Preclinical Evidence That Mesoglycan Unfolds Complex Anti-Aging Effects in Photoaged Female Facial Skin
Abstract
1. Introduction
2. Results
2.1. Rationale for Biomarker Selection
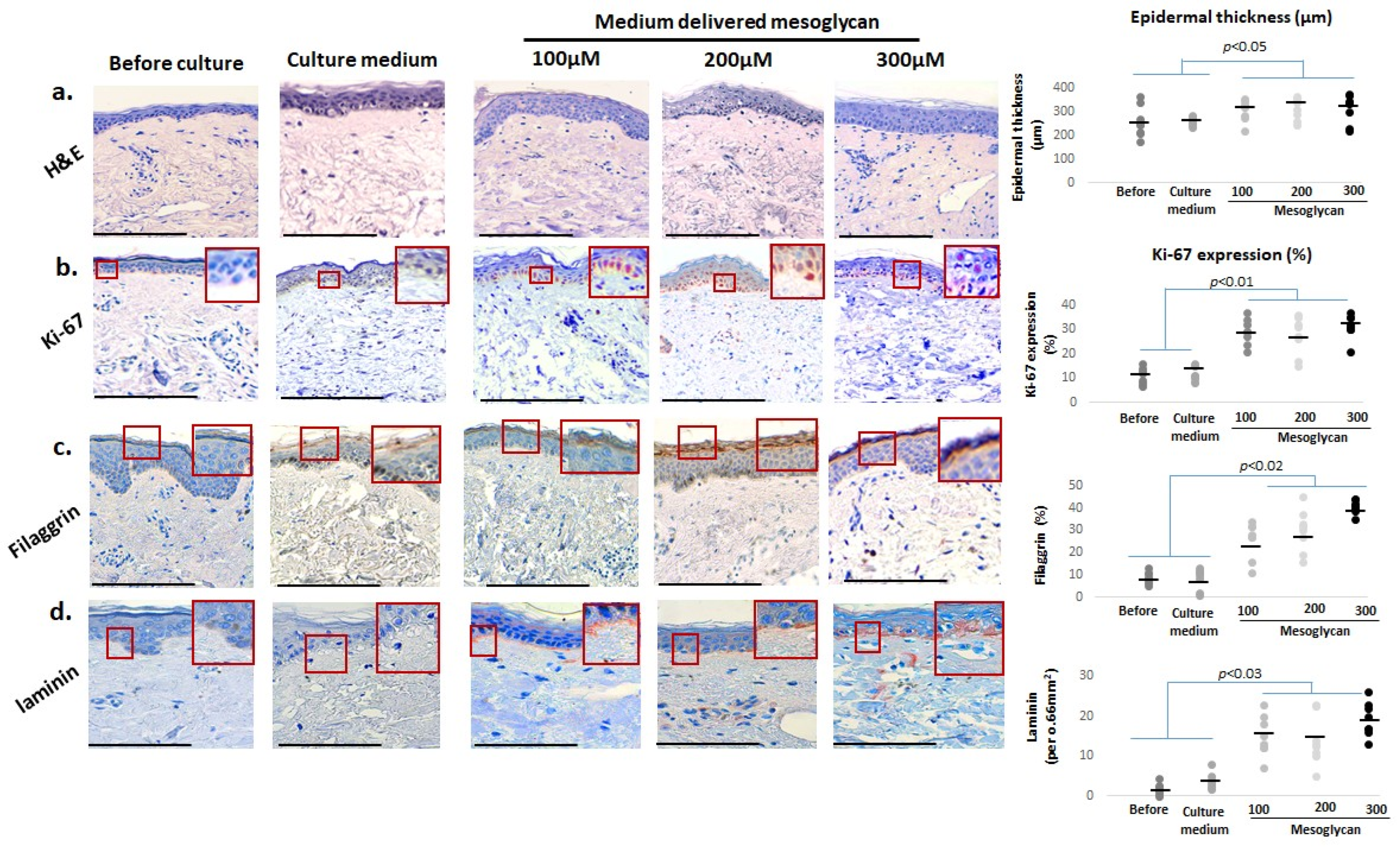
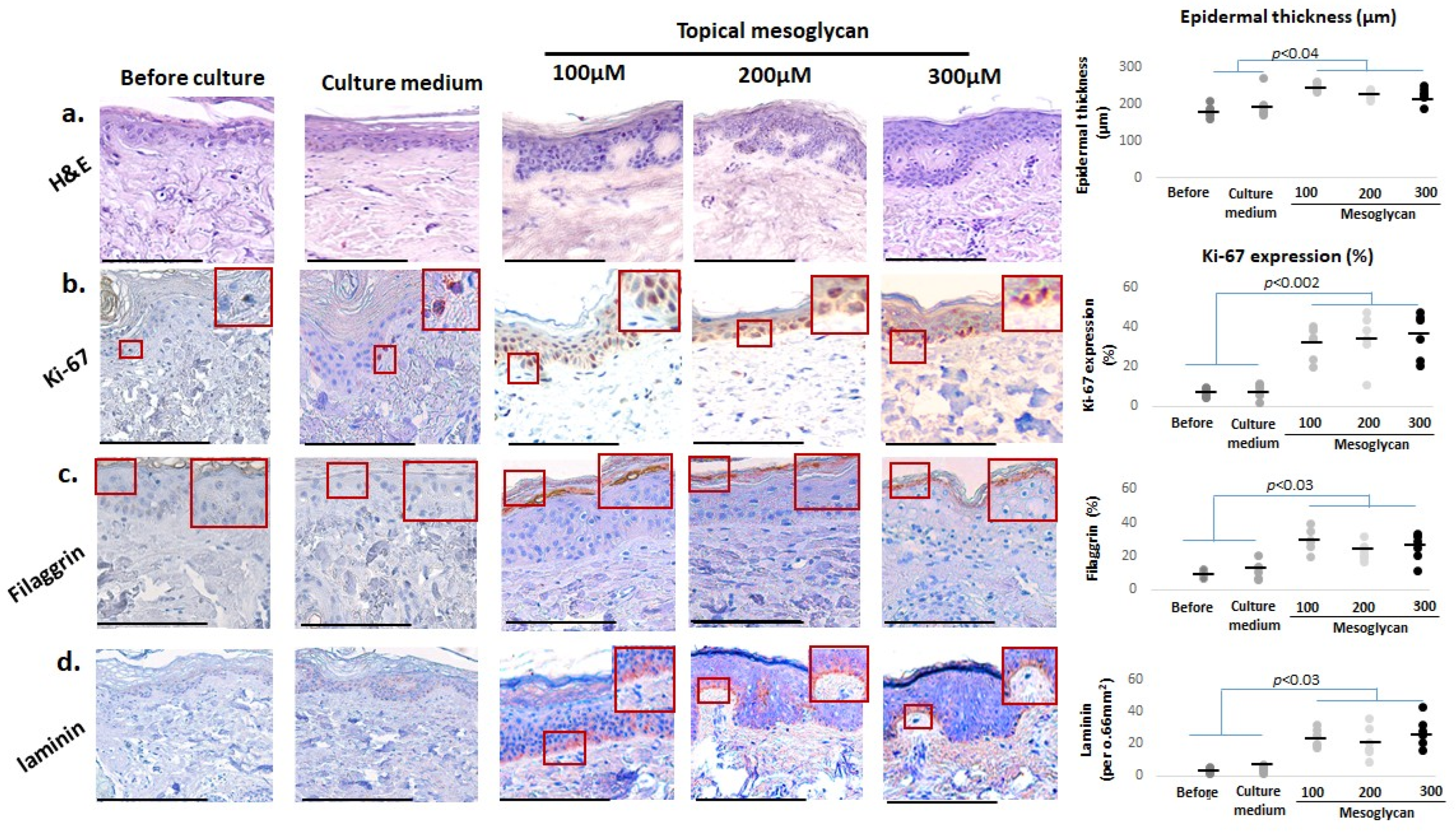
2.2. Medium-Delivered Mesoglycan Improves Epidermal Morphology and Increases Thickness, Basal Layer Proliferation, and Skin Barrier Status in Photoaged Facial Skin
2.3. Medium-Delivered Mesoglycan Enhances Pigment-Associated Markers in the Photoaged Human Epidermis Ex Vivo
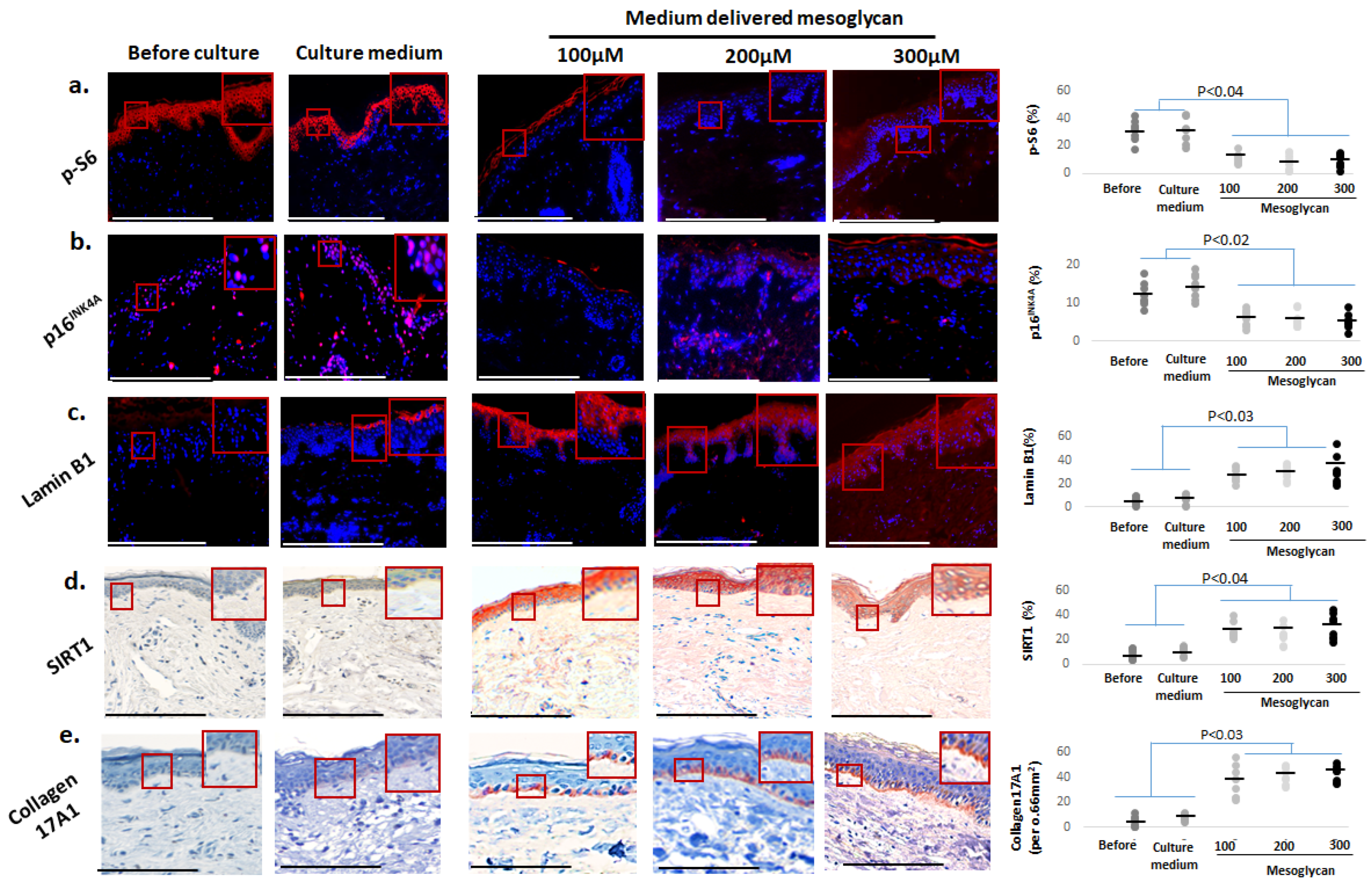
2.4. Medium-Delivered Mesoglycan Improves Several Key Biomarkers of Skin Aging Ex Vivo
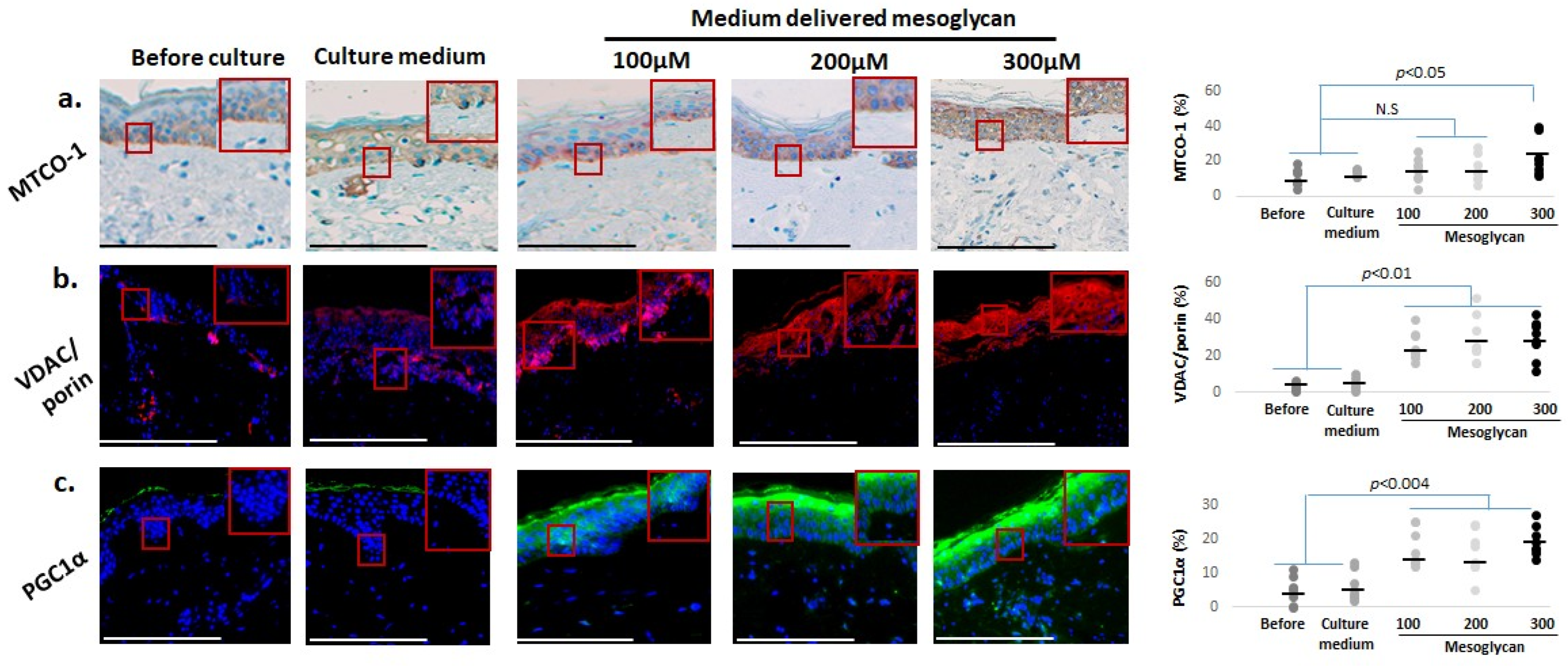
2.5. Mesoglycan Also Improves Mitochondrial Function Parameters and Cutaneous Oxidative Damage Defenses
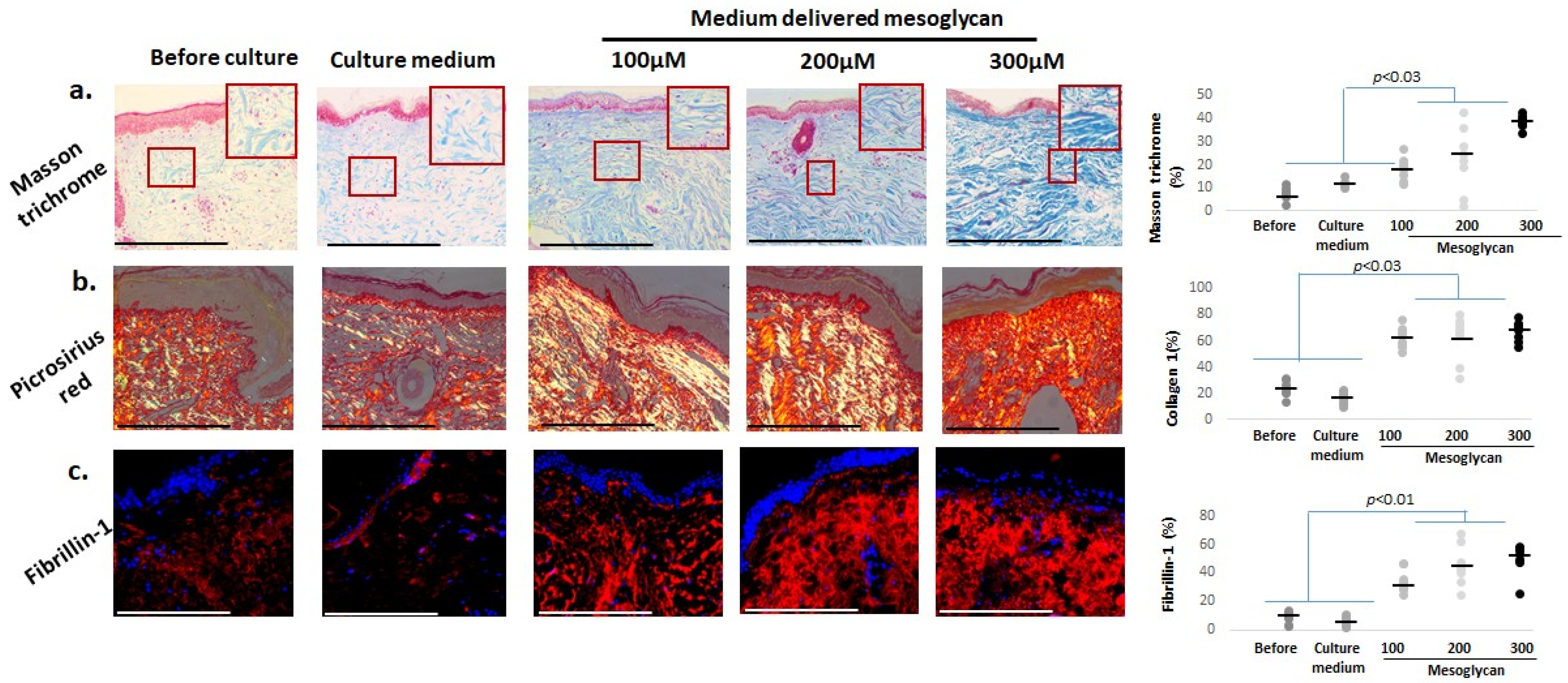
2.6. Mesoglycan Increases Dermal Collagen Staining and Fibrillin-1 Expression in Photoaged Human Skin Ex Vivo
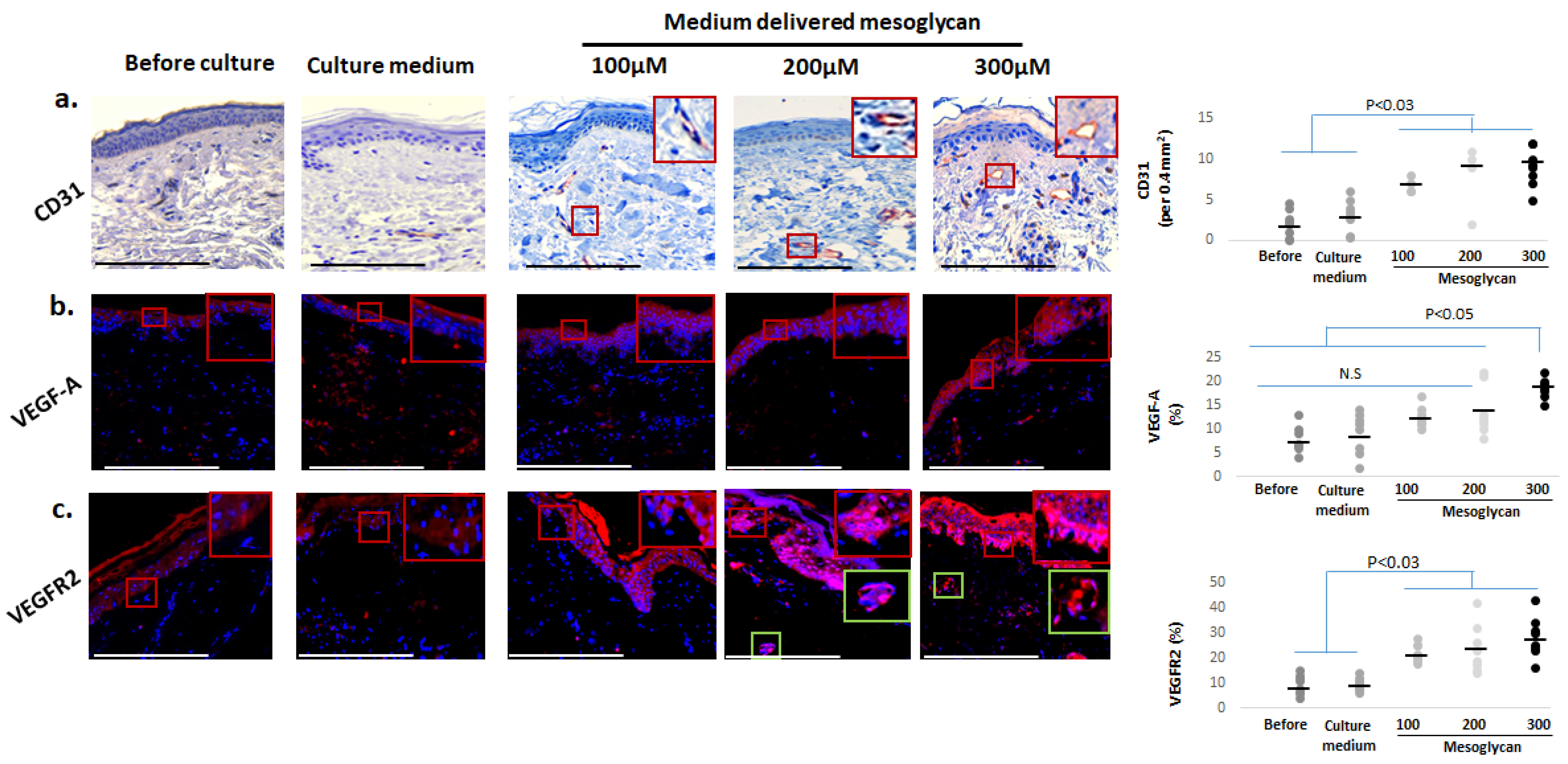
2.7. Medium-Delivered Mesoglycan Increases the Number of Endothelial Cells and VEGF-A/VEGFR2 Protein Expression in Photoaged Dermis
2.8. Topical and Medium-Delivered Mesoglycan Elicit Differential Anti-Skin-Aging Effects Ex Vivo
3. Discussion
4. Materials and Methods
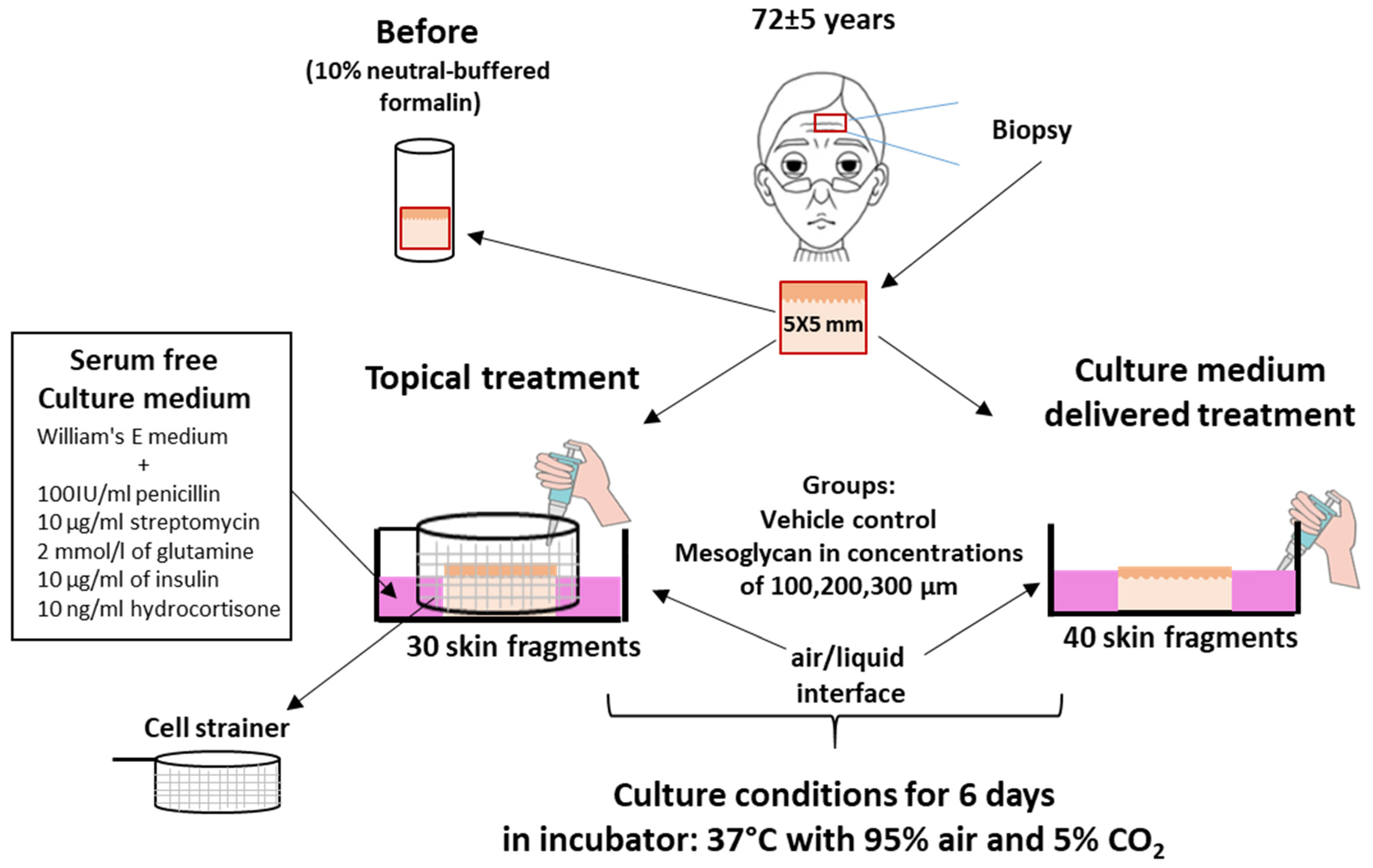
4.1. Human Skin Sourcing
4.2. Skin Organ Culture
- Topical treatment (3 donors; two skin fragments per donor): Skin fragments were placed in a Cell Strainer (SPL, 93040). Mesoglycan was dissolved in PEG 4000 to increase viscosity and then applied to the skin surface via pipette, preventing diffusion into the underlying medium (Figure 9; Supplementary Figures S1–S6).
4.3. Histochemistry and Immunohistochemical and Immunofluorescence Staining
4.4. Masson–Fontana Staining
4.5. Picrosirius Red Staining
4.6. Determination of Epidermal Thickness
4.7. Quantification of Marker Staining
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Watson, R.E.B. Identification of novel skin ageing genes: Evidence from across the pigmentary continuum. Br. J. Dermatol. 2021, 185, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Yu, G.T.; Ganier, C.; Allison, D.B.; Tchkonia, T.; Khosla, S.; Kirkland, J.L.; Lynch, M.D.; Wyles, S.P. Mapping epidermal and dermal cellular senescence in human skin aging. Aging Cell 2025, 24, e14358. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Griffiths, T.W.; Watson, R.E.B.; Langton, A.K. Skin ageing and topical rejuvenation strategies. Br. J. Dermatol. 2023, 189 (Suppl. S1), i17–i23. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Sadick, N.; Pannu, S.; Abidi, Z.; Arruda, S. Topical Treatments for Photoaged Skin. J. Drugs Dermatol. 2023, 22, 867–873. [Google Scholar]
- Lau, M.; Mineroff Gollogly, J.; Wang, J.Y.; Jagdeo, J. Cosmeceuticals for antiaging: A systematic review of safety and efficacy. Arch. Dermatol. Res. 2024, 316, 173. [Google Scholar] [CrossRef]
- Halai, P.; Kiss, O.; Wang, R.; Chien, A.L.; Kang, S.; O’Connor, C.; Bell, M.; Griffiths, C.E.M.; Watson, R.E.B.; Langton, A.K. Retinoids in the treatment of photoageing: A histological study of topical retinoid efficacy in black skin. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1618–1627. [Google Scholar] [CrossRef]
- Mellody, K.T.; Kendall, A.C.; Wray, J.R.; Foster, A.R.; Langton, A.K.; Costello, P.; Newton, V.L.; Bell, M.; Griffiths, C.E.M.; Nicolaou, A.; et al. Influence of menopause and hormone replacement therapy on epidermal ageing and skin biomechanical function. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e576–e580. [Google Scholar] [CrossRef]
- Samra, T.; Gomez-Gomez, T.; Linowiecka, K.; Akhundlu, A.; Lopez de Mendoza, G.; Gompels, M.; Lee, W.W.; Gherardini, J.; Chéret, J.; Paus, R. Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo. Int. J. Mol. Sci. 2023, 24, 15963. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, Calcium, Parathyroid Hormone, and Sex Steroids in Bone Health and Effects of Aging. J. Osteoporos. 2020, 1, 9324505. [Google Scholar] [CrossRef] [PubMed]
- Mekić, S.; Pardo, L.M.; Gunn, D.A.; Jacobs, L.C.; Hamer, M.A.; Ikram, M.A.; Vinke, E.J.; Vernooij, M.W.; Haarman, A.E.G.; Thee, E.F.; et al. Younger facial looks are associate with a lower likelihood of several age-related morbidities in the middle-aged to elderly. Br. J. Dermatol. 2023, 188, 390–395. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Kumar, S.; Sharife, H.; Volinsky, E.; Gileles-Hillel, A.; Licht, T.; Permyakova, A.; Hinden, L.; Azar, S.; Friedmann, Y. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 2021, 373, eabc8479. [Google Scholar] [CrossRef]
- Keren, A.; Bertolini, M.; Keren, Y.; Ullmann, Y.; Paus, R.; Gilhar, A. Human organ rejuvenation by VEGF-A: Lessons from the skin. Sci. Adv. 2022, 8, eabm6756. [Google Scholar] [CrossRef]
- Zhan, H.; Li, H.; Liu, C.; Cheng, L.; Yan, S.; Li, Y. Association of Circulating Vascular Endothelial Growth Factor Levels With Autoimmune Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 674343. [Google Scholar] [CrossRef]
- Sammon, D.; Krueger, A.; Busse-Wicher, M.; Morgan, R.M.; Haslam, S.M.; Schumann, B.; Briggs, D.C.; Hohenester, E. Molecular mechanism of decision-making in glycosaminoglycan biosynthesis. Nat. Commun. 2023, 14, 6425. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vivès, R.R.; Schaefer, L.; Götte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhães, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Morello, S.; Petrella, A. The combination of mesoglycan and VEGF promotes skin wound repair by enhancing the activation of endothelial cells and fibroblasts and their cross-talk. Sci. Rep. 2022, 12, 11041. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Picciariello, A.; Tufano, A.; Camporese, G. Clinical evidence and rationale of mesoglycan to treat chronic venous disease and hemorrhoidal disease: A narrative review. Updates Surg. 2024, 76, 423–434. [Google Scholar] [CrossRef]
- Camporese, G.; Bernardi, E.; Bortoluzzi, C.; Noventa, F.; Simioni, P.; METRO Investigator Study Group. Mesoglycan for the secondary prevention of superficial vein thrombosis: A randomized, controlled, double-blind study (METRO Study)-rationale and protocol. J. Thromb. Thrombolysis 2024, 57, 226–234. [Google Scholar] [CrossRef]
- Mottola, S.; Viscusi, G.; Belvedere, R.; Petrella, A.; De Marco, I.; Gorrasi, G. Production of mono and bilayer devices for wound dressing by coupling of electrospinning and supercritical impregnation techniques. Int. J. Pharm. 2024, 660, 124308. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Zhang, M. Technical development and application of supercritical CO2 foaming technology in PCL foam production. Sci. Rep. 2024, 14, 6825. [Google Scholar] [CrossRef]
- Bizzarro, V.; Belvedere, R.; Pessolano, E.; Parente, L.; Petrella, F.; Perretti, M.; Petrella, A. Mesoglycan induces keratinocyte activation by triggering syndecan-4 pathway and the formation of the annexin A1/S100A11 complex. J. Cell Physiol. 2019, 234, 20174–20192. [Google Scholar] [CrossRef]
- Pessolano, E.; Belvedere, R.; Bizzarro, V.; Franco, P.; Marco, I.; Petrella, F.; Porta, A.; Tosco, A.; Parente, L.; Perretti, M.; et al. Annexin A1 Contained in Extracellular Vesicles Promotes the Activation of Keratinocytes by Mesoglycan Effects: An Autocrine Loop Through FPRs. Cells 2019, 8, 753. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. The Pharmaceutical Device Prisma® Skin Promotes in Vitro Angiogenesis through Endothelial to Mesenchymal Transition during Skin Wound Healing. Int. J. Mol. Sci. 2017, 18, 1614. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. Effects of Prisma® Skin dermal regeneration device containing glycosaminoglycans on human keratinocytes and fibroblasts. Cell Adhes. Migr. 2018, 12, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Mottola, S.; Viscusi, G.; Iannone, G.; Belvedere, R.; Petrella, A.; De Marco, I.; Gorrasi, G. Supercritical Impregnation of Mesoglycan and Lactoferrin on Polyurethane Electrospun Fibers for Wound Healing Applications. Int. J. Mol. Sci. 2023, 24, 9269. [Google Scholar] [CrossRef] [PubMed]
- Valvano, A.; Bosso, G.; Apuzzi, V.; Riccone, F.; Saccà, L.; Oliviero, U. Mesoglycan improves vascular reactivity and insulin sensitivity in patients with metabolic syndrome. Atherosclerosis 2015, 243, 407–413. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, B.; Wakefield, J.S.; Elias, P.M.; Wang, X. The Role and Implications of Epidermal Dysfunction in the Pathogenesis of Inflammaging. J. Investig. Dermatol. 2025; online ahead of print. [Google Scholar]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M., 3rd; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef]
- Pessolano, E.; Belvedere, R.; Novizio, N.; Filippelli, A.; Perretti, M.; Whiteford, J.; Petrella, A. Mesoglycan connects Syndecan-4 and VEGFR2 through Annexin A1 and formyl peptide receptors to promote angiogenesis in vitro. FEBS J. 2021, 288, 6428–6446. [Google Scholar] [CrossRef]
- van Lessen, M.; Mardaryev, A.; Broadley, D.; Bertolini, M.; Edelkamp, J.; Kückelhaus, M.; Funk, W.; Bíró, T.; Paus, R. ‘Speed-ageing’ of human skin in serum-free organ culture ex vivo: An instructive novel assay for preclinical human skin ageing research demonstrates senolytic effects of caffeine and 2,5-dimethylpyrazine. Exp. Dermatol. 2024, 33, e14955. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2019, 21, 73. [Google Scholar] [CrossRef]
- Iriyama, S.; Yasuda, M.; Nishikawa, S.; Takai, E.; Hosoi, J.; Amano, S. Decrease of laminin-511 in the basement membrane due to photoaging reduces epidermal stem/progenitor cells. Sci. Rep. 2020, 10, 12592. [Google Scholar] [CrossRef]
- Byun, K.A.; Oh, S.; Batsukh, S.; Kim, M.J.; Lee, J.H.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. The Extracellular Matrix Vitalizer RATM Increased Skin Elasticity by Modulating Mitochondrial Function in Aged Animal Skin. Antioxidants 2023, 12, 694. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Chang, R.; Ye, T.; Li, Z.; Huang, R.; Wang, Z.; Deng, J.; Xia, H.; Yang, Y.; et al. Dermal Injection of Recombinant Filaggrin-2 Ameliorates UVB-Induced Epidermal Barrier Dysfunction and Photoaging. Antioxidants 2024, 13, 1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Gao, Y.; Li, Q.; Zhang, Y. Targeting the stem cell niche: Role of collagen XVII in skin aging and wound repair. Theranostics 2022, 12, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Nanba, D.; Toki, F.; Asakawa, K.; Matsumura, H.; Shiraishi, K.; Sayama, K.; Matsuzaki, K.; Toki, H.; Nishimura, E.K. EGFR-mediated epidermal stem cell motility drives skin regeneration through COL17A1 proteolysis. J. Cell Biol. 2021, 220, e202012073. [Google Scholar] [CrossRef]
- Endl, E.; Hollmann, C.; Gerdes, J. Antibodies against the Ki-67 protein: Assessment of the growth fraction and tools for cell cycle analysis. Methods Cell Biol. 2001, 63, 399–418. [Google Scholar] [PubMed]
- Schlüter, C.; Duchrow, M.; Wohlenberg, C.; Becker, M.H.; Key, G.; Flad, H.D.; Gerdes, J. The cell proliferation-associated antigen of antibody Ki-67: A very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol. 1993, 123, 513–522. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Chen, H.; Han, M.; Zhang, M.; Liu, K.; Jin, H.; Liu, X.; Shi, M.; Pu, W.; et al. Identifying specific functional roles for senescence across cell types. Cell 2024, 187, 7314–7334. [Google Scholar] [CrossRef]
- Subramanian, P.; Sayegh, S.; Laphanuwat, P.; Devine, O.P.; Fantecelle, C.H.; Sikora, J.; Chambers, E.S.; Karagiannis, S.N.; Gomes, D.C.O.; Kulkarni, A.; et al. Multiple outcomes of the germline p16INK4a mutation affecting senescence and immunity in human skin. Aging Cell 2024, 24. [Google Scholar] [CrossRef]
- Lyons, C.E.; Pallais, J.P.; McGonigle, S.; Mansk, R.P.; Collinge, C.W.; Yousefzadeh, M.J.; Baker, D.J.; Schrank, P.R.; Williams, J.W.; Niedernhofer, L.J.; et al. Chronic social stress induces p16-mediated senescent cell accumulation in mice. Nat. Aging 2025, 5, 48–64. [Google Scholar] [CrossRef]
- Suzuki, T.; Chéret, J.; Scala, F.D.; Akhundlu, A.; Gherardini, J.; Demetrius, D.L.; O’Sullivan, J.D.B.; Kuka Epstein, G.; Bauman, A.J.; Demetriades, C.; et al. mTORC1 activity negatively regulates human hair follicle growth and pigmentation. EMBO Rep. 2023, 24, e56574. [Google Scholar] [CrossRef]
- Gallage, S.; Irvine, E.E.; Barragan Avila, J.E.; Reen, V.; Pedroni, S.M.A.; Duran, I.; Ranvir, V.; Khadayate, S.; Pombo, J.; Brookes, S.; et al. Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome. Nat. Aging 2024, 4, 1544–1561. [Google Scholar] [CrossRef]
- Matias, I.; Diniz, L.P.; Damico, I.V.; Araujo, A.P.B.; Neves, L.D.S.; Vargas, G.; Leite, R.E.P.; Suemoto, C.K.; Nitrini, R.; Jacob-Filho, W.; et al. Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus. Aging Cell 2022, 21, e13521. [Google Scholar] [CrossRef] [PubMed]
- En, A.; Takemoto, K.; Yamakami, Y.; Nakabayashi, K.; Fujii, M. Upregulated expression of lamin B receptor increases cell proliferation and suppresses genomic instability: Implications for cellular immortalization. FEBS J. 2024, 291, 2155–2171. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Wang, C.; Zhou, J.; Feng, X.; Zhang, L.; Fan, Z. Mechanism and role of nuclear laminin B1 in cell senescence and malignant tumors. Cell Death Discov. 2024, 10, 269. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Cui, X.; Mi, T.; Zhang, H.; Gao, P.; Xiao, X.; Lee, J.; Guelakis, M.; Gu, X. Glutathione amino acid precursors protect skin from UVB-induced damage and improve skin tone. J. Eur. Acad. Dermatol. Venereol. 2024, 38 (Suppl. S3), 12–20. [Google Scholar] [CrossRef]
- Sevilla, A.; Chéret, J.; Lee, W.; Paus, R. Concentration-dependent stimulation of melanin production as well as melanocyte and keratinocyte proliferation by melatonin in human eyelid epidermis. Exp. Dermatol. 2023, 32, 684–693. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, J.C.; Kim, Y.; Kim, Y.H.; Park, S.S.; Muther, C.; Tessier, A.; Lee, G.; Gendronneau, G.; Forestier, S.; et al. Senescent melanocytes driven by glycolytic changes are characterized by melanosome transport dysfunction. Theranostics 2023, 13, 3914–3924. [Google Scholar] [CrossRef]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment. Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Everett, E.T.; Uffman, J.; McAndrews-Hill, M.; Cooper, R.; Ryder, J.; Vik, T.; Williams, D.A. Overexpression of human stem cell factor impairs melanocyte, mast cell, and thymocyte development: A role for receptor tyrosine kinase-mediated mitogen activated protein kinase activation in cell differentiation. Blood 1997, 90, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Liu, Y.H.; Brunner, L.M.; Rebling, J.; Ben-Yehuda Greenwald, M.; Werner, S.; Detmar, M.; Razansky, D. Non-invasive longitudinal imaging of VEGF-induced microvascular alterations in skin wounds. Theranostics 2022, 12, 558–573. [Google Scholar] [CrossRef]
- Xiang, T.; Sun, F.; Liu, T.; Zhao, J.; Yang, J.; Ouyang, D.; Chen, H.; Zhu, Q.; Wang, Q.; Li, Y.; et al. EBV-associated epithelial cancers cells promote vasculogenic mimicry formation via a secretory cross-talk with the immune microenvironment. Theranostics 2024, 14, 5123–5140. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Z.; Li, X.; Ye, Y.; Wang, M.; Jiang, J.; Tao, L.; Wang, Y.; Tung, C.-T.; Chung, Y.; et al. Crosstalk between endothelial cells and dermal papilla entails hair regeneration and angiogenesis during aging. J. Adv. Res. 2024, 70, 339–353. [Google Scholar] [CrossRef]
- Baffert, F.; Thurston, G.; Rochon-Duck, M.; Le, T.; Brekken, R.; McDonald, D.M. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ. Res. 2004, 94, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.Q.; Broussy, S.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2024, 14, 1307860. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Souder, D.C.; McGregor, E.R.; Clark, J.P.; Rhoads, T.W.; Porter, T.J.; Eliceiri, K.W.; Moore, D.L.; Puglielli, L.; Anderson, R.M. Neuron-specific isoform of PGC-1α regulates neuronal metabolism and brain aging. Nat. Commun. 2025, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bollag, W.B. The Role of PGC-1α in Aging Skin Barrier Function. Cells 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Chéret, J.; Giesen, M.; Haeger, S.; Alam, M.; Watson, R.E.B.; Langton, A.K.; Klinger, M.; Knuever, J.; Funk, W.; et al. Thyroid Hormones Enhance Mitochondrial Function in Human Epidermis. J. Investig. Dermatol. 2016, 136, 2003–2012. [Google Scholar] [CrossRef]
- Vidali, S.; Knuever, J.; Lerchner, J.; Giesen, M.; Bíró, T.; Klinger, M.; Kofler, B.; Funk, W.; Poeggeler, B.; Paus, R. Hypothalamic-pituitary-thyroid axis hormones stimulate mitochondrial function and biogenesis in human hair follicles. J. Investig. Dermatol. 2014, 134, 33–42. [Google Scholar] [CrossRef]
- Ham, S.J.; Lee, D.; Yoo, H.; Jun, K.; Shin, H.; Chung, J. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl. Acad. Sci. USA 2020, 117, 4281–4291. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Dinkova-Kostova, A.T.; Cohen, G. NRF2 in dermatological disorders: Pharmacological activation for protection against cutaneous photodamage and photodermatosis. Free Radic. Biol. Med. 2022, 188, 262–276. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 system and inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Kanamori, A.; Egawa, N.; Yamasaki, S.; Ikeda, T.; da Rocha, M.J.; Bortolatto, C.F.; Savegnago, L.; Brüning, C.A.; Iwaoka, M. Antioxidative and Antiglycative Stress Activities of Selenoglutathione Diselenide. Pharmaceuticals 2024, 17, 1049. [Google Scholar] [CrossRef]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, Senescence, and Cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Guo, X.; Zheng, J.; Zhang, J.; Han, J.; Shioya, A.; Uramoto, H.; Mochizuki, T.; Yamada, S. PRDX4 Improved Aging-Related Delayed Wound Healing in Mice. J. Investig. Dermatol. 2021, 141, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Pillar, T.; David, M.; Eidelman, S. Melanocytes and Langerhans cells in aged versus young skin before and after transplantation onto nude mice. J. Investig. Dermatol. 1991, 96, 210–214. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.Y.; Dreesen, O.; et al. Nicotinamide Prevents UVB- and Oxidative Stress–Induced Photoaging in Human Primary Keratinocytes. J. Investig. Dermatol. 2022, 142, 1670–1681. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Hiden, U.; Krstic, J.; Panzitt, K.; Wagner, M.; Enzinger, C.; Khalil, M.; Abdellatif, M.; Malle, E.; Madl, T.; et al. Targeting organ-specific mitochondrial dysfunction to improve biological aging. Pharmacol. Ther. 2024, 262, 108710. [Google Scholar] [CrossRef]
- Martic, I.; Papaccio, F.; Bellei, B.; Cavinato, M. Mitochondrial dynamics and metabolism across skin cells: Implications for skin homeostasis and aging. Front. Physiol. 2023, 14, 1284410. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- de Vasconcelos Nasser Caetano, L.; de Oliveira Mendes, T.; Bagatin, E.; Amante Miot, H.; Marques Soares, J.L.; Simoes E Silva Enokihara, M.M.; Abrahao Martin, A. In vivo confocal Raman spectroscopy for intrinsic aging and photoaging assessment. J. Dermatol. Sci. 2017, 88, 199–206. [Google Scholar] [CrossRef]
- O’Reilly, S.; Markiewicz, E.; Idowu, O.C. Aging, senescence, and cutaneous wound healing—A complex relationship. Front. Immunol. 2024, 15, 1429716. [Google Scholar] [CrossRef]
- Thau, H.; Gerjol, B.P.; Hahn, K.; von Gudenberg, R.W.; Knoedler, L.; Stallcup, K.; Emmert, M.Y.; Buhl, T.; Wyles, S.P.; Tchkonia, T.; et al. Senescence as a molecular target in skin aging and disease. Ageing Res. Rev. 2025, 105, 102686. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. NPJ Aging 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Pardo-Pastor, C.; Jenkins, R.G.; Rosenblatt, J. Imperfect wound healing sets the stage for chronic diseases. Science 2024, 386, eadp2974. [Google Scholar] [CrossRef]
- Gansevoort, M.; Oostendorp, C.; Bouwman, L.F.; Tiemessen, D.M.; Geutjes, P.J.; Feitz, W.F.J.; van Kuppevelt, T.H.; Daamen, W.F. Collagen-Heparin-FGF2-VEGF Scaffolds Induce a Regenerative Gene Expression Profile in a Fetal Sheep Wound Model. Tissue Eng. Regen. Med. 2024, 21, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Libby, J.R.; Royce, H.; Walker, S.R.; Li, L. The role of extracellular matrix in angiogenesis: Beyond adhesion and structure. Biomater. Biosyst. 2024, 15, 100097. [Google Scholar] [CrossRef]
- Chocarro-Wrona, C.; Pleguezuelos-Beltrán, P.; López de Andrés, J.; Antich, C.; de Vicente, J.; Jiménez, G.; Arias-Santiago, S.; Gálvez-Martín, P.; López-Ruiz, E.; Marchal, J.A. A bioactive three-layered skin substitute based on ECM components effectively promotes skin wound healing and regeneration. Mater. Today Bio 2025, 31, 101592. [Google Scholar] [CrossRef]
- Sun, J.; Du, J.; Liu, X.; An, J.; Hu, Y.; Wang, J.; Zhu, F.; Feng, H.; Cheng, S.; Tian, H.; et al. Chondroitin sulfate-modified tragacanth gum-gelatin composite nanocapsules loaded with curcumin nanocrystals for the treatment of arthritis. J. Nanobiotechnol. 2024, 22, 270. [Google Scholar] [CrossRef]
- Bogdanowicz, P.; Bensadoun, P.; Noizet, M.; Béganton, B.; Philippe, A.; Alvarez-Georges, S.; Doat, G.; Tourette, A.; Bessou-Touya, S.; Lemaitre, J.M.; et al. Senomorphic activity of a combination of niacinamide and hyaluronic acid: Correlation with clinical improvement of skin aging. Sci. Rep. 2024, 14, 16321. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.B.; Sun, B.K.; Lee, E.Y.; Ren, B. Emerging Techniques in Spatial Multiomics: Fundamental Principles and Applications to Dermatology. J. Investig. Dermatol. 2024, 145, 1017–1032. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. Evaluation of the Effects of Mesoglycan on Some Markers of Endothelial Damage and Walking Distance in Diabetic Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2017, 18, 572. [Google Scholar]
- Castanet, J.; Ortonne, J.P. Pigmentary changes in aged and photoaged skin. Arch. Dermatol. 1997, 133, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. A review of skin ageing and its medical therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- McMullan, R.R.; McAuley, D.F.; O’Kane, C.M.; Silversides, J.A. Vascular leak in sepsis: Physiological basis and potential therapeutic advances. Crit. Care 2024, 28, 97. [Google Scholar] [CrossRef]
- Luengas-Martinez, A.; Ismail, D.; Paus, R.; Young, H.S. Inhibition of vascular endothelial growth factor-A downregulates angiogenesis in psoriasis: A pilot study. Skin Health Dis. 2023, 3, e245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; John, P.; Bhatti, A. Association analysis of Vascular Endothelial Growth Factor-A (VEGF-A) polymorphism in rheumatoid arthritis using computational approaches. Sci. Rep. 2023, 13, 21957. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A. The vascular endothelial growth factor as a candidate biomarker of systemic lupus erythematosus: A GRADE-assessed systematic review and meta-analysis. Clin. Exp. Med. 2024, 24, 218. [Google Scholar] [CrossRef]
- Feliers, D. Vascular endothelial growth factor as a prognostic marker of lupus nephritis. Kidney Int. 2009, 75, 1251–1253. [Google Scholar] [CrossRef]
- Marneros, A.G. Increased VEGF-A promotes multiple distinct aging diseases of the eye through shared pathomechanisms. EMBO Mol. Med. 2016, 8, 208–231. [Google Scholar] [CrossRef]
- Chen, Y.; Tai, Z.; Zhu, C.; Yu, Q.; Zhu, Q.; Chen, Z. Vascular Endothelial Growth Factor A (VEGFA) Inhibition: An Effective Treatment Strategy for Psoriasis. Int. J. Mol. Sci. 2023, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.P.; Bedell, V.M.; Alam, S.K.; O’Gorman, M.; Serres, M.; Hall, S.R.; Pal, K.; Kudgus, R.A.; Mukherjee, P.; Seelig, D.M.; et al. Vascular Endothelial Growth Factor as an Immediate-Early Activator of Ultraviolet-Induced Skin Injury. Mayo Clin Proc. 2022, 97, 154–164. [Google Scholar] [CrossRef]
- Böhm, M.; Stegemann, A.; Paus, R.; Kleszczyński, K.; Maity, P.; Wlaschek, M.; Scharffetter-Kochanek, K. Endocrine Controls of Skin Aging. Endocr. Rev. 2025, 46, 349–375. [Google Scholar] [CrossRef]
- Wang, Z.; Man, M.Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Y.; Han, Z.; Huang, X.; Zhou, S.; Wang, S.; Zhou, Y.; Han, X.; Chen, H. Exploring mechanisms of skin aging: Insights for clinical treatment. Front. Immunol. 2024, 15, 1421858. [Google Scholar] [CrossRef]
- Tufano, A.; Arturo, C.; Cimino, E.; Di Minno, M.N.; Di Capua, M.; Cerbone, A.M.; Di Minno, G. Mesoglycan: Clinical evidences for use in vascular diseases. Int. J. Vasc. Med. 2010, 2010, 390643. [Google Scholar] [CrossRef]
- Kitchens, B.P.; Snyder, R.J.; Cuffy, C.A. A Literature Review of Pharmacological Agents to Improve Venous Leg Ulcer Healing. Wounds 2020, 32, 195–207. [Google Scholar]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Cialdai, F.; Bacci, S.; Zizi, V.; Norfini, A.; Balsamo, M.; Ciccone, V.; Morbidelli, L.; Calosi, L.; Risaliti, C.; Vanhelden, L.; et al. Optimization of an Ex-Vivo Human Skin/Vein Model for Long-Term Wound Healing Studies: Ground Preparatory Activities for the ‘Suture in Space’ Experiment Onboard the International Space Station. Int. J. Mol. Sci. 2022, 23, 14123. [Google Scholar] [CrossRef]
- Gvirtz, R.; Ogen-Shtern, N.; Cohen, G. Kinetic Cytokine Secretion Profile of LPS-Induced Inflammation in the Human Skin Organ Culture. Pharmaceutics 2020, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Tiirikainen, M.L.; Woetmann, A.; Norsgaard, H.; Santamaria-Babí, L.F.; Lovato, P. Ex vivo culture of lesional psoriasis skin for pharmacological testing. J. Dermatol. Sci. 2020, 97, 109–116. [Google Scholar] [CrossRef]
- Liu, Y.; Ilić, T.; Pantelic, I.; Savić, S.; Lunter, D.J. Topically applied lipid-containing emulsions based on PEGylated emulsifiers: Formulation, characterization, and evaluation of their impact on skin properties ex vivo and in vivo. Int. J. Pharm. 2022, 626, 122202. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Alam, M.; Chéret, J.; Kis, N.G.; Hegyi, Z.; Szöllősi, A.G.; Vidali, S.; Bíró, T.; Paus, R. Mitochondrial energy metabolism is negatively regulated by cannabinoid receptor 1 in intact human epidermis. Exp. Dermatol. 2020, 29, 616–622. [Google Scholar] [CrossRef]
- Laufer Britva, R.; Keren, A.; Bertolini, M.; Ullmann, Y.; Paus, R.; Gilhar, A. Involvement of ILC1-like innate lymphocytes in human autoimmunity, lessons from alopecia areata. eLife 2023, 12, e80768. [Google Scholar] [CrossRef]
- Lu, Z.; Hasse, S.; Bodo, E.; Rose, C.; Funk, W.; Paus, R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Exp. Dermatol. 2007, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Asou, T.; Kishi, K. Cistanche deserticola Polysaccharide Reduces Inflammation and Aging Phenotypes in the Dermal Fibroblasts through the Activation of the NRF2/HO-1 Pathway. Int. J. Mol. Sci. 2023, 24, 15704. [Google Scholar] [CrossRef]
- George, M.; Reddy, A.P.; Reddy, P.H.; Kshirsagar, S. Unraveling the NRF2 confusion: Distinguishing nuclear respiratory factor 2 from nuclear erythroid factor 2. Ageing Res. Rev. 2024, 98, 102353. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeltzer, A.; Keren, A.; Paus, R.; Gilhar, A. Preclinical Evidence That Mesoglycan Unfolds Complex Anti-Aging Effects in Photoaged Female Facial Skin. Int. J. Mol. Sci. 2025, 26, 5787. https://doi.org/10.3390/ijms26125787
Zeltzer A, Keren A, Paus R, Gilhar A. Preclinical Evidence That Mesoglycan Unfolds Complex Anti-Aging Effects in Photoaged Female Facial Skin. International Journal of Molecular Sciences. 2025; 26(12):5787. https://doi.org/10.3390/ijms26125787
Chicago/Turabian StyleZeltzer, Assaf, Aviad Keren, Ralf Paus, and Amos Gilhar. 2025. "Preclinical Evidence That Mesoglycan Unfolds Complex Anti-Aging Effects in Photoaged Female Facial Skin" International Journal of Molecular Sciences 26, no. 12: 5787. https://doi.org/10.3390/ijms26125787
APA StyleZeltzer, A., Keren, A., Paus, R., & Gilhar, A. (2025). Preclinical Evidence That Mesoglycan Unfolds Complex Anti-Aging Effects in Photoaged Female Facial Skin. International Journal of Molecular Sciences, 26(12), 5787. https://doi.org/10.3390/ijms26125787







