Self-Assembling Amphiphilic ABA Triblock Copolymers of Hyperbranched Polyglycerol with Poly(tetrahydrofuran) and Their Nanomicelles as Highly Efficient Solubilization and Delivery Systems of Curcumin
Abstract
1. Introduction
2. Results and Discussion
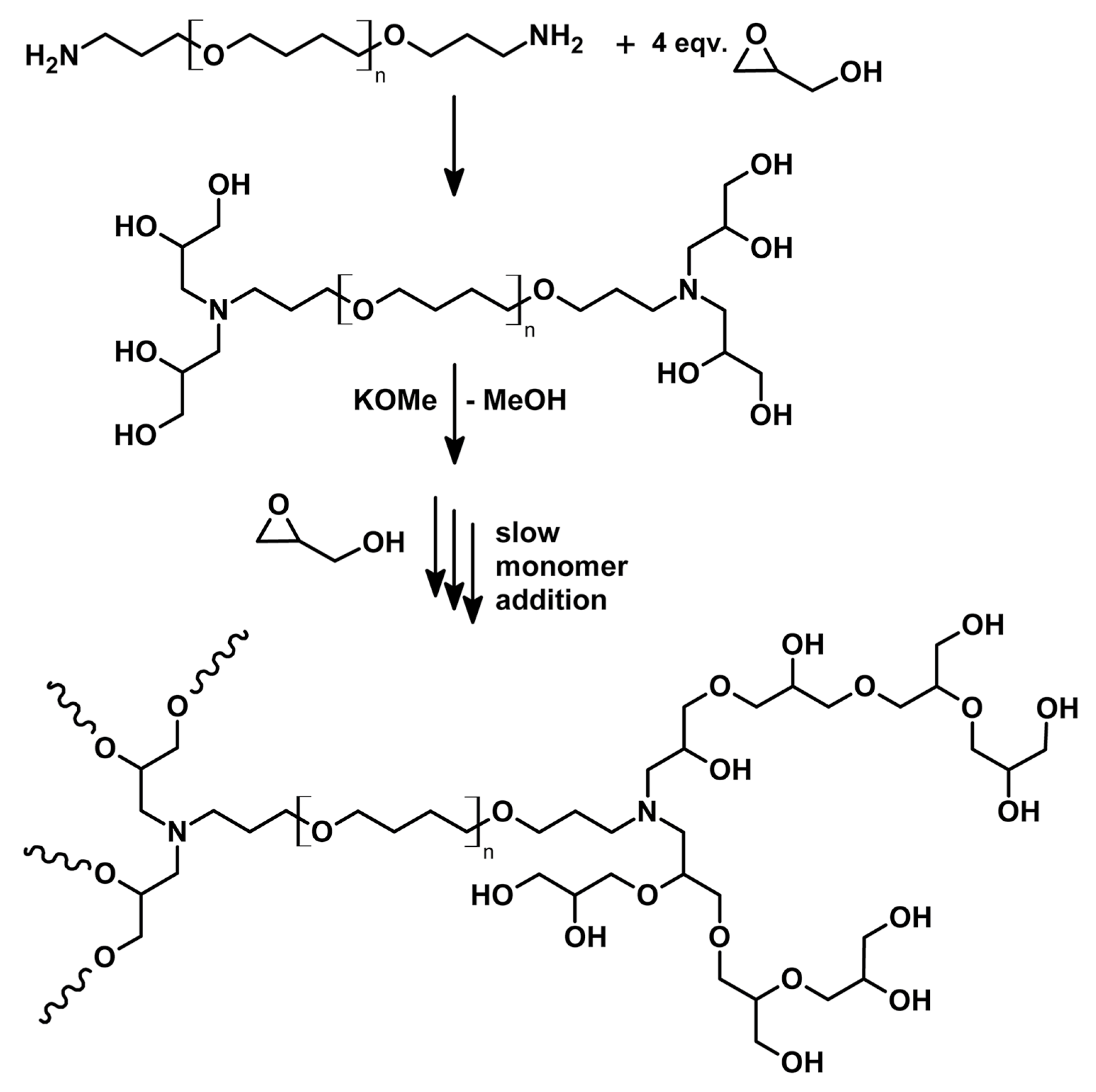
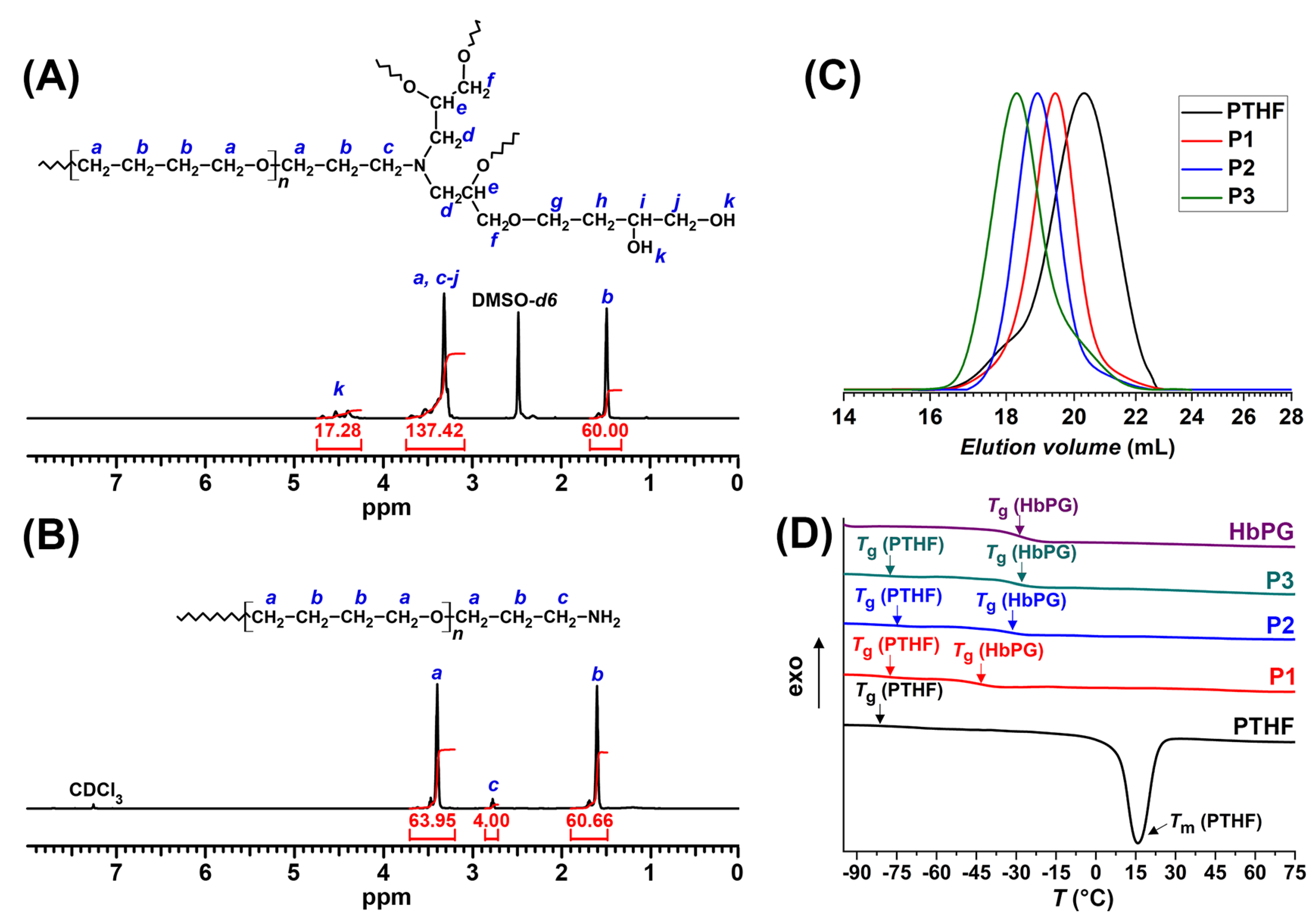
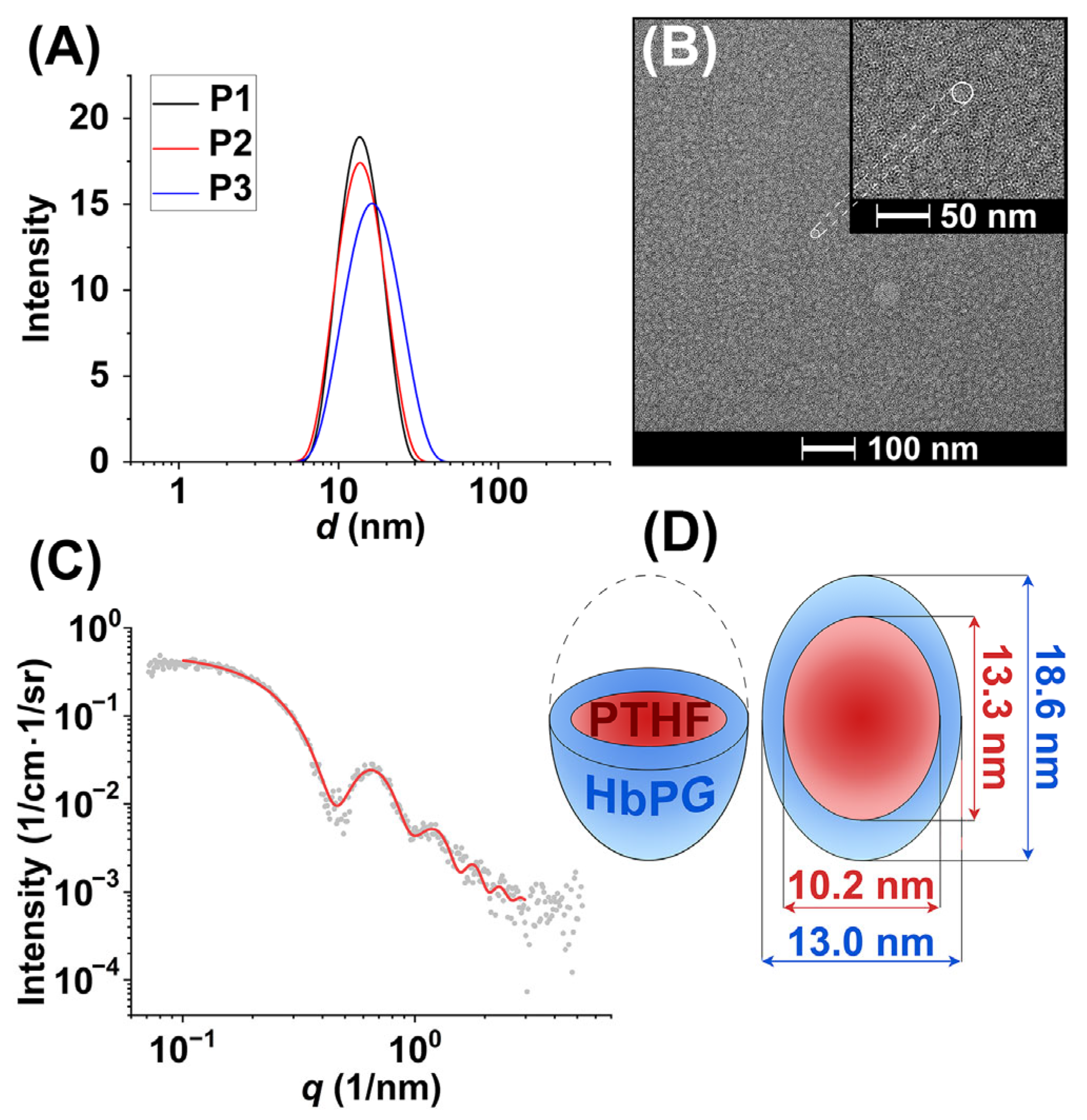
2.1. Synthesis and Nanomicelle Formation of HbPG-PTHF-HbPG Block Copolymers
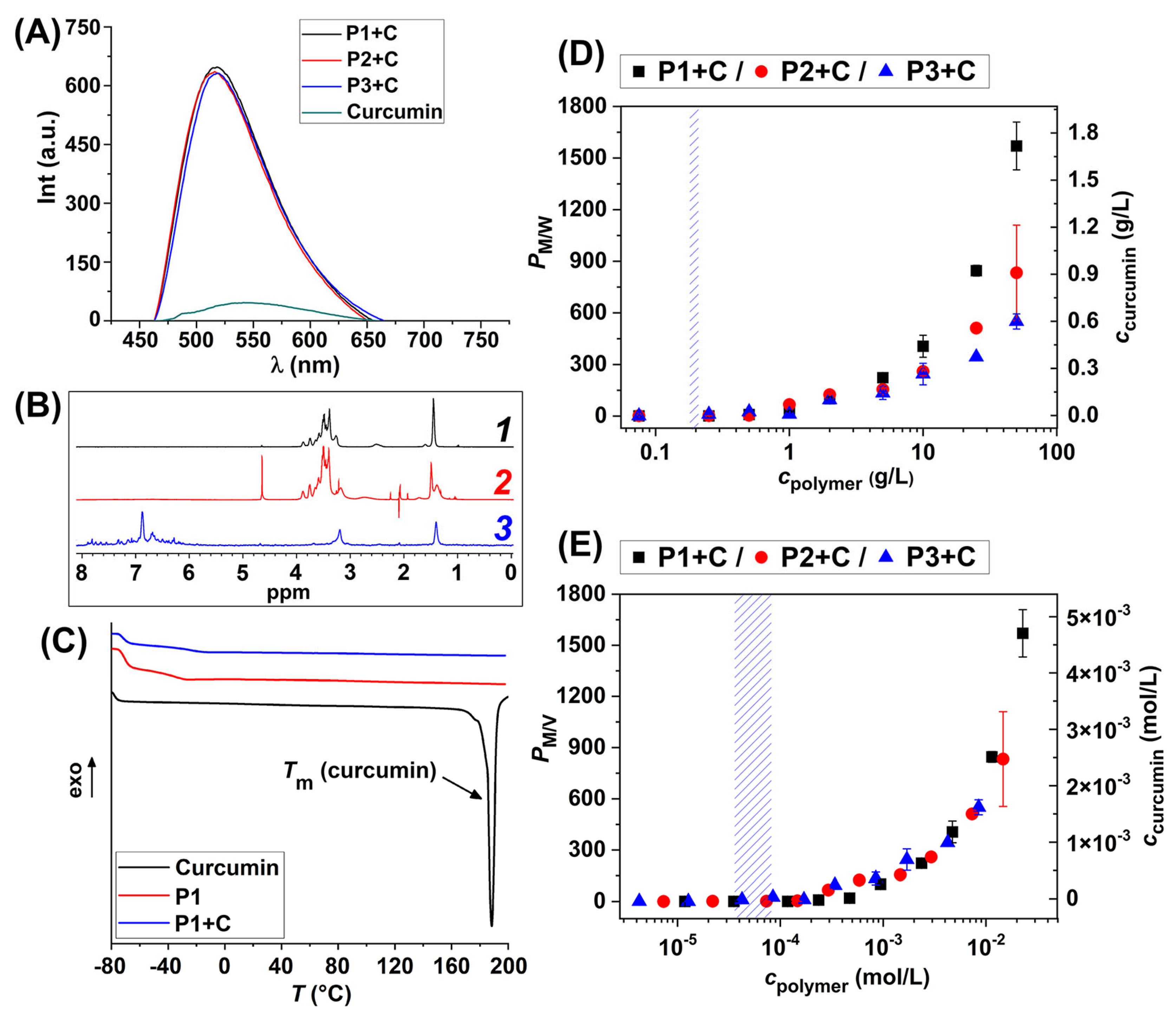
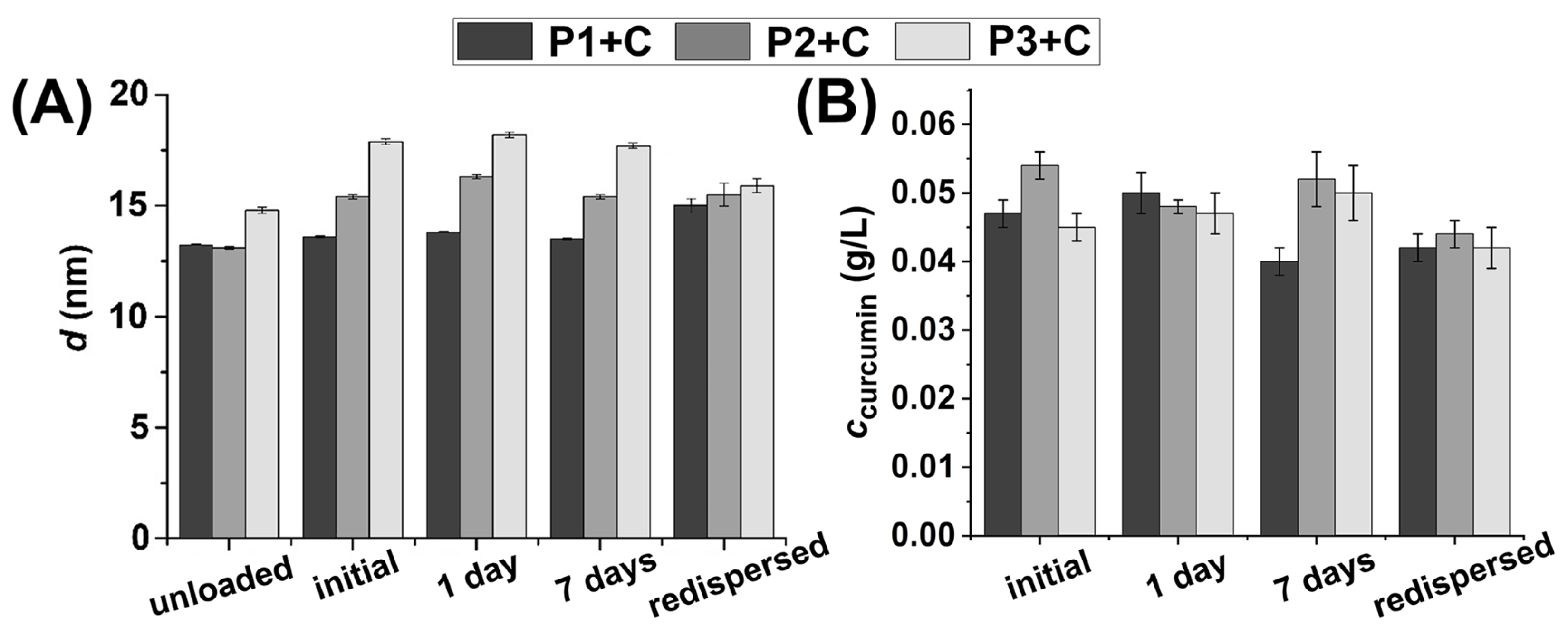
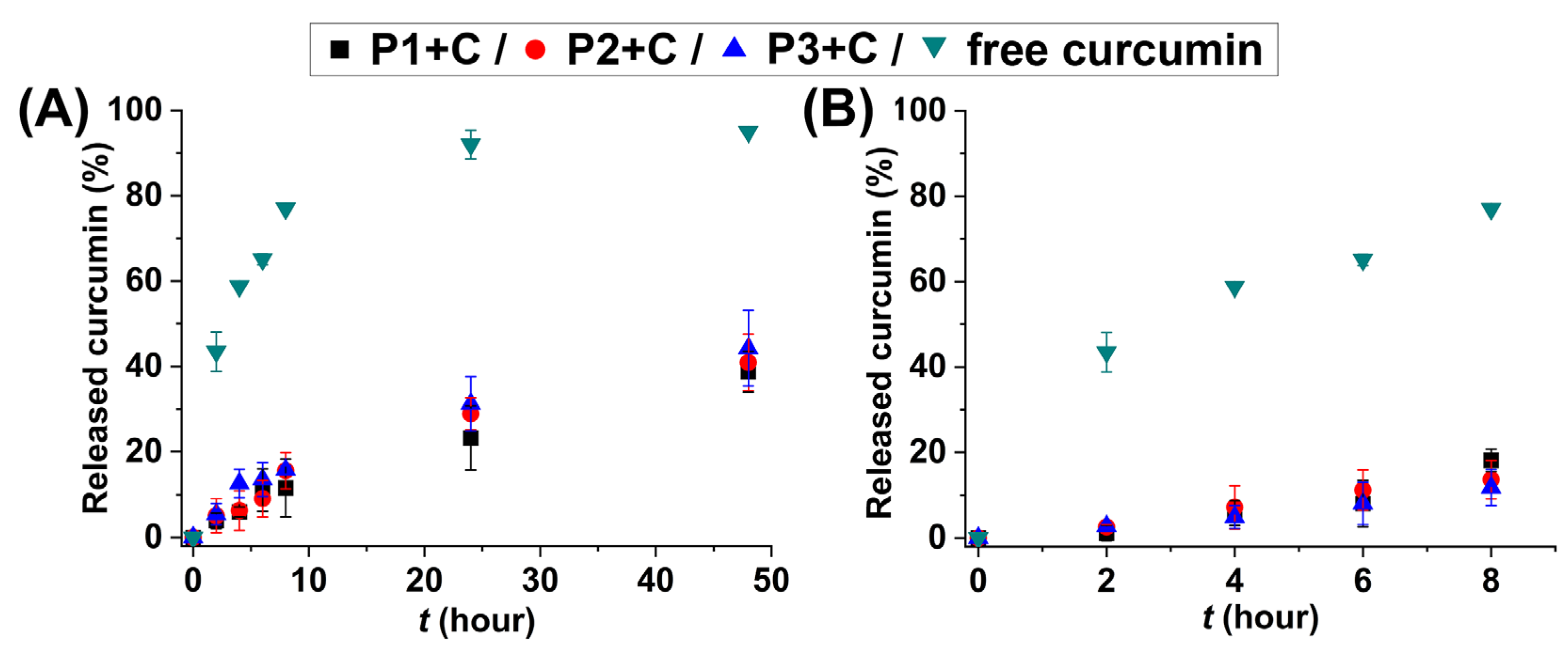
2.2. HbPG-PTHF-HbPG Block Copolymer Nanomicelles as Drug Solubilization and Delivery Systems
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hyperbranched Polyglycerol (HbPG) and Poly(tetrahydrofuran) (PTHF) Containing ABA Triblock Copolymers
3.3. Characterization Methods
3.3.1. Gel Permeation Chromatography
3.3.2. NMR Spectroscopy
3.3.3. Differential Scanning Calorimetry
3.3.4. Determination of the Critical Micelle Concentration by the Pyrene Probe Method
3.3.5. Surface Tension Measurement by the Du Noüy Ring Method
3.3.6. Dynamic Light Scattering
3.3.7. Transmission Electron Microscopy
3.3.8. Small-Angle X-Ray Spectroscopy
3.3.9. Coagulation Experiments
3.4. Encapsulation and Drug Release Experiments
3.4.1. Solubilization of Curcumin
3.4.2. Fluorescence Studies of Curcumin-Loaded Micelles
3.4.3. Drug–Polymer Interaction Study
3.4.4. Stability of the Curcumin-Loaded Micelles
3.4.5. Drug Release Measurements
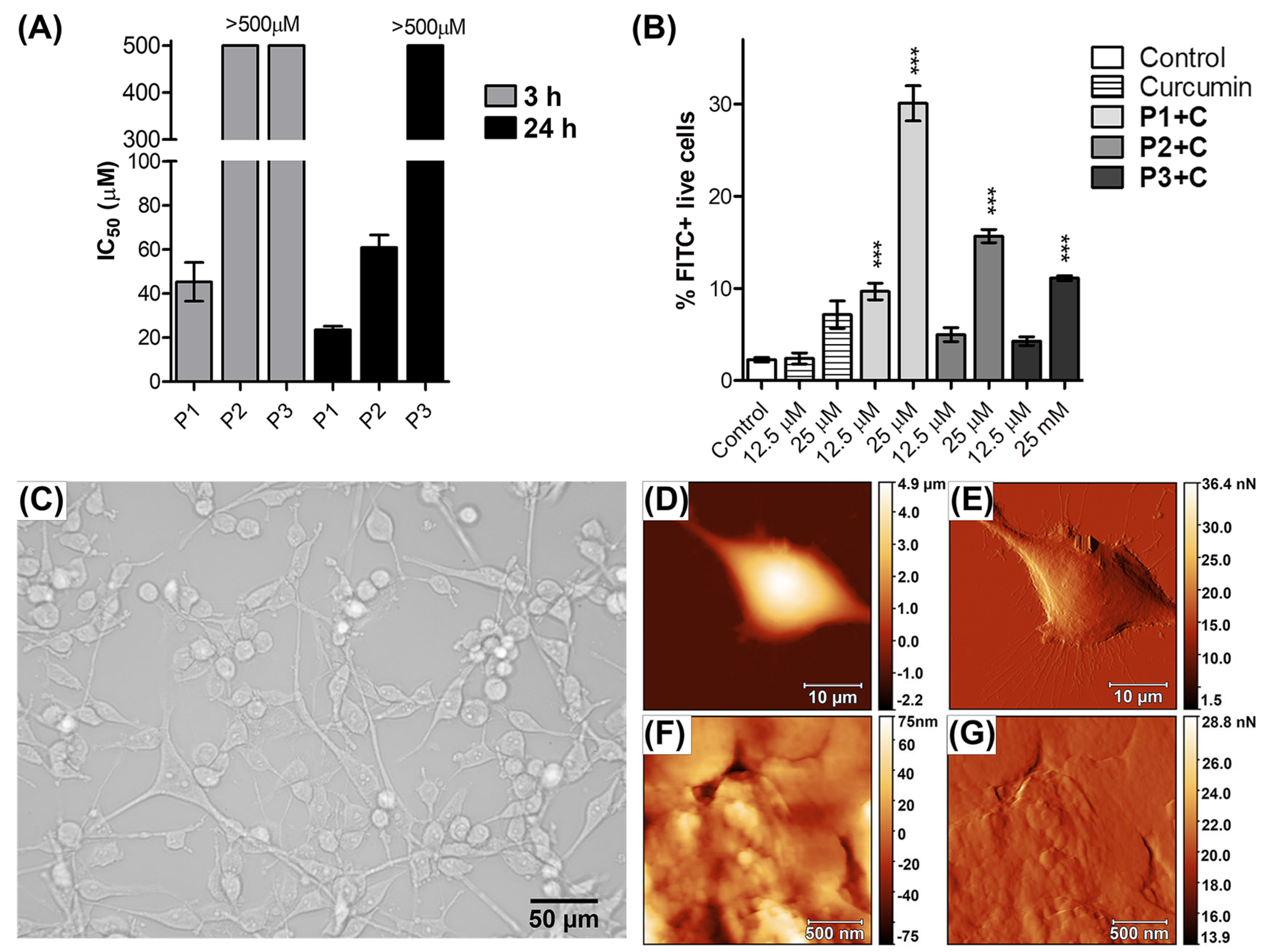
3.5. Biological Activity of the Curcumin-Loaded HbPG-PTHF-HbPG Micelles
3.5.1. Cytotoxicity Assay
3.5.2. Assessment of Cellular Uptake by Flow Cytometry
3.5.3. Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letchford, K.; Burt, H. A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing Amphiphilic Polymeric Micelles for Diagnostic and Therapeutic Applications: Breakthroughs and Bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block Copolymer Solution Self-assembly: Recent Advances, Emerging Trends, and Applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef]
- Lübtow, M.M.; Hahn, L.; Haider, M.S.; Luxenhofer, R. Drug Specificity, Synergy and Antagonism in Ultrahigh Capacity Poly(2-Oxazoline)/Poly(2-Oxazine) Based Formulations. J. Am. Chem. Soc. 2017, 139, 10980–10983. [Google Scholar] [CrossRef]
- Krishnan, A.; Roy, S.; Menon, S. Amphiphilic Block Copolymers: From Synthesis Including Living Polymerization Methods to Applications in Drug Delivery. Eur. Polym. J. 2022, 172, 111224. [Google Scholar] [CrossRef]
- Assiri, A.A.; Glover, K.; Mishra, D.; Waite, D.; Vora, L.K.; Thakur, R.R.S. Block Copolymer Micelles as Ocular Drug Delivery Systems. Drug Discov. Today 2024, 29, 104098. [Google Scholar] [CrossRef]
- Wurm, F.; Frey, H. Linear–Dendritic Block Copolymers: The State of the Art and Exciting Perspectives. Prog. Polym. Sci. 2011, 36, 1–52. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Y.; Xu, W.; Li, L. Linear–Dendritic Block Copolymer for Drug and Gene Delivery. Mater. Sci. Eng. C 2016, 62, 943–959. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and Functionalization of Hyperbranched Polymers for Targeted Drug Delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef]
- Maksimov, A.; Yarullin, B.; Kharlampidi, K.; Kutyrev, G. Advances in Hyperbranched Polymer Chemistry. Iran. Polym. J. 2025, 34, 433–452. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic Polyglycerol: A New Versatile Biocompatible Material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Wilms, D.; Stiriba, S.-E.; Frey, H. Hyperbranched Polyglycerols: From the Controlled Synthesis of Biocompatible Polyether Polyols to Multipurpose Applications. Acc. Chem. Res. 2010, 43, 129–141. [Google Scholar] [CrossRef]
- Kasza, G.; Kali, G.; Domján, A.; Pethő, L.; Szarka, G.; Iván, B. Synthesis of Well-Defined Phthalimide Monofunctional Hyperbranched Polyglycerols and Its Transformation to Various Conjugation Relevant Functionalities. Macromolecules 2017, 50, 3078–3088. [Google Scholar] [CrossRef]
- Quadir, M.; Fehse, S.; Multhaup, G.; Haag, R. Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates. Molecules 2018, 23, 1281. [Google Scholar] [CrossRef]
- Kasza, G.; Stumphauser, T.; Nádor, A.; Osváth, Z.; Szarka, G.; Domján, A.; Mosnáček, J.; Iván, B. Hyperbranched Polyglycerol Nanoparticles Based Multifunctional, Nonmigrating Hindered Phenolic Macromolecular Antioxidants: Synthesis, Characterization and Its Stabilization Effect on Poly(Vinyl Chloride). Polymer 2017, 124, 210–218. [Google Scholar] [CrossRef]
- Ferraro, M.; Silberreis, K.; Mohammadifar, E.; Neumann, F.; Dernedde, J.; Haag, R. Biodegradable Polyglycerol Sulfates Exhibit Promising Features for Anti-Inflammatory Applications. Biomacromolecules 2018, 19, 4524–4533. [Google Scholar] [CrossRef]
- Nie, C.; Pouyan, P.; Lauster, D.; Trimpert, J.; Kerkhoff, Y.; Szekeres, G.P.; Wallert, M.; Block, S.; Sahoo, A.K.; Dernedde, J.; et al. Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions. Angew. Chem. Int. Ed. 2021, 60, 15870–15878. [Google Scholar] [CrossRef]
- Jafari, M.; Abolmaali, S.S.; Najafi, H.; Tamaddon, A.M. Hyperbranched Polyglycerol Nanostructures for Anti-Biofouling, Multifunctional Drug Delivery, Bioimaging and Theranostic Applications. Int. J. Pharm. 2020, 576, 118959. [Google Scholar] [CrossRef]
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Williams, G.R.; Zhou, Y.; Liao, X.; Xiao, Z.; Yu, C.; Liu, Y. Tumor Microenvironment-Responsive Hyperbranched Polymers for Controlled Drug Delivery. J. Pharm. Anal. 2024, 14, 101003. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y. Hyperbranched Polymers: Recent Advances in Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 2222. [Google Scholar] [CrossRef]
- Rafiee, Z.; Omidi, S. Modification of Carbon-Based Nanomaterials by Polyglycerol: Recent Advances and Applications. RSC Adv. 2022, 12, 181–192. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Kumar, S.; Mohr, A.; Sharma, S.K.; Rabe, J.P.; Haag, R. A Bifunctional Nanocarrier Based on Amphiphilic Hyperbranched Polyglycerol Derivatives. J. Mater. Chem. B 2013, 1, 3569. [Google Scholar] [CrossRef]
- Paulus, F.; Steinhilber, D.; Welker, P.; Mangoldt, D.; Licha, K.; Depner, H.; Sigrist, S.; Haag, R. Structure Related Transport Properties and Cellular Uptake of Hyperbranched Polyglycerol Sulfates with Hydrophobic Cores. Polym. Chem. 2014, 5, 5020–5028. [Google Scholar] [CrossRef]
- Misri, R.; Wong, N.K.Y.; Shenoi, R.A.; Lum, C.M.W.; Chafeeva, I.; Toth, K.; Rustum, Y.; Kizhakkedathu, J.N.; Khan, M.K. Investigation of Hydrophobically Derivatized Hyperbranched Polyglycerol with PEGylated Shell as a Nanocarrier for Systemic Delivery of Chemotherapeutics. Nanomed. NBM 2015, 11, 1785–1795. [Google Scholar] [CrossRef]
- Li, N.; Deng, B.; Li, W.; Song, G.; Wang, Y.; Feng, J.; Zhao, D.; Fan, X.; Xu, M. A Universal Multivalent Hyperbranched Delivery Platform for Circumventing Multidrug Resistance via Double Camouflage and Rapid Bonding with Cell. J. Drug Deliv. Sci. Technol. 2023, 81, 104265. [Google Scholar] [CrossRef]
- Jaworska-Krych, D.; Gosecka, M.; Gosecki, M.; Urbaniak, M.; Dzitko, K.; Ciesielska, A.; Wielgus, E.; Kadlubowski, S.; Kozanecki, M. Enhanced Solubility and Bioavailability of Clotrimazole in Aqueous Solutions with Hydrophobized Hyperbranched Polyglycidol for Improved Antifungal Activity. ACS Appl. Mater. Interfaces 2024, 16, 18434–18448. [Google Scholar] [CrossRef]
- Adeli, M.; Namazi, H.; Du, F.; Hönzke, S.; Hedtrich, S.; Keilitz, J.; Haag, R. Synthesis of Multiarm Star Copolymers Based on Polyglycerol Cores with Polylactide Arms and Their Application as Nanocarriers. RSC Adv. 2015, 5, 14958–14966. [Google Scholar] [CrossRef]
- Stefani, S.; Hönzke, S.; Cuellar Camacho, J.L.; Neumann, F.; Prasad, A.K.; Hedtrich, S.; Haag, R.; Servin, P. Hyperbranched Glycerol-Based Core-Amphiphilic Branched Shell Nanotransporters for Dermal Drug Delivery. Polymer 2016, 96, 156–166. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Q.; Wang, C.; Xu, J.; Wu, J.-P.; Kirk, T.B.; Ma, D.; Xue, W. Star-Shaped Amphiphilic Hyperbranched Polyglycerol Conjugated with Dendritic Poly(l-Lysine) for the Codelivery of Docetaxel and MMP-9 siRNA in Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 12609–12619. [Google Scholar] [CrossRef]
- Koeppe, H.; Horn, D.; Scholz, J.; Quaas, E.; Schötz, S.; Reisbeck, F.; Achazi, K.; Mohammadifar, E.; Dernedde, J.; Haag, R. Shell-Sheddable Dendritic Polyglycerol Sulfates Loaded with Sunitinib for Inhibition of Tumor Angiogenesis. Int. J. Pharm. 2023, 642, 123158. [Google Scholar] [CrossRef]
- Tong, R.; Feng, X.; Sun, J.; Ling, Z.; Wang, J.; Li, S.; Yang, B.; Deng, J.; He, G.; Wu, J. Co-Delivery of siNRF2 and Sorafenib by a “Click” Dual Functioned Hyperbranched Nanocarrier for Synergistically Inducing Ferroptosis in Hepatocellular Carcinoma. Small 2024, 20, 2307273. [Google Scholar] [CrossRef]
- Son, S.; Shin, E.; Kim, B.-S. Light-Responsive Micelles of Spiropyran Initiated Hyperbranched Polyglycerol for Smart Drug Delivery. Biomacromolecules 2014, 15, 628–634. [Google Scholar] [CrossRef]
- Kasza, G.; Gyulai, G.; Ábrahám, Á.; Szarka, G.; Iván, B.; Kiss, É. Amphiphilic Hyperbranched Polyglycerols in a New Role as Highly Efficient Multifunctional Surface Active Stabilizers for Poly(Lactic/Glycolic Acid) Nanoparticles. RSC Adv. 2017, 7, 4348–4352. [Google Scholar] [CrossRef]
- Ábrahám, Á.; Katona, M.; Kasza, G.; Kiss, É. Amphiphilic Polymer Layer—Model Cell Membrane Interaction Studied by QCM and AFM. Eur. Polym. J. 2017, 93, 212–221. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Achazi, K.; Wycisk, V.; Licha, K.; Adeli, M.; Haag, R. Fullerene Polyglycerol Amphiphiles as Unimolecular Transporters. Langmuir 2017, 33, 6595–6600. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, G.; Zhan, X.; Zhuo, K.; Wang, J.; Wang, Y. Supramolecular Vector/Drug Coassemblies of Polyglycerol Dendrons and Rutin Enhance the pH Response. Langmuir 2022, 38, 3392–3402. [Google Scholar] [CrossRef]
- Jafari, M.; Abolmaali, S.S.; Borandeh, S.; Najafi, H.; Zareshahrabadi, Z.; Heidari, R.; Azarpira, N.; Zomorodian, K.; Tamaddon, A.M. Amphiphilic Hyperbranched Polyglycerol Nanoarchitectures for Amphotericin B Delivery in Candida Infections. Biomater. Adv. 2022, 139, 212996. [Google Scholar] [CrossRef]
- Rafiee, Z.; Bodaghi, A.; Omidi, S. Fabrication of a Photo- and pH-Sensitive Micelle by Self-Assembly of Azobenzene Polyglycerol for Anticancer Drug Delivery. Monatsh. Chem. 2023, 154, 259–265. [Google Scholar] [CrossRef]
- Pethő, L.; Kasza, G.; Lajkó, E.; Láng, O.; Kőhidai, L.; Iván, B.; Mező, G. Amphiphilic Drug–Peptide–Polymer Conjugates Based on Poly(Ethylene Glycol) and Hyperbranched Polyglycerol for Epidermal Growth Factor Receptor Targeting: The Effect of Conjugate Aggregation on in Vitro Activity. Soft Matter 2020, 16, 5759–5769. [Google Scholar] [CrossRef]
- Istratov, V.; Kautz, H.; Kim, Y.-K.; Schubert, R.; Frey, H. Linear-Dendritic Nonionic Poly(Propylene Oxide)–Polyglycerol Surfactants. Tetrahedron 2003, 59, 4017–4024. [Google Scholar] [CrossRef]
- Oikawa, Y.; Lee, S.; Kim, D.H.; Kang, D.H.; Kim, B.-S.; Saito, K.; Sasaki, S.; Oishi, Y.; Shibasaki, Y. One-Pot Synthesis of Linear-Hyperbranched Amphiphilic Block Copolymers Based on Polyglycerol Derivatives and Their Micelles. Biomacromolecules 2013, 14, 2171–2178. [Google Scholar] [CrossRef]
- Demina, T.V.; Budkina, O.A.; Badun, G.A.; Melik-Nubarov, N.S.; Frey, H.; Müller, S.S.; Nieberle, J.; Grozdova, I.D. Cytotoxicity and Chemosensitizing Activity of Amphiphilic Poly(Glycerol)–Poly(Alkylene Oxide) Block Copolymers. Biomacromolecules 2014, 15, 2672–2681. [Google Scholar] [CrossRef]
- Deng, Y.; Saucier-Sawyer, J.K.; Hoimes, C.J.; Zhang, J.; Seo, Y.-E.; Andrejecsk, J.W.; Saltzman, W.M. The Effect of Hyperbranched Polyglycerol Coatings on Drug Delivery Using Degradable Polymer Nanoparticles. Biomaterials 2014, 35, 6595–6602. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, B.; Hyun, D.C.; Kim, J.W.; Shim, H.-E.; Kang, S.-W.; Jeong, U. Photocrosslinkable Poly(ε-Caprolactone)- b -Hyperbranched Polyglycerol (PCL- b -hbPG) with Improved Biocompatibility and Stability for Drug Delivery. Macromol. Chem. Phys. 2015, 216, 1161–1170. [Google Scholar] [CrossRef]
- Le Kim, T.H.; Yu, J.H.; Jun, H.; Yang, M.Y.; Yang, M.-J.; Cho, J.-W.; Kim, J.W.; Kim, J.S.; Nam, Y.S. Polyglycerolated Nanocarriers with Increased Ligand Multivalency for Enhanced in Vivo Therapeutic Efficacy of Paclitaxel. Biomaterials 2017, 145, 223–232. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-Derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome Formation by Amphiphilic Polyglycerol- b -Polydisulfide- b -Polyglycerol and Glutathione-Triggered Intracellular Drug Delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef]
- Wehr, R.; Gaitzsch, J.; Daubian, D.; Fodor, C.; Meier, W. Deepening the Insight into Poly(Butylene Oxide)- Block -Poly(Glycidol) Synthesis and Self-Assemblies: Micelles, Worms and Vesicles. RSC Adv. 2020, 10, 22701–22711. [Google Scholar] [CrossRef]
- Gosecki, M.; Urbaniak, M.; Martinho, N.; Gosecka, M.; Zloh, M. Evaluation of Encapsulation Potential of Selected Star-Hyperbranched Polyglycidol Architectures: Predictive Molecular Dynamics Simulations and Experimental Validation. Molecules 2023, 28, 7308. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wang, W.; Yang, J.; Hu, Y. High-Flux and Chlorine-Resistant Nanofiltration Membrane Fabricated via Phase Inversion Using Polysulfone-b-Polyglycerol Hyperbranched Block Copolymer. Desalination 2024, 575, 117314. [Google Scholar] [CrossRef]
- Kasza, G.; Fábián, Á.; Fecske, D.; Kardos, A.; Mészáros, R.; Horváti, K.; Iván, B. Hyperbranched Polyglycerol Grafted Poly(N,N-Diethylacrylamide) Thermoresponsive Copolymers as Biocompatible, Highly Efficient Encapsulation and Sustained Release Systems of Curcumin. Eur. Polym. J. 2024, 219, 113378. [Google Scholar] [CrossRef]
- Pol, B.J.M.; Van Der Does, L.; Bantjes, A.; Van Wachem, P.B.; Van Luyn, M.J.A. In Vivo Testing of Crosslinked Polyethers. I. Tissue Reactions and Biodegradation. J. Biomed. Mater. Res. 1996, 32, 307–320. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Deng, J.-R.; Gao, Y.-Z.; Wu, Y.-X. Hemocompatible, Biocompatible and Antifouling Acylated Dextran-g-Polytetrahydrofuran Graft Copolymer with Silver Nanoparticles: Synthesis, Characterization and Properties. Mater. Sci. Eng. C 2021, 123, 111998. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Chen, J.-C.; Cui, Z.; Zhao, C.-L.; Wu, Y.-X. Biocompatible Propylene Glycol Alginate-g-Polytetrahydrofuran Amphiphilic Graft Copolymers for Highly Effective Drug Carriers. Polymer 2022, 246, 124706. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical Applications of Polyurethane Materials and Coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Terban, M.W.; Dabbous, R.; Debellis, A.D.; Pöselt, E.; Billinge, S.J.L. Structures of Hard Phases in Thermoplastic Polyurethanes. Macromolecules 2016, 49, 7350–7358. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Napiwocki, B.N.; Hagerty, B.S.; Chen, G.; Turng, L.-S. Biocompatible, Degradable Thermoplastic Polyurethane Based on Polycaprolactone-Block-Polytetrahydrofuran-Block-Polycaprolactone Copolymers for Soft Tissue Engineering. J. Mater. Chem. B 2017, 5, 4137–4151. [Google Scholar] [CrossRef]
- Sivanesan, D.; Kim, S.; Jang, T.W.; Kim, H.J.; Song, J.; Seo, B.; Lim, C.-S.; Kim, H.-G. Effects of Flexible and Rigid Parts of ε-Caprolactone and Tricyclodecanediol Derived Polyurethane on the Polymer Properties of Epoxy Resin. Polymer 2021, 237, 124374. [Google Scholar] [CrossRef]
- Utaloff, K.; Kothmann, M.H.; Ciesielski, M.; Döring, M.; Neumeyer, T.; Altstädt, V.; Gorman, I.; Henningsen, M. Improvement of Fracture Toughness and Glass Transition Temperature of DGEBA-based Epoxy Systems Using Toughening and Crosslinking Modifiers. Polym. Eng. Sci. 2019, 59, 86–95. [Google Scholar] [CrossRef]
- Fodor, C.; Kali, G.; Iván, B. Poly(N-Vinylimidazole)-l-Poly(Tetrahydrofuran) Amphiphilic Conetworks and Gels: Synthesis, Characterization, Thermal and Swelling Behavior. Macromolecules 2011, 44, 4496–4502. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, W.; Zhang, Y.; Gao, T.; Yang, H.; Cao, Z.; Zhong, J. Ultra-High Stretchability and Shape Fixation Rate Shape Memory Polyurethanes Based on Cyclic Polytetrahydrofuran Molecular Rings. Polymer 2024, 311, 127578. [Google Scholar] [CrossRef]
- Niu, L.; Nagarajan, R.; Guan, F.; Samuelson, L.A.; Kumar, J. Biocatalytic Synthesis of Multi-block Copolymer Composed of Poly(Tetrahydrofuran) and Poly(Ethylene Oxide). J. Macromol. Sci. Part A 2006, 43, 1975–1981. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, M.; Zhang, A.; Feng, Z. Preparation and Evaluation of Novel Amphiphilic Glycopeptide Block Copolymers as Carriers for Controlled Drug Release. Polymer 2008, 49, 446–454. [Google Scholar] [CrossRef]
- Rasolonjatovo, B.; Gomez, J.-P.; Même, W.; Gonçalves, C.; Huin, C.; Bennevault-Celton, V.; Le Gall, T.; Montier, T.; Lehn, P.; Cheradame, H.; et al. Poly(2-Methyl-2-Oxazoline)-b-Poly(Tetrahydrofuran)-b-Poly(2-Methyl-2-Oxazoline) Amphiphilic Triblock Copolymers: Synthesis, Physicochemical Characterizations, and Hydrosolubilizing Properties. Biomacromolecules 2015, 16, 748–756. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Ihara, E.; Saegusa, T. Block Copolymerization of Tetrahydrofuran with Cyclic Imino Ether: Synthesis of a New Nonionic Polymer Surfactant. Macromolecules 1990, 23, 1586–1589. [Google Scholar] [CrossRef]
- Zhou, P.; Dai, X.-G.; Kong, J.; Ling, J. Synthesis of Well-Defined Poly(Tetrahydrofuran)-b-Poly(a-Amino Acid)s via Cationic Ring-Opening Polymerization (ROP) of Tetrahydrofuran and Nucleophilic ROP of N-Thiocarboxyanhydrides. Chin. J. Polym. Sci. 2021, 39, 702–708. [Google Scholar] [CrossRef]
- Lim, S.-H.; Cha, E.-J.; Huh, J.; Ahn, C.-H. Micelle Behavior of Copolymers Composed of Linear and Hyperbranched Blocks in Aqueous Solution. Macromol. Chem. Phys. 2009, 210, 1734–1738. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and Characterization of Curcumin Loaded PLA—Hyperbranched Polyglycerol Electrospun Blend for Wound Dressing Applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Lübtow, M.M.; Nelke, L.C.; Seifert, J.; Kühnemundt, J.; Sahay, G.; Dandekar, G.; Nietzer, S.L.; Luxenhofer, R. Drug Induced Micellization into Ultra-High Capacity and Stable Curcumin Nanoformulations: Physico-Chemical Characterization and Evaluation in 2D and 3D in Vitro Models. J. Control. Release 2019, 303, 162–180. [Google Scholar] [CrossRef]
- Nagy, N.Z.; Varga, Z.; Mihály, J.; Kasza, G.; Iván, B.; Kiss, É. Highly Efficient Encapsulation of Curcumin into and pH-Controlled Drug Release from Poly(ε-Caprolactone) Nanoparticles Stabilized with a Novel Amphiphilic Hyperbranched Polyglycerol. Express Polym. Lett. 2020, 14, 90–101. [Google Scholar] [CrossRef]
- Nagy, N.Z.; Varga, Z.; Mihály, J.; Domján, A.; Fenyvesi, É.; Kiss, É. Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers 2020, 12, 2167. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Chen, J.-C.; Wu, Y.-X. Amphiphilic Graft Copolymers of Quaternized Alginate-g-Polytetrahydrofuran for Anti-Protein Surfaces, Curcumin Carriers, and Antibacterial Materials. ACS Appl. Polym. Mater. 2021, 7, 3465–3477. [Google Scholar] [CrossRef]
- Gerardos, A.M.; Forys, A.; Trzebicka, B.; Pispas, S. Self-Assembly of Hydrophobic Hyperbranched PLMA Homopolymer with –COOH End Groups as Effective Nanocarriers for Bioimaging Applications. Polymers 2024, 16, 2166. [Google Scholar] [CrossRef]
- Datta, S.; Huntosová, V.; Jutková, A.; Seliga, R.; Kronek, J.; Tomkova, A.; Lenkavská, L.; Mácajová, M.; Bilcík, B.; Kundeková, B.; et al. Influence of Hydrophobic Side-Chain Length in Amphiphilic Gradient Copoly(2-oxazoline)s on the Therapeutics Loading, Stability, Cellular Uptake and Pharmacokinetics of Nano-Formulation with Curcumin. Pharmaceutics 2022, 14, 2576. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Kronek, J.; Nadova, Z.; Timulakova, L.; Minarcikova, A.; Miskovsky, P. Effect of polymer architecture on the properties and in vitro cytotoxicity of drug formulation: A case study with mono-and di-gradient amphiphilic poly(2-oxazoline)s. Eur. J. Pharm. Biopharm. 2025, 208, 114635. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior. Int. J. Mol. Sci. 2023, 24, 4621. [Google Scholar] [CrossRef]
- Pantelaiou, M.A.; Vagenas, D.; Pispas, S. Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin. Polymers 2025, 17, 635. [Google Scholar] [CrossRef]
- Ginosati, F.; Vagenas, D.; Gerardos, A.M.; Pispas, S. Multi-Responsive Amphiphilic Hyperbranched Poly[(2-dimethyl aminoethyl methacrylate)-co-(benzyl methacrylate)]copolymers: Self-Assembly and Curcumin Encapsulation in Aqueous Media. Materials 2025, 18, 513. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Song, L.; Shen, Y.; Hou, J.; Lei, L.; Guo, S.; Qian, C. Polymeric micelles for parenteral delivery of curcumin: Preparation, characterization and in vitro evaluation. Colloids Surf. A 2011, 390, 25–32. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the curcumin—casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Lübtow, M.M.; Marciniak, H.; Schmiedel, A.; Roos, M.; Lambert, C.; Luxenhofer, R. Ultra-High to Ultra-Low Drug-Loaded Micelles: Probing Host–Guest Interactions by Fluorescence Spectroscopy. Chem-Eur. J. 2019, 25, 12601–12610. [Google Scholar] [CrossRef]
- Liu, Q.; Han, C.; Tian, Y.; Liu, T. Fabrication of curcumin-loaded zein nanoparticles stabilized by sodium caseinate/sodium alginate: Curcumin solubility, thermal properties, rheology, and stability. Process Biochem. 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: A fluorescence probe study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, X.; Xu, L.; Zhang, L.; Huang, H. Mitochondria-targeted curcumin loaded CTPP– PEG–PCL self-assembled micelles for improving liver fibrosis therapy. RSC Adv. 2021, 11, 5348–5360. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor Targeted Curcumin Delivery by Folate Modified MPEG-PCL Self-Assembly Micelles for Colorectal Cancer Therapy. Int. J. Nanomed. 2020, 15, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Danafar, H.; Taromchi, A.H.; Rakhshbahar, A.; Sharafi, A.; Hasani, V.; Tafvizi, S.; Rostam, M. Co-delivery of methotrexate and curcumin with mPEG-PCL polymeric nanoparticles and evaluation of toxicity effect on MCF7 breast cancer cell line. Inorg. Chem. Commun. 2022, 142, 109715. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Zheng, W.; Chen, J.; Xu, Q.; Liang, Z.; Zhang, H.; Lin, J.; Xie, W.; Zhuo, Y.; et al. Metabolomic and Proteomic Analyses Unveil That Polyethylene Glycol–Polycaprolactone-Loaded Curcumin Nanoparticles Induce Mitochondrial Dysfunction and Metabolic Reprogramming to Suppress Melanoma Growth. ACS Appl. Mater. Interfaces 2025, 17, 33422–33438. [Google Scholar] [CrossRef]
- Liang, H.; Friedman, J.M.; Nacharaju, P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 297–304. [Google Scholar] [CrossRef]
- Kumari, P.; Swami, M.O.; Nadipalli, S.K.; Myneni, S.; Ghosh, B.; Biswas, S. Curcumin Delivery by Poly(Lactide)-Based Co-Polymeric Micelles: An In Vitro Anticancer Study. Pharm. Res. 2016, 33, 826–841. [Google Scholar] [CrossRef]
- Rostami, N.; Faridghiasi, F.; Ghebleh, A.; Noei, H.; Samadzadeh, M.; Gomari, M.M.; Tajiki, A.; Abdouss, M.; Aminoroaya, A.; Kumari, M.; et al. Design, Synthesis, and Comparison of PLA-PEG-PLA and PEG-PLA-PEG Copolymers for Curcumin Delivery to Cancer Cells. Polymers 2023, 15, 3133. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Wu, H.; Yin, T.; Li, K.; Wang, R.; Chen, Y.; Jing, L. Encapsulation property of hyperbranched polyglycerols as prospective drug delivery systems. Polym. Chem. 2018, 9, 300–306. [Google Scholar] [CrossRef]
- Feng, S.S. Nanoparticles of biodegradable polymers for new-concept chemotherapy. Expert Rev. Med. Devic. 2004, 1, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 238428. [Google Scholar] [CrossRef]
- Lee, C.W.; Jang, L.L.; Pan, H.J.; Chen, Y.R.; Chen, C.C.; Lee, C.H. Membrane roughness as a sensitive parameter reflecting the status of neuronal cells in response to chemical and nanoparticle treatments. J. Nanobiotechnol. 2016, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Wang, C.C.; Lee, C.H. Mechanoprofiling on membranes of living cells with atomic force microscopy and optical nano-profilometry. Adv. Phys. 2017, 2, 608–621. [Google Scholar] [CrossRef]
- Kapus, A.; Grinstein, S.; Wasan, S.; Kandasamy, R.; Orlowski, J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J. Biol. Chem. 1994, 269, 23544–23552. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Goddard, E.D.; Turro, N.J.; Kuo, P.L. Fluorescence probes for critical micelle concentration. Langmuir 1985, 1, 352–355. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Moers, C.; Chen, S.; Xu, H.; Wang, Z. Block copolymer aggregates with photo-responsive switches: Towards a controllable supramolecular container. Polymer 2009, 50, 4821–4828. [Google Scholar] [CrossRef]
- Wacha, A.; Varga, Z.; Bóta, A. CREDO: A New General-purpose Laboratory Instrument for Small-angle X-ray Scattering. J. Appl. Crystal. 2014, 47, 1749–1754. [Google Scholar] [CrossRef]
- Wacha, A. Optimized Pinhole Geometry for Small-Angle Scattering. J. Appl. Crystal. 2015, 48, 1843–1848. [Google Scholar] [CrossRef]
- Ponten, J.; Macintyre, E.H. Long term culture of normal and neoplastic human glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med. 2016, 8, 354re3. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.F.; Sawyer, B.; Sträuli, U. Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]

| Polymer | Mtheor (g/mol) | Mn (g/mol) | Đ | Tg1 | Tg2 | ||
|---|---|---|---|---|---|---|---|
| 1H NMR a | 1H NMR b | GPC | (°C) | ||||
| P1 | 2200 | 2190 | 2320 | 4000 | 1.47 | −77 | −43 |
| P2 | 3300 | 3410 | 3500 | 5500 | 1.51 | −74 | −32 |
| P3 | 5500 | 5890 | 6020 | 7000 | 1.64 | −79 | −30 |
| Polymer | PTHF Content (m/m%) | cmc | Micellar Size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pyrene Probe | Surface Tension | DLS | |||||||
| (g/L) | (µmol/L) | (g/L) | (µmol/L) | (g/L) | (µmol/L) | d (nm) | Ð | ||
| P1 | 55 | 0.11 | 50.2 | 0.20 | 91.3 | 0.18 | 82.2 | 13.2 | 0.056 |
| P2 | 35 | 0.13 | 38.1 | 0.34 | 99.7 | 0.21 | 61.4 | 13.1 | 0.070 |
| P3 | 21 | 0.18 | 30.6 | 0.56 | 95.1 | 0.24 | 40.7 | 14.8 | 0.133 |
| Polymer | ccurcumin | PM/W | DLC (%) | ||
|---|---|---|---|---|---|
| (g/L) | (mmol/L) | (g/g) | (mol/mol) | ||
| P1 | 1.72 ± 0.15 | 4.68 ± 0.42 | 1570 ± 139 | 3.3 ± 0.3 | 17.0 ± 1.3 |
| P2 | 0.92 ± 0.30 | 2.48 ± 0.83 | 833 ± 277 | 1.8 ± 0.6 | 14.3 ± 4.0 |
| P3 | 0.60 ± 0.05 | 1.64 ± 0.13 | 550 ± 44 | 1.2 ± 0.1 | 16.2 ± 1.1 |
| Sample | Rα (nm) |
|---|---|
| untreated cell | 14.2 ± 4.8 |
| curcumin-treated cell | 11.4 ± 3.5 |
| P1 + C-treated cell | 10.5 ± 3.7 |
| P2 + C-treated cell | 13.5 ± 4.8 |
| P3 + C-treated cell | 5.7 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fecske, D.; Kasza, G.; Gyulai, G.; Horváti, K.; Szabó, M.; Wacha, A.; Varga, Z.; Szarka, G.; Thomann, Y.; Thomann, R.; et al. Self-Assembling Amphiphilic ABA Triblock Copolymers of Hyperbranched Polyglycerol with Poly(tetrahydrofuran) and Their Nanomicelles as Highly Efficient Solubilization and Delivery Systems of Curcumin. Int. J. Mol. Sci. 2025, 26, 5866. https://doi.org/10.3390/ijms26125866
Fecske D, Kasza G, Gyulai G, Horváti K, Szabó M, Wacha A, Varga Z, Szarka G, Thomann Y, Thomann R, et al. Self-Assembling Amphiphilic ABA Triblock Copolymers of Hyperbranched Polyglycerol with Poly(tetrahydrofuran) and Their Nanomicelles as Highly Efficient Solubilization and Delivery Systems of Curcumin. International Journal of Molecular Sciences. 2025; 26(12):5866. https://doi.org/10.3390/ijms26125866
Chicago/Turabian StyleFecske, Dóra, György Kasza, Gergő Gyulai, Kata Horváti, Márk Szabó, András Wacha, Zoltán Varga, Györgyi Szarka, Yi Thomann, Ralf Thomann, and et al. 2025. "Self-Assembling Amphiphilic ABA Triblock Copolymers of Hyperbranched Polyglycerol with Poly(tetrahydrofuran) and Their Nanomicelles as Highly Efficient Solubilization and Delivery Systems of Curcumin" International Journal of Molecular Sciences 26, no. 12: 5866. https://doi.org/10.3390/ijms26125866
APA StyleFecske, D., Kasza, G., Gyulai, G., Horváti, K., Szabó, M., Wacha, A., Varga, Z., Szarka, G., Thomann, Y., Thomann, R., Mülhaupt, R., Kiss, É., Domján, A., Bősze, S., Bereczki, L., & Iván, B. (2025). Self-Assembling Amphiphilic ABA Triblock Copolymers of Hyperbranched Polyglycerol with Poly(tetrahydrofuran) and Their Nanomicelles as Highly Efficient Solubilization and Delivery Systems of Curcumin. International Journal of Molecular Sciences, 26(12), 5866. https://doi.org/10.3390/ijms26125866












