Macrophage Polarization in Heterotopic Ossification: Inflammation, Osteogenesis, and Emerging Therapeutic Targets
Abstract
1. Introduction
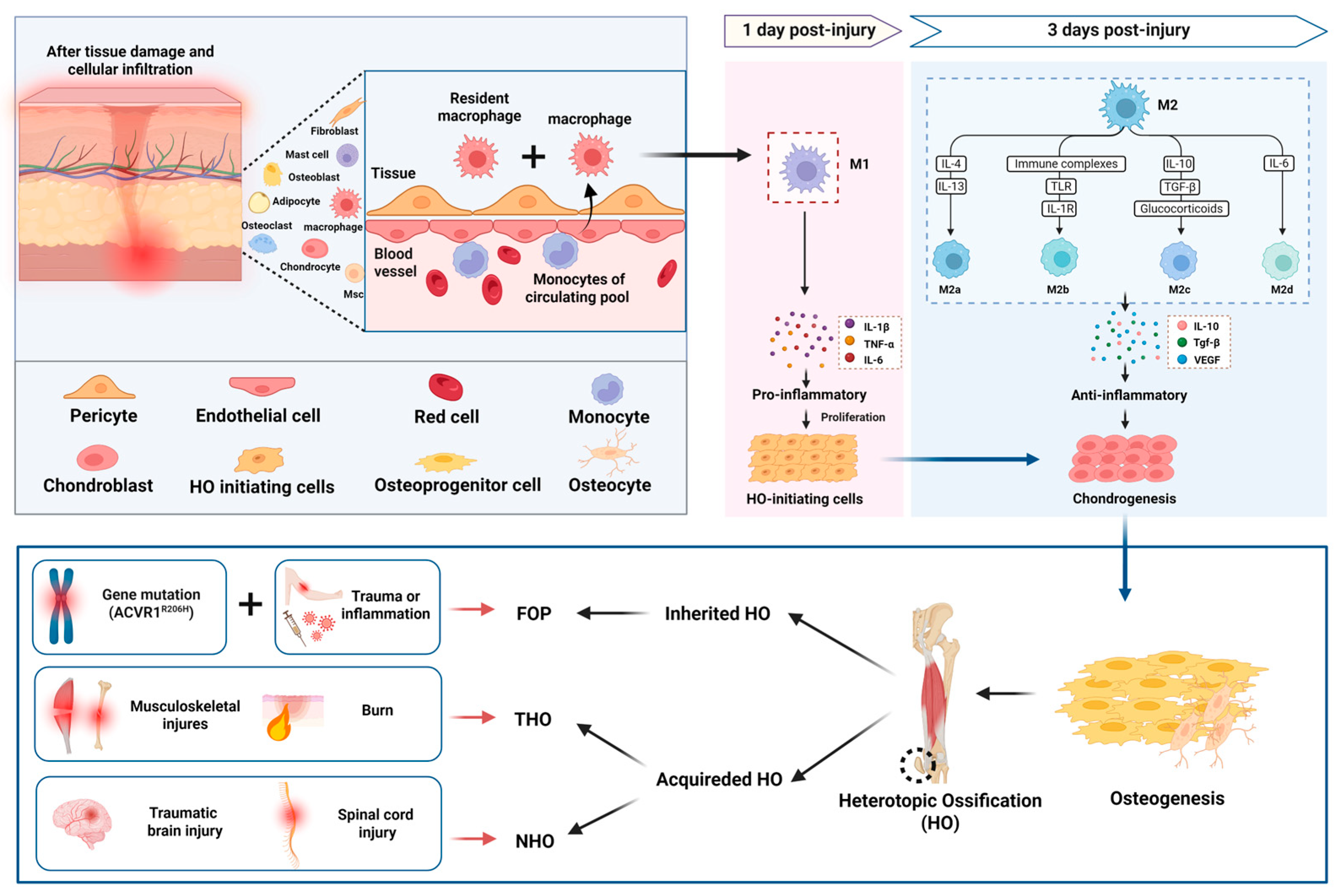
2. Macrophage Polarization in HO
3. Mechanism of Macrophage in HO
3.1. Regulation of Inflammatory Microenvironment
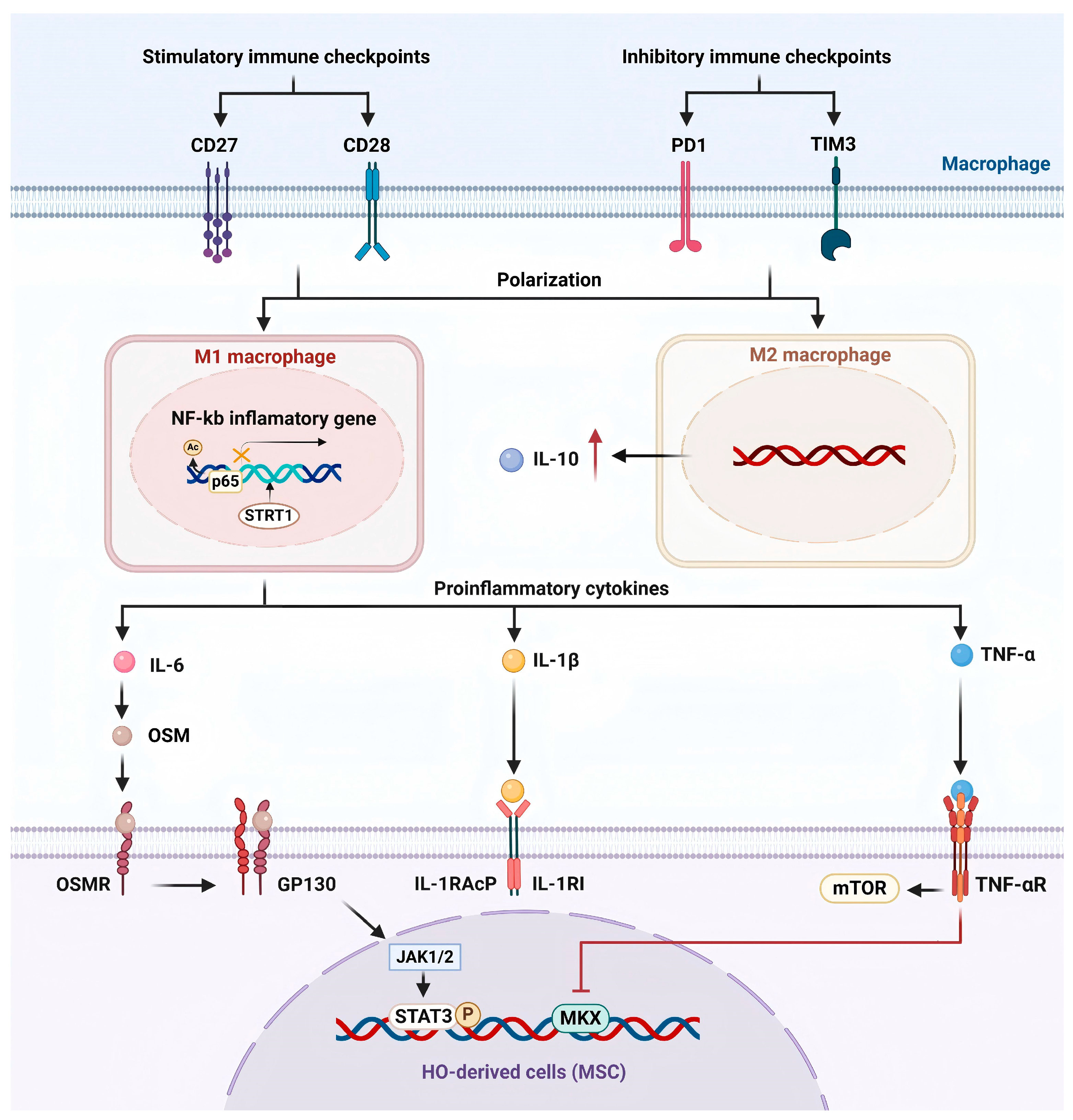
3.1.1. Pro-Inflammatory Cytokines
- IL-6
- IL-1β
- TNF-α
3.1.2. Anti-Inflammatory Cytokines
3.1.3. Immune Checkpoint Proteins
3.1.4. SIRT1/NF-κB
3.1.5. Potential Impact of Inflammation on Chondrogenic Differentiation
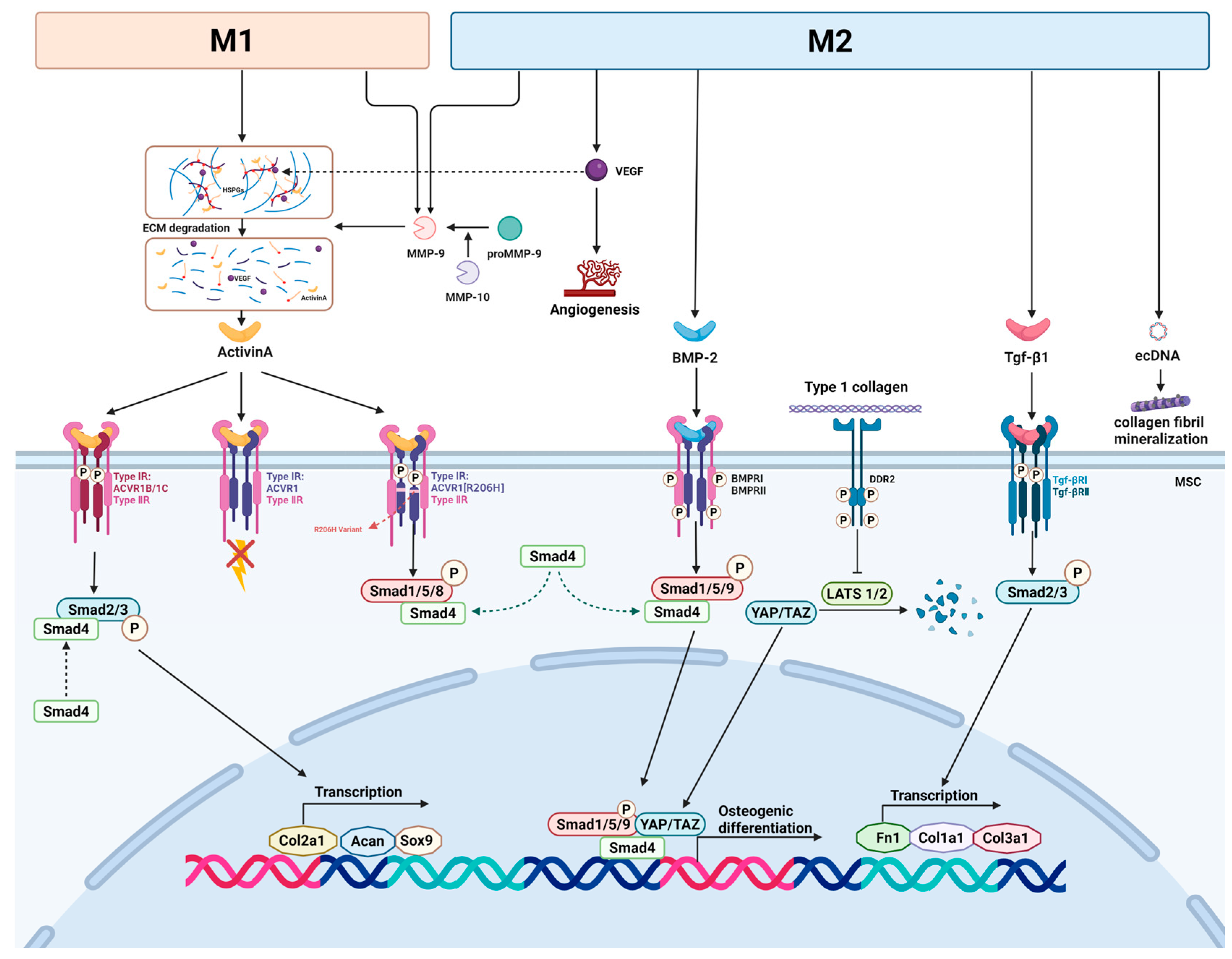
3.2. Macrophage Regulate the Osteogenic Factors and the Osteogenic Pathway
3.2.1. Activin A
3.2.2. Matrix Metalloproteinase
3.2.3. Tgf-β Signaling Pathway
3.2.4. BMP
3.2.5. Macrophage-Derived Extracellular DNA
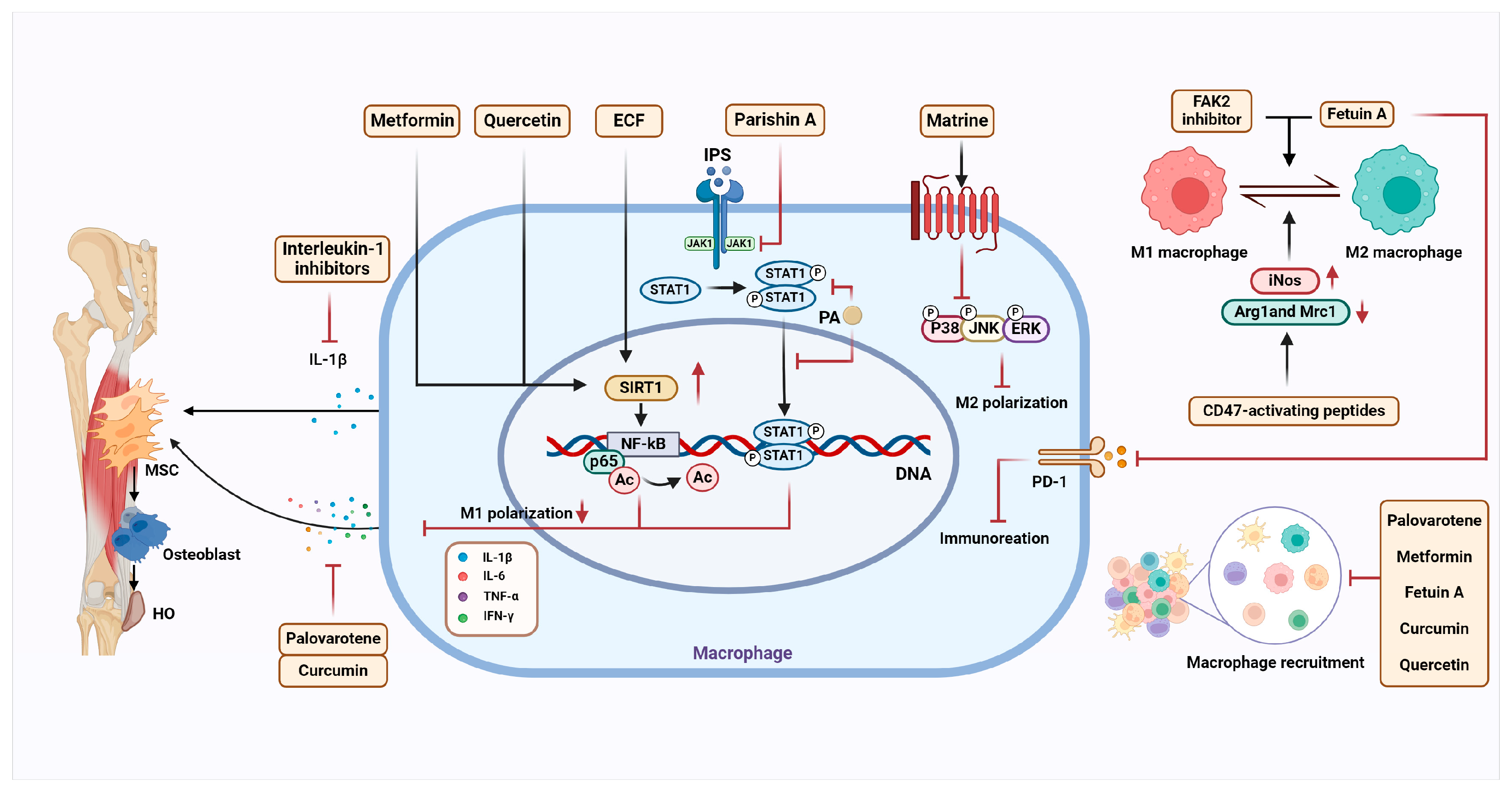
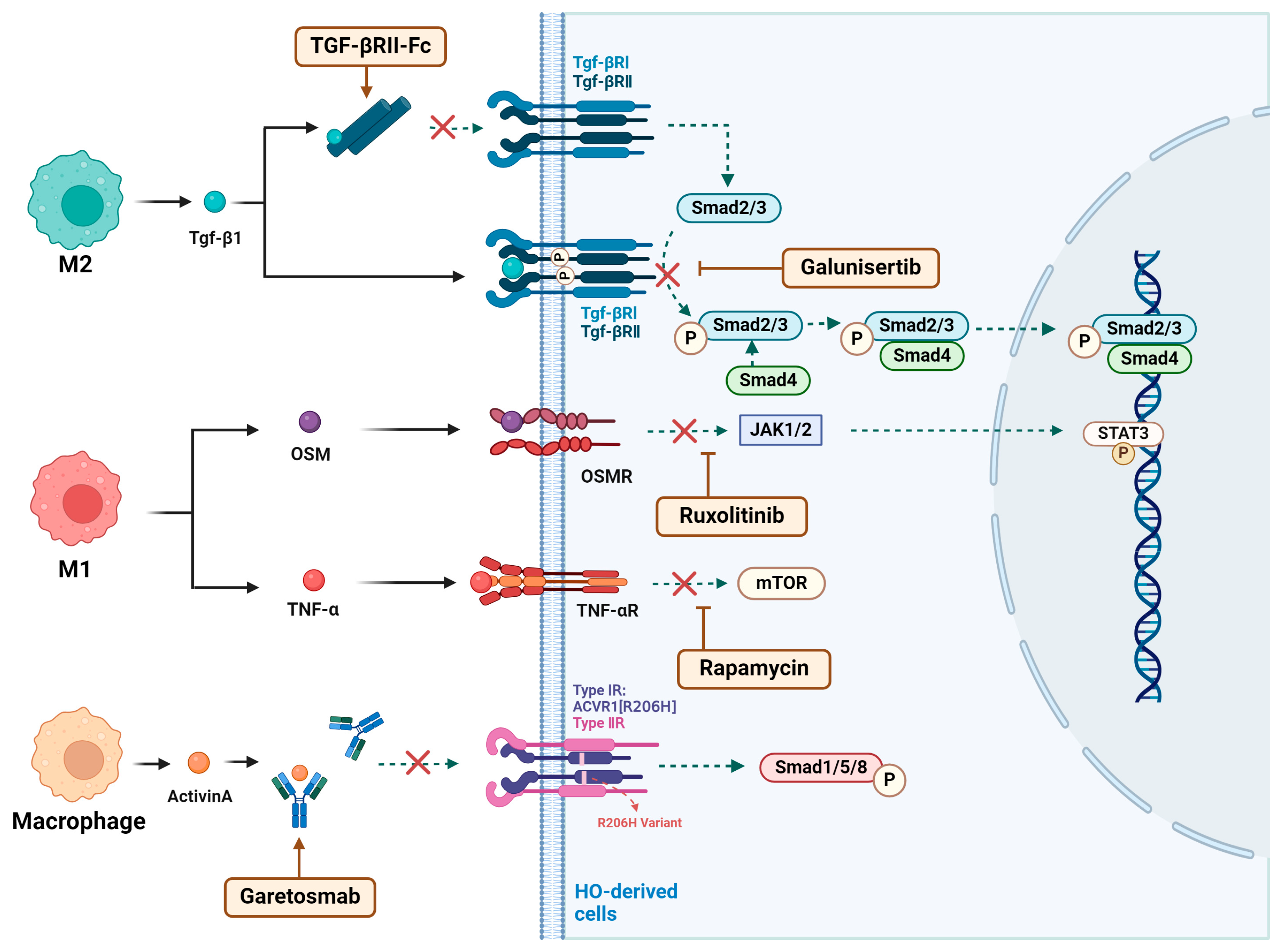
4. HO Treatment Target Macrophage
4.1. Drugs That Modulate the Macrophage and Inflammatory Microenvironment
4.1.1. Interleukin-1 Inhibitors
4.1.2. Palovarotene
4.1.3. Metformin
4.1.4. Parishin A-Loaded Mesoporous Silica Nanoparticles
4.1.5. Matrine
4.1.6. Spray of Hydrogel
4.1.7. Fetuin-A
4.1.8. Quercetin
4.1.9. CD47-Activating Peptides
4.1.10. Focal Adhesion Kinase-2 Inhibitor
4.1.11. Ethyl Caffeate (ECF)
4.2. Drugs That Affect Osteogenic Signaling in Macrophages
4.2.1. TGF-βRII-Fc
4.2.2. Galunisertib
4.2.3. Ruxolitinib
4.2.4. Garetosmab
4.2.5. Rapamycin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMDMs | bone marrow-derived macrophages |
| BMP | bone morphogenetic protein |
| DDR2 | discoidin domain receptor 2 |
| DPI | day post-injury |
| ECF | ethyl caffeate |
| ECM | extracellular matrix |
| eCDNA | extracellular DNA |
| FAK2 | focal adhesion kinase-2 |
| FAPs | fibro-adipogenic progenitors |
| FOP | fibrodysplasia ossificans progressiva |
| FDA | the U.S. Food and Drug Administration |
| GP130 | glycoprotein 130 |
| Gst | galunisertib |
| HO | heterotopic ossification |
| HSPGs | heparan sulfate proteoglycans |
| ICs | immune checkpoints |
| IL-1β | interleukin-1β |
| iMACs | induced pluripotent stem cells-derived macrophages |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | matrix metalloproteinase |
| MPCs | mesenchymal progenitor cells |
| MSCs | mesenchymal stem cells |
| NHO | neurogenic heterotopic ossification |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OSM | oncostatin M |
| OSMR | OSM receptor |
| PA | parishin A |
| POH | progressive ossification heterotopia |
| RARγ | retinoic acid receptor γ |
| SIRT1 | sirtuin 1 |
| SCI | spinal cord injury |
| TBI | traumatic brain injury |
| TDSCs | tendon-derived stem cells |
| TGF-β | transforming growth factor-β |
| TGF-βR1 | TGF-β receptor I |
| TSCs | tendon stem cells |
| TNF | tumor necrosis factor |
References
- Dey, D.; Wheatley, B.M.; Cholok, D.; Agarwal, S.; Yu, P.B.; Levi, B.; Davis, T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. 2017, 186, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tuan, R.S. Mechanism of traumatic heterotopic ossification: In search of injury-induced osteogenic factors. J. Cell. Mol. Med. 2020, 24, 11046–11055. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Hüning, I.; Gillessen-Kaesbach, G. Fibrodysplasia ossificans progressiva: Clinical course, genetic mutations and genotype-phenotype correlation. Mol. Syndr. 2014, 5, 201–211. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Shore, E.M.; Pignolo, R.J. Fibrodysplasia ossificans progressiva emerges from obscurity. Trends Mol. Med. 2024, 31, 106–116. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef]
- Shore, E.M.; Kaplan, F.S. Inherited human diseases of heterotopic bone formation. Nat. Rev. Rheumatol. 2010, 6, 518–527. [Google Scholar] [CrossRef]
- Sullivan, M.P.; Torres, S.J.; Mehta, S.; Ahn, J. Heterotopic ossification after central nervous system trauma: A current review. Bone Jt. Res. 2013, 2, 51–57. [Google Scholar] [CrossRef]
- Wang, H.; Song, D.; Wei, L.; Huang, L.; Wei, D.; Su, Y.; Liang, J.; Lian, H.; Zhao, J.; Liu, Q. Ethyl caffeate inhibits macrophage polarization via SIRT1/NF-κB to attenuate traumatic heterotopic ossification in mice. Biomed. Pharmacother. 2023, 161, 114508. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Zhang, Q.; Liang, Y.; Wu, H. Matrine reduces traumatic heterotopic ossification in mice by inhibiting M2 macrophage polarization through the MAPK pathway. Biomed. Pharmacother. 2024, 177, 117130. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Z.; Luo, G.; Wang, S.; Cui, H.; Yao, Z.; Xiong, H.; He, Y.; Qian, Y.; Fan, C.; et al. Quercetin Attenuates Trauma-Induced Heterotopic Ossification by Tuning Immune Cell Infiltration and Related Inflammatory Insult. Front. Immunol. 2021, 12, 649285. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F., IV; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Kulakova, K.; Lawal, T.R.; McCarthy, E.; Floudas, A. The Contribution of Macrophage Plasticity to Inflammatory Arthritis and Their Potential as Therapeutic Targets. Cells 2024, 13, 1586. [Google Scholar] [CrossRef]
- Anders, C.B.; Lawton, T.M.W.; Smith, H.L.; Garret, J.; Doucette, M.M.; Ammons, M.C.B. Use of integrated metabolomics, transcriptomics, and signal protein profile to characterize the effector function and associated metabotype of polarized macrophage phenotypes. J. Leukoc. Biol. 2022, 111, 667–693. [Google Scholar] [CrossRef]
- Liang, C.; Wu, S.; Xia, G.; Huang, J.; Wen, Z.; Zhang, W.; Cao, X. Engineered M2a macrophages for the treatment of osteoarthritis. Front. Immunol. 2022, 13, 1054938. [Google Scholar] [CrossRef]
- Sezginer, O.; Unver, N. Dissection of pro-tumoral macrophage subtypes and immunosuppressive cells participating in M2 polarization. Inflamm. Res. 2024, 73, 1411–1423. [Google Scholar] [CrossRef]
- Graney, P.L.; Roohani-Esfahani, S.I.; Zreiqat, H.; Spiller, K.L. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J. R. Soc. Interface 2016, 13, 20160346. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Yang, J.; Fan, H.; Dai, Y.; Wang, X.; Chen, R.; Liu, J.; Meng, X.; Wang, W.; Li, G.; et al. Fetuin-A is an immunomodulator and a potential therapeutic option in BMP4-dependent heterotopic ossification and associated bone mass loss. Bone Res. 2022, 10, 62. [Google Scholar] [CrossRef]
- Kan, C.; Tan, Z.; Wang, H.; Wang, W.; Yang, J.; Zhang, Y.; Lu, X.; Cheng, Q.; Chai, L.; Peng, C.; et al. Spatiotemporal Analysis of Mesenchymal Stem Cells Fate Determination by Inflammatory Niche Following Soft Tissue Injury at a Single-Cell Level. Adv. Sci. 2024, 11, e2310282. [Google Scholar] [CrossRef]
- Huang, J.; Lin, J.; Li, C.; Tang, B.; Xiao, H. Palovarotene Can Attenuate Heterotopic Ossification Induced by Tendon Stem Cells by Downregulating the Synergistic Effects of Smad and NF-κB Signaling Pathway following Stimulation of the Inflammatory Microenvironment. Stem Cells Int. 2022, 2022, 1560943. [Google Scholar] [CrossRef]
- Diolintzi, A.; Pervin, M.S.; Hsiao, E.C. Immunologic Aspects in Fibrodysplasia Ossificans Progressiva. Biomolecules 2024, 14, 357. [Google Scholar] [CrossRef]
- Sung Hsieh, H.H.; Chung, M.T.; Allen, R.M.; Ranganathan, K.; Habbouche, J.; Cholok, D.; Butts, J.; Kaura, A.; Tiruvannamalai-Annamalai, R.; Breuler, C.; et al. Evaluation of Salivary Cytokines for Diagnosis of both Trauma-Induced and Genetic Heterotopic Ossification. Front. Endocrinol. 2017, 8, 74. [Google Scholar] [CrossRef]
- Vallés, G.; Bensiamar, F.; Maestro-Paramio, L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 57. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Sinha, S.; Chung, J.; Rux, D.; Catheline, S.E.; Koyama, E.; Qin, L.; Pacifici, M. Activin A promotes the development of acquired heterotopic ossification and is an effective target for disease attenuation in mice. Sci. Signal. 2021, 14, eabd0536. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Luo, G.; Liu, W.; He, Y.; Wang, F.; Qian, Y.; Fan, C. Pharmacological activation of SIRT1 by metformin prevented trauma-induced heterotopic ossification through inhibiting macrophage mediated inflammation. Eur. J. Pharmacol. 2021, 909, 174386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Y.; Jin, S.; Niu, Y.; Yu, M.; Li, Z.; Chen, L.; Wu, X.; Ding, C.; Wu, T.; et al. Parishin A-loaded mesoporous silica nanoparticles modulate macrophage polarization to attenuate tendinopathy. NPJ Regen. Med. 2023, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Lepinski, A.; Chavez, R.D.; Barruet, E.; Pereira, A.; Moody, T.A.; Ton, A.N.; Sharma, A.; Hellman, J.; Tomoda, K.; et al. ACVR1(R206H) extends inflammatory responses in human induced pluripotent stem cell-derived macrophages. Bone 2021, 153, 116129. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xie, Q.; Lin, J.; Dong, H.; Zhuang, X.; Xian, R.; Liang, Y.; Li, S. Immunomodulatory Effects and Mechanisms of Two-Dimensional Black Phosphorus on Macrophage Polarization and Bone Regeneration. Int. J. Nanomed. 2025, 20, 4337–4355. [Google Scholar] [CrossRef]
- Kan, C.; Yang, J.; Na, D.; Xu, Y.; Yang, B.; Zhao, H.; Lu, H.; Li, Y.; Zhang, K.; McGuire, T.L.; et al. Inhibition of immune checkpoints prevents injury-induced heterotopic ossification. Bone Res. 2019, 7, 33. [Google Scholar] [CrossRef]
- Ando, Y.; Tsukasaki, M.; Huynh, N.C.; Zang, S.; Yan, M.; Muro, R.; Nakamura, K.; Komagamine, M.; Komatsu, N.; Okamoto, K.; et al. The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. Int. J. Oral Sci. 2024, 16, 18. [Google Scholar] [CrossRef]
- Guihard, P.; Boutet, M.A.; Brounais-Le Royer, B.; Gamblin, A.L.; Amiaud, J.; Renaud, A.; Berreur, M.; Rédini, F.; Heymann, D.; Layrolle, P.; et al. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am. J. Pathol. 2015, 185, 765–775. [Google Scholar] [CrossRef]
- Torossian, F.; Guerton, B.; Anginot, A.; Alexander, K.A.; Desterke, C.; Soave, S.; Tseng, H.-W.; Arouche, N.; Boutin, L.; Kulina, I.; et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight 2017, 2, e96034. [Google Scholar] [CrossRef]
- Sims, N.A.; Lévesque, J.P. Oncostatin M: Dual Regulator of the Skeletal and Hematopoietic Systems. Curr. Osteoporos. Rep. 2024, 22, 80–95. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, B.; Tang, M.; Jin, D.; Lai, P. Tuberous sclerosis complex 1 targeted depletion in macrophages promotes osteogenesis by modulating secretion of Oncostatin M in the inflammatory stage of bone healing. Int. Immunopharmacol. 2023, 124, 110895. [Google Scholar] [CrossRef]
- Alexander, K.A.; Tseng, H.W.; Fleming, W.; Jose, B.; Salga, M.; Kulina, I.; Millard, S.M.; Pettit, A.R.; Genêt, F.; Levesque, J.-P. Inhibition of JAK1/2 Tyrosine Kinases Reduces Neurogenic Heterotopic Ossification After Spinal Cord Injury. Front. Immunol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Nilkhet, S.; Mongkolpobsin, K.; Sillapachaiyaporn, C.; Wongsirojkul, N.; Tencomnao, T.; Chuchawankul, S. M1 macrophages polarized by crude polysaccharides isolated from Auricularia polytricha exhibit anti-tumor effect on human breast cancer cells. Sci. Rep. 2024, 14, 8179. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.W.; Kulina, I.; Girard, D.; Gueguen, J.; Vaquette, C.; Salga, M.; Fleming, W.; Jose, B.; Millard, S.M.; Pettit, A.R.; et al. Interleukin-1 Is Overexpressed in Injured Muscles Following Spinal Cord Injury and Promotes Neurogenic Heterotopic Ossification. J. Bone Miner. Res. 2022, 37, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Haviv, R.; Moshe, V.; De Benedetti, F.; Prencipe, G.; Rabinowicz, N.; Uziel, Y. Is fibrodysplasia ossificans progressiva an interleukin-1 driven auto-inflammatory syndrome? Pediatr. Rheumatol. 2019, 17, 84. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Yao, Z.; Yuan, F.; Liu, H.; Sun, Z.; Yuan, Z.; Luo, G.; Yao, X.; Cui, H.; et al. NLRP3-Dependent Crosstalk between Pyroptotic Macrophage and Senescent Cell Orchestrates Trauma-Induced Heterotopic Ossification During Aberrant Wound Healing. Adv. Sci. 2023, 10, e2207383. [Google Scholar] [CrossRef]
- Isaji, M.; Horiuchi, K.; Kondo, S.; Nakagawa, T.; Ishizaka, T.; Amako, M.; Chiba, K. Suppression of TNF-α activity by immobilization rescues Mkx expression and attenuates tendon ossification in a mouse Achilles tenotomy model. J. Orthop. Res. 2024, 42, 2140–2148. [Google Scholar] [CrossRef]
- Kushima, Y.; Sato, Y.; Kobayashi, T.; Fukuma, Y.; Matsumoto, M.; Nakamura, M.; Iwamoto, T.; Miyamoto, T. TNFα-dependent mTOR activity is required for tenotomy-induced ectopic ossification in mice. J. Bone Miner. Metab. 2023, 41, 583–591. [Google Scholar] [CrossRef]
- Hess, K.; Ushmorov, A.; Fiedler, J.; Brenner, R.E.; Wirth, T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 2009, 45, 367–376. [Google Scholar] [CrossRef]
- Evans, K.N.; Forsberg, J.A.; Potter, B.K.; Hawksworth, J.S.; Brown, T.S.; Andersen, R.; Dunne, J.R.; Tadaki, D.; Elster, E.A. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J. Orthop. Trauma 2012, 26, e204–e213. [Google Scholar] [CrossRef]
- Barruet, E.; Morales, B.M.; Cain, C.J.; Ton, A.N.; Wentworth, K.L.; Chan, T.V.; Moody, T.A.; Haks, M.C.; Ottenhoff, T.H.M.; Hellman, J.; et al. NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification. JCI Insight 2018, 3, e122958. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, J.; Fan, J.; Tang, Z.; Zhou, W.; Lin, H. Inhibition of NF-κB Signaling-Mediated Crosstalk Between Macrophages and Preosteoblasts by Metformin Alleviates Trauma-Induced Heterotopic Ossification. Inflammation 2023, 46, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, H.; Qian, W. Macrophage Polarization in the Osteoarthritis Pathogenesis and Treatment. Orthop. Surg. 2025, 17, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; de Vries-van Melle, M.L.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; valn Osch, G.J. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Wang, Z.; Zhu, R.; Cheng, L.; Cheng, Q. Low-intensity pulsed ultrasound promotes mesenchymal stem cell transplantation-based articular cartilage regeneration via inhibiting the TNF signaling pathway. Stem Cell Res. Ther. 2023, 14, 93. [Google Scholar] [CrossRef]
- Tu, B.; Li, J.; Sun, Z.; Zhang, T.; Liu, H.; Yuan, F.; Fan, C. Macrophage-Derived TGF-β and VEGF Promote the Progression of Trauma-Induced Heterotopic Ossification. Inflammation 2023, 46, 202–216. [Google Scholar] [CrossRef]
- Heilig, J.; Dietmar, H.F.; Brachvogel, B.; Paulsson, M.; Zaucke, F.; Niehoff, A. Collagen IX deficiency leads to premature vascularization and ossification of murine femoral heads through an imbalance of pro- and antiangiogenic factors. Osteoarthr. Cartil. 2020, 28, 988–999. [Google Scholar] [CrossRef]
- Lounev, V.; Groppe, J.C.; Brewer, N.; Wentworth, K.L.; Smith, V.; Xu, M.; Schomburg, L.; Bhargava, P.; Al Mukaddam, M.; Hsiao, E.C.; et al. Matrix metalloproteinase-9 deficiency confers resilience in fibrodysplasia ossificans progressiva in a man and mice. J. Bone Miner. Res. 2024, 39, 382–398. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J.; Chen, C.; Wang, Y.; Hao, Z.; Chen, T.; Wang, J.; Li, J. Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review. J. Funct. Biomater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Han, X.; Gao, C.; Lu, W.; Yan, J.; Xu, H.; Guo, Z.; Qin, W.; Lu, N.; Gao, J.; Zhu, W.; et al. Macrophage-Derived Extracellular DNA Initiates Heterotopic Ossification. Inflammation 2023, 46, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Arostegui, M.; Goebel, E.J.; Hart, K.N.; Aykul, S.; Lees-Shepard, J.B.; Idone, V.; Economides, A.N.; Hatsell, S.J. How Activin A Became a Therapeutic Target in Fibrodysplasia Ossificans Progressiva. Biomolecules 2024, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef]
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc. Natl. Acad. Sci. USA 2015, 112, 15438–15443. [Google Scholar] [CrossRef]
- Lieu, S.; Hansen, E.; Dedini, R.; Behonick, D.; Werb, Z.; Miclau, T.; Marcucio, R.; Colnot, C. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis. Model. Mech. 2011, 4, 203–211. [Google Scholar] [CrossRef]
- Rodenberg, E.; Azhdarinia, A.; Lazard, Z.W.; Hall, M.; Kwon, S.K.; Wilganowski, N.; Salisbury, E.A.; Merched-Sauvage, M.; Olmsted-Davis, E.A.; Sevick-Muraca, E.M.; et al. Matrix metalloproteinase-9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng. Part A 2011, 17, 2487–2496. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Haupt, J.; Stanley, A.; McLeod, C.M.; Cosgrove, B.D.; Culbert, A.L.; Wang, L.; Mourkioti, F.; Mauck, R.L.; Shore, E.M. ACVR1(R206H) FOP mutation alters mechanosensing and tissue stiffness during heterotopic ossification. Mol. Biol. Cell 2019, 30, 17–29. [Google Scholar] [CrossRef]
- Loomis, T.; Hu, L.-Y.; Wohlgemuth, R.P.; Chellakudam, R.R.; Muralidharan, P.D.; Smith, L.R. Matrix stiffness and architecture drive fibro-adipogenic progenitors’ activation into myofibroblasts. Sci. Rep. 2022, 12, 13582. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yano, M.; Kawao, N.; Tamura, Y.; Okada, K.; Kaji, H. Role of matrix metalloproteinase-10 in the BMP-2 inducing osteoblastic differentiation. Endocr. J. 2013, 60, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Fernández, J.; López-Martínez, T.; Ripalda-Cemboráin, P.; Calvo, I.A.; Sáez, B.; Romero-Torrecilla, J.A.; Aldazabal, J.; Muiños-López, E.; Montiel, V.; Orbe, J.; et al. Molecular and Cellular Mechanisms of Delayed Fracture Healing in Mmp10 (Stromelysin 2) Knockout Mice. J. Bone Miner. Res. 2021, 36, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Matsunobu, T.; Torigoe, K.; Ishikawa, M.; de Vega, S.; Kulkarni, A.B.; Iwamoto, Y.; Yamada, Y. Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev. Biol. 2009, 332, 325–338. [Google Scholar] [CrossRef]
- Patel, N.K.; Nunez, J.H.; Sorkin, M.; Marini, S.; Pagani, C.A.; Strong, A.L.; Hwang, C.D.; Li, S.; Padmanabhan, K.R.; Kumar, R.; et al. Macrophage TGF-β signaling is critical for wound healing with heterotopic ossification after trauma. JCI Insight 2022, 7, e144925. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef]
- Sánchez-Duffhues, G.; Hiepen, C.; Knaus, P.; Ten Dijke, P. Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef]
- Dilling, C.F.; Wada, A.M.; Lazard, Z.W.; Salisbury, E.A.; Gannon, F.H.; Vadakkan, T.J.; Gao, L.; Hirschi, K.; Dickinson, M.E.; Davis, A.R.; et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J. Bone Miner. Res. 2010, 25, 1147–1156. [Google Scholar] [CrossRef]
- Wu, F.; Ge, C.; Pan, H.; Han, Y.; Mishina, Y.; Kaartinen, V.; Franceschi, R.T. Discoidin domain receptor 2 is an important modulator of BMP signaling during heterotopic bone formation. Bone Res. 2025, 13, 7. [Google Scholar] [CrossRef]
- Tirone, M.; Giovenzana, A.; Vallone, A.; Zordan, P.; Sormani, M.; Nicolosi, P.A.; Meneveri, R.; Gigliotti, C.R.; Spinelli, A.E.; Bocciardi, R.; et al. Severe Heterotopic Ossification in the Skeletal Muscle and Endothelial Cells Recruitment to Chondrogenesis Are Enhanced by Monocyte/Macrophage Depletion. Front. Immunol. 2019, 10, 1640. [Google Scholar] [CrossRef]
- Yu, P.B.; Deng, D.Y.; Lai, C.S.; Hong, C.C.; Cuny, G.D.; Bouxsein, M.L.; Hong, D.W.; McManus, P.M.; Katagiri, T.; Sachidanandan, C.; et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 2008, 14, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lin, H.; Shen, H.; Sohn, J.; Alexander, P.G.; Tuan, R.S. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production. J. Orthop. Transl. 2019, 18, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Convente, M.R.; Chakkalakal, S.A.; Yang, E.; Caron, R.J.; Zhang, D.; Kambayashi, T.; Kaplan, F.S.; Shore, E.M. Depletion of Mast Cells and Macrophages Impairs Heterotopic Ossification in an Acvr1(R206H) Mouse Model of Fibrodysplasia Ossificans Progressiva. J. Bone Miner. Res. 2018, 33, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Tseng, H.-W.; Salga, M.; Genêt, F.; Levesque, J.-P. When the Nervous System Turns Skeletal Muscles into Bones: How to Solve the Conundrum of Neurogenic Heterotopic Ossification. Curr. Osteoporos. Rep. 2020, 18, 666–676. [Google Scholar] [CrossRef]
- Muñoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Grüneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity 2019, 51, 443–450.e4. [Google Scholar] [CrossRef]
- Baker, R.; Rogers, K.D.; Shepherd, N.; Stone, N. New relationships between breast microcalcifications and cancer. Br. J. Cancer 2010, 103, 1034–1039. [Google Scholar] [CrossRef]
- Shen, M.J.; Jiao, K.; Wang, C.Y.; Ehrlich, H.; Wan, M.C.; Hao, D.X.; Li, J.; Wan, Q.-Q.; Tonggu, L.; Yan, J.-F.; et al. Extracellular DNA: A Missing Link in the Pathogenesis of Ectopic Mineralization. Adv. Sci. 2022, 9, e2103693. [Google Scholar] [CrossRef]
- Pacifici, M. Acquired and congenital forms of heterotopic ossification: New pathogenic insights and therapeutic opportunities. Curr. Opin. Pharmacol. 2018, 40, 51–58. [Google Scholar] [CrossRef]
- Oni, J.K.; Pinero, J.R.; Saltzman, B.M.; Jaffe, F.F. Effect of a selective COX-2 inhibitor, celecoxib, on heterotopic ossification after total hip arthroplasty: A case-controlled study. HIP Int. 2014, 24, 256–262. [Google Scholar] [CrossRef]
- Honore, T.; Bonan, I.; Salga, M.; Denormandie, P.; Labib, A.; Genet, G.; Grelier, A.; Genet, F. Effectiveness of radiotherapy to prevent recurrence of heterotopic ossification in patients with spinal cord injury and traumatic head injury: A retrospective case-controlled study. J. Rehabil. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Krainer, J.; Siebenhandl, S.; Weinhäusel, A. Systemic autoinflammatory diseases. J. Autoimmun. 2020, 109, 102421. [Google Scholar] [CrossRef] [PubMed]
- Haviv, R.; Zeitlin, L.; Moshe, V.; Ziv, A.; Rabinowicz, N.; De Benedetti, F.; Prencipe, G.; Matteo, V.; De Cunto, C.L.; Hsiao, E.C.; et al. Long-term use of interleukin-1 inhibitors reduce flare activity in patients with fibrodysplasia ossificans progressiva. Rheumatology 2024, 63, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, B.M.; Cilwa, K.E.; Dey, D.; Qureshi, A.T.; Seavey, J.G.; Tomasino, A.M.; Sanders, E.M.; Bova, W.; Boehm, C.A.; Iwamoto, M.; et al. Palovarotene inhibits connective tissue progenitor cell proliferation in a rat model of combat-related heterotopic ossification. J. Orthop. Res. 2018, 36, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Mundy, C.; Yao, L.; Shaughnessy, K.A.; Saunders, C.; Shore, E.M.; Koyama, E.; Pacifici, M. Palovarotene Action Against Heterotopic Ossification Includes a Reduction of Local Participating Activin A-Expressing Cell Populations. JBMR Plus 2023, 7, e10821. [Google Scholar] [CrossRef]
- Hostalek, U.; Gwilt, M.; Hildemann, S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs 2015, 75, 1071–1094. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Hegazy, S.K.; Maher, M.M.; Bahgat, M.M.; Bahaa, M.M. Repurposing metformin as adjuvant therapy in patients with ulcerative colitis treated with mesalamine: A randomized controlled double-blinded study. Int. Immunopharmacol. 2024, 138, 112541. [Google Scholar] [CrossRef]
- Turkistani, A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; Bahaa, M.M.; Al-Windy, S.; Batiha, G.E. Pharmacological characterization of the antidiabetic drug metformin in atherosclerosis inhibition: A comprehensive insight. Immun. Inflamm. Dis. 2024, 12, e1346. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Angiari, S.; Hooftman, A.; Wadhwa, R.; Zhang, Y.; Zheng, Y.; Spina, J.S.; Ruzek, M.C.; Argiriadi, M.A.; McGettrick, A.F.; et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022, 34, 487–501.e8. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Jia, L.-Y.; Rong, Z.; Zhou, X.; Cao, L.-Q.; Li, A.-H.; Guo, M.; Jin, J.; Wang, Y.-D.; Huang, L.; et al. Research Advances on Matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Pan, X.; Rui, Y.; Li, F. Matrine attenuates heterotopic ossification by suppressing TGF-β induced mesenchymal stromal cell migration and osteogenic differentiation. Biomed Pharmacother. 2020, 127, 110152. [Google Scholar] [CrossRef] [PubMed]
- Olmsted-Davis, E.; Mejia, J.; Salisbury, E.; Gugala, Z.; Davis, A.R. A Population of M2 Macrophages Associated with Bone Formation. Front. Immunol. 2021, 12, 686769. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Yao, H.; Wang, F.; Gu, Z.; Yang, X.; Zhao, Y.; Pang, H.; Wang, D.-A.; Chong, H. Curcumin-Encapsulated Co-ZIF-8 for Ulcerative Colitis Therapy: ROS Scavenging and Macrophage Modulation Effects. ACS Omega 2024, 9, 30571–30582. [Google Scholar] [CrossRef]
- Sun, J.; Du, J.; Liu, X.; An, J.; Hu, Y.; Wang, J.; Zhu, F.; Feng, H.; Cheng, S.; Tian, H.; et al. Chondroitin sulfate-modified tragacanth gum-gelatin composite nanocapsules loaded with curcumin nanocrystals for the treatment of arthritis. J. Nanobiotechnol. 2024, 22, 270. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Zamanian, M.Y.; Al-Ani, A.M.; Jabbar, T.L.; Kareem, A.K.; Aghaei, Z.H.; Tahernia, H.; Hjazi, A.; Jissir, S.A.-R.; Hakimizadeh, E. Targeting STAT3 signaling pathway by curcumin and its analogues for breast cancer: A narrative review. Anim. Model. Exp. Med. 2024, 7, 853–867. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Lu, B.; Mei, J.; Xu, L.; Zhang, X.; Su, Z.; Xu, W.; Fang, S.; Zhu, C.; et al. Inflammation-Responsive Hydrogel Spray for Synergistic Prevention of Traumatic Heterotopic Ossification via Dual-Homeostatic Modulation Strategy. Adv. Sci. 2023, 10, e2302905. [Google Scholar] [CrossRef]
- Sardana, O.; Goyal, R.; Bedi, O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD. Inflammopharmacology 2021, 29, 1061–1074. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Tilahun Muche, Z.; Behaile, T.M.A.; Mengie Ayele, T.; Mekonnen Agidew, M.; Teshome Azezew, M.; Zewde, E.A.; Dejenie, T.A.; Mengstie, M.A. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell Dev. Biol. 2022, 10, 945287. [Google Scholar] [CrossRef]
- van den Akker, G.G.H.; Steijns, J.; Stassen, R.; Wasilewski, G.B.; Peeters, L.C.W.; Wijnands, K.A.P.; Schurgers, L.J.; Caron, M.M.J.; van Rhijn, L.W.; Welting, T.J.M. Development of a cyclic-inverso AHSG/Fetuin A-based peptide for inhibition of calcification in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 727–740. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Tilahun Muche, Z.; Behaile, T.M.A.; Mengie Ayele, T.; Mekonnen Agidew, M.; Teshome Azezew, M.; Zewde, E.A.; Asmamaw Dejenie, T. Role of Fetuin-A in the Pathogenesis of Psoriasis and Its Potential Clinical Applications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chu, X.; Cao, J.; Peng, Y. Correlation of serum Klotho, fetuin-A, and MGP levels with coronary artery calcification in maintenance hemodialysis patients. Clinics 2024, 79, 100417. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Dong, G.; Cao, J.; Zhang, J. Association of α2-HS Glycoprotein with Neurogenic Heterotopic Ossification in Patients with Spinal Cord Injury. Med. Sci. Monit. 2017, 23, 5382–5388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.; Xue, F.; Shi, W.Z.; Xiao, H.J. Fetuin A is down-regulated in rats during heterotopic ossification after Achilles tenotomy. Biotech. Histochem. 2016, 91, 229–236. [Google Scholar] [CrossRef]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef]
- Galleggiante, V.; De Santis, S.; Liso, M.; Verna, G.; Sommella, E.; Mastronardi, M.; Campiglia, P.; Chieppa, M.; Serino, G. Quercetin-Induced miR-369-3p Suppresses Chronic Inflammatory Response Targeting C/EBP-β. Mol. Nutr. Food Res. 2019, 63, e1801390. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Shimada, K.; Nakajima, A.; Ikeda, K.; Ishibashi, K.; Shimizu, N.; Ito, K. CD47 regulates the TGF-β signaling pathway in osteoblasts and is distributed in Meckel’s cartilage. J. Oral. Sci. 2011, 53, 169–175. [Google Scholar] [CrossRef]
- Rowe, C.J.; Nwaolu, U.; Salinas, D.; Hong, J.; Nunez, J.; Lansford, J.L.; McCarthy, C.F.; Potter, B.K.; Levi, B.H.; Davis, T.A. Inhibition of focal adhesion kinase 2 results in a macrophage polarization shift to M2 which attenuates local and systemic inflammation and reduces heterotopic ossification after polysystem extremity trauma. Front. Immunol. 2023, 14, 1280884. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Wan, X.; Chowdhury, I.H.; Jie, Z.; Choudhuri, S.; Garg, N.J. Origin of Monocytes/Macrophages Contributing to Chronic Inflammation in Chagas Disease: SIRT1 Inhibition of FAK-NFκB-Dependent Proliferation and Proinflammatory Activation of Macrophages. Cells 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Jeong, K.; Lim, S.S. FAK Family Kinases in Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3630. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Wang, X.; Lin, Y.; Zhao, W.; Wu, D.; Pan, J.; Luo, W.; Wang, Y.; Liang, G. FAK mediates LPS-induced inflammatory lung injury through interacting TAK1 and activating TAK1-NFκB pathway. Cell Death Dis. 2022, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, P.; Nasseri, S.; Mojarrab, M. Antioxidant Activity-guided Phytochemical Investigation of Artemisia aucheri Boiss.: Isolation of Ethyl Caffeate and a Spinacetin Glycoside. Iran. J. Pharm. Res. 2021, 20, 82–90. [Google Scholar]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Ebner, R.; Chen, R.H.; Shum, L.; Lawler, S.; Zioncheck, T.F.; Lee, A.; Lopez, A.R.; Derynck, R. Cloning of a type I TGF-beta receptor and its effect on TGF-beta binding to the type II receptor. Science 1993, 260, 1344–1348. [Google Scholar] [CrossRef]
- Mao, D.; Mi, J.; Pan, X.; Zhao, G.; Rui, Y. Galunisertib attenuates progression of trauma-induced heterotopic ossification via blockage of Smad2/3 signaling in mice. Eur. J. Pharmacol. 2022, 928, 175109. [Google Scholar] [CrossRef]
- Vanhoutte, F.; Liang, S.; Ruddy, M.; Zhao, A.; Drewery, T.; Wang, Y.; DelGizzi, R.; Forleo-Neto, E.; Rajadhyaksha, M.; Herman, G.; et al. Pharmacokinetics and Pharmacodynamics of Garetosmab (Anti-Activin A): Results from a First-in-Human Phase 1 Study. J. Clin. Pharmacol. 2020, 60, 1424–1431. [Google Scholar] [CrossRef]
- Di Rocco, M.; Forleo-Neto, E.; Pignolo, R.J.; Keen, R.; Orcel, P.; Funck-Brentano, T.; Roux, C.; Kolta, S.; Madeo, A.; Bubbear, J.S.; et al. Garetosmab in fibrodysplasia ossificans progressiva: A randomized, double-blind, placebo-controlled phase 2 trial. Nat. Med. 2023, 29, 2615–2624. [Google Scholar] [CrossRef]
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A.; et al. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J. Clin. Investig. 2017, 127, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Choi, S.J.; Lee, Y.H.; Song, G.G.; Ji, J.D. Combined therapeutic application of mTOR inhibitor and vitamin D(3) for inflammatory bone destruction of rheumatoid arthritis. Med. Hypotheses 2012, 79, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Singha, U.K.; Jiang, Y.; Yu, S.; Luo, M.; Lu, Y.; Zhang, J.; Xiao, G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J. Cell. Biochem. 2008, 103, 434–446. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Zhao, W.; Liu, M.; Lin, H. Macrophage Polarization in Heterotopic Ossification: Inflammation, Osteogenesis, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 5821. https://doi.org/10.3390/ijms26125821
Ren Y, Zhao W, Liu M, Lin H. Macrophage Polarization in Heterotopic Ossification: Inflammation, Osteogenesis, and Emerging Therapeutic Targets. International Journal of Molecular Sciences. 2025; 26(12):5821. https://doi.org/10.3390/ijms26125821
Chicago/Turabian StyleRen, Yifei, Wenwen Zhao, Mengchao Liu, and Hui Lin. 2025. "Macrophage Polarization in Heterotopic Ossification: Inflammation, Osteogenesis, and Emerging Therapeutic Targets" International Journal of Molecular Sciences 26, no. 12: 5821. https://doi.org/10.3390/ijms26125821
APA StyleRen, Y., Zhao, W., Liu, M., & Lin, H. (2025). Macrophage Polarization in Heterotopic Ossification: Inflammation, Osteogenesis, and Emerging Therapeutic Targets. International Journal of Molecular Sciences, 26(12), 5821. https://doi.org/10.3390/ijms26125821




