The Roles of tRNA-Derived Fragments in Cancer: Updates and Perspectives
Abstract
1. Introduction
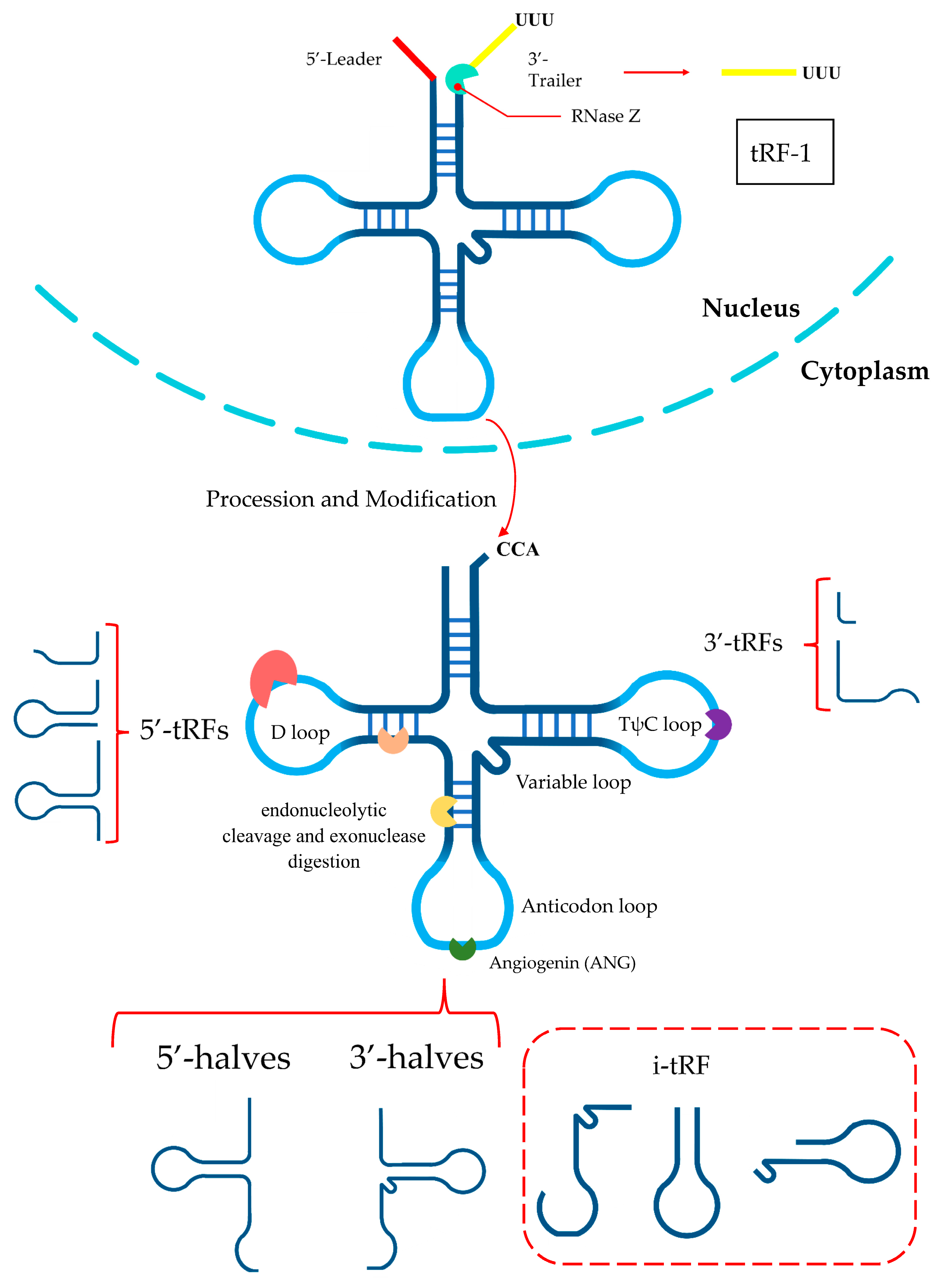
2. tRF Biogenesis and Classification
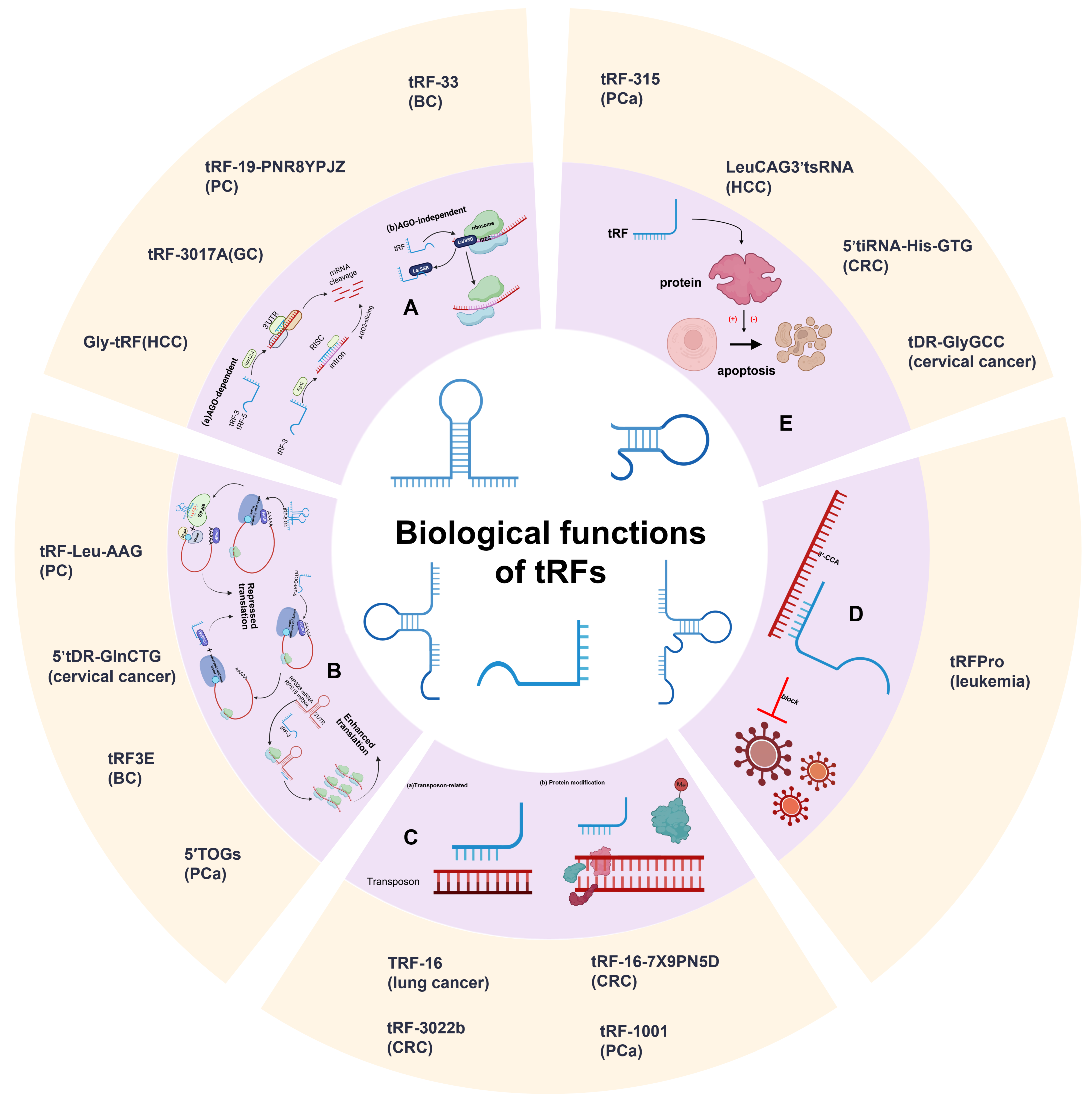
3. Biological Functions of tRFs
3.1. RNA Silencing
3.2. Translational Regulation
3.3. Epigenetic Regulation
3.4. Reverse-Transcriptional Regulation
3.5. Cellular Apoptosis
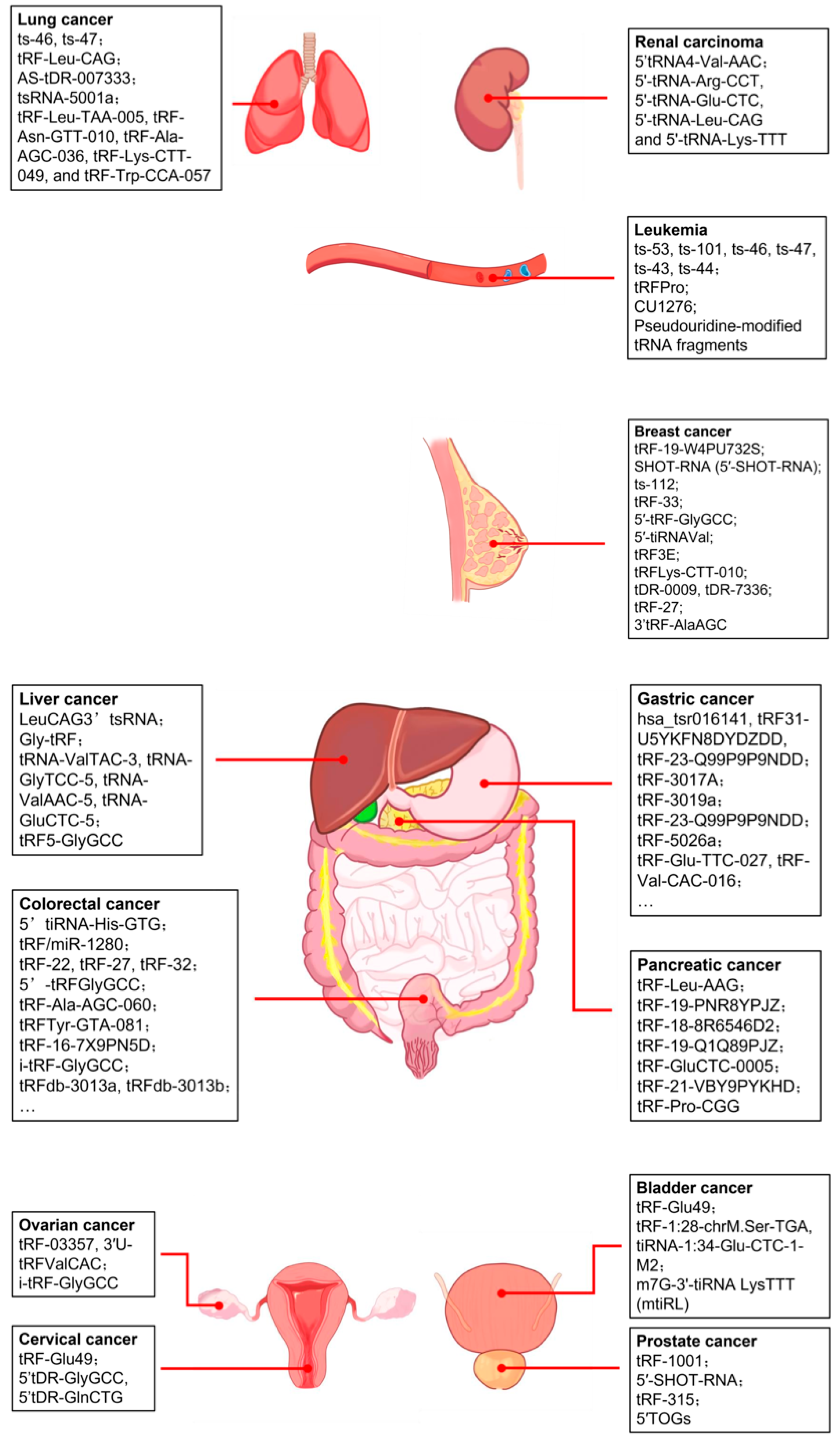
4. tRFs in Cancer
4.1. Breast Cancer
4.2. Prostate Cancer
4.3. Pancreatic Cancer
4.4. Liver Cancer
4.5. Gastric Cancer
4.6. Colorectal Cancer
4.7. Leukemia
4.8. Lung Cancer
4.9. Other Cancers
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, M.; Lu, B.; Zhang, J.; Ding, J.; Liu, P.; Lu, Y. tRNA-derived RNA fragments in cancer: Current status and future perspectives. J. Hematol. Oncol. 2020, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Diao, L.; Han, L. Small non-coding RNAs in human cancer: Function, clinical utility, and characterization. Oncogene 2021, 40, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Zhu, L.; Li, T.; Yan, Z.; Guo, J. Transfer RNA-derived fragments and tRNA halves: Biogenesis, biological functions and their roles in diseases. J. Mol. Med. 2018, 96, 1167–1176. [Google Scholar] [CrossRef]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and Function of Transfer RNA-Related Fragments (tRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef]
- Pan, Y.; Ying, X.; Zhang, X.; Jiang, H.; Yan, J.; Duan, S. The role of tRNA-Derived small RNAs (tsRNAs) in pancreatic cancer and acute pancreatitis. Noncoding RNA Res. 2024, 11, 200–208. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, W.; Qu, B.; Zhang, F.; Wu, Z.; Shi, J.; Chen, X.; Song, Y.; Wang, Z. The tRNA-Derived Fragment-3017A Promotes Metastasis by Inhibiting NELL2 in Human Gastric Cancer. Front. Oncol. 2021, 10, 570916. [Google Scholar] [CrossRef]
- Qiu, P.; Jiang, Q.; Song, H. Unveiling the hidden world of transfer RNA-derived small RNAs in inflammation. J. Inflamm. 2024, 21, 46. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, Y.; Xie, Y.; Yu, X.; Zhang, S.; Ge, J.; Li, Z.; Ye, G.; Guo, J. Extracellular vesicles-associated tRNA-derived fragments (tRFs): Biogenesis, biological functions, and their role as potential biomarkers in human diseases. J. Mol. Med. 2022, 100, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Silva, M.R.; Cabrera-Cabrera, F.; das Neves, R.F.; Souto-Padrón, T.; de Souza, W.; Cayota, A. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: Relevance of tRNA-derived halves. BioMed Res. Int. 2014, 2014, 305239. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ptashkin, R.N.; Chen, Y.; Cheng, Z.; Liu, G.; Phan, T.; Deng, X.; Zhou, J.; Lee, I.; Lee, Y.S.; et al. Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Mol. Ther. 2015, 23, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ender, C.; Meister, G.; Moore, P.S.; Chang, Y.; John, B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012, 40, 6787–6799. [Google Scholar] [CrossRef]
- Schaffer, A.E.; Eggens, V.R.; Caglayan, A.O.; Reuter, M.S.; Scott, E.; Coufal, N.G.; Silhavy, J.L.; Xue, Y.; Kayserili, H.; Yasuno, K.; et al. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 2014, 157, 651–663. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Z.; Wang, C. The Therapeutic Potential of tRNA-derived Small RNAs in Neurodegenerative Disorders. Aging Dis. 2022, 13, 389–401. [Google Scholar] [CrossRef]
- Phan, H.D.; Lai, L.B.; Zahurancik, W.J.; Gopalan, V. The many faces of RNA-based RNase P, an RNA-world relic. Trends Biochem. Sci. 2021, 46, 976–991. [Google Scholar] [CrossRef]
- Maraia, R.J.; Lamichhane, T.N. 3′ processing of eukaryotic precursor tRNAs. Wiley Interdiscip. Rev. RNA 2011, 2, 362–375. [Google Scholar] [CrossRef]
- Xiong, Y.; Steitz, T.A. A story with a good ending: tRNA 3′-end maturation by CCA-adding enzymes. Curr. Opin. Struct. Biol. 2006, 16, 12–17. [Google Scholar] [CrossRef]
- Holley, R.W.; Apgar, J.; Everett, G.A.; Madison, J.T.; Marquisee, M.; Merrill, S.H.; Penswick, J.R.; Zamir, A. Structure of a Ribonucleic Acid. Science 1965, 147, 1462–1465. [Google Scholar] [CrossRef]
- Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021, 22, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Mo, Y.; Ren, D.; Liu, S.; Zeng, Z.; Xiong, W. Transfer RNA-derived small RNAs in tumor microenvironment. Mol. Cancer 2023, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef]
- Fu, B.F.; Xu, C.Y. Transfer RNA-Derived Small RNAs: Novel Regulators and Biomarkers of Cancers. Front. Oncol. 2022, 12, 843598. [Google Scholar] [CrossRef]
- Kumar, P.; Mudunuri, S.B.; Anaya, J.; Dutta, A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2015, 43, D141–D145. [Google Scholar] [CrossRef]
- Tao, E.W.; Cheng, W.Y.; Li, W.L.; Yu, J.; Gao, Q.Y. tiRNAs: A novel class of small noncoding RNAs that helps cells respond to stressors and plays roles in cancer progression. J. Cell. Physiol. 2020, 235, 683–690. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, P.; Zhang, X.; Zeng, L.; Xu, B.; Zhou, P. tRNA-derived fragments: Unveiling new roles and molecular mechanisms in cancer progression. Int. J. Cancer 2024, 155, 1347–1360. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, L.; Yu, X.; Ruan, Y.; Li, Z.; Guo, J. Action mechanisms and research methods of tRNA-derived small RNAs. Signal Transduct. Target. Ther. 2020, 5, 109. [Google Scholar] [CrossRef]
- Cole, C.; Sobala, A.; Lu, C.; Thatcher, S.R.; Bowman, A.; Brown, J.W.; Green, P.J.; Barton, G.J.; Hutvagner, G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA 2009, 15, 2147–2160. [Google Scholar] [CrossRef]
- Telonis, A.G.; Loher, P.; Magee, R.; Pliatsika, V.; Londin, E.; Kirino, Y.; Rigoutsos, I. tRNA Fragments Show Intertwining with mRNAs of Specific Repeat Content and Have Links to Disparities. Cancer Res. 2019, 79, 3034–3049. [Google Scholar] [CrossRef]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 2015, 161, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Ivanov, P.; Hu, G.F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Parker, R. Stressing out over tRNA cleavage. Cell 2009, 138, 215–219. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Ren, Y.; Zhou, J.; Ren, J.; Lee, I.; Bao, X. A tRNA-derived RNA Fragment Plays an Important Role in the Mechanism of Arsenite -induced Cellular Responses. Sci. Rep. 2018, 8, 16838. [Google Scholar] [CrossRef]
- Tao, E.W.; Wang, H.L.; Cheng, W.Y.; Liu, Q.Q.; Chen, Y.X.; Gao, Q.Y. A specific tRNA half, 5′tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef]
- Su, Z.; Kuscu, C.; Malik, A.; Shibata, E.; Dutta, A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J. Biol. Chem. 2019, 294, 16930–16941. [Google Scholar] [CrossRef]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef]
- Haussecker, D.; Huang, Y.; Lau, A.; Parameswaran, P.; Fire, A.Z.; Kay, M.A. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA 2010, 16, 673–695. [Google Scholar] [CrossRef]
- Kim, H.K.; Yeom, J.H.; Kay, M.A. Transfer RNA-Derived Small RNAs: Another Layer of Gene Regulation and Novel Targets for Disease Therapeutics. Mol. Ther. 2020, 28, 2340–2357. [Google Scholar] [CrossRef]
- Sobala, A.; Hutvagner, G. Transfer RNA-derived fragments: Origins, processing, and functions. Wiley Interdiscip. Rev. RNA 2011, 2, 853–862. [Google Scholar] [CrossRef]
- Green, J.A.; Ansari, M.Y.; Ball, H.C.; Haqqi, T.M. tRNA-derived fragments (tRFs) regulate post-transcriptional gene expression via AGO-dependent mechanism in IL-1β stimulated chondrocytes. Osteoarthr. Cartil. 2020, 28, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Di Fazio, A.; Schlackow, M.; Pong, S.K.; Alagia, A.; Gullerova, M. Dicer dependent tRNA derived small RNAs promote nascent RNA silencing. Nucleic Acids Res. 2022, 50, 1734–1752. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lee, W.; Kim, G.W.; Lee, S.H.; Moon, J.S.; Kim, M.; Kim, H.S.; Oh, J.W. Regulation of La/SSB-dependent viral gene expression by pre-tRNA 3′ trailer-derived tRNA fragments. Nucleic Acids Res. 2019, 47, 9888–9901. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Lyons, S.M.; Gudanis, D.; Coyne, S.M.; Gdaniec, Z.; Ivanov, P. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat. Commun. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Ivanov, P.; O’Day, E.; Emara, M.M.; Wagner, G.; Lieberman, J.; Anderson, P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA 2014, 111, 18201–18206. [Google Scholar] [CrossRef]
- Lyons, S.M.; Kharel, P.; Akiyama, Y.; Ojha, S.; Dave, D.; Tsvetkov, V.; Merrick, W.; Ivanov, P.; Anderson, P. eIF4G has intrinsic G-quadruplex binding activity that is required for tiRNA function. Nucleic Acids Res. 2020, 48, 6223–6233. [Google Scholar] [CrossRef]
- Emara, M.M.; Ivanov, P.; Hickman, T.; Dawra, N.; Tisdale, S.; Kedersha, N.; Hu, G.F.; Anderson, P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 2010, 285, 10959–10968. [Google Scholar] [CrossRef]
- Guzzi, N.; Cieśla, M.; Ngoc, P.C.T.; Lang, S.; Arora, S.; Dimitriou, M.; Pimková, K.; Sommarin, M.N.; Munita, R.; Lubas, M.; et al. Pseudouridylation of tRNA-Derived Fragments Steers Translational Control in Stem Cells. Cell 2018, 173, 1204–1216.e26. [Google Scholar] [CrossRef]
- Sobala, A.; Hutvagner, G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013, 10, 553–563. [Google Scholar] [CrossRef]
- Kim, H.K.; Fuchs, G.; Wang, S.; Wei, W.; Zhang, Y.; Park, H.; Roy-Chaudhuri, B.; Li, P.; Xu, J.; Chu, K.; et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 2017, 552, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S.; Greally, J.M. Epigenetics, cellular memory and gene regulation. Curr. Biol. 2016, 26, R644–R648. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Huang, X.; Dou, S.; Tang, X.; Luo, S.; Theurkauf, W.E.; Lu, J.; Weng, Z. A benchmark and an algorithm for detecting germline transposon insertions and measuring de novo transposon insertion frequencies. Nucleic Acids Res. 2021, 49, e44. [Google Scholar] [CrossRef]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Watanabe, T.; Tomizawa, S.; Mitsuya, K.; Totoki, Y.; Yamamoto, Y.; Kuramochi-Miyagawa, S.; Iida, N.; Hoki, Y.; Murphy, P.J.; Toyoda, A.; et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science 2011, 332, 848–852. [Google Scholar] [CrossRef]
- Fields, B.D.; Kennedy, S. Chromatin Compaction by Small RNAs and the Nuclear RNAi Machinery in C. elegans. Sci. Rep. 2019, 9, 9030. [Google Scholar] [CrossRef]
- Couvillion, M.T.; Bounova, G.; Purdom, E.; Speed, T.P.; Collins, K. A Tetrahymena Piwi bound to mature tRNA 3′ fragments activates the exonuclease Xrn2 for RNA processing in the nucleus. Mol. Cell 2012, 48, 509–520. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Liu, C.; Liu, J.; Hu, Q.; Pan, T.; Duan, X.; Liu, B.; Zhang, Y.; Chen, J.; et al. IL-4 Inhibits the Biogenesis of an Epigenetically Suppressive PIWI-Interacting RNA To Upregulate CD1a Molecules on Monocytes/Dendritic Cells. J. Immunol. 2016, 196, 1591–1603. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, Y.; Li, Z.; Hu, K. tRNA-derived small RNAs: Their role in the mechanisms, biomarkers, and therapeutic strategies of colorectal cancer. J. Transl. Med. 2025, 23, 51. [Google Scholar] [CrossRef]
- Schorn, A.J.; Gutbrod, M.J.; LeBlanc, C.; Martienssen, R. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell 2017, 170, 61–71.e11. [Google Scholar] [CrossRef]
- Manivannan, A.C.; Devaraju, V.; Velmurugan, P.; Sathiamoorthi, T.; Sivakumar, S.; Subbiah, S.K.; Ravi, A.V. Tumorigenesis and diagnostic practice applied in two oncogenic viruses: Epstein Barr virus and T-cell lymphotropic virus-1-Mini review. Biomed. Pharmacother. 2021, 142, 111974. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, K.; Guffanti, A.; Corradin, A.; Sharma, V.K.; De Bellis, G.; Corti, G.; Grassi, A.; Zanovello, P.; Bronte, V.; Ciminale, V.; et al. Small noncoding RNAs in cells transformed by human T-cell leukemia virus type 1: A role for a tRNA fragment as a primer for reverse transcriptase. J. Virol. 2014, 88, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Seiki, M.; Hattori, S.; Yoshida, M. Human adult T-cell leukemia virus: Molecular cloning of the provirus DNA and the unique terminal structure. Proc. Natl. Acad. Sci. USA 1982, 79, 6899–6902. [Google Scholar] [CrossRef]
- Mei, Y.; Yong, J.; Liu, H.; Shi, Y.; Meinkoth, J.; Dreyfuss, G.; Yang, X. tRNA binds to cytochrome c and inhibits caspase activation. Mol. Cell 2010, 37, 668–678. [Google Scholar] [CrossRef]
- Mei, Y.; Stonestrom, A.; Hou, Y.M.; Yang, X. Apoptotic regulation and tRNA. Protein Cell 2010, 1, 795–801. [Google Scholar] [CrossRef]
- Hou, Y.M.; Yang, X. Regulation of cell death by transfer RNA. Antioxid. Redox Signal. 2013, 19, 583–594. [Google Scholar] [CrossRef]
- Saikia, M.; Jobava, R.; Parisien, M.; Putnam, A.; Krokowski, D.; Gao, X.H.; Guan, B.J.; Yuan, Y.; Jankowsky, E.; Feng, Z.; et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol. Cell Biol. 2014, 34, 2450–2463. [Google Scholar] [CrossRef]
- Lu, S.; Wei, X.; Tao, L.; Dong, D.; Hu, W.; Zhang, Q.; Tao, Y.; Yu, C.; Sun, D.; Cheng, H. A novel tRNA-derived fragment tRF-3022b modulates cell apoptosis and M2 macrophage polarization via binding to cytokines in colorectal cancer. J. Hematol. Oncol. 2022, 15, 176. [Google Scholar] [CrossRef]
- Pan, L.; Huang, X.; Liu, Z.X.; Ye, Y.; Li, R.; Zhang, J.; Wu, G.; Bai, R.; Zhuang, L.; Wei, L.; et al. Inflammatory cytokine-regulated tRNA-derived fragment tRF-21 suppresses pancreatic ductal adenocarcinoma progression. J. Clin. Investig. 2021, 131, e148130. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Balatti, V.; Palamarchuk, A.; Rizzotto, L.; Veneziano, D.; Nigita, G.; Rassenti, L.Z.; Pass, H.I.; Kipps, T.J.; Liu, C.G.; et al. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, J.; Chen, Y.; Wang, D.; Wang, T.; Weng, Y.; Zhu, Z.; Peng, R.; Wang, Y.; Yan, F. Evaluation of 5′-tRF-His-GTG As a Molecular Biomarker in Breast Cancer Diagnoses and Prognosis. Cancer Biother. Radiopharm. 2024, 39, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, G.; Ge, H.; Han, X.; Mao, X.; Wang, X.; Veeramootoo, J.S.; Xia, T.; Liu, X.; Wang, S. Circulating tRNA-derived small RNAs (tsRNAs) signature for the diagnosis and prognosis of breast cancer. NPJ Breast Cancer 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Balatti, V.; Nigita, G.; Veneziano, D.; Drusco, A.; Stein, G.S.; Messier, T.L.; Farina, N.H.; Lian, J.B.; Tomasello, L.; Liu, C.-G.; et al. tsRNA signatures in cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 8071–8076. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Zhao, W.; Zhao, X.; Tao, Y. tRF-19-W4PU732S promotes breast cancer cell malignant activity by targeting inhibition of RPL27A (ribosomal protein-L27A). Bioengineered 2022, 13, 2087–2098. [Google Scholar] [CrossRef]
- Honda, S.; Loher, P.; Shigematsu, M.; Palazzo, J.P.; Suzuki, R.; Imoto, I.; Rigoutsos, I.; Kirino, Y. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl. Acad. Sci. USA 2015, 112, E3816–E3825. [Google Scholar] [CrossRef]
- Farina, N.H.; Scalia, S.; Adams, C.E.; Hong, D.; Fritz, A.J.; Messier, T.L.; Balatti, V.; Veneziano, D.; Lian, J.B.; Croce, C.M.; et al. Identification of tRNA-derived small RNA (tsRNA) responsive to the tumor suppressor, RUNX1, in breast cancer. J. Cell Physiol. 2020, 235, 5318–5327. [Google Scholar] [CrossRef]
- Lou, Y.; Fu, B.; Liu, L.; Song, J.; Zhu, M.; Xu, C. The tRF-33/IGF1 axis dysregulates mitochondrial homeostasis in HER2-negative breast cancer. Am. J. Physiol. Cell Physiol. 2025, 328, C627–C638. [Google Scholar] [CrossRef]
- Chen, F.; Song, C.; Meng, F.; Zhu, Y.; Chen, X.; Fang, X.; Ma, D.; Wang, Y.; Zhang, C. 5′-tRF-GlyGCC promotes breast cancer metastasis by increasing fat mass and obesity-associated protein demethylase activity. Int. J. Biol. Macromol. 2023, 226, 397–409. [Google Scholar] [CrossRef]
- Jürchott, K.; Kuban, R.J.; Krech, T.; Blüthgen, N.; Stein, U.; Walther, W.; Friese, C.; Kiełbasa, S.M.; Ungethüm, U.; Lund, P.; et al. Identification of Y-box binding protein 1 as a core regulator of MEK/ERK pathway-dependent gene signatures in colorectal cancer cells. PLoS Genet. 2010, 6, e1001231. [Google Scholar] [CrossRef]
- Uchiumi, T.; Fotovati, A.; Sasaguri, T.; Shibahara, K.; Shimada, T.; Fukuda, T.; Nakamura, T.; Izumi, H.; Tsuzuki, T.; Kuwano, M.; et al. YB-1 is important for an early stage embryonic development: Neural tube formation and cell proliferation. J. Biol. Chem. 2006, 281, 40440–40449. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Jiang, P.; Yang, Y.; Mao, X.; Tan, X.; Tang, X.; Wei, D.; Li, B.; Wang, X.; Tang, L.; et al. A tRNA fragment, 5′-tiRNAVal, suppresses the Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019, 457, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Giangrossi, M.; Zabaleta, M.E.; Wang, J.; Gambini, V.; Tilio, M.; Bencardino, D.; Occhipinti, S.; Belletti, B.; Laudadio, E.; et al. A novel 3′-tRNAGlu-derived fragment acts as a tumor suppressor in breast cancer by targeting nucleolin. FASEB J. 2019, 33, 13228–13240. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Li, Y.; Chu, J.; Li, J.; Zhang, Y.; Ding, X.; Fu, Z.; Li, W.; Huang, X.; Yin, Y. Identification of tRNA-derived small noncoding RNAs as potential biomarkers for prediction of recurrence in triple-negative breast cancer. Cancer Med. 2018, 7, 5130–5144. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, J.; Zhi, X.; Zhou, Y.; Wang, X.; Wang, C.; Gao, Y.; Zhang, X.; Yu, J.; Sun, Y.; et al. tRNA-derived fragment tRFLys-CTT-010 promotes triple-negative breast cancer progression by regulating glucose metabolism via G6PC. Carcinogenesis 2021, 42, 1196–1207, Erratum in Carcinogenesis 2022, 43, 813. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, Y.; Wu, X.; Zheng, M.; Xia, Y.; Fu, Z.; Ge, H.; Wang, S.; Xie, H. Hypoxia-induced tRNA-derived fragments, novel regulatory factor for doxorubicin resistance in triple-negative breast cancer. J. Cell Physiol. 2019, 234, 8740–8751. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Gong, J.; Yang, F.; Sun, C.; Yan, X.; Duan, N.; Hua, Y.; Zeng, T.; Fu, Z.; et al. tRF-27 competitively Binds to G3BPs and Activates MTORC1 to Enhance HER2 Positive Breast Cancer Trastuzumab Tolerance. Int. J. Biol. Sci. 2024, 20, 3923–3941. [Google Scholar] [CrossRef]
- Mo, D.; Tang, X.; Ma, Y.; Chen, D.; Xu, W.; Jiang, N.; Zheng, J.; Yan, F. tRNA-derived fragment 3′tRF-AlaAGC modulates cell chemoresistance and M2 macrophage polarization via binding to TRADD in breast cancer. J. Transl. Med. 2024, 22, 706. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Martens-Uzunova, E.S.; Jalava, S.E.; Dits, N.F.; van Leenders, G.J.; Møller, S.; Trapman, J.; Bangma, C.H.; Litman, T.; Visakorpi, T.; Jenster, G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012, 31, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Olvedy, M.; Scaravilli, M.; Hoogstrate, Y.; Visakorpi, T.; Jenster, G.; Martens-Uzunova, E.S. A comprehensive repertoire of tRNA-derived fragments in prostate cancer. Oncotarget 2016, 7, 24766–24777. [Google Scholar] [CrossRef]
- Magee, R.G.; Telonis, A.G.; Loher, P.; Londin, E.; Rigoutsos, I. Profiles of miRNA Isoforms and tRNA Fragments in Prostate Cancer. Sci. Rep. 2018, 8, 5314. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef]
- Chiang, K.C.; Tsui, K.H.; Chung, L.C.; Yeh, C.N.; Feng, T.H.; Chen, W.T.; Chang, P.L.; Chiang, H.Y.; Juang, H.H. Cisplatin modulates B-cell translocation gene 2 to attenuate cell proliferation of prostate carcinoma cells in both p53-dependent and p53-independent pathways. Sci. Rep. 2014, 4, 5511. [Google Scholar] [CrossRef]
- Yang, C.; Lee, M.; Song, G.; Lim, W. tRNALys-Derived Fragment Alleviates Cisplatin-Induced Apoptosis in Prostate Cancer Cells. Pharmaceutics 2021, 13, 55. [Google Scholar] [CrossRef]
- García-Vílchez, R.; Añazco-Guenkova, A.M.; Dietmann, S.; López, J.; Morón-Calvente, V.; D’Ambrosi, S.; Nombela, P.; Zamacola, K.; Mendizabal, I.; García-Longarte, S.; et al. METTL1 promotes tumorigenesis through tRNA-derived fragment biogenesis in prostate cancer. Mol. Cancer 2023, 22, 119. [Google Scholar] [CrossRef]
- Sui, S.; Wang, Z.; Cui, X.; Jin, L.; Zhu, C. The biological behavior of tRNA-derived fragment tRF-Leu-AAG in pancreatic cancer cells. Bioengineered 2022, 13, 10617–10628. [Google Scholar] [CrossRef]
- Cao, W.; Dai, S.; Ruan, W.; Long, T.; Zeng, Z.; Lei, S. Pancreatic stellate cell-derived exosomal tRF-19-PNR8YPJZ promotes proliferation and mobility of pancreatic cancer through AXIN2. J. Cell. Mol. Med. 2023, 27, 2533–2546. [Google Scholar] [CrossRef]
- Lan, S.; Liu, S.; Wang, K.; Chen, W.; Zheng, D.; Zhuang, Y.; Zhang, S. tRNA-derived RNA fragment, tRF-18-8R6546D2, promotes pancreatic adenocarcinoma progression by directly targeting ASCL2. Gene 2024, 927, 148739. [Google Scholar] [CrossRef]
- Cao, W.; Zeng, Z.; Lei, S. 5′-tRF-19-Q1Q89PJZ Suppresses the Proliferation and Metastasis of Pancreatic Cancer Cells via Regulating Hexokinase 1-Mediated Glycolysis. Biomolecules 2023, 13, 1513. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Jiang, S.; Zhou, X.; Yao, L.; Di, Y.; Jiang, Y.; Gu, J.; Mao, Y.; Li, J.; et al. WD repeat-containing protein 1 maintains β-Catenin activity to promote pancreatic cancer aggressiveness. Br. J. Cancer 2020, 123, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Peng, W.; Wang, R.; Bai, S.; Cao, M.; Xiong, S.; Li, Y.; Yang, Y.; Liang, J.; Liu, L.; et al. Exosome-derived tRNA fragments tRF-GluCTC-0005 promotes pancreatic cancer liver metastasis by activating hepatic stellate cells. Cell Death Dis. 2024, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, L.; Gao, Y.; Gao, P.; Ma, L.; Zhu, B.; Yin, X.; Sui, S.; Chen, S.; Jiang, Z.; et al. Low expression of tRF-Pro-CGG predicts poor prognosis in pancreatic ductal adenocarcinoma. J. Clin. Lab. Anal. 2021, 35, e23742. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Liu, L.; Yan, M.; Zhang, Q.; Song, X.; Lin, Y.; Zhu, D.; Wei, Y.; Fu, Z.; et al. Gly-tRF enhances LCSC-like properties and promotes HCC cells migration by targeting NDFIP2. Cancer Cell Int. 2021, 21, 502. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Wang, J.; Zhang, L.; Sun, Z.; Chen, S.; Ding, Y.; Wang, W. Transfer RNA-derived fragment 5′tRF-Gly promotes the development of hepatocellular carcinoma by direct targeting of carcinoembryonic antigen-related cell adhesion molecule 1. Cancer Sci. 2022, 113, 3476–3488. [Google Scholar] [CrossRef]
- Rui, T.; Zhu, K.; Mao, Z.; Wu, J.; Pan, Y.; Ye, Q.; Chen, C.; Xiang, A.; Guo, J.; Tang, N.; et al. A Novel tRF, HCETSR, Derived From tRNA-Glu/TTC, Inhibits HCC Malignancy by Regulating the SPBTN1-catenin Complex Axis. Adv. Sci. 2025, 12, e2415229. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, S.; Yan, L.; Hu, L.; Bowler, S.; Zitello, E.; Huang, G.; Deng, Y. Development of a tRNA-derived small RNA diagnostic and prognostic signature in liver cancer. Genes Dis. 2021, 9, 393–400. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.Q.; et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef]
- Zuo, L.; Shi, S.; Fan, J.; Tian, Z.; Yu, D. MRI-based preoperative markers combined with narrow-margin hepatectomy result in higher early recurrence. Eur. J. Radiol. 2022, 157, 110521. [Google Scholar] [CrossRef]
- Herrera, F.G.; Ronet, C.; Ochoa de Olza, M.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Schneider, H.; Silginer, M.; Steinle, A.; Pruschy, M.; Polić, B.; Weller, M.; Roth, P. NKG2D-Dependent Antitumor Effects of Chemotherapy and Radiotherapy against Glioblastoma. Clin. Cancer Res. 2018, 24, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Donlon, N.E.; Power, R.; Hayes, C.; Reynolds, J.V.; Lysaght, J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021, 502, 84–96. [Google Scholar] [CrossRef]
- Ji, J.; Ding, K.; Cheng, B.; Zhang, X.; Luo, T.; Huang, B.; Yu, H.; Chen, Y.; Xu, X.; Lin, H.; et al. Radiotherapy-Induced Astrocyte Senescence Promotes an Immunosuppressive Microenvironment in Glioblastoma to Facilitate Tumor Regrowth. Adv. Sci. 2024, 11, e2304609. [Google Scholar] [CrossRef]
- Gong, Y.; Zeng, F.; Zhang, F.; Liu, X.; Li, Z.; Chen, W.; Liu, H.; Li, X.; Cheng, Y.; Zhang, J.; et al. Radiotherapy plus a self-gelation powder encapsulating tRF5-GlyGCC inhibitor potentiates natural kill cell immunity to prevent hepatocellular carcinoma recurrence. J. Nanobiotechnol. 2025, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ma, S.; Liang, B.; Ju, S. Serum hsa_tsr016141 as a Kind of tRNA-Derived Fragments Is a Novel Biomarker in Gastric Cancer. Front. Oncol. 2021, 11, 679366. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Qin, X.; Huang, Y.; Ju, S. Evaluation of serum tRF-23-Q99P9P9NDD as a potential biomarker for the clinical diagnosis of gastric cancer. Mol. Med. 2022, 28, 63. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Gu, X.; Qin, S.; Zheng, M.; Shi, X.; Peng, C.; Ju, S. Elucidating the Role of Serum tRF-31-U5YKFN8DYDZDD as a Novel Diagnostic Biomarker in Gastric Cancer (GC). Front. Oncol. 2021, 11, 723753. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.; Wu, Z.; Gao, P.; Zhang, W.; Qu, B.; Wang, X.; Song, Y.; Wang, Z. A 3′-tRNA-derived fragment enhances cell proliferation, migration and invasion in gastric cancer by targeting FBXO47. Arch. Biochem. Biophys. 2020, 690, 108467. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Li, Y.; Li, X.; Huang, Y.; Ju, S. Transfer RNA-derived fragment tRF-23-Q99P9P9NDD promotes progression of gastric cancer by targeting ACADSB. J. Zhejiang Univ. Sci. B 2024, 25, 438–450. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Ruan, Y.; Li, Z.; Xie, Y.; Yan, Z.; Guo, J. Global profile of tRNA-derived small RNAs in gastric cancer patient plasma and identification of tRF-33-P4R8YP9LON4VDP as a new tumor suppressor. Int. J. Med. Sci. 2021, 18, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xie, Y.; Yu, X.; Zhang, S.; Wen, Q.; Ye, G.; Guo, J. Clinical diagnostic values of transfer RNA-derived fragment tRF-19-3L7L73JD and its effects on the growth of gastric cancer cells. J. Cancer 2021, 12, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Yu, X.; Ruan, Y.; Shen, Y.; Shao, Y.; Zhang, X.; Ye, G.; Guo, J. The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2021, 12, 418. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, B.; Wang, J.; Tang, L.; Hu, Q.; Wang, J.; Chen, H.; Zheng, J.; Yan, F.; Chen, H. tRNA-Derived Fragment tRF-Glu-TTC-027 Regulates the Progression of Gastric Carcinoma via MAPK Signaling Pathway. Front. Oncol. 2021, 11, 733763. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, J.; Wang, X.; Zhou, B.; Chen, H.; Li, G.; Yan, F. tRF-Val-CAC-016 modulates the transduction of CACNA1d-mediated MAPK signaling pathways to suppress the proliferation of gastric carcinoma. Cell Commun. Signal. 2022, 20, 68. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Peng, L.; Liu, L.; Xie, X.; Zhang, Y.; Gao, Z.; Zhang, C.; Yu, X.; Hu, Y.; et al. A novel 5′tRNA-derived fragment tRF-Tyr inhibits tumor progression by targeting hnRNPD in gastric cancer. Cell Commun. Signal. 2025, 23, 88. [Google Scholar] [CrossRef]
- Ding, R.; Li, Y.; Zhang, Y.; Li, X.; Song, Y.; Gu, X.; Shen, X.; Ju, S. Comprehensive assessment of serum 3′-tRFArg as a novel diagnostic biomarker for gastric cancer. Transl. Oncol. 2025, 54, 102338. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Wu, H.; Du, F.; Xie, X.; Zeng, S.; Zhang, Z.; Dong, K.; Shang, L.; Jing, C.; et al. A novel 3′tRNA-derived fragment tRF-Val promotes proliferation and inhibits apoptosis by targeting EEF1A1 in gastric cancer. Cell Death Dis. 2022, 13, 471. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Huang, B.; Yang, H.; Cheng, X.; Wang, D.; Fu, S.; Shen, W.; Zhang, Q.; Zhang, L.; Xue, Z.; Li, Y.; et al. tRF/miR-1280 Suppresses Stem Cell-like Cells and Metastasis in Colorectal Cancer. Cancer Res. 2017, 77, 3194–3206. [Google Scholar] [CrossRef]
- Xu, R.; Du, A.; Deng, X.; Du, W.; Zhang, K.; Li, J.; Lu, Y.; Wei, X.; Yang, Q.; Tang, H. tsRNA-GlyGCC promotes colorectal cancer progression and 5-FU resistance by regulating SPIB. J. Exp. Clin. Cancer Res. 2024, 43, 230. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, M.; Liu, X.; Gowrikumar, S.; Fatima, I.; Fisher, K.W.; Bastola, D.; Vecchio, A.J.; Singh, A.B.; Dhawan, P. Claudin-1 interacts with EPHA2 to promote cancer stemness and chemoresistance in colorectal cancer. Cancer Lett. 2023, 579, 216479. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Cheng, F.; Huang, L.; Wang, K.; Zhong, L.; Lu, Y.; Ouyang, M. New plasma diagnostic markers for colorectal cancer: Transporter fragments of glutamate tRNA origin. J. Cancer 2024, 15, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, X.; Jiang, G.; Zhang, H.; Ge, L.; Chen, F.; Li, J.; Liu, H.; Wang, H. 5′-tRF-GlyGCC: A tRNA-derived small RNA as a novel biomarker for colorectal cancer diagnosis. Genome Med. 2021, 13, 20. [Google Scholar] [CrossRef]
- Dang, Y.; Dai, L.; Xu, J.; Zhou, W.; Xu, Y.; Ji, G. tRNA-derived fragments are promising biomarkers for screening of early colorectal cancer. MedComm 2023, 4, e227. [Google Scholar] [CrossRef]
- Christodoulou, S.; Katsaraki, K.; Vassiliu, P.; Danias, N.; Michalopoulos, N.; Tzikos, G.; Sideris, D.C.; Arkadopoulos, N. High Intratumoral i-tRF-GlyGCC Expression Predicts Short-Term Relapse and Poor Overall Survival of Colorectal Cancer Patients, Independent of the TNM Stage. Biomedicines 2023, 11, 1945. [Google Scholar] [CrossRef]
- Tan, L.; Wu, X.; Tang, Z.; Chen, H.; Cao, W.; Wen, C.; Zou, G.; Zou, H. The tsRNAs (tRFdb-3013a/b) serve as novel biomarkers for colon adenocarcinomas. Aging 2024, 16, 4299–4326. [Google Scholar] [CrossRef]
- Peng, J.; Bu, F.; Duan, L.; Song, A.; Wang, G.; Zhang, Z. Serum extracellular vesicles 3′tRF-ThrCGTand 3′tRF-mtlleGAT combined with tumor markers can serve as minimally invasive diagnostic predictors for colorectal cancer. Front. Oncol. 2024, 14, 1474095. [Google Scholar] [CrossRef]
- Huang, T.; Chen, C.; Du, J.; Zheng, Z.; Ye, S.; Fang, S.; Liu, K. A tRF-5a fragment that regulates radiation resistance of colorectal cancer cells by targeting MKNK1. J. Cell. Mol. Med. 2023, 27, 4021–4033. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Cao, K.Y.; Pan, Y.; Yan, T.M.; Tao, P.; Xiao, Y.; Jiang, Z.H. Antitumor Activities of tRNA-Derived Fragments and tRNA Halves from Non-pathogenic Escherichia coli Strains on Colorectal Cancer and Their Structure-Activity Relationship. mSystems 2022, 7, e0016422. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.A.; Maghiar, T.A.; Alaya, A.; Olah, N.K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, J.; Park, S.; Ham, J.; Park, W.; Park, H.; An, G.; Hong, T.; Kim, H.S.; Song, G.; et al. Targeting Thymidylate Synthase and tRNA-Derived Non-Coding RNAs Improves Therapeutic Sensitivity in Colorectal Cancer. Antioxidants 2022, 11, 2158. [Google Scholar] [CrossRef] [PubMed]
- Balatti, V.; Rizzotto, L.; Miller, C.; Palamarchuk, A.; Fadda, P.; Pandolfo, R.; Rassenti, L.Z.; Hertlein, E.; Ruppert, A.S.; Lozanski, A.; et al. TCL1 targeting miR-3676 is codeleted with tumor protein p53 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 2169–2174. [Google Scholar] [CrossRef]
- Veneziano, D.; Tomasello, L.; Balatti, V.; Palamarchuk, A.; Rassenti, L.Z.; Kipps, T.J.; Pekarsky, Y.; Croce, C.M. Dysregulation of different classes of tRNA fragments in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2019, 116, 24252–24258. [Google Scholar] [CrossRef]
- Katsaraki, K.; Adamopoulos, P.G.; Papageorgiou, S.G.; Pappa, V.; Scorilas, A.; Kontos, C.K. A 3′ tRNA-derived fragment produced by tRNALeuAAG and tRNALeuTAG is associated with poor prognosis in B-cell chronic lymphocytic leukemia, independently of classical prognostic factors. Eur. J. Haematol. 2021, 106, 821–830. [Google Scholar] [CrossRef]
- Syu, Y.C.; Hatterschide, J.; Budding, C.R.; Tang, Y.; Musier-Forsyth, K. Human T-cell leukemia virus type 1 uses a specific tRNAPro isodecoder to prime reverse transcription. RNA 2024, 30, 967–976. [Google Scholar] [CrossRef]
- Maute, R.L.; Schneider, C.; Sumazin, P.; Holmes, A.; Califano, A.; Basso, K.; Dalla-Favera, R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1404–1409. [Google Scholar] [CrossRef]
- Guzzi, N.; Muthukumar, S.; Cieśla, M.; Todisco, G.; Ngoc, P.C.T.; Madej, M.; Munita, R.; Fazio, S.; Ekström, S.; Mortera-Blanco, T.; et al. Pseudouridine-modified tRNA fragments repress aberrant protein synthesis and predict leukaemic progression in myelodysplastic syndrome. Nat. Cell Biol. 2022, 24, 299–306. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, W.; Shen, F.; Zhou, J.; Gu, Y.; Chen, Y. tRNA-derived fragment tRF-Glu49 inhibits cell proliferation, migration and invasion in cervical cancer by targeting FGL1. Oncol. Lett. 2022, 24, 334. [Google Scholar] [CrossRef]
- Skeparnias, I.; Anastasakis, D.; Grafanaki, K.; Kyriakopoulos, G.; Alexopoulos, P.; Dougenis, D.; Scorilas, A.; Kontos, C.K.; Stathopoulos, C. Contribution of miRNAs, tRNAs and tRFs to Aberrant Signaling and Translation Deregulation in Lung Cancer. Cancers 2020, 12, 3056. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, Y.; Shao, Z.; Wu, Y.; Li, Y.; Fang, S.; Wu, S. TRF-16 Inhibits Lung Cancer Progression by Hindering the N6-Methyladenosine Modification of CPT1A mRNA. J. Cell. Mol. Med. 2024, 28, e70291. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Niu, Y.; Mao, X.; Cui, J.; Wu, X.; Simone, C.B., 2nd; Kang, H.S.; Qin, W.; Jiang, L. tsRNA-5001a promotes proliferation of lung adenocarcinoma cells and is associated with postoperative recurrence in lung adenocarcinoma patients. Transl. Lung Cancer Res. 2021, 10, 3957–3972. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, C.; Fang, L.; Yu, W. Serum transfer RNA-derived fragment tRF-31-79MP9P9NH57SD acts as a novel diagnostic biomarker for non-small cell lung cancer. J. Clin. Lab. Anal. 2022, 36. [Google Scholar] [CrossRef]
- Gu, W.; Shi, J.; Liu, H.; Zhang, X.; Zhou, J.J.; Li, M.; Zhou, D.; Li, R.; Lv, J.; Wen, G.; et al. Peripheral blood non-canonical small non-coding RNAs as novel biomarkers in lung cancer. Mol. Cancer 2020, 19, 159. [Google Scholar] [CrossRef]
- Zheng, B.; Song, X.; Wang, L.; Zhang, Y.; Tang, Y.; Wang, S.; Li, L.; Wu, Y.; Song, X.; Xie, L. Plasma exosomal tRNA-derived fragments as diagnostic biomarkers in non-small cell lung cancer. Front. Oncol. 2022, 12, 1037523. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Wang, J.; He, W.; Li, Y.; Li, H.; Wei, Z.; Cao, Y. tRNA-derived fragment tRF-03357 promotes cell proliferation, migration and invasion in high-grade serous ovarian cancer. Onco Targets Ther. 2019, 12, 6371–6383. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Magkou, P.; Dreyer, T.; Dorn, J.; Obermayr, E.; Mahner, S.; van Gorp, T.; Braicu, I.; Magdolen, V.; Zeillinger, R.; et al. tRNA-derived small RNA 3′U-tRFValCAC promotes tumour migration and early progression in ovarian cancer. Eur. J. Cancer 2023, 180, 134–145. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Dreyer, T.; Dorn, J.; Obermayr, E.; Mahner, S.; Gorp, T.V.; Braicu, I.; Zeillinger, R.; Magdolen, V.; Avgeris, M.; et al. tRNAGlyGCC-Derived Internal Fragment (i-tRF-GlyGCC) in Ovarian Cancer Treatment Outcome and Progression. Cancers 2021, 14, 24. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, M.; Shen, B.; Luo, G.; Wu, Y.; Li, J.; Lu, Z.; Zheng, Z.; Dai, Q.; Wang, H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019, 47, 2533–2545. [Google Scholar] [CrossRef]
- Papadimitriou, M.A.; Avgeris, M.; Levis, P.; Papasotiriou, E.C.; Kotronopoulos, G.; Stravodimos, K.; Scorilas, A. tRNA-Derived Fragments (tRFs) in Bladder Cancer: Increased 5′-tRF-LysCTT Results in Disease Early Progression and Patients’ Poor Treatment Outcome. Cancers 2020, 12, 3661. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, J.; Bai, M.; Liu, T.; Zhan, S.; Li, J.; Ma, Y.; Zhang, Y.; Wu, L.; Zhao, Z.; et al. Plasma tsRNA Signatures Serve as a Novel Biomarker for Bladder Cancer. Cancer Sci. 2025, 116, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Hu, W.; Huang, Y.; Lv, Y.; Ji, D.; Chen, C.; Yang, B.; Zhang, C.; Liang, Y.; Zhang, H.; et al. A Novel tsRNA, m7G-3′ tiRNA LysTTT, Promotes Bladder Cancer Malignancy Via Regulating ANXA2 Phosphorylation. Adv. Sci. 2024, 11, e2400115. [Google Scholar] [CrossRef] [PubMed]
- Nientiedt, M.; Deng, M.; Schmidt, D.; Perner, S.; Müller, S.C.; Ellinger, J. Identification of aberrant tRNA-halves expression patterns in clear cell renal cell carcinoma. Sci. Rep. 2016, 6, 37158. [Google Scholar] [CrossRef]
- Zhao, C.; Tolkach, Y.; Schmidt, D.; Kristiansen, G.; Müller, S.C.; Ellinger, J. 5′-tRNA Halves are Dysregulated in Clear Cell Renal Cell Carcinoma. J. Urol. 2018, 199, 378–383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Sun, Z.; Li, H. The Roles of tRNA-Derived Fragments in Cancer: Updates and Perspectives. Int. J. Mol. Sci. 2025, 26, 5822. https://doi.org/10.3390/ijms26125822
Geng J, Sun Z, Li H. The Roles of tRNA-Derived Fragments in Cancer: Updates and Perspectives. International Journal of Molecular Sciences. 2025; 26(12):5822. https://doi.org/10.3390/ijms26125822
Chicago/Turabian StyleGeng, Jiamian, Zhaoyuan Sun, and Hang Li. 2025. "The Roles of tRNA-Derived Fragments in Cancer: Updates and Perspectives" International Journal of Molecular Sciences 26, no. 12: 5822. https://doi.org/10.3390/ijms26125822
APA StyleGeng, J., Sun, Z., & Li, H. (2025). The Roles of tRNA-Derived Fragments in Cancer: Updates and Perspectives. International Journal of Molecular Sciences, 26(12), 5822. https://doi.org/10.3390/ijms26125822




