The ADCK Kinase Family: Key Regulators of Bioenergetics and Mitochondrial Function and Their Implications in Human Cancers
Abstract
1. Bioenergetics and Mitochondrial Function
2. Aarf Domain-Containing Kinases (ADCK)
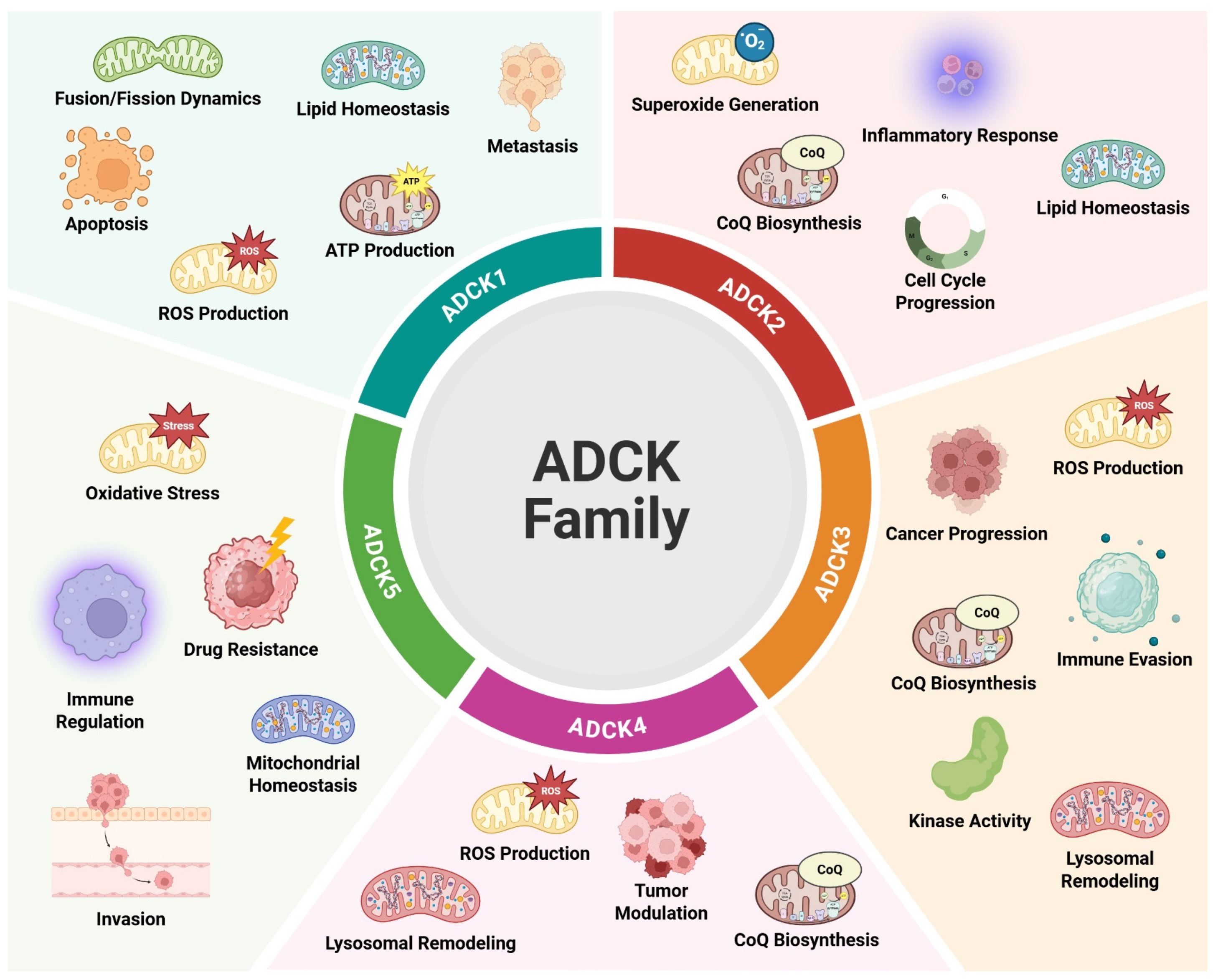
3. ADCK1
4. ADCK2
5. ADCK3
6. ADCK4
7. ADCK5
8. Therapeutic Potential of ADCK Kinases
9. Conclusions
Funding
Conflicts of Interest
References
- Garcia, J. A Short Review on Bioenergetics and Its Applications in Biology. Adv. Tech. Biol. Med. 2021, 9, 288. [Google Scholar] [CrossRef]
- Rigoulet, M.; Bouchez, C.L.; Paumard, P.; Ransac, S.; Cuvellier, S.; Duvezin-Caubet, S.; Mazat, J.P.; Devin, A. Cell Energy Metabolism: An Update. Biochim. Biophys. Acta (BBA)—Bioenerg. 2020, 1861, 148276. [Google Scholar] [CrossRef]
- Hill, B.G.; Shiva, S.; Ballinger, S.; Zhang, J.; Darley-Usmar, V.M. Bioenergetics and Translational Metabolism: Implications for Genetics, Physiology and Precision Medicine. Biol. Chem. 2019, 401, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yang, P.; Singh, S.; Zhuang, H.; Xu, L.; Chen, W.-H.; Dolfing, J.; Li, D.; Zhang, Y.; Zeng, H.; et al. A Review on the Bioenergetics of Anaerobic Microbial Metabolism Close to the Thermodynamic Limits and Its Implications for Digestion Applications. Bioresour. Technol. 2018, 247, 1095–1106. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Metabolic Regulation of Mitochondrial Dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Marmisolle, I.; Tarallo, D.; Quijano, C. Mitochondrial Bioenergetics and Dynamics in Secretion Processes. Front. Endocrinol. 2020, 11, 319. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics & Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Martinez, B.A.; Petersen, D.A.; Gaeta, A.L.; Stanley, S.P.; Caldwell, G.A.; Caldwell, K.A. Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. Elegans Models of Parkinson’s Disease. J. Neurosci. 2017, 37, 11085–11100. [Google Scholar] [CrossRef]
- Belenguer, P.; Duarte, J.M.N.; Schuck, P.F.; Ferreira, G.C. Mitochondria and the Brain: Bioenergetics and Beyond. Neurotox. Res. 2019, 36, 219–238. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jȩdrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Eickelmann, C.; Kleinbongard, P. Mitochondrial Kinase Signaling for Cardioprotection. Int. J. Mol. Sci. 2024, 25, 4491. [Google Scholar] [CrossRef]
- Horbinski, C.; Chu, C.T. Kinase Signaling Cascades in the Mitochondrion: A Matter of Life or Death. Free. Radic. Biol. Med. 2005, 38, 2–11. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Tang, M.; Zhong, G.; Si, Y.; Li, H.; Zhu, F.; Liao, Q.; Li, L.; Zhao, J.; et al. A Subcellular Map of the Human Kinome. Elife 2021, 10, e64943. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.J.; Vazquez Fonseca, L.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q Biosynthesis in Health and Disease. Biochim. Biophys. Acta 2016, 1857, 1079–1085. [Google Scholar] [CrossRef]
- Wisidagama, D.R.; Thomas, S.M.; Lam, G.; Thummel, C.S. Functional Analysis of Aarf Domain-Containing Kinase 1 in Drosophila Melanogaster. Dev. Dyn. 2019, 248, 762–770. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Liu, J.; Xu, Y.-X.; Pu, W.-Y.; Shen, M.-J.; Jiang, K.-Q.; Yang, Y.-L.; Lu, J.; Deng, Z.; Yang, Y.; et al. Identification of the Mitochondrial Protein ADCK2 as a Therapeutic Oncotarget of NSCLC. Int. J. Biol. Sci. 2022, 18, 6163–6175. [Google Scholar] [CrossRef]
- Wheeler, B.; Jia, Z. Preparation and Characterization of Human ADCK3, a Putative Atypical Kinase. Protein Expr. Purif. 2015, 108, 13–17. [Google Scholar] [CrossRef]
- Daehn, I.S. Mitochondria Matter: A Critical Role of ADCK4 in Stabilizing the CoQ Complex in Podocytes in Steroid-Resistant Nephrotic Syndrome. J. Am. Soc. Nephrol. 2020, 31, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Li, G.; Wang, P.; Li, X.; Lai, F.; Luo, R.; Liu, B.; Lin, J. aarF Domain Containing Kinase 5 Gene Promotes Invasion and Migration of Lung Cancer Cells through ADCK5-SOX9-PTTG1 Pathway. Exp. Cell Res. 2020, 392, 112002. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Fernández-Ayala, D.J.M.; Vicente-García, C.; Navas-Enamorado, I.; López-Lluch, G.; Oliva, C.; Artuch, R.; Garcia-Villoria, J.; Ribes, A.; de Cabo, R.; et al. Calorie Restriction Rescues Mitochondrial Dysfunction in Adck2-Deficient Skeletal Muscle. Front. Physiol. 2022, 13, 898792. [Google Scholar] [CrossRef]
- Stefely, J.A.; Reidenbach, A.G.; Ulbrich, A.; Oruganty, K.; Floyd, B.J.; Jochem, A.; Saunders, J.M.; Johnson, I.E.; Minogue, C.E.; Wrobel, R.L.; et al. Mitochondrial ADCK3 Employs an Atypical Protein Kinase-like Fold to Enable Coenzyme Q Biosynthesis. Mol. Cell 2015, 57, 83–94. [Google Scholar] [CrossRef]
- Zhuo, B.-B.; Zhu, L.-Q.; Yao, C.; Wang, X.-H.; Li, S.-X.; Wang, R.; Li, Y.; Ling, Z.-Y. ADCK1 Is a Potential Therapeutic Target of Osteosarcoma. Cell Death Dis. 2022, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.K.; Abdul Murad, N.; Yeo, A.; McKenzie, M.; Ward, M.; Chong, K.L.; Schieber, N.L.; Parton, R.G.; Lim, Y.C.; Wolvetang, E.; et al. AarF Domain Containing Kinase 3 (ADCK3) Mutant Cells Display Signs of Oxidative Stress, Defects in Mitochondrial Homeostasis and Lysosomal Accumulation. PLoS ONE 2016, 11, e0148213. [Google Scholar] [CrossRef][Green Version]
- Ashraf, S.; Gee, H.Y.; Woerner, S.; Xie, L.X.; Vega-Warner, V.; Lovric, S.; Fang, H.; Song, X.; Cattran, D.C.; Avila-Casado, C.; et al. ADCK4 Mutations Promote Steroid-Resistant Nephrotic Syndrome through CoQ10 Biosynthesis Disruption. J. Clin. Investig. 2013, 123, 5179–5189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, H.; Wang, T.; Yao, C. Identification of Mitochondria-Related Biomarkers in Childhood Allergic Asthma. BMC Med. Genom. 2024, 17, 141. [Google Scholar] [CrossRef]
- Khadria, A.S.; Mueller, B.K.; Stefely, J.A.; Tan, C.H.; Pagliarini, D.J.; Senes, A. A Gly-Zipper Motif Mediates Homodimerization of the Transmembrane Domain of the Mitochondrial Kinase ADCK3. J. Am. Chem. Soc. 2014, 136, 14068–14077. [Google Scholar] [CrossRef]
- Schirinzi, T.; Favetta, M.; Romano, A.; Sancesario, A.; Summa, S.; Minosse, S.; Zanni, G.; Castelli, E.; Bertini, E.; Petrarca, M.; et al. One-Year Outcome of Coenzyme Q10 Supplementation in ADCK3 Ataxia (ARCA2). Cerebellum Ataxias 2019, 6, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Jiang, Y.; Lv, X.; Yu, Y.; Dai, Q.; Ao, L.; Tao, L.; Peng, Z. Urinary Coenzyme Q10 as a Diagnostic Biomarker and Predictor of Remission in a Patient with ADCK4-Associated Glomerulopathy: A Case Report. BMC Nephrol. 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Goto-Yamaguchi, L.; Yamamoto-Ibusuki, M.; Yamamoto, Y.; Fujiki, Y.; Tomiguchi, M.; Sueta, A.; Takeshita, T.; Iwase, H. Therapeutic Predictors of Neoadjuvant Endocrine Therapy Response in Estrogen Receptor-Positive Breast Cancer with Reference to Optimal Gene Expression Profiling. Breast Cancer Res. Treat. 2018, 172, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, Y.; Sun, C.; Yu, L.; Wang, Z.; Du, X.; Yang, W.; Zhang, C.; Tao, C.; Wang, J.; et al. ADCK1 Activates the β-Catenin/TCF Signaling Pathway to Promote the Growth and Migration of Colon Cancer Cells. Cell Death Dis. 2021, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Schoolmeesters, A.; Brown, D.D.; Fedorov, Y. Kinome-Wide Functional Genomics Screen Reveals a Novel Mechanism of TNFα-Induced Nuclear Accumulation of the HIF-1α Transcription Factor in Cancer Cells. PLoS ONE 2012, 7, e31270. [Google Scholar] [CrossRef]
- Vierthaler, M.; Sun, Q.; Wang, Y.; Steinfass, T.; Poelchen, J.; Hielscher, T.; Novak, D.; Umansky, V.; Utikal, J. ADCK2 Knockdown Affects the Migration of Melanoma Cells via MYL6. Cancers 2022, 14, 1071. [Google Scholar] [CrossRef]
- Yoon, W.; Hwang, S.-H.; Lee, S.-H.; Chung, J. Drosophila ADCK1 Is Critical for Maintaining Mitochondrial Structures and Functions in the Muscle. PLoS Genet. 2019, 15, e1008184. [Google Scholar] [CrossRef]
- Li, Q.; Wineinger, N.E.; Fu, D.-J.; Libiger, O.; Alphs, L.; Savitz, A.; Gopal, S.; Cohen, N.; Schork, N.J. Genome-Wide Association Study of Paliperidone Efficacy. Pharmacogenet Genom. 2017, 27, 7–18. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Palmini, G.; Perigli, G.; Santoro, R.; Brandi, M.L. Genetics and Epigenetics of Parathyroid Carcinoma. Front. Endocrinol. 2022, 13, 834362. [Google Scholar] [CrossRef]
- Kemmerer, Z.A.; Robinson, K.P.; Schmitz, J.M.; Manicki, M.; Paulson, B.R.; Jochem, A.; Hutchins, P.D.; Coon, J.J.; Pagliarini, D.J. UbiB Proteins Regulate Cellular CoQ Distribution in Saccharomyces Cerevisiae. Nat. Commun. 2021, 12, 4769. [Google Scholar] [CrossRef]
- Karihtala, P.; Porvari, K.; Roininen, N.; Voutilainen, S.; Mattson, J.; Heikkilä, P.; Haapasaari, K.-M.; Selander, K. Comparison of the Mutational Profiles of Neuroendocrine Breast Tumours, Invasive Ductal Carcinomas and Pancreatic Neuroendocrine Carcinomas. Oncogenesis 2022, 11, 53. [Google Scholar] [CrossRef]
- Vázquez-Fonseca, L.; Schäefer, J.; Navas-Enamorado, I.; Santos-Ocaña, C.; Hernández-Camacho, J.D.; Guerra, I.; Cascajo, M.V.; Sánchez-Cuesta, A.; Horvath, Z.; Siendones, E.; et al. ADCK2 Haploinsufficiency Reduces Mitochondrial Lipid Oxidation and Causes Myopathy Associated with CoQ Deficiency. J. Clin. Med. 2019, 8, 1374. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Camacho, J.D.; Vicente-García, C.; Ardila-García, L.; Padilla-Campos, A.; López-Lluch, G.; Santos-Ocaña, C.; Zammit, P.S.; Carvajal, J.J.; Navas, P.; Fernández-Ayala, D.J.M. Prenatal and Progressive Coenzyme Q10 Administration to Mitigate Muscle Dysfunction in Mitochondrial Disease. J. Cachexia Sarcopenia Muscle 2024, 15, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Brough, R.; Frankum, J.R.; Sims, D.; Mackay, A.; Mendes-Pereira, A.M.; Bajrami, I.; Costa-Cabral, S.; Rafiq, R.; Ahmad, A.S.; Cerone, M.A.; et al. Functional Viability Profiles of Breast Cancer. Cancer Discov. 2011, 1, 260–273. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Murray, N.H.; Pagliarini, D.J. ADCK3/COQ8A: The Choice Target of the UbiB Protein Kinase-like Family. Nat. Rev. Drug Discov. 2019, 18, 815. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Tazir, M.; López, L.C.; Quinzii, C.M.; Assoum, M.; Drouot, N.; Busso, C.; Makri, S.; Ali-Pacha, L.; Benhassine, T.; et al. ADCK3, an Ancestral Kinase, Is Mutated in a Form of Recessive Ataxia Associated with Coenzyme Q10 Deficiency. Am. J. Hum. Genet. 2008, 82, 661–672. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Zhou, J.; Wang, D. Genome-Wide CRISPR Screening Reveals ADCK3 as a Key Regulator in Sensitizing Endometrial Carcinoma Cells to MPA Therapy. Br. J. Cancer 2023, 129, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Stefely, J.A.; Licitra, F.; Laredj, L.; Reidenbach, A.G.; Kemmerer, Z.A.; Grangeray, A.; Jaeg-Ehret, T.; Minogue, C.E.; Ulbrich, A.; Hutchins, P.D.; et al. Cerebellar Ataxia and Coenzyme Q Deficiency through Loss of Unorthodox Kinase Activity. Mol. Cell 2016, 63, 608–620. [Google Scholar] [CrossRef]
- Chang, A.; Ruiz-Lopez, M.; Slow, E.; Tarnopolsky, M.; Lang, A.E.; Munhoz, R.P. ADCK3-related Coenzyme Q10 Deficiency: A Potentially Treatable Genetic Disease. Mov. Disord. Clin. Pract. 2018, 5, 635–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, X.; Wang, Y.; Wu, L.D.; Luo, R.L.; Xia, R.P. Tumor Purity–Associated Genes Influence Hepatocellular Carcinoma Prognosis and Tumor Microenvironment. Front. Oncol. 2023, 13, 1197898. [Google Scholar] [CrossRef]
- Widmeier, E.; Yu, S.; Nag, A.; Chung, Y.W.; Nakayama, M.; Fernández-del-Río, L.; Hugo, H.; Schapiro, D.; Buerger, F.; Choi, W.-I.; et al. ADCK4 Deficiency Destabilizes the Coenzyme Q Complex, Which Is Rescued by 2,4-Dihydroxybenzoic Acid Treatment. J. Am. Soc. Nephrol. 2020, 31, 1191–1211. [Google Scholar] [CrossRef]
- Egashira, S.; Jinnin, M.; Makino, K.; Ajino, M.; Shimozono, N.; Okamoto, S.; Tazaki, Y.; Hirano, A.; Ide, M.; Kajihara, I.; et al. Recurrent Fusion Gene ADCK4-NUMBL in Cutaneous Squamous Cell Carcinoma Mediates Cell Proliferation. J. Investig. Dermatol. 2019, 139, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Chłopek, M.; Lasota, J.; Thompson, L.D.R.; Szczepaniak, M.; Kuźniacka, A.; Hińcza, K.; Kubicka, K.; Kaczorowski, M.; Newford, M.; Liu, Y.; et al. Alterations in Key Signaling Pathways in Sinonasal Tract Melanoma. A Molecular Genetics and Immunohistochemical Study of 90 Cases and Comprehensive Review of the Literature. Mod. Pathol. 2022, 35, 1609–1617. [Google Scholar] [CrossRef]
- Atmaca, M.; Gulhan, B.; Korkmaz, E.; Inozu, M.; Soylemezoglu, O.; Candan, C.; Bayazıt, A.K.; Elmacı, A.M.; Parmaksiz, G.; Duzova, A.; et al. Follow-up Results of Patients with ADCK4 Mutations and the Efficacy of CoQ10 Treatment. Pediatr. Nephrol. 2017, 32, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Gülhan, B.; Atayar, E.; Bayazıt, A.K.; Candan, C.; Arıcı, M.; Topaloğlu, R.; Özaltın, F. Long-Term Follow-up Results of Patients with ADCK4 Mutations Who Have Been Diagnosed in the Asymptomatic Period: Effects of Early Initiation of CoQ10 Supplementation. Turk. J. Pediatr. 2019, 61, 657–663. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dörflein, I.; Ren, X.; Pfeffer, S.; Britzen-Laurent, N.; Grützmann, R.; Duan, X.; Pilarsky, C. Impact of CRISPR/Cas9-Mediated CD73 Knockout in Pancreatic Cancer. Cancers 2023, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Afaq, F.; Karthikeyan, S.K.; Athar, M.; Shrestha, S.; Singh, R.; Manne, U.; Varambally, S. Bromodomain Inhibitor Treatment Leads to Overexpression of Multiple Kinases in Cancer Cells. Neoplasia 2024, 57, 101046. [Google Scholar] [CrossRef]
- Han, C.; Deng, Y.; Yang, B.; Hu, P.; Hu, B.; Wang, T.; Liu, J.; Xia, Q.; Liu, X. Identification of a Novel Senescence-Associated Signature to Predict Biochemical Recurrence and Immune Microenvironment for Prostate Cancer. Front. Immunol. 2023, 14, 1126902. [Google Scholar] [CrossRef]
- ADCK1 aarF Domain Containing Kinase 1 [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/57143 (accessed on 14 April 2025).
- Tan, T.; Özbalci, C.; Brügger, B.; Rapaport, D.; Dimmer, K.S. Mcp1 and Mcp2, Two Novel Proteins Involved in Mitochondrial Lipid Homeostasis. J. Cell Sci. 2013, 126, 3563–3574. [Google Scholar] [CrossRef]
- Tamura, Y.; Kawano, S.; Endo, T. Lipid Homeostasis in Mitochondria. Biol. Chem. 2020, 401, 821–833. [Google Scholar] [CrossRef]
- Su, Y.-A.; Bousman, C.; Li, Q.; Li, J.-T.; Lin, J.-Y.; Si, T.-M. Genetic Variations in the ADCK1 Gene Predict Paliperidone Palmitate Efficacy in Han Chinese Patients with Schizophrenia. J. Neural Transm. 2019, 126, 19–25. [Google Scholar] [CrossRef]
- Sirp, A.; Shubina, A.; Tuvikene, J.; Tamberg, L.; Kiir, C.S.; Kranich, L.; Timmusk, T. Expression of Alternative Transcription Factor 4 mRNAs and Protein Isoforms in the Developing and Adult Rodent and Human Tissues. Front. Mol. Neurosci. 2022, 15, 1033224. [Google Scholar] [CrossRef] [PubMed]
- ADCK2 aarF Domain Containing Kinase 2 [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/90956 (accessed on 14 April 2025).
- Cherniack, A.; Shen, H.; Walter, V.; Stewart, C.; Murray, B.; Bowlby, R.; Hu, X.; Ling, S.; Soslow, R.A.; Broaddus, R.R.; et al. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017, 31, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- COQ8A Coenzyme Q8A [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/56997 (accessed on 7 May 2025).
- Rhee, H.-W.; Zou, P.; Udeshi, N.D.; Martell, J.D.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging. Science 2013, 339, 1328–1331. [Google Scholar] [CrossRef]
- Floyd, B.J.; Wilkerson, E.M.; Veling, M.T.; Minogue, C.E.; Xia, C.; Beebe, E.T.; Wrobel, R.L.; Cho, H.; Kremer, L.S.; Alston, C.L.; et al. Mitochondrial Protein Interaction Mapping Identifies New Regulators of Respiratory Chain Function. Mol. Cell 2016, 63, 621–632. [Google Scholar] [CrossRef]
- COQ8B Coenzyme Q8B [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/79934 (accessed on 14 April 2025).
- Malaga-Dieguez, L.; Susztak, K. ADCK4 “Reenergizes” Nephrotic Syndrome. J. Clin. Investig. 2013, 123, 4996–4999. [Google Scholar] [CrossRef]
- ADCK5 aarF Domain Containing Kinase 5 [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/203054 (accessed on 14 April 2025).
- Gao, P.; Tambe, M.; Chen, C.Z.; Huang, W.; Tawa, G.J.; Hirschhorn, T.; Stockwell, B.R.; Zheng, W.; Shen, M. Identification of Potent ADCK3 Inhibitors through Structure-Based Virtual Screening. J. Chem. Inf. Model. 2024, 64, 6072–6080. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Berger, B.-T.; Wan, J.; Bennett, J.M.; Capuzzi, S.J.; Crona, D.J.; Drewry, D.H.; East, M.P.; Elkins, J.M.; Fedrov, O.; et al. SGC-GAK-1: A Chemical Probe for Cyclin G Associated Kinase (GAK). J. Med. Chem. 2019, 62, 2830–2836. [Google Scholar] [CrossRef]
- Murray, N.H.; Asquith, C.R.M.; Fang, Z.; East, M.P.; Ptak, N.; Smith, R.W.; Vasta, J.D.; Zimprich, C.A.; Corona, C.R.; Robers, M.B.; et al. Small Molecule Inhibition of the Archetypal UbiB Protein COQ8. Nat. Chem. Biol. 2023, 19, 230–238. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Khan, Y.S.; Rasheed, Z. Cancer Bioenergetics and Tumor Microenvironments—Enhancing Chemotherapeutics and Targeting Resistant Niches through Nanosystems. Cancers 2023, 15, 3836. [Google Scholar] [CrossRef]
| Protein | Functions | Proposed Targets/Interactions | Localization | Pathological Implications | Potential Oncogenic Role |
|---|---|---|---|---|---|
| ADCK1 | Mitochondrial Maintenance/ Homeostasis [18,36] | Directly- COQ3-4-5-6-9, PDSS2, MT-CO1, NDFUS3, NDFUV2, YME1L1 Indirectly-OPA1, IMMT, TCF4, B-Catenin [18,25,33,36] | Mitochondrial Membrane [36] | Colon Cancer, Osteosarcoma, Parathyroid Cancer, Schizophrenia [33,36,37,38] | Tumor Growth/Cell Proliferation, ATP Production, Cellular Migration, Apoptosis, Colony Formation, Metastasis [33,38] |
| ADCK2 | CoQ Biosynthesis, Mitochondrial Metabolism/Maintenance [23,39] | Directly- COQ3-4-5-9, Akt Indirectly- MYL6/TNFa-HIF-1a axis/RELB-dependent NF-KB signaling pathway, Akt-mTOR signaling pathway, S6K [17,19,23,34,35,40] | Mitochondrial Matrix [41] | Breast Cancer, CoQ Deficiency, Melanoma, Osteo- sarcoma, Non- Small Cell Lung Cancer [34,35,40,42,43] | Tumor Progression, Oxidative Stress, ROS Production, Inflammatory Response, Tumor Growth/Cell Proliferation, Metabolic Adaptation, Cellular Motility, Invasion, Cell Cycle Progression, Cell Survival [32,34,35,40] |
| ADCK3 | CoQ Biosynthesis, Kinase Activity, Mitochondrial Maintenance/ Homeostasis [26,29] | Directly- COQ2-3-4-5-6-7-9- 10, PDSS1, PDSS2, MBP, p53, ALOX15, UNC-CA157, IMM Indirectly-PI3K/Akt, MPA, OX- PHOS [20,24,26,44,45,46] | Mitochondrial Cristae, Inner Mitochondrial Membrane/Cristae [26,29] | Autosomal Recessive Cerebellar Ataxia 2, Chronic Kidney Disease, Endometrial Carcinoma, Hepatocellular Carcinoma, CoQ Dysfunction [44,45,46,47,48,49] | Tumor Purity, Tumor Progression, Tumor Growth/Cell Proliferation, Immune Evasion, Drug Resistance, MPA-Induced Cell Death [46,49] |
| ADCK4 | CoQ Biosynthesis, Mitochondrial Metabolism/ Maintenance [27,29] | Directly- COQ2-3,4,5,6,7,9,10A, PDSS1, PDSS2, NUMBL Indirectly- BRAF, RAS [21,27,50,51,52] | Inner Mitochondrial Membrane/Cristae Mitochondria and Cytosol [21,29] | Focal Segmental Glomerulosclerosis, Nephropathy, Sinonasal Tract Mucosal Melanoma, Steroid-Resistant Nephrotic Syndrome CoQ Dysfunction [31,50,51,52,53,54] | Tumor Modulation, Tumor Growth/Cell Proliferation [51,52] |
| ADCK5 | Mitochondrial Maintenance/ Homeostasis [28] | Directly- SOX9, SCLC52A2, MFN1, CD73 Indirectly- PTTG, NBR1, BNIP3, STX17 [22,28,55,56] | Mitochondria [16] | Asthma, Lung Cancer, Pancreatic Cancer, Prostate Cancer [55,56,57] | Tumor Growth/Cell Proliferation, Tumor Progression, Metastasis, Cell Cycle Regulation, Cellular Differentiation, Cell Survival, Drug Resistance [55,56,57] |
| References: [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacquet, N.; Zhao, Y. The ADCK Kinase Family: Key Regulators of Bioenergetics and Mitochondrial Function and Their Implications in Human Cancers. Int. J. Mol. Sci. 2025, 26, 5783. https://doi.org/10.3390/ijms26125783
Jacquet N, Zhao Y. The ADCK Kinase Family: Key Regulators of Bioenergetics and Mitochondrial Function and Their Implications in Human Cancers. International Journal of Molecular Sciences. 2025; 26(12):5783. https://doi.org/10.3390/ijms26125783
Chicago/Turabian StyleJacquet, Noel, and Yunfeng Zhao. 2025. "The ADCK Kinase Family: Key Regulators of Bioenergetics and Mitochondrial Function and Their Implications in Human Cancers" International Journal of Molecular Sciences 26, no. 12: 5783. https://doi.org/10.3390/ijms26125783
APA StyleJacquet, N., & Zhao, Y. (2025). The ADCK Kinase Family: Key Regulators of Bioenergetics and Mitochondrial Function and Their Implications in Human Cancers. International Journal of Molecular Sciences, 26(12), 5783. https://doi.org/10.3390/ijms26125783






