Grigoletto, M.E.; Rangel-Zúñiga, O.A.; Landaeta-Díaz, L.L.; Caballero-Villarraso, J.; López-Miranda, J.; Pérez-Jiménez, F.; Fuentes-Jiménez, F. Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin. Sci. 2012, 123, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, J.C.; Martínez-Sánchez, M.A.; Bernal-López, M.R.; Muñoz-Garach, A.; Martínez-González, M.A.; Fitó, M.; Salas-Salvadó, J.; Tinahones, F.J.; Ramos-Molina, B. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on the serum polyamine metabolome in individuals at high cardiovascular disease risk: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghoshal, U.C.; Sonthalia, N.; Roy, A.; Goenka, M.K. Metabolic Syndrome and Gastroesophageal Reflux Disease: Clinical Remission With Treatment, Beyond an Epidemiological Association. J. Neurogastroenterol. Motil. 2025, 31, 1–2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapa, K.; Khan, H.; Chahuan, S.; Dhankhar, S.; Kaur, A.; Garg, N.; Saini, M.; Singh, T.G. Insights into therapeutic approaches for the treatment of neurodegenerative diseases targeting metabolic syndrome. Mol. Biol. Rep. 2025, 52, 260. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Ramirez, A.; Hernández-Vásquez, A.; Comandé, D.; Azañedo, D. Impact of subgingival periodontal treatment on systemic markers of inflammation in patients with metabolic syndrome: A systematic review of randomized clinical trials. Front. Oral Health 2025, 5, 1465820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barcot, O.; Ivanda, M.; Buljan, I.; Pieper, D.; Puljak, L. Enhanced access to recommendations from the Cochrane Handbook for improving authors’ judgments about risk of bias: A randomized controlled trial. Res. Synth. Methods 2021, 12, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Bajerska, J.; Chmurzynska, A.; Muzsik, A.; Krzyżanowska, P.; Mądry, E.; Malinowska, A.M.; Walkowiak, J. Weight loss and metabolic health effects from energy-restricted Mediterranean and Central-European diets in postmenopausal women: A randomized controlled trial. Sci. Rep. 2018, 8, 11170, Erratum in Sci. Rep. 2019, 9, 16077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortin, A.; Rabasa-Lhoret, R.; Lemieux, S.; Labonté, M.E.; Gingras, V. Comparison of a Mediterranean to a low-fat diet intervention in adults with type 1 diabetes and metabolic syndrome: A 6-month randomized trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konieczna, J.; Ruiz-Canela, M.; Galmes-Panades, A.M.; Abete, I.; Babio, N.; Fiol, M.; Martín-Sánchez, V.; Estruch, R.; Vidal, J.; Buil-Cosiales, P.; et al. An Energy-Reduced Mediterranean Diet, Physical Activity, and Body Composition: An Interim Subgroup Analysis of the PREDIMED-Plus Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2337994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez-Huelgas, R.; Jansen-Chaparro, S.; Baca-Osorio, A.J.; Mancera-Romero, J.; Tinahones, F.J.; Bernal-López, M.R. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur. J. Intern. Med. 2015, 26, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Silva, J.; NNavarrete, N.; Peralta-Ramírez, M.I.; García-Sánchez, A.; Ferrer-González, M.Á.; Caballo, V.E. Efficacy of Cognitive Behavioral Therapy in Adherence to the Mediterranean Diet in Metabolic Syndrome Patients: A Randomized Controlled Trial. J. Nutr. Educ. Behav. 2018, 50, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Bibiloni, M.D.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.Á.; PREDIMED Study Investigators. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Chrysoula, L.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 1514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasanisi, P.; Gargano, G.; Gaetana Di Mauro, M.; Cortellini, M.; Casagrande, A.; Villarini, A.; Bruno, E.; Roveda, E.; Saibene, G.; Venturelli, E.; et al. A randomized controlled trial of Mediterranean diet and metformin to prevent age-related diseases in people with metabolic syndrome. Tumori J. 2018, 104, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
- Daidone, M.; Di Chiara, T.; Del Cuore, A.; Casuccio, A.; Salamone, G.; Di Raimondo, D.; Tuttolomondo, A. Mediterranean diet and hypertension: Relationship between adherence to a Mediterranean diet and arterial hypertension. BMC Nutr. 2025, 11, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scaglione, S.; Di Chiara, T.; Daidone, M.; Tuttolomondo, A. Effects of the Mediterranean Diet on the Components of Metabolic Syndrome Concerning the Cardiometabolic Risk. Nutrients 2025, 17, 358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Lee, J.W.; Lee, Y.; Nam, C.M.; Kwon, Y.J. Impact of Mediterranean Diet Adherence on the Incidence of New-Onset Hypertension in Adults With Obesity in Korea: A Nationwide Cohort Study. J. Clin. Hypertens. 2025, 27, e14951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Micó-Pérez, R.M.; Hernández Segura, N.; Martín-Sánchez, V.; Barquilla-García, A.; Velilla-Zancada, S.M.; Polo-García, J.; Prieto-Díaz, M.Á.; Pallares-Carratala, V.; Segura-Fragoso, A.; Ginel-Mendoza, L.; et al. Physical activity and metabolic syndrome in primary care patients in Spain. PLoS ONE 2025, 20, e0317593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tárraga Marcos, P.J.; López-González, Á.A.; Martínez-Almoyna Rifá, E.; Paublini Oliveira, H.; Martorell Sánchez, C.; Tárraga López, P.J.; Ramírez-Manent, J.I. The Prevalence of Metabolic Syndrome and Hypertriglyceridemic Waist Based on Sociodemographic Variables and Healthy Habits in Healthcare Workers: A Retrospective Study. Life 2025, 15, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornali, K.; Di Lauro, M.; Marrone, G.; Masci, C.; Montalto, G.; Giovannelli, A.; Schievano, C.; Tesauro, M.; Pieri, M.; Bernardini, S.; et al. The Effects of a Food Supplement, Based on Co-Micronized Palmitoylethanolamide (PEA)-Rutin and Hydroxytyrosol, in Metabolic Syndrome Patients: Preliminary Results. Nutrients 2025, 17, 413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pranjić, I.; Sila, S.; Lulić Kujundžić, S.; Dodig, M.; Vestergaard Larsen, A.; Kranjčec, I. Metabolic Sequelae and Quality of Life in Early Post-Treatment Period in Adolescents with Hodgkin Lymphoma. J. Clin. Med. 2025, 14, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Author | Country | Population | |
|---|---|---|---|
| Sample Size (n) | Patients Mean (SD) | ||
| Velázquez-López 2014 [19] | Mexico | CG: 25 EG: 24 | CG: 11.4 (2.9) EG: 11.2 (2.7) Children with obesity |
| Asoudeh 2023 [3] | Iran | CG: 35 EG: 35 | CG: 14 (1) EG: 14 (1) |
| Konieczna 2023 [20] | Spain | CG: 761 EG: 760 | CG: 65.4 (4.9) EG: 65.1 (5.1) older adults with overweight or obesity and metabolic syndrome. |
| Bajerska 2018 [17] | Poland | EG: 72 EC: 72 | EG: 60.5 EC:60.5 |
| Mayneris 2014 [4] | Spain | CG: 141 EG1 (MD + VOO): 142 EG2 (MD + nuts): 141 | CG: EG1 (MD + VOO): EG2 (MD + nuts): The age of each group is not specified as such, only the difference between individuals with MS 67.7 (5.8) and individuals without MS 67.5 (6.1) was considered. |
| Fernández 2020 [8] | Spain | EG1 (MedDiet + PA): 38 EG2 (MedDiet): 37 | EG1 (MedDiet + PA): 63.3 (4.4) EG2 (MedDiet): 65.6 (4.1) |
| Garcia 2018 [22] | Spain | Total: 79 EG: 48 CG: 31 | Total: 55.7 + −7.3 EG: 55.4 + −8.4 CG: 55.9 + −6.5 |
| Gomez 2015 [21] | Spain | Total: 601 EG: 298 CG: 303 | EG: 2.39 (1.44) CG: it does not specify |
| Babio 2014 [1] | Spain | Total: 5801 EG1: 1982 EG2: 1885 CG: 1934 | EG1: 67.1 EG2: 66.7 CG: 67.3 |
| Esposito 2004 [2] | Italy | Total: 180 EG: 90 CG: 90 | EG: 44.3 CG: 43.5 |
| Sureda 2016 [23] | Spain | Total: 75 EG1 (Mediterranean + extra virgin olive oil): 25 | EG1: EG2: CG: 55 to 80 years. The inclusion criteria were subjects without history of CVD and either the presence of Type 2 Diabetes Mellitus (T2DM) or the presence of three or more CVD risk factors. |
| Fortin 2018 [18] | Canada | EG: 14 CG: 14 | EG: 52.1 CG: 49.8 |
| Author | Intervention | Outcomes | Follow-Up | Results | |
|---|---|---|---|---|---|
| Intervention | Characteristics/Dose | ||||
| Velázquez-López 2014 [19] | CG: Standard diet EG: Mediterranean diet | CG: Standard diet group was distributed with 55–60% of carbohydrates (45–50% complex and no more than 10% refined and processed sugars), 25–30% lipids, and 15% proteins. For 16 weeks. EG: Mediterranean diet: 60% carbohydrates (50% complex and no more than 10% refined and processed sugars), 25% lipids, and 15% proteins. | Weight (Kg) Height (m) BMI (kg/m2) Waist (cm) Hip (cm) Ratio MAC (cm) TSF (mm) Fat Mass (Kg) Lean Mass (Kg) SBP (mmHg) DBP (mmHg) Glucose (mg/dL) TC (mg/dL) TG (mg/dL) HDLC (mg/dL) LDLC (mg/dL) | 16 weeks | Weight (Kg) p = 0.369 Height (m) p = 0.001 BMI (kg/m2) p = 0.001 Waist (cm) p = 0.0826 Hip (cm) p = 0.303 Ratio p = 0.600 MAC (cm) p = 0.222 TSF (mm) p = 0.056 Fat Mass (Kg) p = 0.001 Lean Mass (Kg) p = 0.001 SBP (mmHg) p = 0.229 DBP (mmHg) p = 0.435 Glucose (mg/dL) p = 0.001 TC (mg/dL) p = 0.001 TG (mg/dL) p = 0.001 HDLC (mg/dL) p = 0.001 LDLC (mg/dL) p = 0.001 |
| Asoudeh 2023 [3] | CG: Diet supported by dietary advice based on the food pyramid EG: Prescribed Mediterranean diet | CG: Diet supported by dietary advice based on the food pyramid. Participants in the control group were only advised verbally to follow healthy eating tips recommended according to the usual Iranian diet EG: Mediterranean Diet. Energy requirements were calculated for each participant based on their weight. Diets were planned to include 53–58% of energy from carbohydrates, 15–18% from proteins and 26–30% from fats. Participants were encouraged to increase consumption of olive oil, fruits, vegetables, sweets and red meat. Refined grains were replaced with whole grains. Exchange lists were provided, and participants were instructed on their use | Weight (kg) BMI (kg/m2) WC (cm) SBP (mmHg) DBP (mmHg) FBS (mg/dL) TG (mg/dL) TC (mg/dL) HDL (mg/dL) LDL (mg/dL) Insulin (μIU/mL) HOMA-IR | 12 weeks | Weight (kg) p = <0.001 BMI (kg/m2) p = <0.001 WC (cm) p = <0.001 SBP (mmHg) p = <0.001 DBP (mmHg) p = 0.64 FBS (mg/dL) p = <0.001 TG (mg/dL) p = <0.001 TC (mg/dL) p = 0.05 HDL (mg/dL) p = <0.001 LDL (mg/dL) p = <0.001 Insulin (μIU/mL) p = 0.13 HOMA-IR p = 0.02 |
| Konieczna 2023 [20] | CG: Usual care, with advice to follow an ad libitum MedDiet, but no physical activity promotion. EG:energy-reduced Mediterranean diet (MedDiet) and increased physical activity (PA) | CG: Usual Care. They were given general advice to follow ad libitum the traditional MedDiet, without PA promotions, during group sessions twice a year. EG: Energy-reduced MedDiet. In addition to 30% energy reduction, the consumption of some foods (processed meats, butter, sweetened beverages, added sugar, biscuits, and bread and other refined cereals; whole grains were promoted) was limited. Participants were encouraged to progressively increase aerobic PA, aiming for 45 min of walking per day or more or the equivalent during 6 days per week, along with strength, flexibility, and balance exercises. Participants from the intervention group received support from dietitians 3 times a month (group session, individual session, and call) in the first year, along with behavioral and motivational support strategies including self-monitoring, goal setting, and problem solving. | Total fat mass (%) Total lean mass (%) Visceral fat mass (g) Total fat mass (g) Total lean mass (g) Visceral fat mass (%) Android to gynoid fat mass. Total lean mass to total fat. | 3 years | Total fat mass (%) p = <0.001 Total lean mass (%) p = <0.001 Visceral fat mass (g) p = <0.001 Total fat mass (g) p = <0.001 Total lean mass (g) p = <0.001 Visceral fat mass (%) p = 0.37 Android to gynoid fat mass. p = 0.1 Total lean mass to total fat. p = <0.001 |
| Bajerska 2018 [17] | EG: Mediterranean diet EC: Central European Diet | EG: The diet consists of: Total Fat: Approximately 37% of total energy. Monounsaturated Fatty Acids (MUFAs): 20% of total energy. Polyunsaturated Fatty Acids (PUFAs): 9% of total energy. Saturated Fatty Acids (SFAs): 8% of total energy. Protein: 18% of total energy. Carbohydrates: 45% of total energy. Dietary Fiber (DF): The ratio of soluble to insoluble fiber is 20 to 80%. Characteristic Foods: Includes typical Mediterranean products, such as olive oil (used at every meal) and nuts (five to seven per day). EC: The diet consists of: Total Fat: Approximately 27% of total energy. Monounsaturated Fatty Acids (MUFAs): 10% of total energy. Polyunsaturated Fatty Acids (PUFAs): 9% of total energy. Saturated Fatty Acids (SFAs): 8% of total energy. Protein: 18% of total energy. Carbohydrates: 55% of total energy. Dietary Fiber (DF): The ratio of soluble to insoluble fiber is 35 to 65%. Characteristic Foods: Based on typical Central European foods, such as cereals (oats and barley), legumes (peas and beans), vegetables (roots and cruciferous), and fruits (apples and plums). | Δ Body weight (kg) Δ WC (cm) Δ FM (kg) Δ FFM (kg) Δ VF (kg) Δ GLU (mg/dL) Δ INS (μU/mL) Δ HOMA2-IR Δ T-C (mg/dL) Δ LDL-C (mg/dL) Δ HDL-C (mg/dL) Δ TG (mg/dL) Δ Hcy (μM) Δ SBP (mmHg) Δ DBP (mmHg) | 16 weeks | Δ Body weight (kg) p = <0.001 Δ WC (cm) p = 0.014 Δ FM (kg) p = 0.020 Δ FFM (kg) p = 0.002 Δ VF (kg) p = 0.285 Δ GLU (mg/dL) p = 0.432 Δ INS (μU/mL) p = 0.397 Δ HOMA2-IR p = 0.456 Δ T-C (mg/dL) p = 0.676 Δ LDL-C (mg/dL) p = 0.922 Δ HDL-C (mg/dL) p = 0.400 Δ TG (mg/dL) p = 0.110 Δ Hcy (μM) p = 0.367 Δ SBP (mmHg) p= 0.938 Δ DBP (mmHg) p = 0.519 |
| Mayneris 2014 [4] | CG: Low-Fat Diet. EG1 (MD + VOO): Mediterranean Diet supplemented with Virgin Olive Oil. EG2 (MD + nuts): Mediterranean Diet supplemented with Nuts. | CG: They were advised to reduce all types of fats and increase the consumption of healthy foods such as vegetables and fruits. EG1 (MD + VOO): They were provided with a 1-L bottle of extra virgin olive oil every week. They were given recommendations to increase the consumption of vegetables, fruits, legumes, fish, seafood, and white meats. They were advised to limit the intake of red and processed meats, high-fat dairy products, sweets, and sugary drinks. EG2 (MD + nuts): They were provided with 30 g of mixed nuts daily (15 g of walnuts, 7.5 g of hazelnuts, and 7.5 g of almonds). They received the same dietary recommendations as the MD + VOO group. | Weight (kg) BMI (kg/m2) Overweight or obese (%) C Hypertension (%) D Dyslipidemia (%) E Type 2 diabetes mellitus (%) Family history of premature CHD (%) F Current smoker (%) * MetS components (%) G Elevated WC Elevated TG Reduced HDL-C Elevated BP Elevated fasting glucose * Medications (%) Aspirin or antiplatelet drugs Antihypertensive agents Hypolipidemic agents Insulin Hypoglycemic agents * Occupation (%) Worker Unemployed or unfit Retired * Education level (%) None Primary school Secondary school University | 52 weeks | peso p = <0.001 BMI (kg/m2) p = <0.001 Overweight or obese (%) C p = <0.001 Hypertension (%)D p = 0.854 Dyslipidemia (%)E p = <0.001 Type 2 diabetes mellitus (%) p = 0.001 Family history of premature CHD (%)F p = 0.805 Current smoker (%) p = 0.118 Elevated WC p = <0.001 Elevated TG p = <0.001 Reduced HDL-C p = <0.001 Elevated BP p = <0.001 Elevated fasting glucose p = <0.001 Aspirin or antiplatelet drugs p = 0.593 Antihypertensive agents p = 0.110 Hipolypidemic agents p = 0.001 Insulin p = 0.783 Hypoglycemic agents p = 0.001 Worker p = 0.078 Unemployed or unfit p = 0.141 Retired p = 0.745 None p = 0.237 Primary school p = 0.116 Secondary school p = 0.012 University p = 0.995 |
| Fernández 2020 [8] | EG1 (er-MedDiet + PA): Mediterranean diet with caloric restriction combined with the promotion of physical activity and behavioral support. EG2 (MedDiet): Mediterranean diet without caloric restriction and received traditional healthcare. | EG1 (er-MedDiet + PA): The Energy-Restricted Mediterranean Diet combined the principles of the Mediterranean diet with caloric restriction to promote weight loss. It included high consumption of fruits, vegetables, legumes, whole grains, fish, and extra virgin olive oil, with reduced consumption of red/processed meats, added sugars, and high-fat dairy. Participants engaged in increased physical activity levels, focusing on aerobic and resistance exercises. Personalized counseling, behavioral support and group sessions were offered to improve adherence to the diet and exercise program. EG2 (MedDiet): The Mediterranean Diet without Caloric Restriction followed the traditional Mediterranean diet principles, focusing on a high intake of fruits, vegetables, legumes, whole grains, fish, and virgin olive oil, while limiting red/processed meats, added sugars, and high-fat dairy. Participants received standard healthcare with general recommendations but no specific focus on caloric restriction, structured physical activity, or behavioral support. | BMI, kg/m2 Serum glucose, mg/dL Serum HbA1c, % Serum HDL cholesterol, mg/dL Serum triglycerides, mg/dL | 26 weeks | BMI, kg/m2 p = <0.001 Serum glucose, mg/dL p = 0.708 Serum HbA1c, % p = 0.444 Serum HDL cholesterol, mg/dL p = 0.205 Serum triglycerides, mg/dL p = 0.635 |
| Garcia 2018 [22] | CG: Single workshop providing basic information about MetS and associated cardiovascular risks. EG: CBT | EG: Cognitive Behavioral Therapy (CBT) was delivered in 12 weekly 90-min sessions (10–12 participants) led by a psychologist. The program addressed factors related to Metabolic Syndrome (MetS) and CBT, negative beliefs about diet and exercise, problem solving for lifestyle changes, strategies to manage impulsivity, stress and anger, assertiveness training, confidence building and strategies for long-term adherence to healthy habits with support from family and professionals. CG: A 90-min workshop was conducted once for each of the four subgroups, each consisting of approximately 10 to 15 participants. It provided fundamental information on MetS and its associated cardiovascular risks, presenting standard therapeutic strategies in accordance with the Nutrition, Physical Activity, and Obesity Prevention Strategy of the Spanish Agency for Food Safety and Nutrition. | IMC (kg/m2) PA (mmHg) HDLc (mg/dL) MedDiet MEDAS-14 MMSE | 12 weeks | Waist circumference p= 0.001 Triglycerides p = 0.018 Mediterranean diet p = 0.000 |
| Gomez 2015 [21] | EG: Mediterranean Diet and physical activity CG: General recommendations on healthy diet and physical exercise | EG: The Mediterranean-style diet emphasized olive oil, vegetables, fruits, legumes, and fish with limited red or processed meats, dairy milk, sugary drinks, and sweets. Overweight or obese patients followed a 600 kcal/day deficit diet, aiming for a ≥5% weight loss, calculated using the Harris-Benedict equation. Daily exercise was encouraged, with a minimum of 150 min of walking per week. A total of 27 visits were conducted throughout the study, including 9 medical visits and 18 nursing visits. CG: General recommendations on heart-healthy diet and physical exercise. Participants received at least 4 medical consultations and 4 nursing visits per year. | Waist circumference (cm) Weight (kg) Normoweight (%) Overweight (%) Obesity (%) BMI (kg/m2) SBP (mmHg)DBP (mmHg) Low educational level (%) Sedentary lifestyle (%) Smoking (%) Glycemia (mg/dL) HbA1c (%) Creatinine (mg/dL) Uric acid (mg/dL) Total cholesterol (mg/dL) LDL cholesterol (mg/dL) HDL cholesterol (mg/dL) Triglycerides (mg/dL) Type 2 diabetes mellitus, n (%) Cardiovascular disease, n (%) | 3 years | Waist circumference (cm) p = 0.001 Weight (kg) p = ns BMI (kg/m2) p = ns SBP (mmHg) p = 0.004 DBP (mmHg) p = <0.001 Glycemia (mg/dL) p = ns HbA1c (%) p = ns Total cholesterol (mg/dL) p = ns LDL cholesterol (mg/dL) p = ns HDL cholesterol (mg/dL) p = 0.05 Triglycerides (mg/dL) p = ns Metabolic syndrome, n (%) p = ns Number of components of metabolic syndrome p = 0.02 Antihypertensive drugs, p = ns Lipid-lowering drugs, p = ns Antidiabetic drugs p = ns Type 2 Diabetes mellitus p = ns Cardiovascular disease p = ns |
| Babio 2014 [1] | EG1: Mediterranean diet and extra-virgin olive oil EG2: Mediterranean diet and nuts CG: low-fat diet | EG1: Mediterranean diet with an emphasis on the consumption of extra virgin olive oil, 1 L per week. Participants were given sessions to provide information on the Mediterranean diet, meal plans, and recipes. EG2: Mediterranean diet with an emphasis on the consumption of a mixed nut blend (30 g/day): 15 g of walnuts, 7.5 g of hazelnuts, and 7.5 g of almonds. Participants were given sessions to provide information on the Mediterranean diet, meal plans, and recipes. CG: Diet low in both animal and vegetable fats. Participants were given sessions to receive recommendations on reducing fats in their diet. | Former smoker (%) Current smoker (%) BMI (kg/m2) Waist circumference (cm) Leisure time physical activity (METs/min per d) Mediterranean diet score (0–14) T2DM (%) Metabolic syndrome (%) Central obesity (%) Hypertriglyceridemia (%) Low HDL cholesterol (%) Hypertension (%) High fasting plasma glucose (%) Insulin (%) Hypoglycemic agents (%) Fibrates (%) Statins (%) Aspirin (%) Antihypertensive agents (%) | 4.8 years | Former smoker p = 0.08 Current smoker p = 0.8 BMI (kg/m2) p = <0.001 Waist circumference (cm) p = 0.08 Leisure time physical activity (METs/min per d) p = <0.001 Mediterranean diet score (0–14) p = <0.001 T2DM p = 0.1 Metabolic syndrome p = 0.07 Central obesity p = <0.001 Hypertriglyceridemia p = 0.4 Low HDL cholesterol 0.8 Hypertension p = 0.3 High fasting plasma glucose p = 0.6 Insulin p = 0.1 Hypoglycemic agents p = 0.02 Fibrates p = 0.3 Statins p = 0.2 Aspirin p = 0.5 Antihypertensive agents p = 0.3 |
| Esposito 2004 [2] | EG: Mediterranean diet CG: macronutrient composition similar to mediterranean diet | EG: Carbohydrates 50–60%, proteins 15–20%, total fat less than 30%, saturated fat less than 10%, cholesterol consumption less than 300 mg per day. Patients were advised to consume at least 250 to 300 g of fruits, 125 to 150 g of vegetables and 25 to 50 g of walnuts per day. They were also encouraged to consume 400 g of whole grains (legumes, rice, maize and wheat) daily and to increase their consumption of olive oil. Patients had monthly sessions with the nutritionist for the first year and bi-monthly sessions for the second year. CG: Carbohydrates 50–60%, proteins 15–20%, total fat less than 30%. Patients had bi-monthly sessions with study personnel for 2 years. | No. of components of the metabolic syndrome (%) Body weight (kg) BMI (kg/m2) Waist circumference (cm) Plasma glucose (mg/dL) Serum insulin (μU/mL) HOMA score (%) Total cholesterol (mg/dL) HDL-C (mg/dL) Triglycerides (mg/dL) Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) Endothelial function score hs-CRP (mg/L) IL-6 (pg/mL) IL-7 (pg/mL) IL-18 (pg/mL) | 2 years | No. of components of the metabolic syndrome p = 0.34 Body weight p = 0.36 BMI (kg/m2) p = 0.55 Waist circumference (cm) p = 0.62 Plasma glucose (mg/dL) p = 0.43 Serum insulin (μU/mL) p = 0.15 HOMA score p = 0.18 Total cholesterol (mg/dL) p = 0.18 HDL-C (mg/dL) p = 0.35 Triglycerides (mg/dL) p = 0.24 Systolic blood pressure (mmHg) p = 0.11 Diastolic blood pressure (mmHg) p = 0.21 Endothelial function score p = 0.13 hs-CRP (mg/L) p = 0.25 IL-6 (pg/mL) p = 0.14 IL-7 (pg/mL) p = 0.12 IL-18 (pg/mL) p = 0.1 |
| Sureda 2016 [23] | EG1: MeDiet supplemented with extra virgin olive oil (MeDiet + EVOO), EG2: MeDiet with nuts (MeDiet + nuts) CG: Low-fat diet | EG: Participants in the MeDiet groups received free EVOO (15 L for 3 months) or mixed nuts (15 g walnuts, 7.5 g hazelnuts, 7.5 g almonds daily for 3 months) with excess provided for family needs. A 1-h group session (up to 20 participants) for each MeDiet group with a dietician was held to improve compliance, focusing on increasing the MeDiet 14-item score. Recommendations included using olive oil for cooking, increasing intake of vegetables, nuts, fish and white meat, preparing home-made sauces to dress vegetables, pasta, rice and other dishes, and moderate red wine consumption for drinkers. No energy restrictions or physical activity interventions were implemented. CG: Participants allocated to a low-fat diet were advised to reduce all types of fat and were given written recommendations according to the American Heart Association guidelines. | Superoxide dismutase activity (pkat/L) Catalase activity (k/L) Myeloperoxidase activity (μkat/L) Xanthine oxidase activity (U/L) Superoxide dismutase protein level (%) Catalase protein level (%) Myeloperoxidase protein level (%) Xanthine oxidase protein level (%) (Datos de arriba en el plasma) Superoxide dismutase activity (pkat/mL) Catalase Activity (k/mL) (Red blood cells) | 5 years | Superoxide dismutase activity (pkat/L) p < 0.003 Catalase activity (k/L) p < 0.004 Myeloperoxidase activity (μkat/L) p = 0.690 Xanthine oxidase activity (U/L) p = 0.008 Superoxide dismutase protein level (%) p = 0.033 Catalase protein level (%) p = 0.823 Myeloperoxidase protein level (%)p = 0.241 Xanthine oxidase protein level (%) p = 0.296 (Datos de arriba en el plasma) Superoxide dismutase activity (pkat/mL) p = 0.233 Catalase Activity (k/mL) p = 0.225 (Red blood cells) |
| Fortin 2018 [18] | EG: Mediterranean diet CG: Low-fat diet | EG: included using olive oil as the main source of fat, multiple servings of fish per week and a limited intake of red and processed meat. CG: Low-fat diet included lean cuts of meat and limited fried and cholesterol-rich foods. Each group received dietary teaching sessions, once a week for the first month, bi-weekly for the second month, and then monthly. | Body weight (kg) Waist circumference (cm) BMI (kg/m2) Body fat (%) HbA1c (%) Estimated glucose disposal rate (eGDR) Hypoglycemia episode (n per day) Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) Total cholesterol (mmol/L) HDL-cholesterol (mmol/L) LDL-cholesterol (mmol/L) Triglycerides (mmol/L) Apolipoprotein-B (g/L) C-reactive protein (mg/L) | 6 meses | Body weight (kg) p = 0.96 Waist circumference (cm) p = 0.93 BMI (kg/m2) p = 0.24 Body fat p = 0.27 HbA1c p = 0.7 Estimated glucose disposal rate (eGDR) p = 0.19 Hypoglycemia episode (n per day) p = 85 Systolic blood pressure (mmHg) p = 0.21 Diastolic blood pressure (mmHg) p = 0.76 Total cholesterol (mmol/L) p = 0.61 HDL-cholesterol (mmol/L) p = 0.1 LDL-cholesterol (mmol/L) p = 0.89 Triglycerides (mmol/L) p = 0.61 Apolipoprotein-B (g/L) p = 0.1 C-reactive protein (mg/L) p = 0.84 |
| Parameter | Normal Reference Values | Reference Values in Diabetics | Values Reported by the Articles (Average) | Reference Values for Metabolic Syndrome (Expected to Increase) | Additional Considerations |
|---|---|---|---|---|---|
| Body weight | It depends on weight and height | It depends on weight and height | 77 | It depends on weight and height | No present |
| BMI (IMC) | 18.5–24.9 kg/m2 | 18.5–24.9 kg/m2 | 28.4 kg/m2 | >30 kg/m2 | No present |
| Waist circumference | <88 cm (women) <102 cm (males) | <88 cm (women) <102 cm (males) | 97 cm | >90 cm (women) >102 cm (males) | No present |
| SBP | <120 mmHg | 130–139 mmHg | 122 mmHg | >130 mmHg | No present |
| DBP | <80 mmHg | 80–85 mmHg | 76 mmHg | >85 mmH | No present |
| Triglycerides | <150 mg/dL | <135 mg/dL | 162 mg/dL | >150 mg/dL | The stated goals apply to diabetic patients without coronary heart disease. |
| Glucose | <100 mg/dL | <140 mg/dL | 110 mg/dL | >110 mg/dL | Goals vary depending on the person’s condition, age, life expectancy, and presence of other comorbidities. |
| Total cholesterol | <200 mg/dL | >240 mg/dL | 186.53 mg/dL | >240 mg/dL | The stated goals apply to diabetic patients without coronary heart disease. |
| HDL | >50 mg/dL (women) >40 mg/dL (men) | <50 mg/dL (women) <40 mg/dL (men) | 48.45 mg/dL | <50 mg/ dL (women) <40 mg/dL (men) | The stated goals apply to diabetic patients without coronary heart disease. |
| LDL | <100 | >130 mg/dL | 107.43 mg/dL | >130 mg/dL | The goal varies depending on whether the person has a history of cardiovascular disease (CVD). |
| Body fat | 10–20% (men) 20–30% (women) | There are no official reference values; these vary depending on the person and their health status. | 34.03 kg | >25% (men) >35% (women) | Reference values should be interpreted in the context of overall health, physical activity level, and other indicators such as body mass index (BMI) and age. |
| HOMA-IR | <2.5 | >2.5 | 2.98 | >2.5 | It may vary depending on each case |
| Insulin | 2–25 µU/ mL | Insulin levels may be elevated, especially if you are in a phase of insulin resistance | 0.0894 mg/dL | >25 µU/mL | Glucose reference values vary depending on factors such as physiological status (pregnancy or not), time elapsed since last food intake, and individual patient characteristics. |
| Outcome | Moderator | B | 95% LLCI | 95% ULCI | p-Value |
|---|---|---|---|---|---|
| Body weight | Age | −0.04 | −0.17 | 0.09 | 0.55077 |
| Body weight | Calories | 0.00 | 0.00 | 0.00 | 0.50502 |
| Body weight | Diet weeks | −2.05 | −3.67 | −0.43 | 0.01316 |
| Body weight | Frequency per week | −0.08 | −0.62 | 0.47 | 0.78602 |
| BMI | Age | 0.01 | 0.00 | 0.01 | 0.07544 |
| BMI | Calories | 0.00 | 0.00 | 0.00 | 0.09745 |
| BMI | Diet weeks | 0.02 | 0.00 | 0.05 | 0.10371 |
| BMI | Frequency per week | 0.14 | −0.15 | 0.42 | 0.34744 |
| Cholesterol | Age | 0.00 | −0.66 | 0.67 | 0.98971 |
| Cholesterol | Calories | 0.00 | −0.02 | 0.01 | 0.73085 |
| Cholesterol | Diet weeks | −0.74 | −2.85 | 1.38 | 0.49607 |
| Cholesterol | Frequency per week | 6.34 | −4.06 | 16.75 | 0.23222 |
| DBP | Age | −0.03 | −0.12 | 0.05 | 0.44586 |
| DBP | Calories | 0.00 | 0.00 | 0.00 | 0.03633 |
| DBP | Diet weeks | −0.15 | −0.39 | 0.10 | 0.24880 |
| DBP | Frequency per week | −0.75 | −3.01 | 1.51 | 0.51456 |
| Glucose | Age | 0.03 | −0.23 | 0.29 | 0.83103 |
| Glucose | Calories | 0.00 | 0.00 | 0.00 | 0.00179 |
| Glucose | Diet weeks | −0.37 | −1.24 | 0.50 | 0.40457 |
| Glucose | Frequency per week | 0.94 | −3.66 | 5.55 | 0.68765 |
| HOMA−IR | Age | 0.00 | −0.05 | 0.05 | 0.96735 |
| HOMA−IR | Calories | 0.00 | 0.00 | 0.00 | 0.00001 |
| HOMA−IR | Diet weeks | −0.05 | −0.19 | 0.10 | 0.51892 |
| Insulin | Age | −0.03 | −0.13 | 0.08 | 0.62624 |
| Insulin | Calories | 0.00 | 0.00 | 0.00 | 0.28858 |
| Insulin | Diet weeks | 0.01 | −0.38 | 0.40 | 0.95117 |
| LDL | Age | 0.42 | 0.08 | 0.76 | 0.01563 |
| LDL | Calories | −0.01 | −0.03 | 0.02 | 0.60745 |
| LDL | Diet weeks | 0.94 | −1.63 | 3.51 | 0.47353 |
| LDL | Frequency per week | 2.64 | −10.50 | 15.78 | 0.69337 |
| HDL | Age | −0.08 | −0.16 | 0.01 | 0.08473 |
| HDL | Calories | 0.00 | 0.00 | 0.00 | 0.92310 |
| HDL | Diet weeks | −0.20 | −0.50 | 0.10 | 0.18456 |
| HDL | Frequency per week | 1.39 | 0.36 | 2.41 | 0.00834 |
| Triglycerides | Age | 0.70 | −0.33 | 1.72 | 0.18370 |
| Triglycerides | Calories | −0.01 | −0.04 | 0.02 | 0.57344 |
| Triglycerides | Diet weeks | 0.80 | −4.68 | 6.28 | 0.77523 |
| Triglycerides | Frequency per week | 20.52 | −8.20 | 49.24 | 0.16132 |
| SBP | Age | −0.01 | −0.20 | 0.18 | 0.92662 |
| SBP | Calories | 0.00 | 0.00 | 0.00 | 0.39526 |
| SBP | Diet weeks | 0.01 | −0.75 | 0.77 | 0.97900 |
| SBP | Frequency per week | −2.11 | −5.13 | 0.91 | 0.17148 |
| Waist circumference | Age | 0.03 | −0.04 | 0.11 | 0.36890 |
| Waist circumference | Calories | 0.00 | 0.00 | 0.00 | 0.30175 |
| Waist circumference | Diet weeks | 0.01 | −0.30 | 0.32 | 0.94285 |
| Waist circumference | Frequency per week | 0.47 | −1.43 | 2.36 | 0.62964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruna-Mejias, A.; San Martin, J.; Arciniegas-Diaz, D.; Meneses-Caroca, T.; Salamanca-Cerda, A.; Beas-Gambi, A.; Paola-Loaiza-Giraldo, J.; Ortiz-Ahumada, C.; Nova-Baeza, P.; Oyanedel-Amaro, G.; et al. Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 5887. https://doi.org/10.3390/ijms26125887
Bruna-Mejias A, San Martin J, Arciniegas-Diaz D, Meneses-Caroca T, Salamanca-Cerda A, Beas-Gambi A, Paola-Loaiza-Giraldo J, Ortiz-Ahumada C, Nova-Baeza P, Oyanedel-Amaro G, et al. Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(12):5887. https://doi.org/10.3390/ijms26125887
Chicago/Turabian StyleBruna-Mejias, Alejandro, Jessica San Martin, Danna Arciniegas-Diaz, Trinidad Meneses-Caroca, Amelia Salamanca-Cerda, Antonia Beas-Gambi, Jessica Paola-Loaiza-Giraldo, Cynthia Ortiz-Ahumada, Pablo Nova-Baeza, Gustavo Oyanedel-Amaro, and et al. 2025. "Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 12: 5887. https://doi.org/10.3390/ijms26125887
APA StyleBruna-Mejias, A., San Martin, J., Arciniegas-Diaz, D., Meneses-Caroca, T., Salamanca-Cerda, A., Beas-Gambi, A., Paola-Loaiza-Giraldo, J., Ortiz-Ahumada, C., Nova-Baeza, P., Oyanedel-Amaro, G., Orellana-Donoso, M., Suazo-Santibáñez, A., Sanchis-Gimeno, J., & Valenzuela-Fuenzalida, J. J. (2025). Comparison of the Mediterranean Diet and Other Therapeutic Strategies in Metabolic Syndrome: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(12), 5887. https://doi.org/10.3390/ijms26125887