Body Circumference and Cognitive Function: Role of Apolipoprotein E ε4 in the Elderly
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
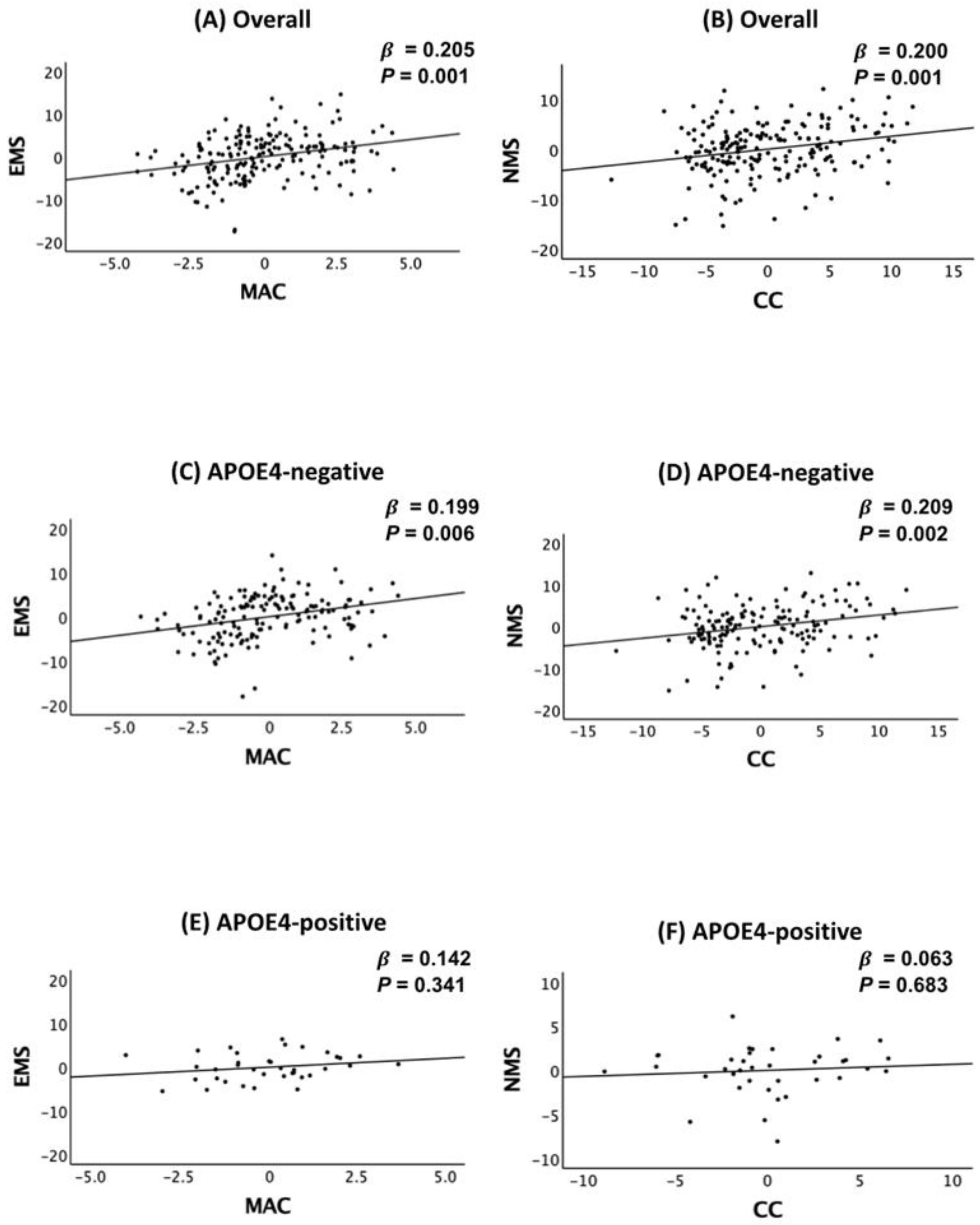
2.2. Association of Body Circumference with Cognition
2.3. APOE4 Moderation of the Association Between Body Circumference and Cognition
2.4. Sensitivity Analyses
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Standard Protocol Approvals, Registrations, and Participants Consent
4.3. Clinical Assessments
4.4. Measuring Body Circumferences and Blood Biomarkers
4.5. APOE4 Genotyping
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Wang, S.M.; Kang, D.W.; Um, Y.H.; Yoon, H.M.; Lee, S.; Choe, Y.S.; Kim, R.E.; Kim, D.; Lee, C.U.; et al. Development of a prediction model for cognitive impairment of sarcopenia using multimodal neuroimaging in non-demented older adults. Alzheimers Dement 2024, 20, 4868–4878. [Google Scholar] [CrossRef] [PubMed]
- Oudbier, S.J.; Goh, J.; Looijaard, S.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.; Van Ancum, J.M.; Verlaan, S.; Scheerman, K.; Meskers, C.G.M.; Maier, A.B. Lower Cognitive Function in Older Patients with Lower Muscle Strength and Muscle Mass. Dement. Geriatr. Cogn. Disord. 2018, 45, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef]
- Seo, Y.K.; Won, C.W.; Soh, Y. Associations between body composition and cognitive function in an elderly Korean population: A cohort-based cross-sectional study. Medicine 2021, 100, e25027. [Google Scholar] [CrossRef]
- Booranasuksakul, U.; Macdonald, I.A.; Stephan, B.C.M.; Siervo, M. Body Composition, Sarcopenic Obesity, and Cognitive Function in Older Adults: Findings From the National Health and Nutrition Examination Survey (NHANES) 1999–2002 and 2011–2014. J. Am. Nutr. Assoc. 2024, 43, 539–552. [Google Scholar] [CrossRef]
- Samuelsson, J.; Marseglia, A.; Wallengren, O.; Lindberg, O.; Dartora, C.; Cedres, N.; Shams, S.; Kern, S.; Zettergren, A.; Westman, E.; et al. Association of body composition with neuroimaging biomarkers and cognitive function; a population-based study of 70-year-olds. EBioMedicine 2025, 112, 105555. [Google Scholar] [CrossRef]
- Kim, J.; Suh, S.I.; Park, Y.J.; Kang, M.; Chung, S.J.; Lee, E.S.; Jung, H.N.; Eo, J.S.; Koh, S.B.; Oh, K.; et al. Sarcopenia is a predictor for Alzheimer’s continuum and related clinical outcomes. Sci. Rep. 2024, 14, 21074. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef]
- Spangler, H.B.; Lynch, D.H.; Howard, A.G.; Tien, H.C.; Du, S.; Zhang, B.; Wang, H.; Gordon Larsen, P.; Batsis, J.A. Association Between Mid-arm Muscle Circumference and Cognitive Function: A Longitudinal Study of Chinese Adults. J. Geriatr. Psychiatry Neurol. 2024, 37, 272–281. [Google Scholar] [CrossRef]
- Low, S.; Ng, T.P.; Lim, C.L.; Moh, A.; Ang, S.F.; Wang, J.; Goh, K.S.; Ang, K.; Tang, W.E.; Kwan, P.Y.; et al. Association between Lower Extremity Skeletal Muscle Mass and Impaired Cognitive Function in Type 2 Diabetes. Sci. Rep. 2020, 10, 2956. [Google Scholar] [CrossRef] [PubMed]
- Weisgraber, K.H.; Mahley, R.W. Human apolipoprotein E: The Alzheimer’s disease connection. FASEB J. 1996, 10, 1485–1494. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Huang, Y. Apolipoprotein e sets the stage: Response to injury triggers neuropathology. Neuron 2012, 76, 871–885. [Google Scholar] [CrossRef]
- Haney, M.S.; Palovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, W.; Liang, W.; Guo, T.; Hu, K.; Su, W.; Chen, Y.; Ning, M.; Liu, Y. Sarcopenia and cognitive impairment in older adults: Long-term prognostic implications based on the National Health and Nutrition Examination Survey (2011–2014). Exp. Gerontol. 2024, 196, 112561. [Google Scholar] [CrossRef]
- Cabett Cipolli, G.; Sanches Yassuda, M.; Aprahamian, I. Sarcopenia Is Associated with Cognitive Impairment in Older Adults: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 525–531. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Khalil, M.H. The BDNF-Interactive Model for Sustainable Hippocampal Neurogenesis in Humans: Synergistic Effects of Environmentally-Mediated Physical Activity, Cognitive Stimulation, and Mindfulness. Int. J. Mol. Sci. 2024, 25, 12924. [Google Scholar] [CrossRef]
- Danieli, K.; Guyon, A.; Bethus, I. Episodic Memory formation: A review of complex Hippocampus input pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 126, 110757. [Google Scholar] [CrossRef]
- Ekstrom, A.D.; Ranganath, C. Space, time, and episodic memory: The hippocampus is all over the cognitive map. Hippocampus 2018, 28, 680–687. [Google Scholar] [CrossRef]
- Meysami, S.; Raji, C.A.; Glatt, R.M.; Popa, E.S.; Ganapathi, A.S.; Bookheimer, T.; Slyapich, C.B.; Pierce, K.P.; Richards, C.J.; Lampa, M.G.; et al. Handgrip Strength Is Related to Hippocampal and Lobar Brain Volumes in a Cohort of Cognitively Impaired Older Adults with Confirmed Amyloid Burden. J. Alzheimers Dis. 2023, 91, 999–1006. [Google Scholar] [CrossRef]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef]
- Guiney, H.; Lucas, S.J.; Cotter, J.D.; Machado, L. Evidence cerebral blood-flow regulation mediates exercise-cognition links in healthy young adults. Neuropsychology 2015, 29, 1–9. [Google Scholar] [CrossRef]
- Tarumi, T.; Zhang, R. Cerebral hemodynamics of the aging brain: Risk of Alzheimer disease and benefit of aerobic exercise. Front. Physiol. 2014, 5, 6. [Google Scholar] [CrossRef]
- Steves, C.J.; Mehta, M.M.; Jackson, S.H.; Spector, T.D. Kicking Back Cognitive Ageing: Leg Power Predicts Cognitive Ageing after Ten Years in Older Female Twins. Gerontology 2016, 62, 138–149. [Google Scholar] [CrossRef]
- Jerez-Salas, F.; Campos-Jara, C.; Araya Sierralta, S.; Jerez-Mayorga, D.; Ramirez-Campillo, R.; Contreras-Diaz, G.; Carrasco-Alarcon, V.; Martinez-Cortes, H.; Arellano-Roco, C.; Hernandez-Cifuentes, V.; et al. Effects of Resistance Training on Executive Functions of Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis Protocol. Healthcare 2025, 13, 165. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Aerobic-Exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019, 37, 100572. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef]
- de Laat, K.F.; Tuladhar, A.M.; van Norden, A.G.; Norris, D.G.; Zwiers, M.P.; de Leeuw, F.E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2011, 134 Pt 1, 73–83. [Google Scholar] [CrossRef]
- Ko, Y.W.; Kim, S.M.; Kang, K.D.; Han, D.H. Changes in Functional Connectivity Between Default Mode Network and Attention Network in Response to Changes in Aerobic Exercise Intensity. Psychiatry. Investig. 2023, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, J.P.D.; Mensink, R.P.; Ivanov, D.; Adam, J.J.; Uludag, K.; Joris, P.J. Aerobic Exercise Training Improves Cerebral Blood Flow and Executive Function: A Randomized, Controlled Cross-Over Trial in Sedentary Older Men. Front. Aging Neurosci. 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Tai, L.M.; Ghura, S.; Koster, K.P.; Liakaite, V.; Maienschein-Cline, M.; Kanabar, P.; Collins, N.; Ben-Aissa, M.; Lei, A.Z.; Bahroos, N.; et al. APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: Current landscape, novel data, and future perspective. J. Neurochem. 2015, 133, 465–488. [Google Scholar] [CrossRef]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE4 and Abeta Oligomers Reduce BDNF Expression via HDAC Nuclear Translocation. J. Neurosci. 2015, 35, 7538–7551. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.; Sible, I.; Kapoor, A.; Engstrom, A.C.; Shenasa, F.; Alitin, J.P.M.; Gaubert, A.; Rodgers, K.E.; Bradford, D.; Mather, M.; et al. Blood Pressure Variability, Central Autonomic Network Dysfunction, and Cerebral Small-Vessel Disease in APOE4 Carriers. J. Am. Heart Assoc. 2024, 13, e034116. [Google Scholar] [CrossRef]
- Luo, X.; Jiaerken, Y.; Yu, X.; Huang, P.; Qiu, T.; Jia, Y.; Li, K.; Xu, X.; Shen, Z.; Guan, X.; et al. Associations between APOE genotype and cerebral small-vessel disease: A longitudinal study. Oncotarget 2017, 8, 44477–44489. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf circumference as a surrogate indicator for detecting low muscle mass in hospitalized geriatric patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Heal. 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Cournot, M.; Nourhashemi, F.; Reynish, W.; Riviere, D.; Vellas, B.; Grandjean, H. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J. Am. Geriatr. Soc. 2003, 51, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, Q.; Bi, G.; Wang, Y.; Yang, G.; Zhang, C.; Song, Y.; Wu, Y.; Gong, X.; Bi, Q. Comparison of different MRI-based unsupervised segmentation algorithms in predicting sarcopenia. Eur. J. Radiol. 2024, 181, 111748. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip Strength and the Risk of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Front. Aging Neurosci. 2021, 13, 625551. [Google Scholar] [CrossRef]
- Moreau, J.; Ordan, M.A.; Barbe, C.; Mazza, C.; Perrier, M.; Botsen, D.; Brasseur, M.; Portefaix, C.; Renard, Y.; Talliere, B.; et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 3677–3684. [Google Scholar] [CrossRef]
- Scott, D.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Hirani, V. Sarcopenia prevalence and functional outcomes in older men with obesity: Comparing the use of the EWGSOP2 sarcopenia versus ESPEN-EASO sarcopenic obesity consensus definitions. Clin. Nutr. 2023, 42, 1610–1618. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Mehrnezhad, A.; Razaviarab, N.; Barbosa-Silva, T.G.; Heymsfield, S.B. Calf circumference: Cutoff values from the NHANES 1999–2006. Am. J. Clin. Nutr. 2021, 113, 1679–1687. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, J.Y.; Hong, D.; Kim, D.; Min, J.Y.; Min, K.B. Sex differences in the association between sarcopenia and mild cognitive impairment in the older Korean population. BMC Geriatr. 2023, 23, 332. [Google Scholar] [CrossRef]
- Nagano, M.; Kabayama, M.; Ohata, Y.; Kido, M.; Rakugi, H.; Kamide, K. Sex differences in reduction of trunk muscle mass related to falls and cognitive function during the COVID-19 pandemic in older adults. Geriatr. Gerontol. Int. 2024, 24, 1060–1066. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [PubMed]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and neuropsychological assessment batteries. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, 47–53. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.U.; Lee, J.H.; Kim, K.W.; Jhoo, J.H.; Kim, S.Y.; Yoon, J.C.; Woo, S.I.; Ha, J.; Woo, J.I. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc. 2004, 10, 72–81. [Google Scholar] [CrossRef]
- Howieson, D.B.; Dame, A.; Camicioli, R.; Sexton, G.; Payami, H.; Kaye, J.A. Cognitive markers preceding Alzheimer’s dementia in the healthy oldest old. J. Am. Geriatr. Soc. 1997, 45, 584–589. [Google Scholar] [CrossRef]
- Grober, E.; Lipton, R.B.; Hall, C.; Crystal, H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 2000, 54, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tromp, D.; Dufour, A.; Lithfous, S.; Pebayle, T.; Despres, O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2015, 24 Pt B, 232–262. [Google Scholar] [CrossRef]
- Backman, L.; Small, B.J.; Fratiglioni, L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 2001, 124 Pt 1, 96–102. [Google Scholar] [CrossRef]
- Laakso, M.P.; Hallikainen, M.; Hanninen, T.; Partanen, K.; Soininen, H. Diagnosis of Alzheimer’s disease: MRI of the hippocampus vs delayed recall. Neuropsychologia 2000, 38, 579–584. [Google Scholar] [CrossRef]
- Backman, L.; Jones, S.; Berger, A.K.; Laukka, E.J.; Small, B.J. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology 2005, 19, 520–531. [Google Scholar] [CrossRef]
- Ferman, T.J.; Smith, G.E.; Kantarci, K.; Boeve, B.F.; Pankratz, V.S.; Dickson, D.W.; Graff-Radford, N.R.; Wszolek, Z.; Van Gerpen, J.; Uitti, R.; et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013, 81, 2032–2038. [Google Scholar] [CrossRef]
- Seo, E.H.; Lee, D.Y.; Lee, J.H.; Choo, I.H.; Kim, J.W.; Kim, S.G.; Park, S.Y.; Shin, J.H.; Do, Y.J.; Yoon, J.C.; et al. Total scores of the CERAD neuropsychological assessment battery: Validation for mild cognitive impairment and dementia patients with diverse etiologies. Am. J. Geriatr. Psychiatry 2010, 18, 801–809. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, C.; Mungas, D.; Harvey, D.; Reed, B.; Weiner, M.; Chui, H.; Jagust, W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004, 63, 220–227. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.H.; Lee, J.J.; Huh, Y.; Lee, S.B.; Han, S.K.; Choi, S.W.; Lee, D.Y.; Kim, K.W.; Woo, J.I. Standardization of the korean version of the geriatric depression scale: Reliability, validity, and factor structure. Psychiatry Investig. 2008, 5, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.-A.; KJJeon, M.; Chae, Y.-R. Evaluation of the Korean Version of Physical Activity Scale for the Elderly (K-PASE). Korean J. Women Health Nurs. 2010, 16, 47. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.E.; Chumlea, W.; Salva, A.; Rubenstein, L.Z.; et al. Overview of the MNA--Its history and challenges. J. Nutr. Health Aging 2006, 10, 456–463. [Google Scholar]
- Ma, W.Y.; Yang, C.Y.; Shih, S.R.; Hsieh, H.J.; Hung, C.S.; Chiu, F.C.; Lin, M.S.; Liu, P.H.; Hua, C.H.; Hsein, Y.C.; et al. Measurement of Waist Circumference: Midabdominal or iliac crest? Diabetes Care 2013, 36, 1660–1666. [Google Scholar] [CrossRef]
- Oguaju, B.; Lau, D.; Padwal, R.; Ringrose, J. Inter-observer reliability and anatomical landmarks for arm circumference to determine cuff size for blood pressure measurement. J. Clin. Hypertens. 2024, 26, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Rose Berlin Piodena-Aportadera, M.; Lau, S.; Chew, J.; Lim, J.P.; Ismail, N.H.; Ding, Y.Y.; Lim, W.S. Calf Circumference Measurement Protocols for Sarcopenia Screening: Differences in Agreement, Convergent Validity and Diagnostic Performance. Ann. Geriatr. Med. Res. 2022, 26, 215–224. [Google Scholar] [CrossRef] [PubMed]
| Overall | APOE4-Negative | APOE4-Positive | p | |
|---|---|---|---|---|
| n | 196 | 156 | 40 | |
| Age, y | 72.65 (5.95) | 72.95 (5.96) | 71.50 (5.86) | 0.170 a |
| Female, n (%) | 138 (70.41) | 106 (67.95) | 32 (80.00) | 0.136 b |
| Education, y | 9.62 (4.51) | 9.61 (4.55) | 9.68 (4.38) | 0.934 a |
| MCI, n (%) | 113 (57.65) | 88 (56.41) | 25 (62.50) | 0.487 b |
| VRS, % | 23.98 (18.58) | 23.93 (19.14) | 24.17 (16.43) | 0.943 a |
| MMSE | 25.58 (3.45) | 25.52 (3.46) | 25.83 (3.43) | 0.618 a |
| Body circumference index | ||||
| Measures | ||||
| MAC, cm | 25.66 (3.28) | 25.60 (3.31) | 25.90 (3.19) | 0.612 a |
| CC, cm | 36.41 (5.18) | 36.76 (5.18) | 34.97 (5.00) | 0.053 a |
| WC, cm | 87.98 (8.97) | 87.98 (8.68) | 87.95 (10.17) | 0.983 a |
| Ratio | ||||
| MAC/WC | 0.29 (0.04) | 0.29 (0.04) | 0.30 (0.04) | 0.535 a |
| CC/WC | 0.42 (0.05) | 0.42 (0.05) | 0.40 (0.06) | 0.050 a |
| MAC/CC | 0.72 (0.13) | 0.71 (0.13) | 0.75 (0.13) | 0.060 a |
| Body mass index | 24.83 (3.41) | 24.78 (3.41) | 25.03 (3.40) | 0.680 a |
| PASE | 64.77 (46.21) | 64.45 (47.19) | 66.04 (42.70) | 0.847 a |
| Decrease in food intake over the past three months | 1.00 c | |||
| No, n (%) | 182 (92.86) | 145 (92.95) | 37 (92.50) | |
| Yes, n (%) | 14 (7.14) | 37 (23.72) | 3 (7.50) | |
| Serum nutritional markers | ||||
| Albumin, g/dL | 4.57 (0.26) | 4.57 (0.26) | 4.60 (0.25) | 0.465 a |
| Glucose, fasting, mg/dL | 108.15 (19.94) | 108.46 (21.02) | 106.87 (14.87) | 0.660 a |
| HDL-Cholesterol, mg/dL | 54.64 (12.96) | 54.51 (12.89) | 55.21 (13.38) | 0.765 a |
| LDL-Cholesterol, mg/dL | 96.41 (33.82) | 96.10 (35.42) | 97.68 (26.64) | 0.796 a |
| Cognition | ||||
| Memory score | ||||
| EMS | 35.10 (9.48) | 35.17 (9.47) | 34.83 (9.67) | 0.840 a |
| Non-memory score | ||||
| NMS | 34.25 (6.62) | 34.06 (6.92) | 35.00 (5.26) | 0.423 a |
| Global cognition | ||||
| TS | 69.98 (15.61) | 70.00 (16.15) | 69.90 (13.52) | 0.971 a |
| MAC | CC | WC | MAC/WC | CC/WC | MAC/CC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | p | |||||||
| EMS | ||||||||||||
| Model 1 | 0.196 | 0.004 | −0.026 | 0.583 | −0.131 | 0.023 | 0.167 | <0.001 | 0.046 | 0.347 | 0.083 | 0.082 |
| Model 2 | 0.205 | 0.001 | −0.037 | 0.414 | −0.084 | 0.139 | 0.148 | 0.002 | 0.005 | 0.911 | 0.095 | 0.040 |
| NMS | ||||||||||||
| Model 1 | 0.106 | 0.249 | 0.194 | 0.002 | −0.071 | 0.364 | 0.076 | 0.256 | 0.247 | <0.001 | 0.083 | 0.082 |
| Model 2 | 0.128 | 0.152 | 0.200 | 0.001 | −0.031 | 0.692 | 0.069 | 0.296 | 0.225 | <0.001 | 0.095 | 0.040 |
| TS | ||||||||||||
| Model 1 | 0.155 | 0.041 | 0.032 | 0.540 | −0.123 | 0.058 | 0.137 | 0.013 | 0.102 | 0.062 | 0.025 | 0.643 |
| Model 2 | 0.177 | 0.016 | 0.026 | 0.614 | −0.084 | 0.192 | 0.131 | 0.017 | 0.070 | 0.194 | 0.038 | 0.472 |
| MAC | CC | MAC/WC | CC/WC | MAC/CC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | ||||||
| EMS | ||||||||||
| BC | 0.209 | <0.001 | −0.047 | 0.352 | 0.144 | 0.002 | 0.002 | 0.976 | 0.097 | 0.056 |
| APOE4-positivity | 0.206 | 0.005 | −0.225 | 0.477 | 0.201 | 0.007 | −0.132 | 0.680 | −0.048 | 0.848 |
| BC APOE4-positivity | −0.265 | <0.001 | 0.145 | 0.647 | −0.264 | <0.001 | 0.055 | 0.863 | −0.043 | 0.866 |
| NMS | ||||||||||
| BC | 0.130 | 0.149 | 0.265 | <0.001 | 0.065 | 0.330 | 0.282 | <0.001 | −0.117 | 0.093 |
| APOE4-positivity | 0.118 | 0.249 | 0.352 | 0.004 | 0.136 | 0.197 | 0.378 | 0.002 | −0.093 | 0.788 |
| BC APOE4-positivity | −0.122 | 0.245 | −0.342 | 0.005 | −0.142 | 0.185 | −0.448 | <0.001 | 0.102 | 0.769 |
| TS | ||||||||||
| BC | 0.181 | 0.014 | 0.037 | 0.519 | 0.131 | 0.017 | 0.097 | 0.101 | 0.036 | 0.534 |
| APOE4-positivity | 0.122 | 0.149 | 0.113 | 0.754 | 0.122 | 0.157 | 0.347 | 0.336 | −0.031 | 0.913 |
| BC APOE4-positivity | −0.174 | 0.045 | −0.164 | 0.648 | −0.180 | 0.041 | −0.396 | 0.271 | −0.026 | 0.928 |
| MAC | CC | MAC/WC | CC/WC | MAC/CC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | ||||||
| EMS | ||||||||||
| APOE4-negative | ||||||||||
| Model 1 | 0.200 | 0.006 | −0.041 | 0.421 | 0.185 | <0.001 | 0.032 | 0.535 | 0.089 | 0.079 |
| Model 2 | 0.199 | 0.006 | −0.058 | 0.255 | 0.172 | 0.002 | −0.008 | 0.883 | 0.106 | 0.036 |
| APOE4-positive | ||||||||||
| Model 1 | 0.170 | 0.418 | 0.093 | 0.501 | 0.058 | 0.667 | 0.134 | 0.384 | −0.026 | 0.855 |
| Model 2 | 0.142 | 0.341 | −0.110 | 0.302 | 0.113 | 0.324 | −0.108 | 0.388 | 0.100 | 0.326 |
| NMS | ||||||||||
| APOE4-negative | ||||||||||
| Model 1 | 0.148 | 0.138 | 0.208 | 0.002 | 0.134 | 0.069 | 0.278 | <0.001 | −0.108 | 0.116 |
| Model 2 | 0.172 | 0.086 | 0.209 | 0.002 | 0.140 | 0.064 | 0.260 | <0.001 | −0.103 | 0.139 |
| APOE4-positive | ||||||||||
| Model 1 | −0.145 | 0.598 | 0.163 | 0.367 | −0.215 | 0.223 | 0.083 | 0.683 | −0.154 | 0.400 |
| Model 2 | −0.160 | 0.458 | 0.063 | 0.683 | −0.276 | 0.087 | −0.103 | 0.569 | −0.094 | 0.528 |
| TS | ||||||||||
| APOE4-negative | ||||||||||
| Model 1 | 0.173 | 0.033 | 0.032 | 0.571 | 0.177 | 0.003 | 0.114 | 0.046 | 0.027 | 0.630 |
| Model 2 | 0.197 | 0.016 | 0.022 | 0.697 | 0.185 | 0.003 | 0.086 | 0.138 | 0.042 | 0.460 |
| APOE4-positive | ||||||||||
| Model 1 | 0.046 | 0.848 | 0.127 | 0.418 | −0.081 | 0.597 | 0.084 | 0.631 | −0.087 | 0.585 |
| Model 2 | −0.043 | 0.790 | −0.041 | 0.722 | −0.093 | 0.456 | −0.130 | 0.334 | −0.004 | 0.968 |
| MAC | CC | MAC/WC | CC/WC | MAC/CC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | ||||||
| EMS | ||||||||||
| Overall | ||||||||||
| Model 1 | 0.176 | 0.008 | −0.041 | 0.383 | 0.158 | 0.001 | 0.029 | 0.554 | 0.083 | 0.080 |
| Model 2 | 0.174 | 0.006 | −0.046 | 0.320 | 0.127 | 0.009 | −0.010 | 0.838 | 0.088 | 0.057 |
| APOE4-negative | ||||||||||
| Model 1 | 0.183 | 0.009 | −0.060 | 0.228 | 0.177 | 0.001 | 0.009 | 0.866 | 0.098 | 0.049 |
| Model 2 | 0.176 | 0.012 | −0.073 | 0.150 | 0.158 | 0.005 | −0.030 | 0.568 | 0.110 | 0.029 |
| APOE4-positive | ||||||||||
| Model 1 | 0.162 | 0.449 | 0.101 | 0.477 | 0.053 | 0.699 | 0.141 | 0.371 | −0.035 | 0.810 |
| Model 2 | 0.089 | 0.538 | −0.100 | 0.327 | 0.092 | 0.390 | −0.078 | 0.519 | 0.076 | 0.436 |
| NMS | ||||||||||
| Overall | ||||||||||
| Model 1 | 0.064 | 0.487 | 0.199 | 0.002 | 0.066 | 0.343 | 0.265 | <0.001 | −0.130 | 0.048 |
| Model 2 | 0.087 | 0.347 | 0.214 | 0.001 | 0.055 | 0.437 | 0.252 | <0.001 | −0.134 | 0.043 |
| APOE4-negative | ||||||||||
| Model 1 | 0.116 | 0.246 | 0.213 | 0.002 | 0.135 | 0.079 | 0.298 | <0.001 | −0.123 | 0.082 |
| Model 2 | 0.142 | 0.167 | 0.225 | 0.002 | 0.145 | 0.078 | 0.294 | <0.001 | −0.123 | 0.092 |
| APOE4-positive | ||||||||||
| Model 1 | −0.217 | 0.439 | 0.205 | 0.270 | −0.252 | 0.152 | 0.116 | 0.578 | −0.210 | 0.262 |
| Model 2 | −0.229 | 0.303 | 0.075 | 0.635 | −0.298 | 0.063 | −0.077 | 0.682 | −0.122 | 0.420 |
| TS | ||||||||||
| Overall | ||||||||||
| Model 1 | 0.123 | 0.102 | 0.021 | 0.701 | 0.127 | 0.024 | 0.095 | 0.087 | 0.018 | 0.733 |
| Model 2 | 0.142 | 0.058 | 0.024 | 0.658 | 0.113 | 0.049 | 0.068 | 0.224 | 0.022 | 0.685 |
| APOE4-negative | ||||||||||
| Model 1 | 0.145 | 0.066 | 0.019 | 0.735 | 0.170 | 0.005 | 0.105 | 0.067 | 0.026 | 0.638 |
| Model 2 | 0.170 | 0.036 | 0.019 | 0.746 | 0.177 | 0.007 | 0.084 | 0.163 | 0.035 | 0.549 |
| APOE4-positive | ||||||||||
| Model 1 | 0.008 | 0.972 | 0.150 | 0.347 | −0.101 | 0.511 | 0.104 | 0.562 | −0.118 | 0.467 |
| Model 2 | −0.104 | 0.516 | −0.032 | 0.781 | −0.111 | 0.348 | −0.102 | 0.443 | −0.030 | 0.785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Choe, Y.M.; Choi, H.J.; Lee, B.C.; Suh, G.-H.; Kim, S.G.; Kim, H.S.; Hwang, J.; Yi, D.; Kim, J.W. Body Circumference and Cognitive Function: Role of Apolipoprotein E ε4 in the Elderly. Int. J. Mol. Sci. 2025, 26, 5831. https://doi.org/10.3390/ijms26125831
Kim J-H, Choe YM, Choi HJ, Lee BC, Suh G-H, Kim SG, Kim HS, Hwang J, Yi D, Kim JW. Body Circumference and Cognitive Function: Role of Apolipoprotein E ε4 in the Elderly. International Journal of Molecular Sciences. 2025; 26(12):5831. https://doi.org/10.3390/ijms26125831
Chicago/Turabian StyleKim, Ji-Hyun, Young Min Choe, Hye Ji Choi, Boung Chul Lee, Guk-Hee Suh, Shin Gyeom Kim, Hyun Soo Kim, Jaeuk Hwang, Dahyun Yi, and Jee Wook Kim. 2025. "Body Circumference and Cognitive Function: Role of Apolipoprotein E ε4 in the Elderly" International Journal of Molecular Sciences 26, no. 12: 5831. https://doi.org/10.3390/ijms26125831
APA StyleKim, J.-H., Choe, Y. M., Choi, H. J., Lee, B. C., Suh, G.-H., Kim, S. G., Kim, H. S., Hwang, J., Yi, D., & Kim, J. W. (2025). Body Circumference and Cognitive Function: Role of Apolipoprotein E ε4 in the Elderly. International Journal of Molecular Sciences, 26(12), 5831. https://doi.org/10.3390/ijms26125831







