Strontium-Substituted Calcium Orthophosphates: Structure, Stability, Morphology, and Biomedical Applications
Abstract
1. Introduction
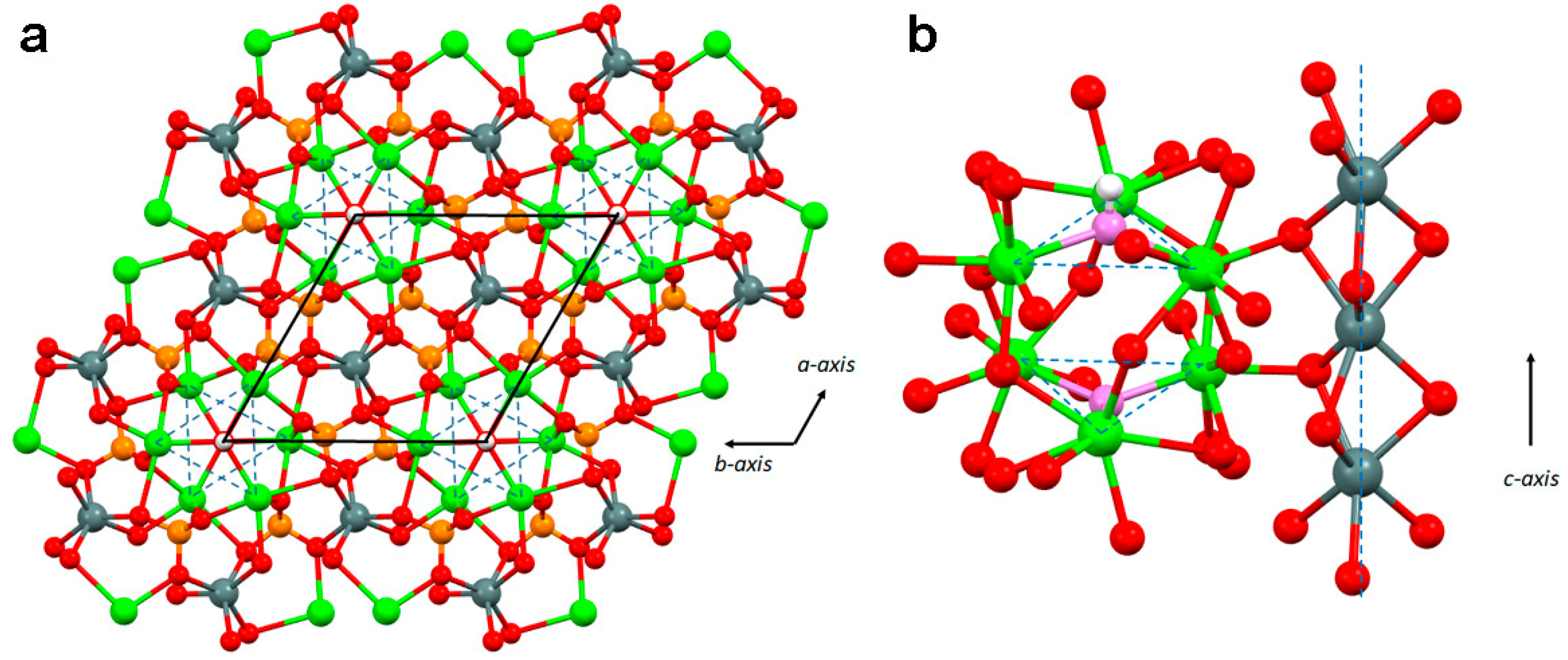
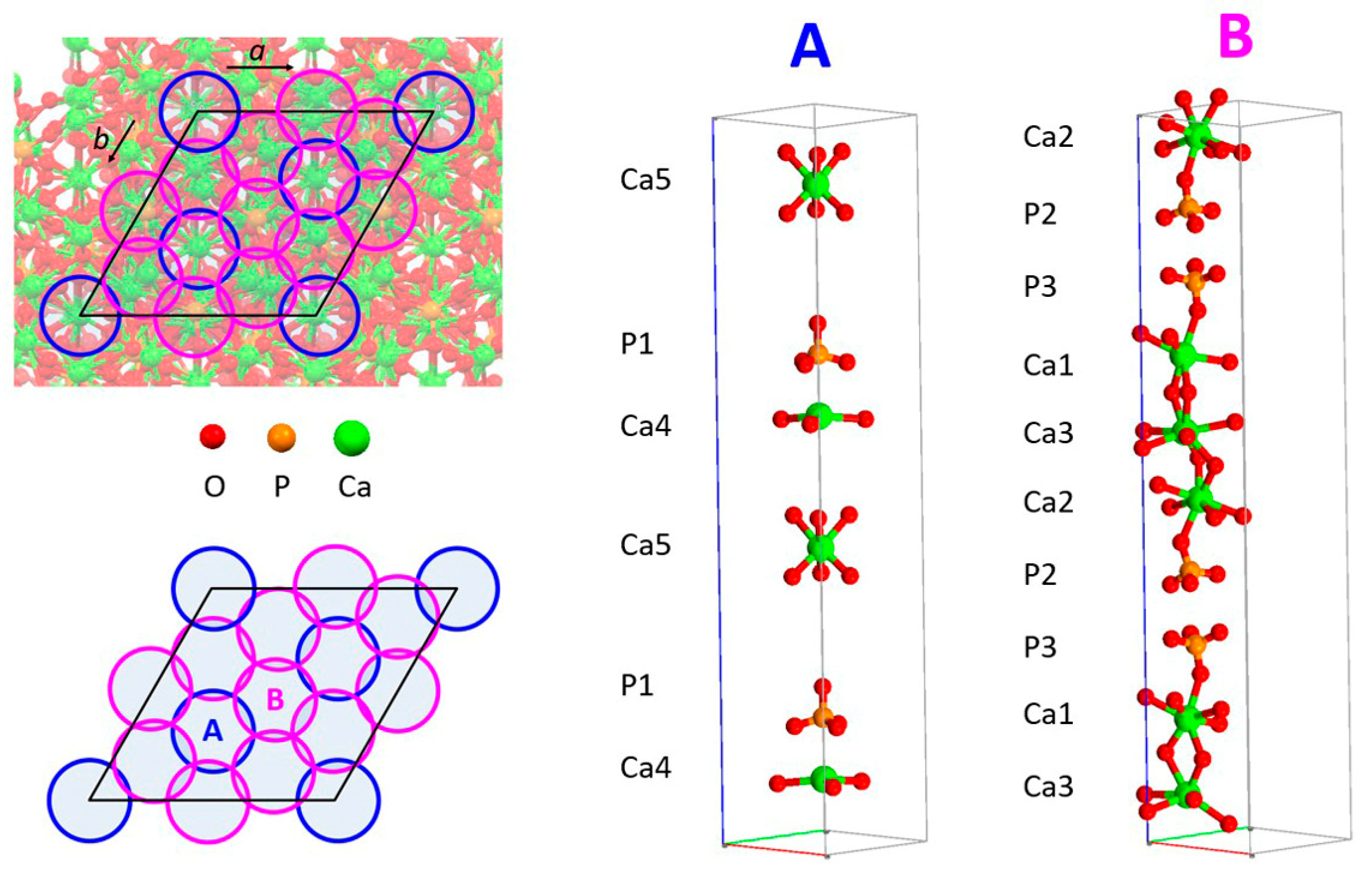
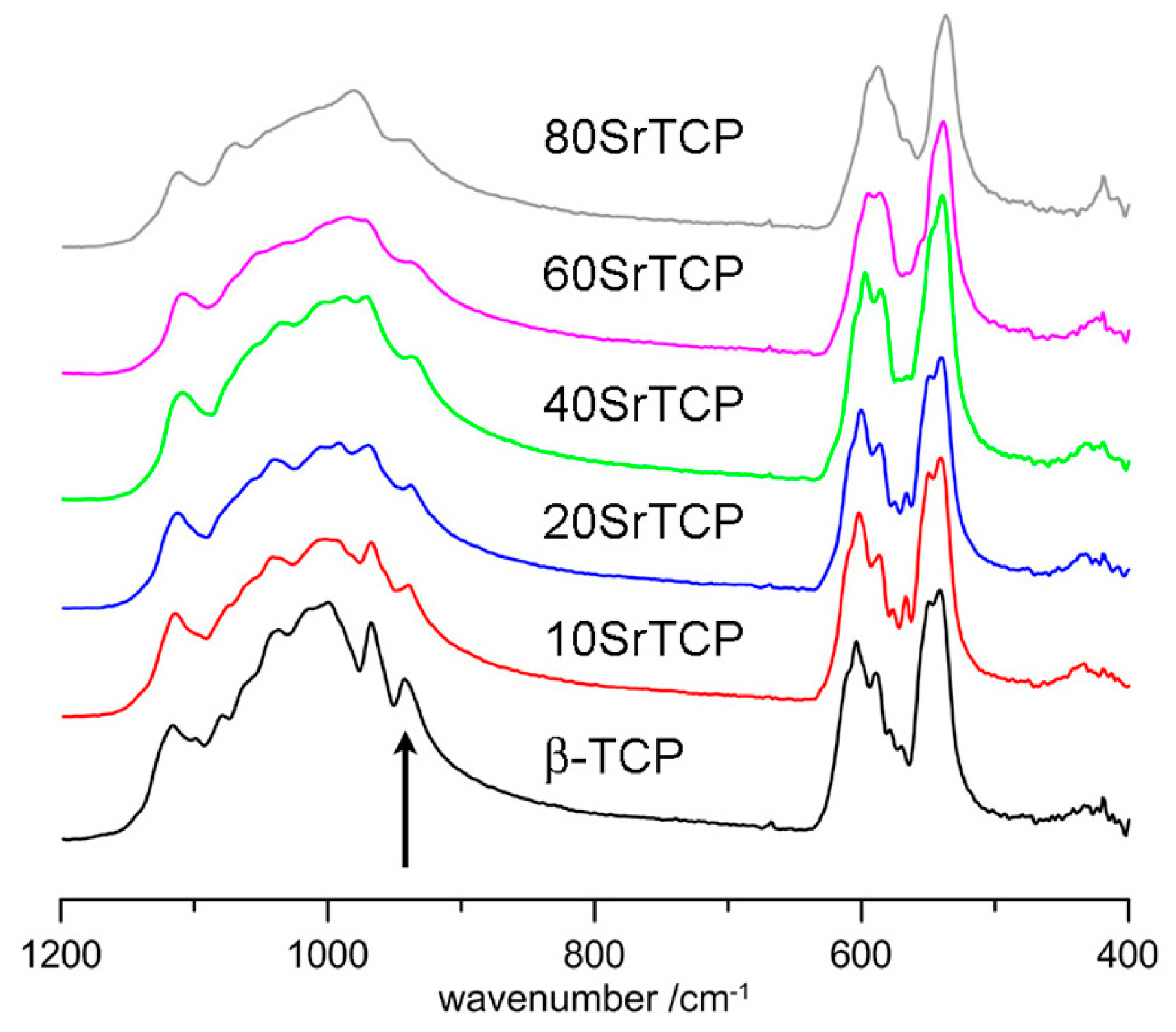
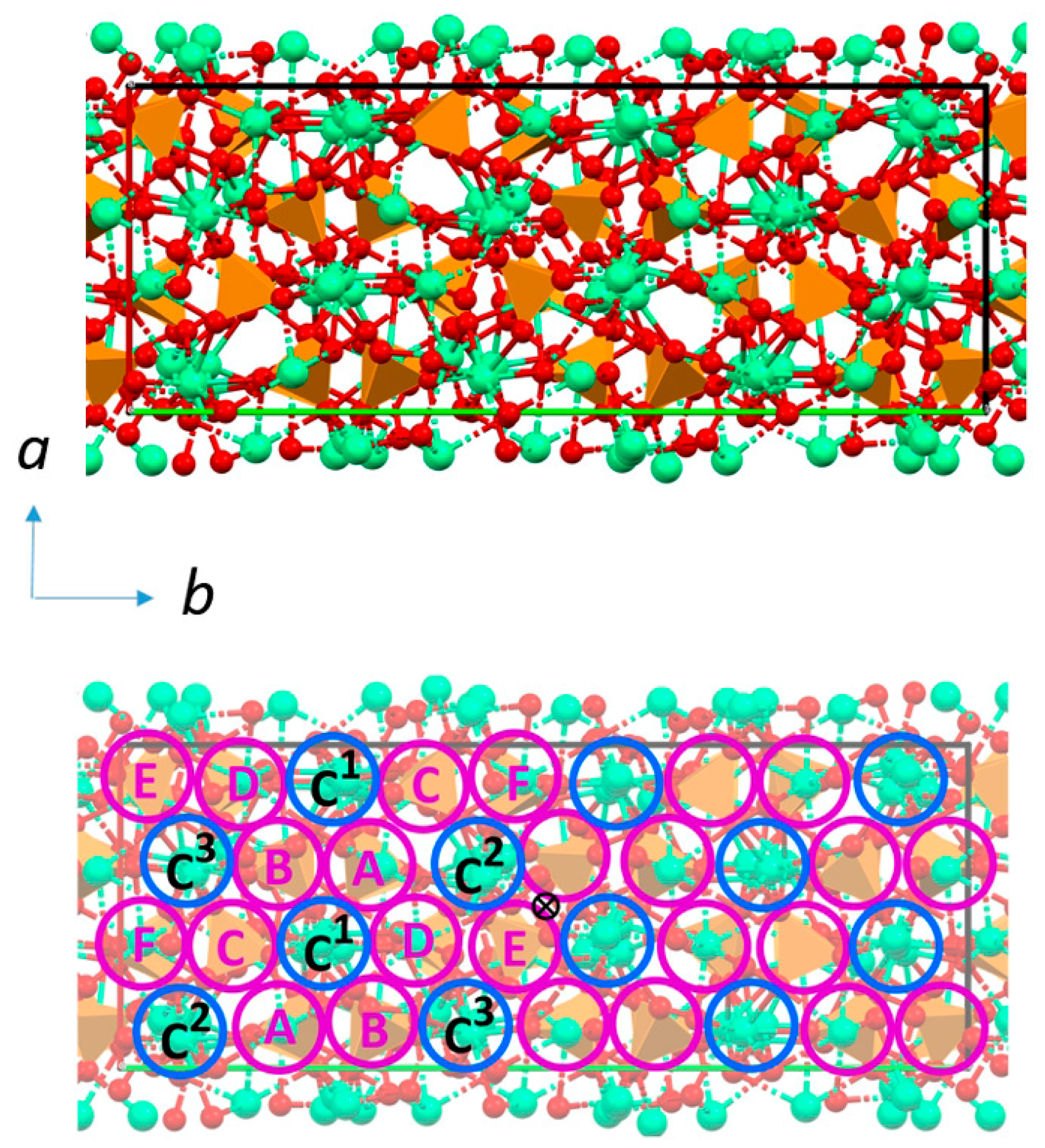
2. Structure and Stability
3. Morphology
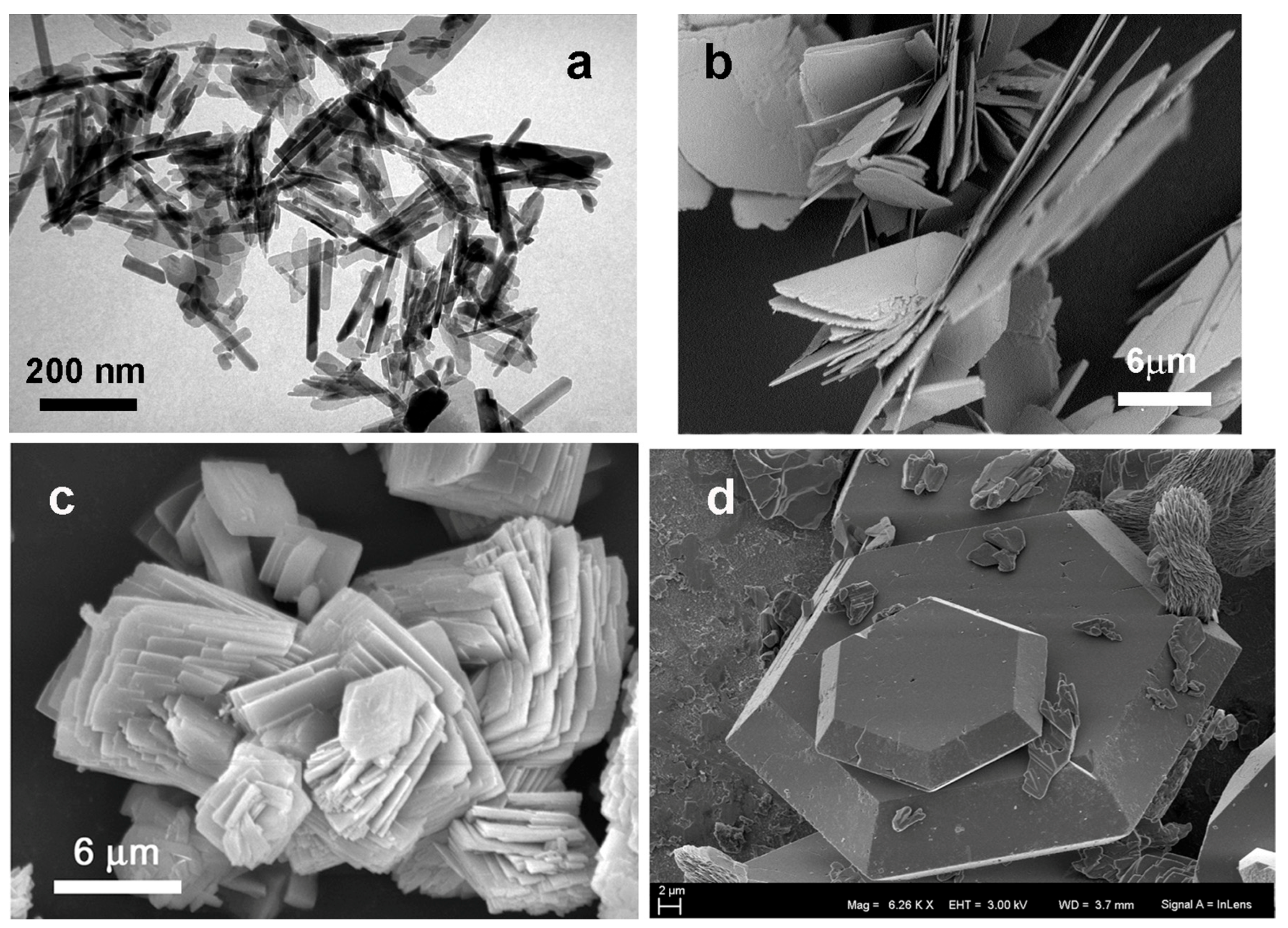
4. Applications for Biomaterials
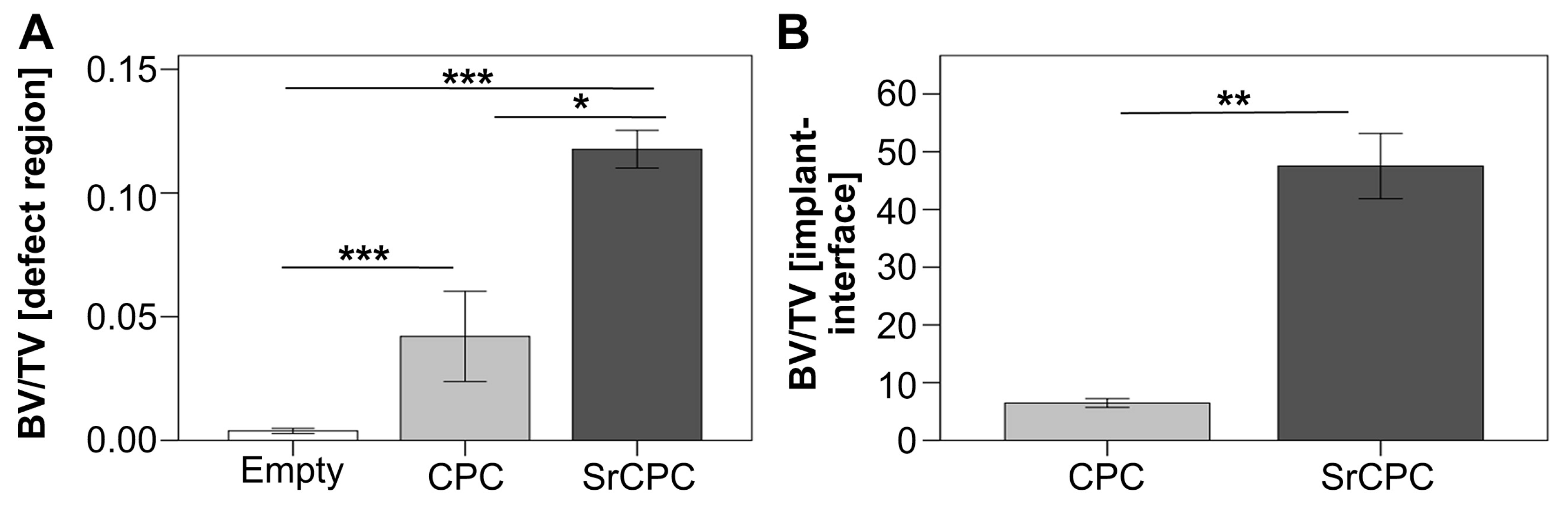
4.1. Cements
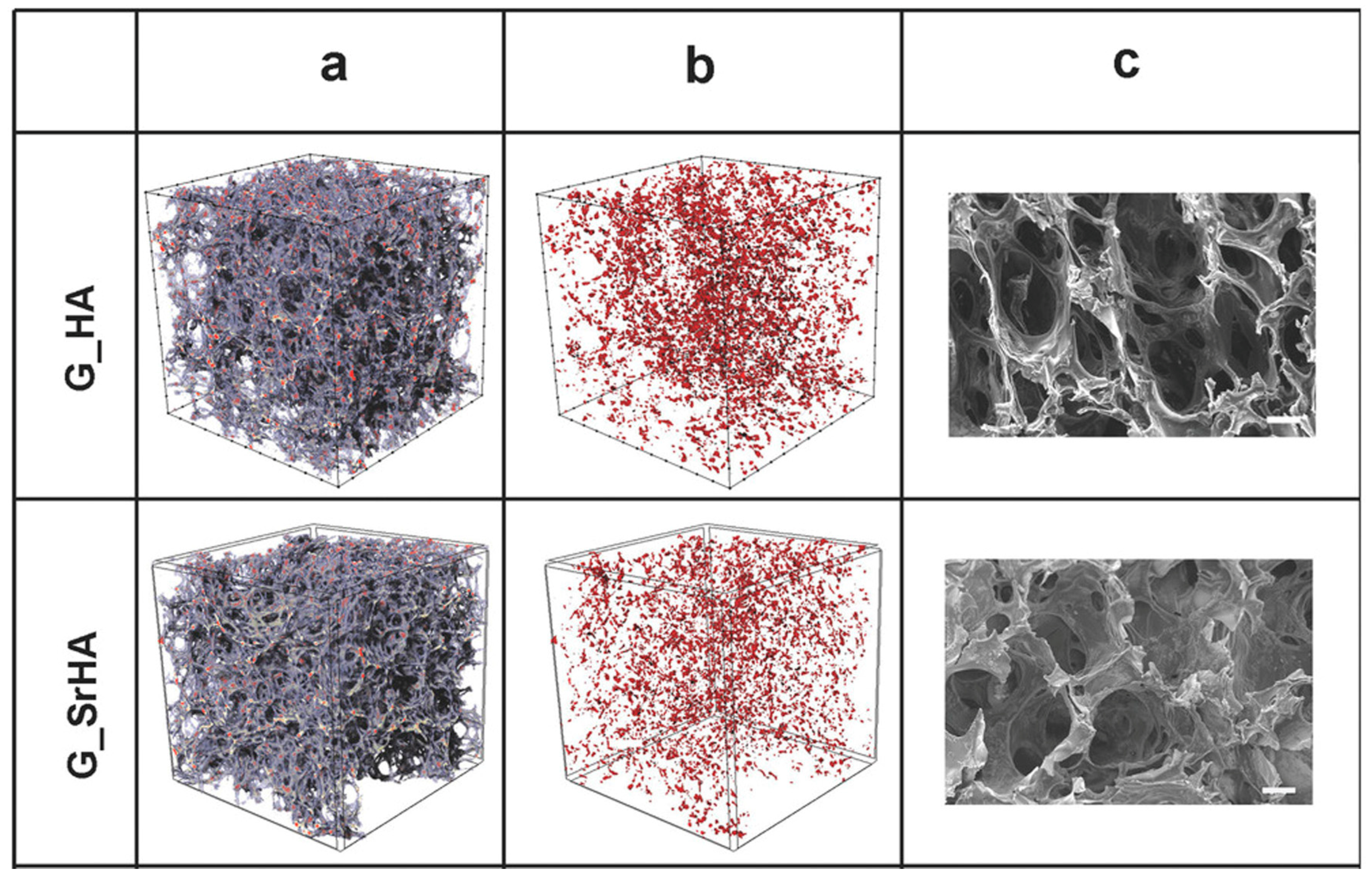
4.2. Scaffolds
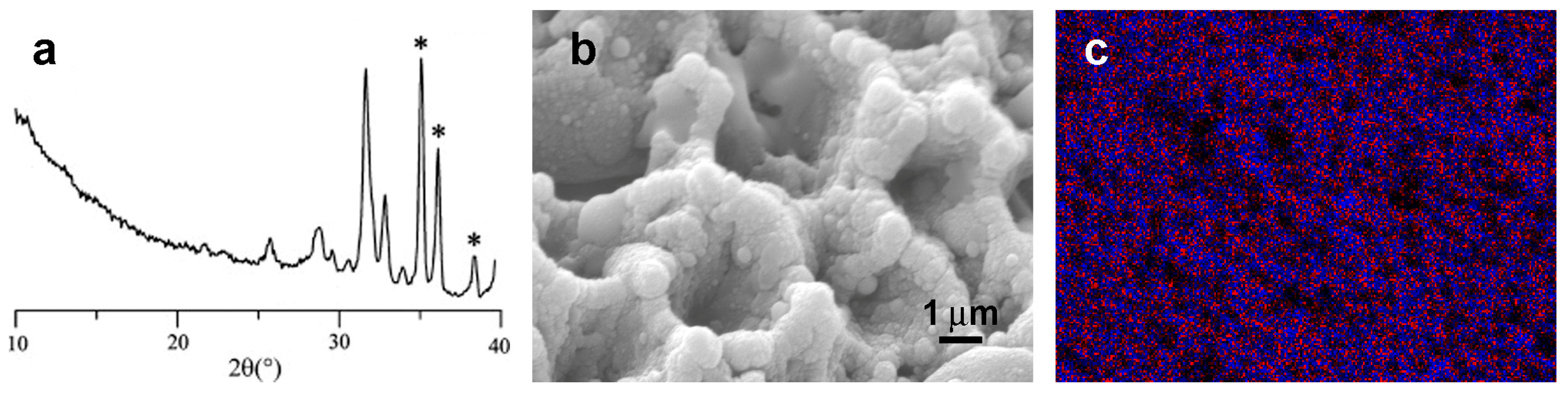
4.3. Coatings
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Musgrove, M.L. The occurrence and distribution of strontium in U.S. roundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Boivin, G.; Deloffre, P.; Perrat, B.; Panczer, G.; Boudeulle, M.; Mauras, Y.; Allain, P.; Tsouderos, Y.; Meunier, P.J. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J. Bone Miner. Res. 2009, 11, 1302–1311. [Google Scholar] [CrossRef]
- Dahl, S.G.; Allain, P.; Marie, P.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Bussola Tovani, C.; Divoux, T.; Manneville, S.; Azaïs, T.; Laurent, G.; de Frutos, M.; Gloter, A.; Ciancaglini, P.; Ramos, A.P.; Nassif, N. Strontium-driven physiological to pathological transition of bone-like architecture: A dose-dependent investigation. Acta Biomater. 2023, 169, 579–588. [Google Scholar] [CrossRef]
- Uskokovic, V.; Jankovic-Castvan, I.; Wu, V.M. Bone mineral crystallinity governs the orchestration of ossification and resorption during bone remodeling. ACS Biomater. Sci. Eng. 2019, 5, 3483–3498. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, A.; Jiang, U.; Liaqat, K.; Lin, K.; Sun, W.; Yuan, C. Promoting vascularized bone regeneration via strontium-incorporated hydroxyapatite bioceramic. Mater. Des. 2023, 234, 112313. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef]
- Marie, P.J.; Hott, M.; Modrowski, D.; De Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res. 1993, 8, 607–615. [Google Scholar] [CrossRef]
- Chou, J.; Valenzuela, S.M.; Santos, J.; Bishop, D.; Milthorpe, B.; Green, D.W.; Otsuka, M.; Ben-Nissan, B. Strontium- and magnesium-enriched biomimetic β-TCP macrospheres with potential for bone tissue morphogenesis. J. Tissue Eng. Regen. Med. 2014, 8, 771–778. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Borciani, G.; Ciapetti, C.; Vitale-Brovarone, N.; Baldini, N. Strontium functionalization of biomaterials for bone tissue engineering purposes: A biological point of view. Materials 2022, 15, 1724. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Christensen, T.E.; Berglund Davidsen, M.; Van Malderen, S.; Garrevoet, J.; Offermanns, V.; Andersen, O.Z.; Foss, M.; Birkedal, H. Local release of strontium from sputter-deposited coatings at implants increases the strontium-to-calcium ratio in peri-implant bone. ACS Biomater. Sci. Eng. 2022, 8, 620–625. [Google Scholar] [CrossRef]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced applications of strontium-containing biomaterials in bone tissue engineering. Materials Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Gazzano, M. Ion substitution in biological and synthetic apatites. In Biomineralization and Biomaterials, Foundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing (Imprint Elsevier): Cambridge, UK, 2015; pp. 235–266. ISBN 9781782423386. [Google Scholar] [CrossRef]
- Shah, F.A. Revisiting the physical and chemical nature of the mineral component of bone. Acta Biomater. 2025, 196, 1–16. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- LeGeros, Z.R.; Ito, A.; Ishikawa, K.; Sakae, T.; LeGeros, J.P. Fundamentals of hydroxyapatite and related calcium phosphates. In Advanced Biomaterials: Fundamentals, Processing, and Applications; Basu, B., Katti, D.S., Kumar, A., Eds.; Wiley: Newark, NJ, USA, 2009; pp. 19–52. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalized biomimetic calcium phosphates for bone tissue repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Reactivity of alpha-tricalcium phosphate. J. Mater. Sci. 2002, 37, 963–969. [Google Scholar] [CrossRef]
- Boanini, E.; Panzavolta, S.; Rubini, K.; Gandolfi, M.; Bigi, A. Effect of strontium and gelatin on the reactivity of α-tricalcium phosphate. Acta Biomater. 2010, 6, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Putnis, C.V. Underlying role of brushite in pathological mineralization of hydroxyapatite. J. Phys. Chem. B 2019, 123, 2874–2881. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalization of octacalcium phosphate for bone replacement. In Octacalcium Phosphate Biomaterials Understanding of Bioactive Properties and Application; Suzuki, O., Insley, G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 37–54. ISBN 978-0-08-102511-6. [Google Scholar]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Tung, M.S.; Tomazic, B.; Brown, W.E. The effects of magnesium and fluoride on the hydrolysis of octacalcium phosphate. Arch. Oral Biol. 1992, 37, 585–591. [Google Scholar] [CrossRef]
- Chow, L.C.; Markovic, M.; Frukhtbeyn, S.A.; Takagi, S. Hydrolysis of tetracalcium phosphate under a near-constant-composition condition—Effects of pH and particle size. Biomaterials 2005, 26, 393–401. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Capuccini, C.; Gazzano, M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta 2007, 360, 1009–1016. [Google Scholar] [CrossRef]
- Zhu, K.; Yanagisawa, K.; Shimanouchi, R.; Onda, A.; Kajiyoshi, K. Preferential occupancy of metal ions in the hydroxyapatite solid solutions synthesized by hydrothermal method. J. Eur. Ceram. Soc. 2006, 26, 509–513. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Fredholm, Y.; de Rouffignac, A.; Hill, R.G. Structural analysis of a series of strontium-substituted apatites. Acta Biomater. 2008, 4, 1455–1464. [Google Scholar] [CrossRef]
- Zeglinski, J.; Nolan, M.; Bredol, M.; Schattec, A.; Tofail, S.A.M. Unravelling the specific site preference in doping of calcium hydroxyapatite with strontium from ab initio investigations and Rietveld analyses. Phys. Chem. Chem. Phys. 2012, 14, 3435–3443. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yamazaki, A.; Otsuka, R.; Akao, M.; Aoki, H. Crystal structure of Sr-substituted hydroxyapatite synthesized by hydrothermal method. J. Solid State Chem. 1994, 113, 373–378. [Google Scholar] [CrossRef]
- Baldassarre, F.; Altomare, A.; Mesto, E.; Lacalamita, M.; Dida, B.; Mele, A.; Bauer, E.M.; Puzone, M.; Tempesta, E.; Capelli, D.; et al. Structural characterization of low-Sr-doped hydroxyapatite obtained by solid-state synthesis. Crystals 2023, 13, 117. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H. Strontium substitution in bioactive calcium phosphates: A first-principles study. J. Phys. Chem. B 2009, 113, 3584–3589. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Tian, Y.; Zhu, Y. Anisotropic expansion effect of Sr doping on the crystal structure of hydroxyapatite. CrystEngComm 2022, 24, 1546–1555. [Google Scholar] [CrossRef]
- Pan, H.B.; Li, Z.Y.; Lam, W.M.; Wong, J.C.; Darvell, B.W.; Luk, K.D.; Lu, W.W. Solubility of strontium-substituted apatite by solid titration. Acta Biomater. 2009, 5, 1678–1685. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Y.; Yin, H.; Ruan, Y.; Sun, Y.; Lin, K. Effects of strontium substitution on the structural distortion of hydroxyapatite by Rietveld refinement and Raman spectroscopy. Ceram. Int. 2019, 45, 11073–11078. [Google Scholar] [CrossRef]
- Shi, H.; He, F.; Ye, J. Synthesis and structure of iron- and strontium-substituted octacalcium phosphate: Effects of ionic charge and radius. J. Mater. Chem. B 2016, 4, 1712–1719. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Rubini, K.; Bigi, A. Collapsed octacalcium phosphate stabilized by ionic substitutions. Cryst. Growth Des. 2010, 10, 3612–3617. [Google Scholar] [CrossRef]
- Teterina, A.Y.; Smirnov, I.V.; Fadeeva, I.S.; Fadeev, R.S.; Smirnova, P.V.; Minaychev, V.V.; Kobyakova, M.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Octacalcium phosphate for bone tissue engineering: Synthesis, modification, and in vitro biocompatibility assessment. Int. J. Mol. Sci. 2021, 22, 12747. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Wu, T.; Zhang, J.; Ye, J. Regulating the physicochemical and biological properties in vitro of octacalcium phosphate by substitution with strontium in a large doping range. Mater. Today Chem. 2017, 5, 81–91. [Google Scholar] [CrossRef]
- Ressler, A.; Cvetnic, M.; Antunovic, M.; Marijanovic, I.; Ivankovic, M.; Ivankovic, H. Strontium substituted biomimetic calcium phosphate system derived from cuttlefish bone. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1697–1709. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Zhang, J.; Wu, T.; Yu, T.; Zhou, C.; Ye, J. A thermostability perspective on enhancing physicochemical and cytological characteristics of octacalcium phosphate by doping iron and strontium. Bioact. Mater. 2021, 6, 1267–1282. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Kannan, S.; Pina, S.; Ferreira, J.M.F. Formation of strontium-stabilized β-tricalcium phosphate from calcium-deficient apatite. J. Am. Ceram. Soc. 2006, 89, 3277–3280. [Google Scholar] [CrossRef]
- Renaudin, G.; Laquerriere, P.; Filinchuk, Y.; Jallot, E.; Nedelec, J.M. Structural characterization of sol–gel derived Sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J. Mater. Chem. 2008, 18, 3593–3600. [Google Scholar] [CrossRef]
- Belik, A.A.; Izumi, F.; Stefanovich, S.Y.; Malakho, A.P.; Lazoryak, B.I.; Leonidov, I.A.; Leonidova, O.N.; Davydov, S.A. Polar and centrosymmetric phases in solid solutions Ca3−xSrx(PO4)2 (0 ≤ x ≤ 16/7). Chem. Mater. 2002, 14, 3197–3205. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Ajmal, Z.; Bouddouch, A.; Bakiz, B.; Navío, J.A.; Albourine, A.; Valmalette, J.-C.; Ezahri, M.; Benlhachemi, A. Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2. J. Colloid Interf. Sci. 2020, 572, 269–280. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Isomorphous substitutions in β-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Nervi, C.; Chierotti, M.R.; Rubini, K.; Gobetto, R.; Bigi, A. Strontium and zinc substitution in β-tricalcium phosphate: An X-ray diffraction, solid state NMR and ATR-FTIR study. J. Funct. Biomater. 2019, 10, 20. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Deyneko, D.V.; Forysenkova, A.A.; Morozov, V.A.; Akhmedova, S.A.; Kirsanova, V.A.; Sviridova, I.K.; Sergeeva, N.S.; Rodionov, S.A.; Udyanskaya, I.L.; et al. Strontium substituted β-tricalcium phosphate ceramics: Physiochemical properties and cytocompatibility. Molecules 2022, 27, 6085. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Curto, H.; Urruth, G.; Thuault, A.; Petit, F.; Leriche, A. Influence of dopants on thermal stability and densification of β-tricalcium phosphate powders. Open Ceram. 2021, 7, 100168. [Google Scholar] [CrossRef]
- Saint-Jean, S.J.; Camiré, C.L.; Nevsten, P.; Hansen, S.; Ginebra, M.P. Study of the reactivity and in vitro bioactivity of Sr-substituted α-TCP cements. J. Mater. Sci.-Mater. Med. 2005, 16, 993–1001. [Google Scholar] [CrossRef]
- Gazzano, M.; Rubini, K.; Bigi, A.; Boanini, E. Strontium-substituted α-TCP: Structure, stability, and reactivity in solution. Cryst. Growth Des. 2023, 23, 5690–5698. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kubota, T.; Toyoura, K.; Nakamura, A. First-principles calculations of divalent substitution of Ca2+ in tricalcium phosphates. Acta Biomater. 2015, 23, 329–337. [Google Scholar] [CrossRef]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements—Approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Sayahi, M.; Santos, J.; El-Feki, H.; Charvillat, C.; Bosc, F.; Karacan, I.; Milthorpe, B.; Drouet, C. Brushite (Ca,M)HPO4 2H2O doping with bioactive ions (M = Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): A new path to functional biomaterials? Mater. Today Chem. 2020, 16, 100230. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and hydrolysis of brushite (DCPD): The role of ionic substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. II. Preparation of normal and isotopically substituted calcium, strontium, and barium hydroxyapatites and spectra-structure-composition correlations. Inorg. Chem. 1974, 13, 207–214. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Rubini, K.; Mazzeo, P.P.; Bigi, A. Structural interplay between strontium and calcium in α-CaHPO4 and β-SrHPO4. Ceram. Int. 2021, 47, 24412–24420. [Google Scholar] [CrossRef]
- Catti, M.; Ferraris, G.; Filhol, A. Hydrogen bonding in the crystalline state. CaHPO4 (Monetite), P-1 or PI? A novel neutron diffraction study. Acta Crystallogr. B 1977, 33, 1223–1229. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, T.S.S.; Mahalaxmi, S.; Abburi, S.; Rubaiya, Y.; Doble, M. Dentin remineralizing ability and enhanced antibacterial activity of strontium and hydroxyl ion co-releasing radiopaque hydroxyapatite cement. J. Mater. Sci. Mater. Med. 2017, 28, 95. [Google Scholar] [CrossRef]
- Delgado López, J.M.; Frison, R.; Cervellino, A.; Gómez-Morales, J.; Guagliardi, A.; Masciocchi, N. Crystal size, morphology, and growth mechanism in bio-inspired apatite nanocrystals. Adv. Funct. Mater. 2014, 24, 1090–1099. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Lu, W.W.; Yang, X. Synthesis, characterization and the formation mechanism of magnesium- and strontium-substituted hydroxyapatite. J. Mater. Chem. B 2015, 3, 3738–3746. [Google Scholar] [CrossRef]
- Kurzyk, A.; Szwed-Georgiou, A.; Pagacz, J.; Antosik, A.; Tymowicz-Grzyb, P.; Gerle, A.; Szterner, P.; Włodarczyk, M.; Płociński, P.; Urbaniak, M.M.; et al. Calcination and ion substitution improve physicochemical and biological properties of nanohydroxyapatite for bone tissue engineering applications. Sci. Rep. 2023, 13, 15384. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Gazzano, M.; Rubini, K. Structural and morphological modifications of hydroxyapatite-polyaspartate composite crystals induced by heat treatment. Cryst. Res. Technol. 2005, 40, 1094–1098. [Google Scholar] [CrossRef]
- Jokić, B.; Mitrić, M.; Radmilović, V.; Drmanić, S.; Petrović, R.; Janaćković, D. Synthesis and characterization of monetite and hydroxyapatite whiskers obtained by a hydrothermal method. Ceram. Int. 2011, 37, 167–173. [Google Scholar] [CrossRef]
- Stojanović, Z.S.; Ignjatović, N.; Wu, V.; Žunič, V.; Veselinović, L.; Škapin, S.; Miljković, M.; Uskoković, V.; Uskoković, D. Hydrothermally processed 1D hydroxyapatite: Mechanism of formation and biocompatibility studies. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 68, 746–757. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Velázquez-Castillo, R.; Rivera-Muñoz, E.M.; Bucio-Galindo, L.; Mondragón-Galicia, G.; Manzano-Ramírez, A.; Ángel Ocampo, M.; Apátiga-Castro, L.M. Crystal growth and structural analysis of hydroxyapatite nanofibers synthesized by the hydrothermal microwave-assisted method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Ren, X.; Liang, Z.; Zhao, X. Preparation of hydroxyapatite nanofibers by using ionic liquids as template and application in enhancing hydrogel performance. Front. Bioeng. Biotechnol. 2023, 11, 1247448. [Google Scholar] [CrossRef]
- Ye, Z.; Qi, Y.; Zhang, A.; Karels, B.J.; Aparicio, C. Biomimetic mineralization of fibrillar collagen with strontium-doped hydroxyapatite. ACS Macro Lett. 2023, 12, 408–414. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Falini, G.; Panzavolta, S.; Roveri, N. Effect of sodium polyacrylate on the hydrolysis of octacalcium phosphate. J. Inorg. Biochem. 2000, 78, 227–233. [Google Scholar] [CrossRef]
- Sinusaite, L.; Grigoraviciute-Puroniene, I.; Popov, A.; Ishikawa, K.; Kareiva, A.; Zarkov, A. Controllable synthesis of tricalcium phosphate (TCP) polymorphs by wet precipitation: Effect of washing procedure. Ceram. Int. 2019, 45, 12423–12428. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Olivares-Pinto, U.; Aguilar-Reyes, E.A.; López-Juárez, R.; Alfonso, I. Characterization of β-tricalcium phosphate powders synthesized by sol–gel and mechanosynthesis. Bol. Soc. Esp. Ceram. Vidr. 2018, 57, 213–220. [Google Scholar] [CrossRef]
- Yuan, Z.; Bi, J.; Wang, W.; Sun, X.; Wang, L.; Mao, J.; Yang, F. Synthesis and properties of Sr2+ doping α-tricalcium phosphate at low temperature. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800021996999. [Google Scholar] [CrossRef]
- Švecová, M.; Bartůněk, V. Facile synthesis of monetite nanoparticles from basic raw materials. Ceram. Int. 2018, 44, 16079–16082. [Google Scholar] [CrossRef]
- Chen, S.; Krumova, M.; Cölfen, H.; Sturm, E.V. Synthesis of fiber-like monetite without organic additives and its transformation to hydroxyapatite. Chem. Mater. 2019, 31, 1543–1551. [Google Scholar] [CrossRef]
- Adawy, A.; Diaz, R. Probing the structure, cytocompatibility, and antimicrobial efficacy of silver-, strontium-, and zinc-doped monetite. ACS Appl. Bio Mater. 2022, 5, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Rabelo Neto, J.S.; Ricardo, P.C.; Valério, M.E.G.; Xia, W.; Engqvist, H.; Fredel, M.C. The influence of strontium doping on the crystal morphology of synthetic calcium phosphates. J. Mol. Struct. 2024, 1316, 139030. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Calcium phosphates as delivery systems for bisphosphonates. J. Funct. Biomater. 2018, 9, 6. [Google Scholar] [CrossRef]
- Zeng, Y.; Hoque, J.; Varghese, S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019, 93, 152–168. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Lukaviciute, L.; Ganceviciene, R.; Tsuru, K.; Ishikawa, K.; Yang, J.C.; Grigoraviciute, I.; Kareiva, A. Cationic substitution effects in phosphate-based bioceramics—A way towards superior bioproperties. Ceram. Int. 2024, 50, 34479–34509. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef]
- Schumacher, M.; Wagner, A.S.; Kokesch-Himmelreich, J.; Bernhardt, A.; Rohnke, M.; Wenisch, S.; Gelinsky, M. Strontium substitution in apatitic CaP cements effectively attenuates osteoclastic resorption but does not inhibit osteoclastogenesis. Acta Biomater. 2016, 37, 184–194. [Google Scholar] [CrossRef]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef]
- Tadier, S.; Bareille, R.; Siadous, R.; Marsan, O.; Charvillat, C.; Cazalbou, S.; Amedee, J.; Rey, C.; Combes, C. Strontium-loaded mineral bone cements as sustained release systems: Compositions, release properties, and effects on human osteoprogenitor cells. J. Biomed. Mater. Res. Part B 2012, 100B, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, K.; Zhao, X.; Han, Y. Development of a strontium-containing hydroxyapatite bone cement. Biomaterials 2005, 26, 4073–4083. [Google Scholar] [CrossRef]
- Hamdan Alkhraisat, M.; Tamimi Mariño, F.; Rueda Rodríguez, C.; Blanco Jerez, L.; Lopez Cabarcos, E. Combined effect of strontium and pyrophosphate on the properties of brushite cements. Acta Biomater. 2008, 4, 664–670. [Google Scholar] [CrossRef]
- Hamdan Alkhraisat, M.; Moseke, C.; Blanco, L.; Barralet, J.E.; Lopez-Carbacos, E.; Gbureck, U. Strontium modified biocements with zero order release kinetics. Biomaterials 2008, 29, 4691–4697. [Google Scholar] [CrossRef]
- Hamdan Alkhraisat, M.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Tamimi Mariño, F.; Torres García-Denche, J.; Blanco Jerez, L.; Gbureck, U.; Lopez Cabarcos, E. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010, 6, 1522–1528. [Google Scholar] [CrossRef]
- Taha, A.; Akram, M.; Jawad, Z.; Alshemary, A.Z.; Hussain, R. Strontium doped injectable bone cement for potential drug delivery applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 93–101. [Google Scholar] [CrossRef]
- Rau, J.V.; Fadeeva, I.V.; Forysenkova, A.A.; Davydova, G.A.; Fosca, M.; Filippov, Y.Y.; Antoniac, I.V.; Antoniac, A.; D’Arco, A.; Di Fabrizio, M.; et al. Strontium substituted tricalcium phosphate bone cement: Short and long-term time-resolved studies and in vitro properties. Adv. Mater. Interfaces 2022, 9, 2200803. [Google Scholar] [CrossRef]
- Hamdan Alkhraisat, M.; Rueda, C.; López Cabarcos, E. Strontium ions substitution in brushite crystals: The role of strontium chloride. J. Funct. Biomater. 2011, 2, 31–38. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2010, 6, 928–935. [Google Scholar] [CrossRef]
- Silingardi, F.; Salamanna, F.; Español, M.; Maglio, M.; Sartori, M.; Giavaresi, G.; Bigi, A.; Ginebra, M.P.; Boanini, E. Regulation of osteogenesis and angiogenesis by cobalt, manganese and strontium doped apatitic materials for functional bone tissue regeneration. Biomater. Adv. 2024, 163, 213968. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Fu, Y.; Chen, D.; Sun, Z. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 124, 112052. [Google Scholar] [CrossRef]
- Neves, N.; Linhares, D.; Costa, G.; Ribeiro, C.C.; Barbosa, M.A. In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: A systematic review. Bone Joint Res. 2017, 6, 366–375. [Google Scholar] [CrossRef]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Müller, R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef]
- Panzavolta, S.; Fini, M.; Nicoletti, A.; Bracci, B.; Rubini, K.; Giardino, R.; Bigi, A. Porous composite scaffolds based on gelatin and partially hydrolyzed alpha-tricalcium phosphate. Acta Biomater. 2009, 5, 636–643. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Li, J.; Li, J.; Lin, T.; Liu, M.; Sang, H.; Hong, X.; Pu, J.; Huang, W. Advancements in 3D printing technologies for personalized treatment of osteonecrosis of the femoral head. Mater. Today Bio 2025, 31, 101531. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-substituted hydroxyapatite-gelatin biomimetic scaffolds modulate bone cell response. Macromol. Biosci. 2018, 18, e1800096. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, Z.; Ke, Q.; Yin, W.; Chen, Y.; Zhang, C.; Guo, Y. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ehret, C.; Aid-Launais, R.; Sagardoy, T.; Siadous, R.; Bareille, R.; Rey, S.; Pechev, S.; Etienne, L.; Kalisky, J.; de Mones, E.; et al. Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLoS ONE 2017, 12, e0184663. [Google Scholar] [CrossRef]
- Quade, M.; Schumacher, M.; Bernhardt, A.; Lode, A.; Kampschulte, M.; Voß, A.; Simon, P.; Uckermann, O.; Kirsch, M.; Gelinsky, M. Strontium-modification of porous scaffolds from mineralized collagen for potential use in bone defect therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 159–167. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lin, W.Y.; Yang, C.Y.; Lee, T.M. Fabrication of gelatin-strontium substituted calcium phosphate scaffolds with unidirectional pores for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 152. [Google Scholar] [CrossRef]
- Shaikh, S.; Mehrotra, S.; van Bochove, B.; Teotia, A.K.; Singh, P.; Laurén, I.; Lindfors, N.C.; Seppälä, J.; Kumar, A. Strontium-substituted nanohydroxyapatite containing biodegradable 3D printed composite scaffolds for bone regeneration. ACS Appl. Mater. Interfaces 2024, 16, 65378–65393. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Fischetti, T.; Borciani, G.; Avnet, S.; Rubini, K.; Baldini, N.; Graziani, G.; Boanini, E. Incorporation/enrichment of 3D bioprinted constructs by biomimetic nanoparticles: Tuning printability and cell behavior in bone models. Nanomaterials 2023, 13, 2040. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Xiong, J.; Zhang, C.; Gan, J.; Fu, Q.; Li, Y.; Wen, R.; He, F.; Shi, H. Fabrication of 3D-printed Ca2Sr(PO4)2-based composite ceramic scaffolds as potential bone regenerative biomaterials. Ceram. Int. 2024, 50, 41703–41710. [Google Scholar] [CrossRef]
- M’Pemba Hennebert, P.; Amirthalingam, S.; Hoon Kang, T.; So, K.H.; Hwang, N.S. Strontium-doped whitlockite scaffolds for enhanced bone regeneration. ACS Appl. Mater. Interfaces 2024, 16, 65822–65836. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572–1589. [Google Scholar] [CrossRef]
- Tao, Z.S.; Zhou, W.S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Huang, Z.L.; Tu, K.K.; Li, H.; Sun, T.; et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Hosick, H.L.; Bandyopadhyay, A.; Bose, S.; Ding, C.; Luk, K.D.K.; Cheung, K.M.C.; Lu, W.W. Preparation and cell–materials interactions of plasma sprayed strontium-containing hydroxyapatite coating. Surf. Coat. Technol. 2007, 201, 4685–4693. [Google Scholar] [CrossRef]
- Ullah, I.; Xu, Q.; Ullah Jan, H.; Ren, L.; Yang, K. Effects of strontium and zinc substituted plasma sprayed hydroxyapatite coating on bone-like apatite layer formation and cell-material interaction. Mat. Chem. Phys. 2022, 275, 125219. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Plasma-sprayed hydroxyapatite-strontium coating for improved corrosion resistance and surface properties of biodegradable AZ31 Mg alloy for biomedical applications. J. Mater. Eng. Perform. 2021, 30, 1768–1779. [Google Scholar] [CrossRef]
- Ghezzi, D.; Graziani, G.; Cappelletti, M.; Fadeeva, I.V.; Montesissa, M.; Sassoni, E.; Borciani, G.; Barbaro, K.; Boi, M.; Baldini, N.; et al. New strontium-based coatings show activity against pathogenic bacteria in spine infection. Front. Bioeng. Biotechnol. 2024, 12, 1347811. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Reis, R.L.; Li, P. Strontium-substituted apatite coating grown on Ti6Al4V substrate through biomimetic synthesis. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 258–265. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Borchardt, P.; Ballo, A.; Thomsen, P.; Engqvist, H. Biomineralized strontium-substituted apatite/titanium dioxide coating on titanium surfaces. Acta Biomater. 2010, 6, 1591–1600. [Google Scholar] [CrossRef]
- Navarro da Rocha, D.; de Oliveira Cruz, L.R.; de Campos, J.B.; Lopes dos Santos, J.; Santana Blazutti Marçal, R.L.; Mijares, D.Q.; Maza Barbosa, R.; Coelho, P.G.; Prado da Silva, M.H. Bioactivity of strontium-monetite coatings for biomedical applications. Ceram. Int. 2019, 45, 7568–7579. [Google Scholar] [CrossRef]
- Wen, J.; Gu, G.C.; Wang, K.; Xiao, G.Y.; Lu, Y.P.; Liu, B. Synthesis and characterization of strontium substituted monetite coatings via mild phosphate chemical conversion. Surf. Coat. Technol. 2024, 478, 130394. [Google Scholar] [CrossRef]
- Ozeki, K.; Goto, T.; Aoki, H.; Masuzawa, T. Characterization of Sr-substituted hydroxyapatite thin film by sputtering technique from mixture targets of hydroxyapatite and strontium apatite. Biomed. Mater. Eng. 2014, 24, 1447–1456. [Google Scholar] [CrossRef]
- Boyd, A.R.; Rutledge, L.; Randolph, L.D.; Meenan, B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 290–300. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Pulsed electrodeposition for the synthesis of strontium-substituted calcium phosphate coatings with improved dissolution properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4260–4265. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Zhang, Z.; Lian, J.; Guo, Y.; Ren, L. Effect of strontium-substituted calcium phosphate coatings prepared by one-step electrodeposition at different temperatures on corrosion resistance and biocompatibility of AZ31 magnesium alloys. ACS Biomater. Sci. Eng. 2024, 10, 326–337. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Pereiro, I.; Rodríguez-Valencia, C.; Serra, C.; Solla, E.L.; Serra, J.; González, P. Pulsed laser deposition of strontium-substituted hydroxyapatite coatings. Appl. Surf. Sci. 2012, 258, 9192–9197. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Fini, M.; Sima, F.; Serban, N.; Mihailescu, I.N.; Bigi, A. Magnesium and strontium doped octacalcium phosphate thin films by matrix assisted pulsed laser evaporation. J. Inorg. Biochem. 2012, 107, 65–72. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium and zoledronate hydroxyapatites graded composite coatings for bone prostheses. J. Colloid Interface Sci. 2015, 448, 1–7. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Gradient coatings of strontium hydroxyapatite/zinc β-tricalcium phosphate as a tool to modulate osteoblast/osteoclast response. J. Inorg. Biochem. 2018, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Formula | Name (Mineral) | Abbreviation |
|---|---|---|
| Ca10(PO4)6(OH)2 | Hydroxyapatite | HA |
| Ca8H2(PO4)6·5H2O | Octacalcium phosphate | OCP |
| β-Ca3(PO4)2 | Beta-tricalcium phosphate (whitlockite) | β-TCP |
| α-Ca3(PO4)2 | Alpha-tricalcium phosphate | α-TCP |
| Ca(H2PO4)2·H2O | Monocalcium phosphate monohydrate | MCPM |
| Ca(H2PO4)2 | Anhydrous monocalcium phosphate | MCPA |
| CaHPO4·2H2O | Dicalcium phosphate dihydrate (brushite) | DCPD |
| CaHPO4 | Anhydrous dicalcium phosphate (monetite) | DCPA |
| Ca4(PO4)2 | Tetracalcium phosphate | TTCP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigi, A.; Boanini, E. Strontium-Substituted Calcium Orthophosphates: Structure, Stability, Morphology, and Biomedical Applications. Int. J. Mol. Sci. 2025, 26, 5886. https://doi.org/10.3390/ijms26125886
Bigi A, Boanini E. Strontium-Substituted Calcium Orthophosphates: Structure, Stability, Morphology, and Biomedical Applications. International Journal of Molecular Sciences. 2025; 26(12):5886. https://doi.org/10.3390/ijms26125886
Chicago/Turabian StyleBigi, Adriana, and Elisa Boanini. 2025. "Strontium-Substituted Calcium Orthophosphates: Structure, Stability, Morphology, and Biomedical Applications" International Journal of Molecular Sciences 26, no. 12: 5886. https://doi.org/10.3390/ijms26125886
APA StyleBigi, A., & Boanini, E. (2025). Strontium-Substituted Calcium Orthophosphates: Structure, Stability, Morphology, and Biomedical Applications. International Journal of Molecular Sciences, 26(12), 5886. https://doi.org/10.3390/ijms26125886







