Noradrenaline and Adrenoreceptors Promote Prostaglandin F2α Generation in Lipopolysaccharide-Exposed Endometrial Epithelial Cells of Pigs (Sus scrofa domesticus)
Abstract
1. Introduction
2. Results
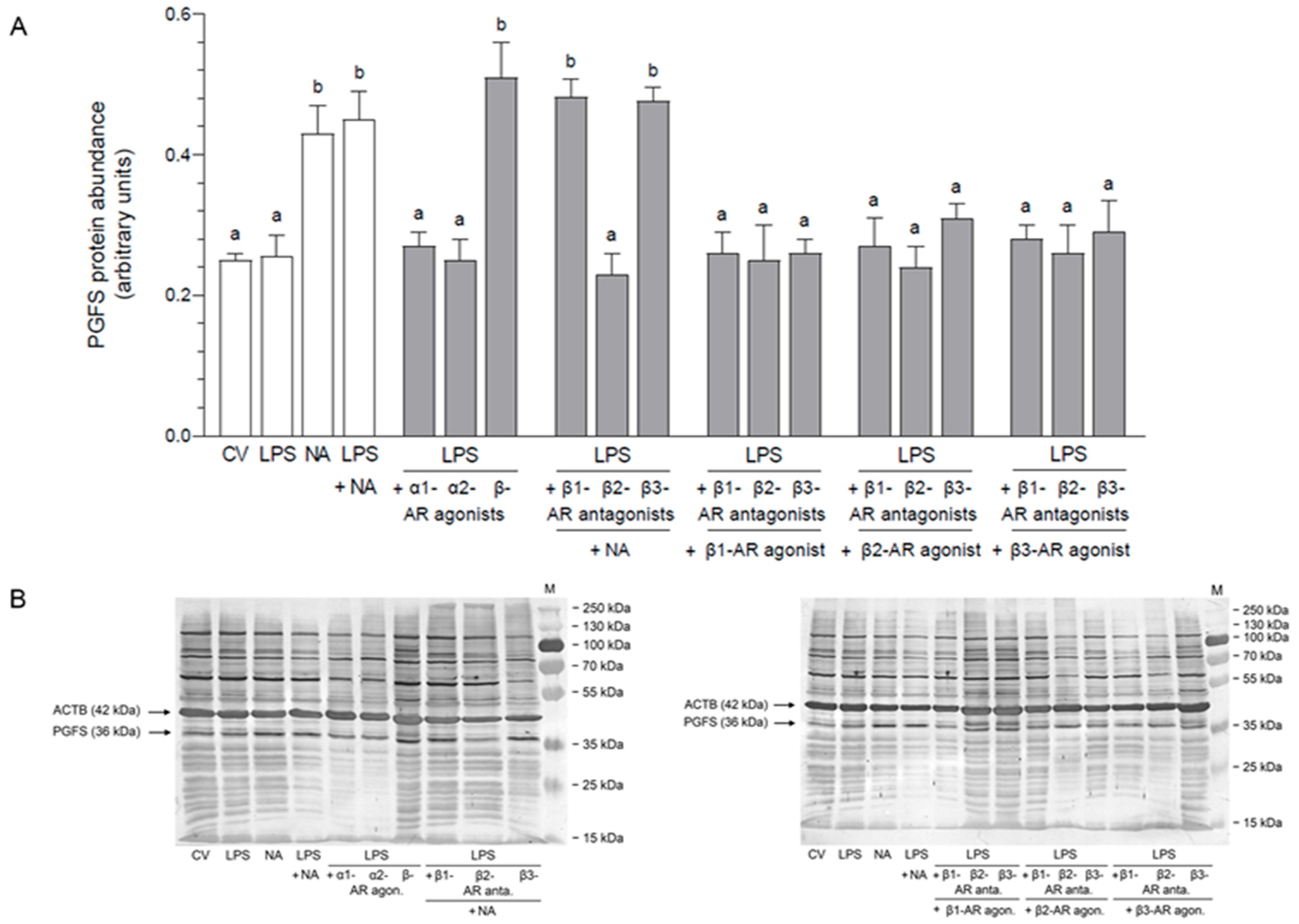
2.1. Impact of NA, Agonists and Antagonists of ARs on the Abundance of PGFS Protein in the LPS-Stimulated Epithelial Cells of the Endometrium
2.1.1. AR Agonists and Antagonists Alone
2.1.2. LPS Alone, NA Alone, LPS with NA or AR Agonists, Antagonists
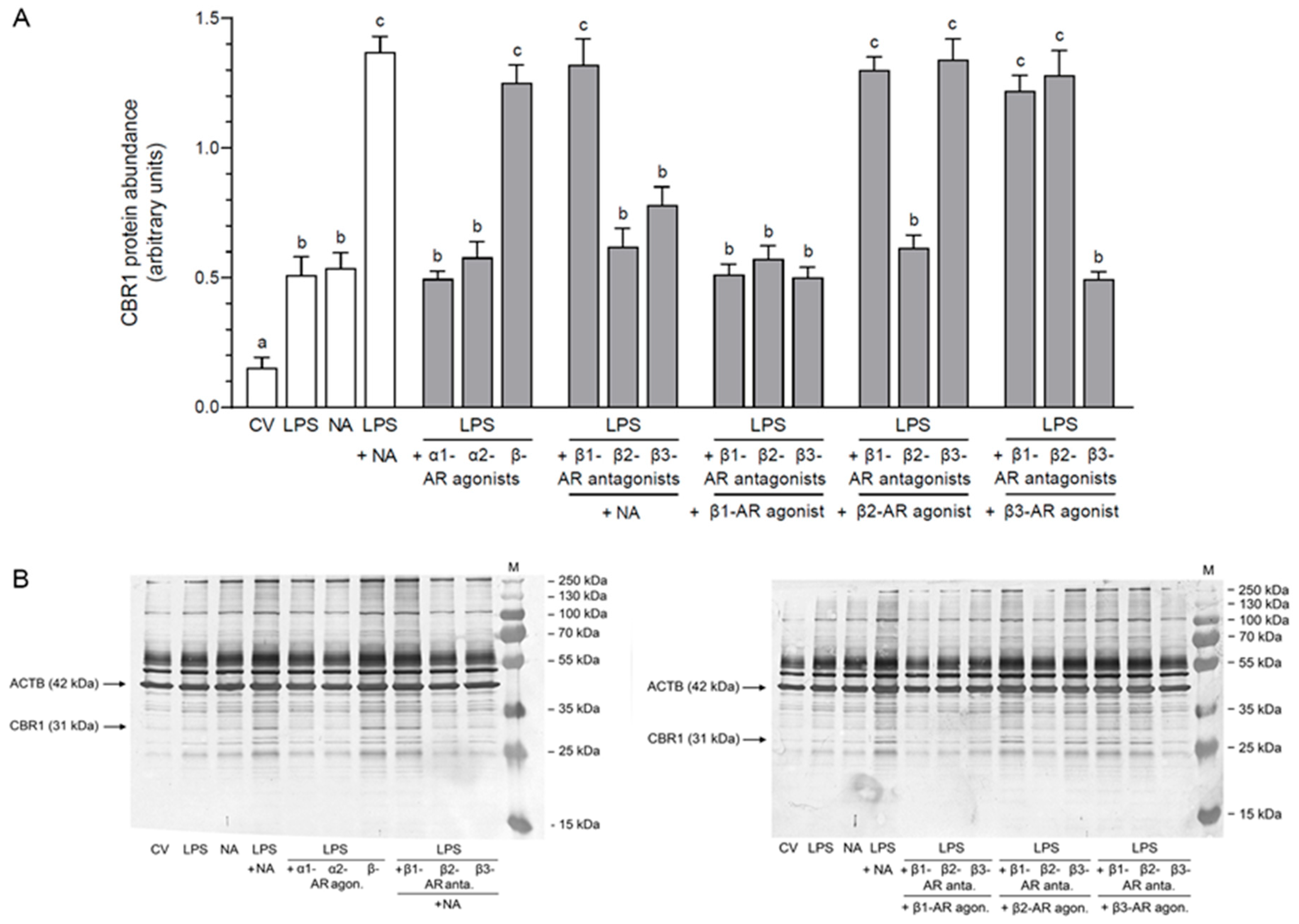
2.2. Impact of NA, Agonists and Antagonists of ARs on the Abundance of CBR1 Protein in the LPS-Stimulated Epithelial Cells of the Endometrium
2.2.1. AR Agonists and Antagonists Alone
2.2.2. LPS Alone, NA Alone, LPS with NA or AR Agonists, Antagonists
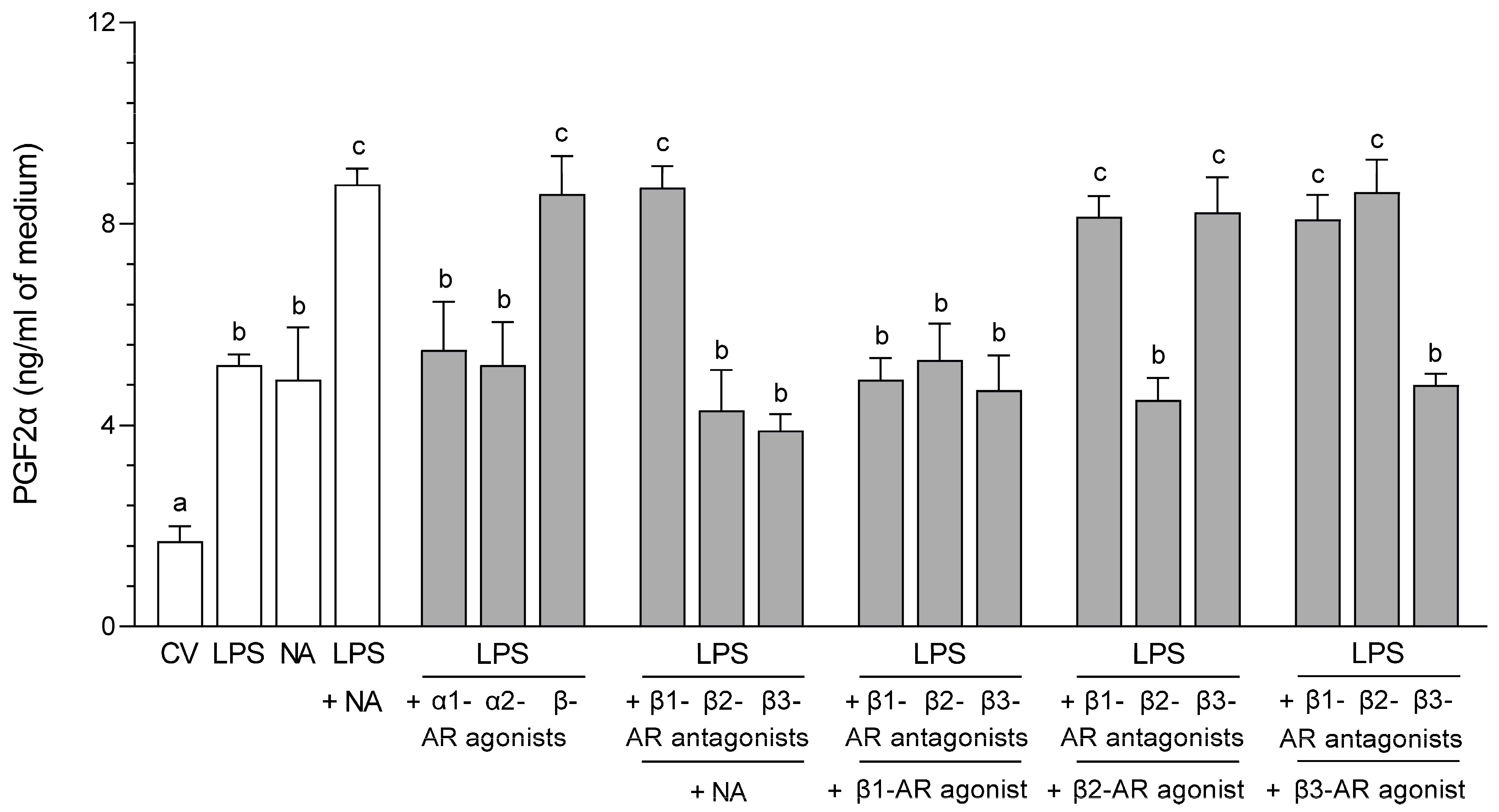
2.3. Impact of NA, Agonists and Antagonists of ARs on the Concentration in the Medium of PGF2α Following Culture of the LPS-Stimulated Epithelial Cells of the Endometrium
2.3.1. AR Agonists and Antagonists Alone
2.3.2. LPS Alone, NA Alone, LPS with NA or AR Agonists, Antagonists
3. Discussion
4. Material and Methods
4.1. Collection of Uteri from Gilts
4.2. Isolation of Epithelial Cells from the Endometrium
4.3. Adding LPS, NA, Agonists and Antagonists of ARs to the Endometrial Epithelial Cells
4.4. Western Blot Analysis
4.5. ELISA Method
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheong, S.H.; Sá Filho, O.G.; Absalon-Medina, V.A.; Schneider, A.; Butler, W.R.; Gilbert, R.O. Uterine and Systemic Inflammation Influences Ovarian Follicular Function in Postpartum Dairy Cows. PLoS ONE 2017, 12, e0177356. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.-E.; Turner, M.L. Innate Immunity and the Sensing of Infection, Damage and Danger in the Female Genital Tract. J. Reprod. Immunol. 2017, 119, 67–73. [Google Scholar] [CrossRef]
- Tummaruk, P.; Kesdangsakonwut, S.; Prapasarakul, N.; Kaeoket, K. Endometritis in Gilts: Reproductive Data, Bacterial Culture, Histopathology, and Infiltration of Immune Cells in the Endometrium. Comp. Clin. Pathol. 2010, 19, 575–584. [Google Scholar] [CrossRef]
- De Winter, P.J.J.; Verdonck, M.; De Kruif, A.; Devriese, L.A.; Haesebrouck, F. Bacterial Endometritis and Vaginal Discharge in the Sow: Prevalence of Different Bacterial Species and Experimental Reproduction of the Syndrome. Anim. Reprod. Sci. 1995, 37, 325–335. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-Like Receptor 4 and MYD88-Dependent Signaling Mechanisms of the Innate Immune System Are Essential for the Response to Lipopolysaccharide by Epithelial and Stromal Cells of the Bovine Endometrium1. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. Lipopolysaccharide Appears to Activate Human Endometrial Endothelial Cells Through TLR-4-Dependent and TLR-4-Independent Mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef]
- Turner, M.; Healey, G.; Sheldon, I. Immunity and Inflammation in the Uterus. Reprod. Domest. Anim. 2012, 47, 402–409. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of Immune Function in the Bovine Uterus Peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, M.; Pfarrer, C.; Górriz Martín, L.; Schmicke, M.; Hoedemaker, M.; Bollwein, H.; Heppelmann, M. In Vitro Effects of Lipopolysaccharides on Bovine Uterine Contractility. Reprod. Domest. Anim. 2021, 56, 172–182. [Google Scholar] [CrossRef]
- Barański, W.; Łukasik, K.; Skarżyński, D.; Sztachańska, M.; Zduńczyk, S.; Janowski, T. Secretion of Prostaglandins and Leukotrienes by Endometrial Cells in Cows with Subclinical and Clinical Endometritis. Theriogenology 2013, 80, 766–772. [Google Scholar] [CrossRef]
- Manns, J.G.; Nkuuhe, J.R.; Bristol, F. Prostaglandin Concentrations in Uterine Fluid of Cows with Pyometra. Can. J. Comp. Med. 1985, 49, 436–438. [Google Scholar] [PubMed]
- Mateus, L.; Lopes Da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between Endotoxin and Prostaglandin (PGE2 and PGFM) Concentrations and Ovarian Function in Dairy Cows with Puerperal Endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tobolski, D.; Zwierzchowski, G.; Lukasik, K.; Skarżyński, D.J.; Pascottini, O.B.; Opsomer, G.; Barański, W. Progesterone-Independent Endometrial mRNA Expression in Dairy Cows with Clinical or Subclinical Endometritis. Theriogenology 2024, 216, 146–154. [Google Scholar] [CrossRef]
- Janowski, T.; Barański, W.; Łukasik, K.; Skarżyński, D.; Zduńczyk, S.; Malinowska, K. Endometrial mRNA Expression of Prostaglandin Synthase Enzymes PTGS 2, PTGFS and mPTGES 1 in Repeat-Breeding Cows with Cytologically Determined Endometritis. Acta Vet. Hung. 2017, 65, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Kucharski, J.; Dzienis, A.; Deptuła, K. Changes in Prostaglandin Production and Ovarian Function in Gilts during Endometritis Induced by Escherichia Coli Infection. Anim. Reprod. Sci. 2007, 97, 137–150. [Google Scholar] [CrossRef]
- Jana, B.; Kozłowska, A.; Koszykowska, M.; Majewski, M. Expression of Cyclooxygenase-2 in the Inflammatory Changed Porcine Uterus. Pol. J. Vet. Sci. 2009, 12, 1–8. [Google Scholar]
- Koszykowska, M.; Kozłowska, A.; Jana, B. Expression of the Enzymes Participating in the Prostaglandin F2a and E2 Synthesis in Inflamed Porcine Uterus. In Proceedings of the XIII Congresses of the Polish Society of Veterinary Sciences, Olsztyn, Poland, 18 September 2008. [Google Scholar]
- Roongsitthichai, A.; Srisuwatanasagul, S.; Koonjaenak, S.; Tummaruk, P. Expression of Cyclooxygenase-2 in the Endometrium of Gilts with Different Stages of Endometritis. J. Vet. Med. Sci. 2011, 73, 1425–1431. [Google Scholar] [CrossRef]
- Jana, B.; Kucharski, J.; Ziecik, A.J. Effect of Intrauterine Infusion of Escherichia coli on Hormonal Patterns in Gilts during the Oestrous Cycle. Reprod. Nutr. Dev. 2004, 44, 37–48. [Google Scholar] [CrossRef]
- Kucharski, J.; Jaroszewski, J.; Jana, B.; Górska, J.; Kozłowska, A.; Markiewicz, W. The Influence of Inflammatory Process On Prostaglandin F2α Contractile Activity in Porcineuterus. J. Anim. Feed Sci. 2007, 16, 654–667. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; You, X.; Leimert, K.; Popowycz, K.; Fang, X.; Wood, S.L.; Slater, D.M.; Sun, Q.; Gu, H.; et al. PGF2α Modulates the Output of Chemokines and Pro-Inflammatory Cytokines in Myometrial Cells from Term Pregnant Women through Divergent Signaling Pathways. Mol. Hum. Reprod. 2015, 21, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Bosu, W.T.K.; Peter, A.T. The Effect of Endotoxin on Luteal Function in Holstein Heifers. Theriogenology 1990, 33, 645–651. [Google Scholar] [CrossRef]
- Peter, A.T.; Bosu, W.T.K.; Gilbert, R.O. Absorption of Endotoxin (Lipopolysaccharide) from the Uteri of Postpartum Dairy Cows. Theriogenology 1990, 33, 1011–1014. [Google Scholar] [CrossRef]
- Majewski, M.; Sienkiewicz, W.; Kaleczyc, J.; Mayer, B.; Czaja, K.; Lakomy, M. The Distribution and Co-Localization of Immunoreactivity to Nitric Oxide Synthase, Vasoactive Intestinal Polypeptide and Substance P within Nerve Fibres Supplying Bovine and Porcine Female Genital Organs. Cell Tissue Res. 1995, 281, 445–464. [Google Scholar] [CrossRef]
- Wa̧sowicz, K.; Majewski, M.; Łakomy, M. Distribution of Neurons Innervating the Uterus of the Pig. J. Auton. Nerv. Syst. 1998, 74, 13–22. [Google Scholar] [CrossRef]
- Bóta, J.; Hajagos-Tóth, J.; Ducza, E.; Samavati, R.; Borsodi, A.; Benyhe, S.; Gáspár, R. The Effects of Female Sexual Hormones on the Expression and Function of α1A- and α1D-Adrenoceptor Subtypes in the Late-Pregnant Rat Myometrium. Eur. J. Pharmacol. 2015, 769, 177–184. [Google Scholar] [CrossRef]
- Ducza, E.; Gáspár, R.; Falkay, G. Altered Levels of mRNA Expression and Pharmacological Reactivity of α1-adrenergic Receptor Subtypes in the Late-pregnant Rat Myometrium. Mol. Reprod. Dev. 2002, 62, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Gal, A.; Galik, M.; Ducza, E.; Minorics, R.; Kolarovszkisipiczki, Z.; Klukovits, A.; Falkay, G. Different Roles of A2-Adrenoceptor Subtypes in Non-Pregnant and Late-Pregnant Uterine Contractility in Vitro in the Rat. Neurochem. Int. 2007, 51, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Limon-Boulez, I.; Mhaouty-Kodja, S.; Coudouel, N.; Benoit De Coignac, A.; Legrand, C.; Maltier, J.P. The Alpha1B-Adrenergic Receptor Subtype Activates the Phospholipase C Signaling Pathway in Rat Myometrium at Parturition1. Biol. Reprod. 1997, 57, 1175–1182. [Google Scholar] [CrossRef]
- Meller, K.A.; Całka, J.; Kaczmarek, M.; Jana, B. Expression of Alpha and Beta Adrenergic Receptors in the Pig Uterus during Inflammation. Theriogenology 2018, 119, 96–104. [Google Scholar] [CrossRef]
- Ontsouka, E.C.; Reist, M.; Graber, H.; Blum, J.W.; Steiner, A.; Hirsbrunner, G. Expression of Messenger RNA Coding for 5-HT Receptor, Alpha and Beta Adrenoreceptor (Subtypes) during Oestrus and Dioestrus in the Bovine Uterus. J. Vet. Med. Ser. A 2004, 51, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Uttam Singh, T.; Ravi Prakash, V.; Mishra, S.K. Molecular and Functional Characteristics of Β3-Adrenoceptors in Late Pregnant Mouse Uterus: A Comparison with Β2-Adrenoceptors. Eur. J. Pharmacol. 2013, 700, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Taneike, T.; Narita, T.; Kitazawa, T.; Bando, S.; Teraoka, H.; Ohga, A. Binding and Functional Characterization of Alpha-2 Adrenoceptors in Isolated Swine Myometrium. J. Auton. Pharmacol. 1995, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Quaas, L.; Zahradnik, H.P. The Effects of α- and β-Adrenergic Stimulation on Contractility and Prostaglandin (Prostaglandins E2 and F2α and 6-Keto-Prostaglandin F1α) Production of Pregnant Human Myometrial Strips. Am. J. Obstet. Gynecol. 1985, 152, 852–856. [Google Scholar] [CrossRef]
- Ishikawa, M.; Fuchs, A.-R. Effects of Epinephrine and Oxytocin on the Release of Prostaglandin F from the Rat Uterus In Vitro. Prostaglandins 1978, 15, 89–101. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Palus, K.; Witek, K. Noradrenaline and Adrenoreceptors Regulate Prostaglandin F2A Formation in Endometrium after Experimentally-Induced Inflammation in the Pig. Ann. Anim. Sci. 2024, 24, 453–463. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Uenoyama, Y.; Kotwica, J.; Okuda, K. Noradrenaline Stimulates the Production of Prostaglandin F2α in Cultured Bovine Endometrial Cells1. Biol. Reprod. 1999, 60, 277–282. [Google Scholar] [CrossRef]
- Chaud, M.A.; Frenchi, A.M.; Gonzalez, E.T.; Gimeno, M.F.; Gimeno, A.L. Norepinephrine Alters Output Ratio in Isolated Uterus from Ovariectomized Rats. Prostaglandins Leukot. Med. 1986, 22, 201–210. [Google Scholar] [CrossRef]
- Gimeno, M.F.; Franchi, A.M.; Faletti, A.; Gonzalez, E.T.; Gimeno, A.L. On the Synthesis of Prostaglandins E1,E2 and F2α by Sow Oviducts. Differential Modulation of 1 and 2 Series of Prostaglandins by Norepinephrine. Prostaglandins Leukot. Med. 1985, 17, 61–70. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J. Endometritis Changes the Neurochemical Characteristics of the Caudal Mesenteric Ganglion Neurons Supplying the Gilt Uterus. Animals 2020, 10, 891. [Google Scholar] [CrossRef]
- Miciński, B.; Jana, B.; Całka, J. Endometritis Decreases the Population of Uterine Neurons in the Paracervical Ganglion and Changes the Expression of Sympathetic Neurotransmitters in Sexually Mature Gilts. BMC Vet. Res. 2021, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Meller, K.; Całka, J.; Palus, K.; Jana, B. Inflammation-Induced Changes in Expression of DβH, SP and GAL in the Porcine Uterine Nerve Fibres. In Proceedings of the 4th Winter Workshop of the Society for Biology of Reproduction “Central and local Regulations of Reproductive Processes”, Kraków, Poland, 3 February 2016. [Google Scholar]
- Asselin, E.; Drolet, P.; Fortier, M.A. Cellular Mechanisms Involved during Oxytocin-Induced Prostaglandin F2α Production in Endometrial Epithelial Cells in Vitro: Role of Cyclooxygenase-2. Endocrinology 1997, 138, 4798–4805. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, D.J.; Miyamoto, Y.; Okuda, K. Production of Prostaglandin F2α by Cultured Bovine Endometrial Cells in Response to Tumor Necrosis Factor α: Cell Type Specificity and Intracellular Mechanisms1. Biol. Reprod. 2000, 62, 1116–1120. [Google Scholar] [CrossRef]
- MacKintosh, S.B.; Schuberth, H.-J.; Healy, L.L.; Sheldon, I.M. Polarised Bovine Endometrial Epithelial Cells Vectorially Secrete Prostaglandins and Chemotactic Factors under Physiological and Pathological Conditions. Reproduction 2013, 145, 57–72. [Google Scholar] [CrossRef]
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Eicosanoid Pathway Expression in Bovine Endometrial Epithelial and Stromal Cells in Response to Lipopolysaccharide, Interleukin 1 Beta, and Tumor Necrosis Factor Alpha. Reprod. Biol. 2018, 18, 390–396. [Google Scholar] [CrossRef]
- Blitek, A.; Ziecik, A. Prostaglandins F2α and E2 Secretion by Porcine Epithelial and Stromal Endometrial Cells on Different Days of the Oestrous Cycle. Reprod. Domest. Anim. 2004, 39, 340–346. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial Lipopolysaccharide Induces an Endocrine Switch from Prostaglandin F2α to Prostaglandin E2 in Bovine Endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Penrod, L.V.; Allen, R.E.; Turner, J.L.; Limesand, S.W.; Arns, M.J. Effects of Oxytocin, Lipopolysaccharide (LPS), and Polyunsaturated Fatty Acids on Prostaglandin Secretion and Gene Expression in Equine Endometrial Explant Cultures. Domest. Anim. Endocrinol. 2013, 44, 46–55. [Google Scholar] [CrossRef]
- Korzekwa, A.J.; Łupicka, M.; Socha, B.M.-; Szczepańska, A.A.; Piotrowicz, E.; Barański, W. In Vitro Cow Uterine Response to Escherichia Coli, Leukotrienes and Cytokines. Vet. Immunol. Immunopathol. 2016, 182, 59–62. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Andronowska, A.; Mówińska, A.; Witek, K.; Palus, K. Noradrenaline and Adrenoreceptors Are Involved in the Regulation of Prostaglandin I2 Production in the Porcine Endometrium after Experimentally Induced Inflammation. Int. J. Mol. Sci. 2024, 25, 6313. [Google Scholar] [CrossRef]
- Paisley, L.G.; Mickelsen, W.D.; Anderson, P.B. Mechanisms and Therapy for Retained Fetal Membranes and Uterine Infections of Cows: A Review. Theriogenology 1986, 25, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Novel Cyclooxygenase-catalyzed Bioactive Prostaglandin F2α from Physiology to New Principles in Inflammation. Med. Res. Rev. 2007, 27, 435–468. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Stabenfeldt, G.H.; Kindahl, H.; Kennedy, P.C.; Edqvist, L.E.; Neely, D.P.; Schalm, O.W. Pyometra in the Mare. J. Reprod. Fertil. 1979, (Suppl. 27), 321–329. [Google Scholar]
- Akins, E.L.; Morrissette, M.C. Gross Ovarian Changes during Estrous Cycle of Swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- Blitek, A.; Morawska, E.; Kiewisz, J.; Ziecik, A.J. Effect of Conceptus Secretions on HOXA10 and PTGS2 Gene Expression, and PGE2 Release in Co-Cultured Luminal Epithelial and Stromal Cells of the Porcine Endometrium at the Time of Early Implantation. Theriogenology 2011, 76, 954–966. [Google Scholar] [CrossRef]
- Złotkowska, A.; Andronowska, A. Variable Chemokine Expression in Porcine Trophoblasts and Endometrium during the Peri-Implantation Period. Theriogenology 2019, 131, 16–27. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Treatment | PGFS | CBR1 | PGF2α |
|---|---|---|---|
| (Protein Abundances, Arbitrary Units) | (ng/mL of Medium) | ||
| CV | 0.25 ± 0.01 a | 0.15 ± 0.04 a | 1.72 ± 0.31 a |
| NA | 0.43 ± 0.04 b | 0.53 ± 0.06 b | 4.91 ± 1.04 b |
| α1 agon. | 0.28 ± 0.03 a | 0.14 ± 0.06 a | 1.51 ± 0.22 a |
| α2 agon. | 0.21 ± 0.04 a | 0.19 ± 0.03 a | 1.43 ± 0.25 a |
| β agon. | 0.66 ± 0.03 c | 0.58 ± 0.05 b | 6.09 ± 1.01 b |
| β1 agon. | 0.29 ± 0.03 a | 0.22 ± 0.03 a | 1.32 ± 0.51 a |
| β2 agon. | 0.83 ± 0.05 c | 0.81 ± 0.04 d | 6.31 ± 1.25 b |
| β3 agon. | 0.22 ± 0.03 a | 0.79 ± 0.02 d | 6.23 ± 1.19 b |
| β1 anta. | 0.19 ± 0.06 a | 0.14 ± 0.03 a | 1.43 ± 0.11 a |
| β2 anta. | 0.24 ± 0.03 a | 0.23 ± 0.02 a | 1.61 ± 0.22 a |
| β3 anta. | 0.26 ± 0.05 a | 0.17 ± 0.04 a | 1.52 ± 0.39 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jana, B.; Całka, J.; Mówińska, A. Noradrenaline and Adrenoreceptors Promote Prostaglandin F2α Generation in Lipopolysaccharide-Exposed Endometrial Epithelial Cells of Pigs (Sus scrofa domesticus). Int. J. Mol. Sci. 2025, 26, 5874. https://doi.org/10.3390/ijms26125874
Jana B, Całka J, Mówińska A. Noradrenaline and Adrenoreceptors Promote Prostaglandin F2α Generation in Lipopolysaccharide-Exposed Endometrial Epithelial Cells of Pigs (Sus scrofa domesticus). International Journal of Molecular Sciences. 2025; 26(12):5874. https://doi.org/10.3390/ijms26125874
Chicago/Turabian StyleJana, Barbara, Jarosław Całka, and Aleksandra Mówińska. 2025. "Noradrenaline and Adrenoreceptors Promote Prostaglandin F2α Generation in Lipopolysaccharide-Exposed Endometrial Epithelial Cells of Pigs (Sus scrofa domesticus)" International Journal of Molecular Sciences 26, no. 12: 5874. https://doi.org/10.3390/ijms26125874
APA StyleJana, B., Całka, J., & Mówińska, A. (2025). Noradrenaline and Adrenoreceptors Promote Prostaglandin F2α Generation in Lipopolysaccharide-Exposed Endometrial Epithelial Cells of Pigs (Sus scrofa domesticus). International Journal of Molecular Sciences, 26(12), 5874. https://doi.org/10.3390/ijms26125874






