Role of Extracellular Vesicles of Stem Cells from Human Exfoliated Deciduous Teeth in Osteogenesis
Abstract
1. Introduction
2. Results
2.1. Identification of EVs Extracted from SHED
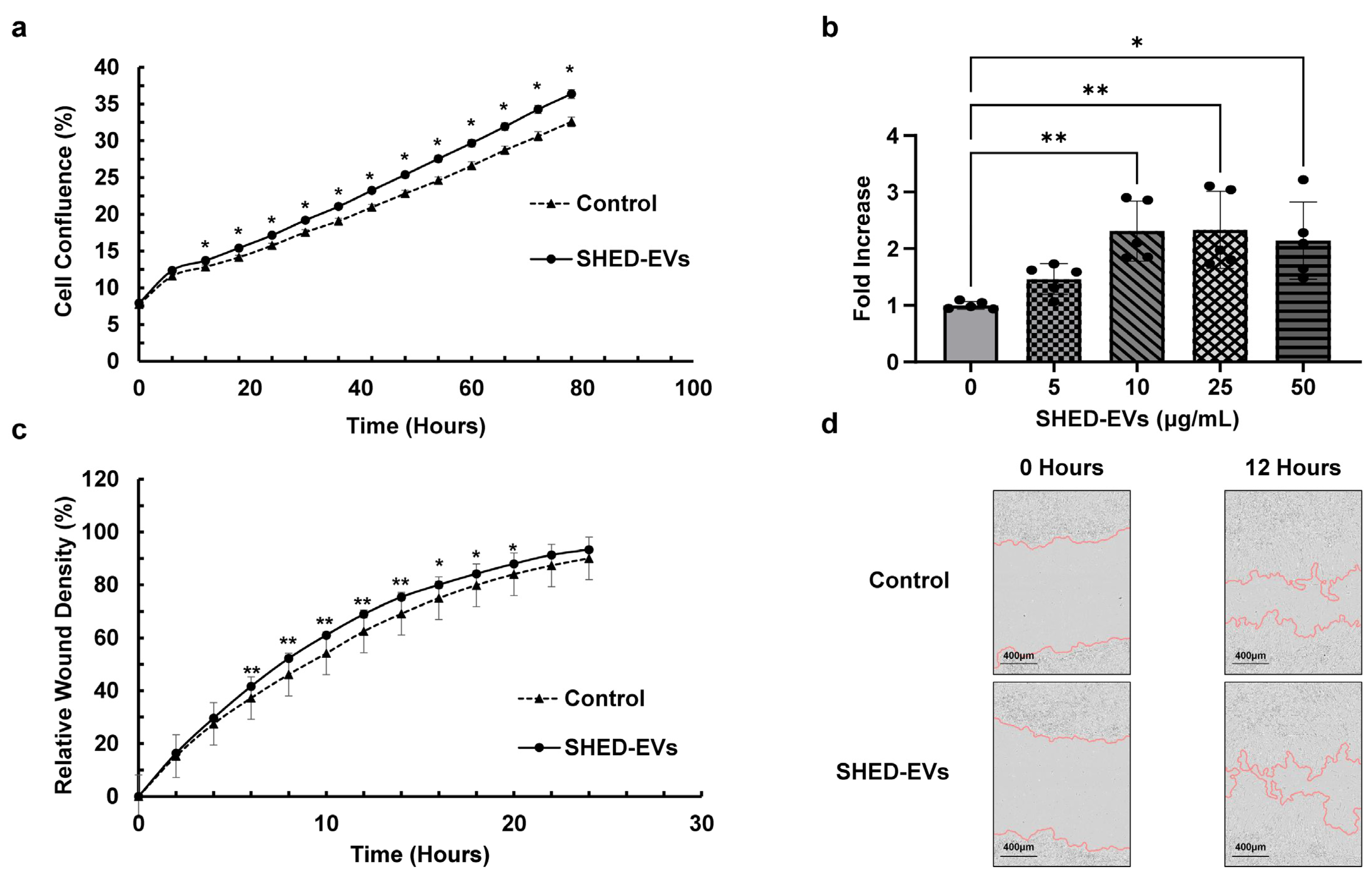
2.2. Cell Proliferation and Migration Ability of SHED-EVs
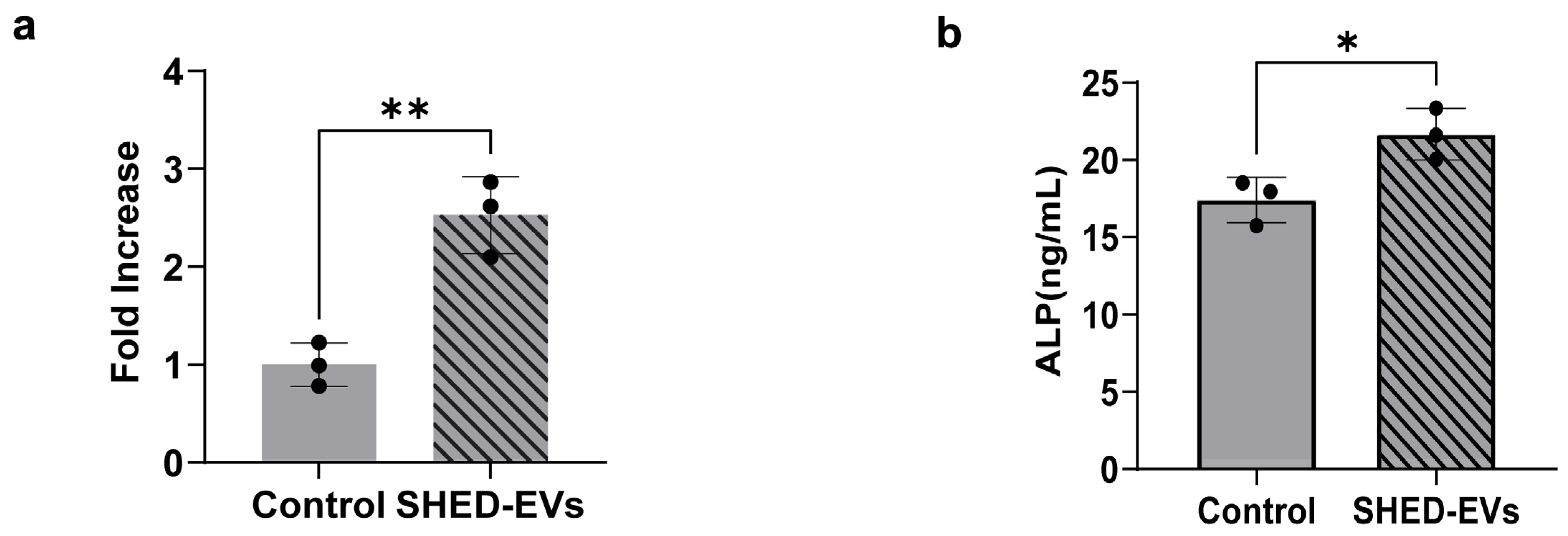
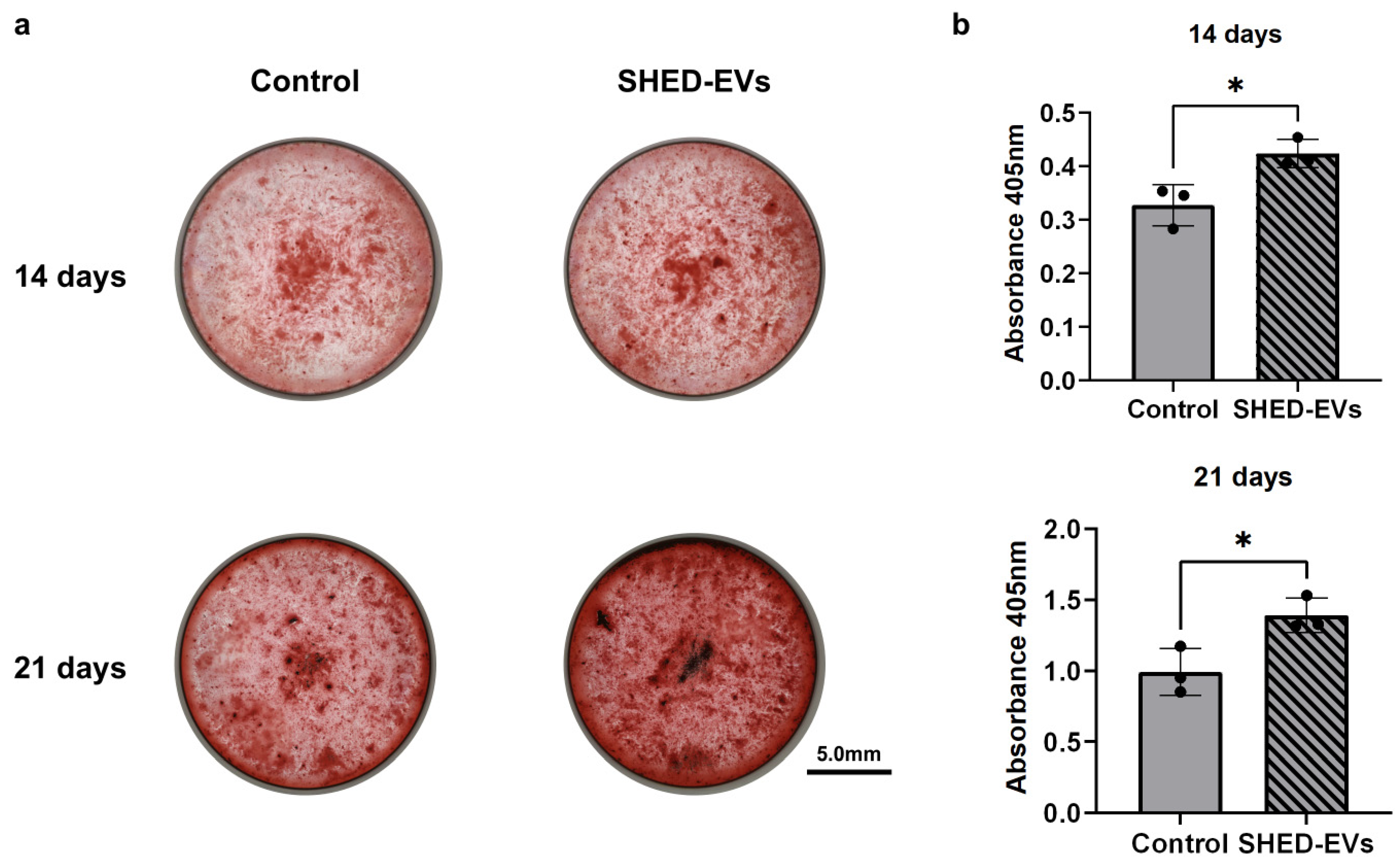
2.3. Bone Regeneration Potential of SHED-Evs
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of SHED and HCOs
4.2. Isolation and Purification of EVs Derived from SHED
4.3. Identification of EVs Derived from SHED
4.3.1. Transmission Electron Microscopy
4.3.2. Nanoparticle Tracking Analysis of EVs
4.4. Cell Proliferation Test
4.4.1. Observation of Cell Proliferative Potential Using Live Cell Imaging System
4.4.2. Evaluation of the Cell Proliferative Potential via BrdU
4.4.3. Scratch Wound Assay
4.5. Evaluation of Alkaline Phosphatase Expression
4.5.1. Real-Time Polymerase Chain Reaction
4.5.2. ALP Quantification
4.5.3. Alizarin Red S Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSC | mesenchymal stem cells |
| DPSC | dental pulp stem cells |
| SHEDs | stem cells from human exfoliated deciduous teeth |
| BMSCs | bone mesenchymal stem cells |
| BMP | bone morphogenetic protein |
| ALP | alkaline phosphatase |
| ARS | Alizarin Red S |
References
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Li, A.; Guo, F.; Pan, Q.; Chen, S.; Chen, J.; Liu, H.F.; Pan, Q. Mesenchymal stem cell therapy: Hope for patients with systemic lupus erythematosus. Front. Immunol. 2021, 12, 728190. [Google Scholar] [CrossRef]
- Rodríguez-Pallares, J.; García-Garrote, M.; Parga, J.A.; Labandeira-García, J.L. Combined cell-based therapy strategies for the treatment of Parkinson’s disease: Focus on mesenchymal stromal cells. Neural Regen. Res. 2023, 18, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.M.; Deng, K.Q.; Zhang, M.; Wu, X.C.; Yang, R.L.; Fu, S.P.; Lin, F.Q.; Zhang, Q.; Ao, J.; Zhang, T. Multiple strategies enhance the efficacy of MSCs transplantation for spinal cord injury. Biomed. Pharmacother. 2023, 157, 114011. [Google Scholar] [CrossRef]
- Luchetti, F.; Carloni, S.; Nasoni, M.G.; Reiter, R.J.; Balduini, W. Melatonin, tunneling nanotubes, mesenchymal cells, and tissue regeneration. Neural Regen. Res. 2023, 18, 760–762. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wang, S.; Xu, L.; Zhang, M.; Wang, G.; Jin, Y.; Zhang, X.; Jiang, X. Comparison of the use of adipose tissue-derived and bone marrow-derived stem cells for rapid bone regeneration. J. Dent. Res. 2013, 92, 1136–1141. [Google Scholar] [CrossRef]
- Kunkel, N.; Wagner, A.; Gehwolf, R.; Heimel, P.; Tempfer, H.; Korntner, S.; Augat, P.; Resch, H.; Redl, H.; Betz, O.; et al. Comparing the osteogenic potential of bone marrow and tendon-derived stromal cells to repair a critical-sized defect in the rat femur. J. Tissue Eng. Regen. Med. 2017, 11, 2014–2023. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regulation: Systematic review of preclinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mat. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef]
- Li, J.J.; Hosseini-Beheshti, E.; Grau, G.E.; Zreiqat, H.; Little, C.B. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials 2019, 9, 261. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Svennerholm, K.; Shelke, G.V.; Bandeira, E.; Lässer, C.; Jang, S.C.; Chandode, R.; Gribonika, I.; Lötvall, J. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res. Ther. 2019, 10, 231. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, R.; Wu, C.; Wang, L.; Zhang, C. Exosome: A novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 2016, 17, 712. [Google Scholar] [CrossRef]
- Wang, X.; Thomsen, P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin. Pharmacol. Toxicol. 2021, 128, 18–36. [Google Scholar] [CrossRef]
- He, X.; Chu, X.Y.; Chen, X.; Xiang, Y.L.; Li, Z.L.; Gao, C.Y.; Luan, Y.Y.; Yang, K.; Zhang, D.L. Dental pulp stem cell-derived extracellular vesicles loaded with hydrogels promote osteogenesis in rats with alveolar bone defects. Mol. Med. Rep. 2025, 31, 29. [Google Scholar] [CrossRef]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in platelet rich plasma-derived extracellular vesicles for regenerative medicine: A systematic-narrative review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, L.; Wang, T.; Xian, P.; Wang, H.; Long, Q. Inhalation of MSC-EVs is a noninvasive strategy for ameliorating acute lung injury. J. Control. Release 2022, 345, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 3402. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Feng, D.; Li, Z.; Yang, L.; Liang, H.; He, H.; Liu, L.; Zhang, W. BMSC-EV-derived lncRNA NORAD facilitates migration, invasion, and angiogenesis in osteosarcoma cells by regulating CREBBP via delivery of miR-877-3p. Oxidative Med. Cell. Longev. 2022, 2022, 8825784. [Google Scholar] [CrossRef]
- Lan, Q.; Xiao, X.; Bi, X.; Gu, Y.; Ai, Y. Effects of periodontal ligament stem cell-derived exosomes on osteoblastic proliferation, migration, differentiation, apoptosis, and signaling pathways. Oral Dis. 2024, 30, 710–718. [Google Scholar] [CrossRef]
- Murshed, M.; Harmey, D.; Millán, J.L.; McKee, M.D.; Karsenty, G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005, 19, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Balbaied, T.; Moore, E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: Recent approaches in cells culture techniques. Biosensors 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X.; et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Chaput, N.; Théry, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- de Miguel Pérez, D.; Rodriguez Martínez, A.; Ortigosa Palomo, A.; Delgado Ureña, M.; Garcia Puche, J.L.; Serrano, M.J. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413. [Google Scholar] [CrossRef] [PubMed]
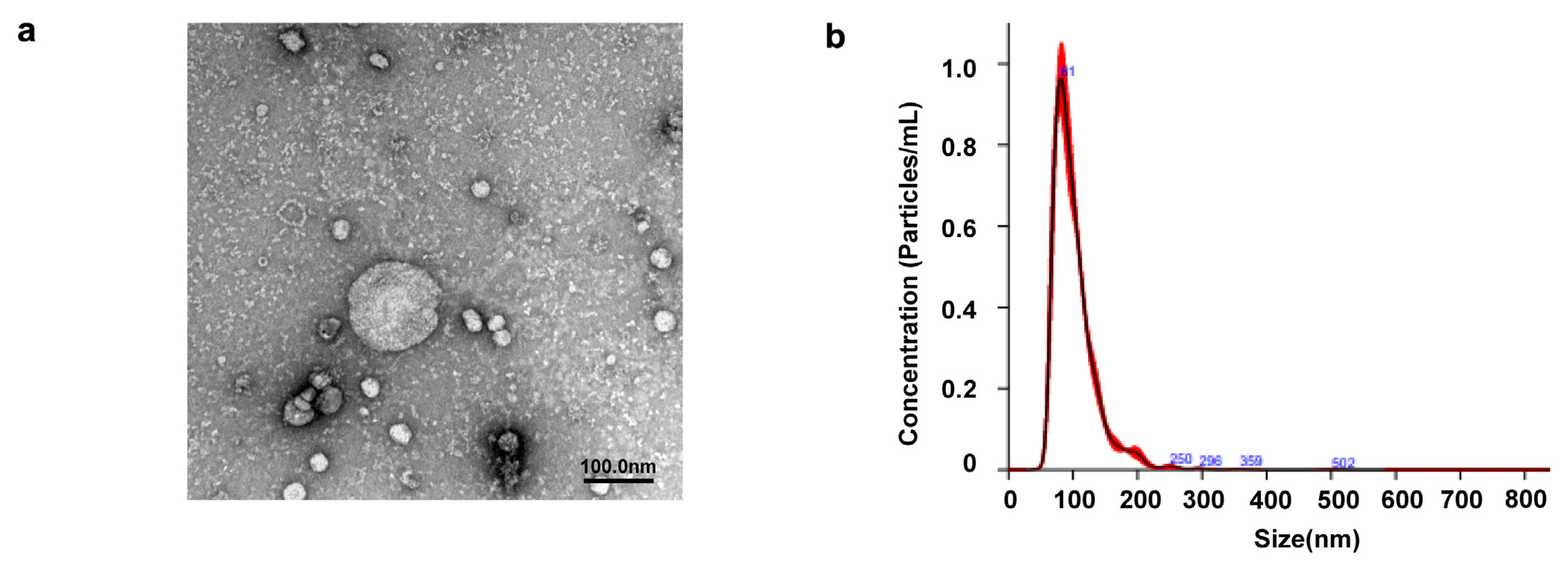
| Parameters | Merged Data (nm) | Standard Error of the Mean (nm) |
|---|---|---|
| Mean | 102.2 | 1.4 |
| Mode | 79.9 | 3.2 |
| SD | 36 | 1.1 |
| D10 | 70.6 | 1.4 |
| D50 | 93.5 | 1.4 |
| D90 | 142.3 | 2.1 |
| Gene | Sequence (5′→3′) |
|---|---|
| GAPDH (Forward) | GGCCTCCAAGGAGTAAGACC |
| GAPDH (Reverse) | AGGGGTCTACATGGCAACTG |
| ALP (Forward) | AGAATCTGGTGCAGGAATGG |
| ALP (Reverse) | CATGAGATGGGTCACAGACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, R.; Kunimatsu, R.; Ito, S.; Ogasawara, T.; Ogashira, S.; Nakatani, A.; Rikitake, K.; Odo, A.; Hirabae, A.; Koyanagi, I.; et al. Role of Extracellular Vesicles of Stem Cells from Human Exfoliated Deciduous Teeth in Osteogenesis. Int. J. Mol. Sci. 2025, 26, 5841. https://doi.org/10.3390/ijms26125841
Shibata R, Kunimatsu R, Ito S, Ogasawara T, Ogashira S, Nakatani A, Rikitake K, Odo A, Hirabae A, Koyanagi I, et al. Role of Extracellular Vesicles of Stem Cells from Human Exfoliated Deciduous Teeth in Osteogenesis. International Journal of Molecular Sciences. 2025; 26(12):5841. https://doi.org/10.3390/ijms26125841
Chicago/Turabian StyleShibata, Rio, Ryo Kunimatsu, Shota Ito, Tomohiro Ogasawara, Shintaro Ogashira, Ayaka Nakatani, Kodai Rikitake, Ayaka Odo, Akira Hirabae, Io Koyanagi, and et al. 2025. "Role of Extracellular Vesicles of Stem Cells from Human Exfoliated Deciduous Teeth in Osteogenesis" International Journal of Molecular Sciences 26, no. 12: 5841. https://doi.org/10.3390/ijms26125841
APA StyleShibata, R., Kunimatsu, R., Ito, S., Ogasawara, T., Ogashira, S., Nakatani, A., Rikitake, K., Odo, A., Hirabae, A., Koyanagi, I., Abe, T., Hiraki, T., Sakata, S., Yoshimi, Y., & Tanimoto, K. (2025). Role of Extracellular Vesicles of Stem Cells from Human Exfoliated Deciduous Teeth in Osteogenesis. International Journal of Molecular Sciences, 26(12), 5841. https://doi.org/10.3390/ijms26125841







