Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance
Abstract
1. Introduction
2. Results
2.1. Study Population Characteristics and Dietary Adherence of Patients with PKU
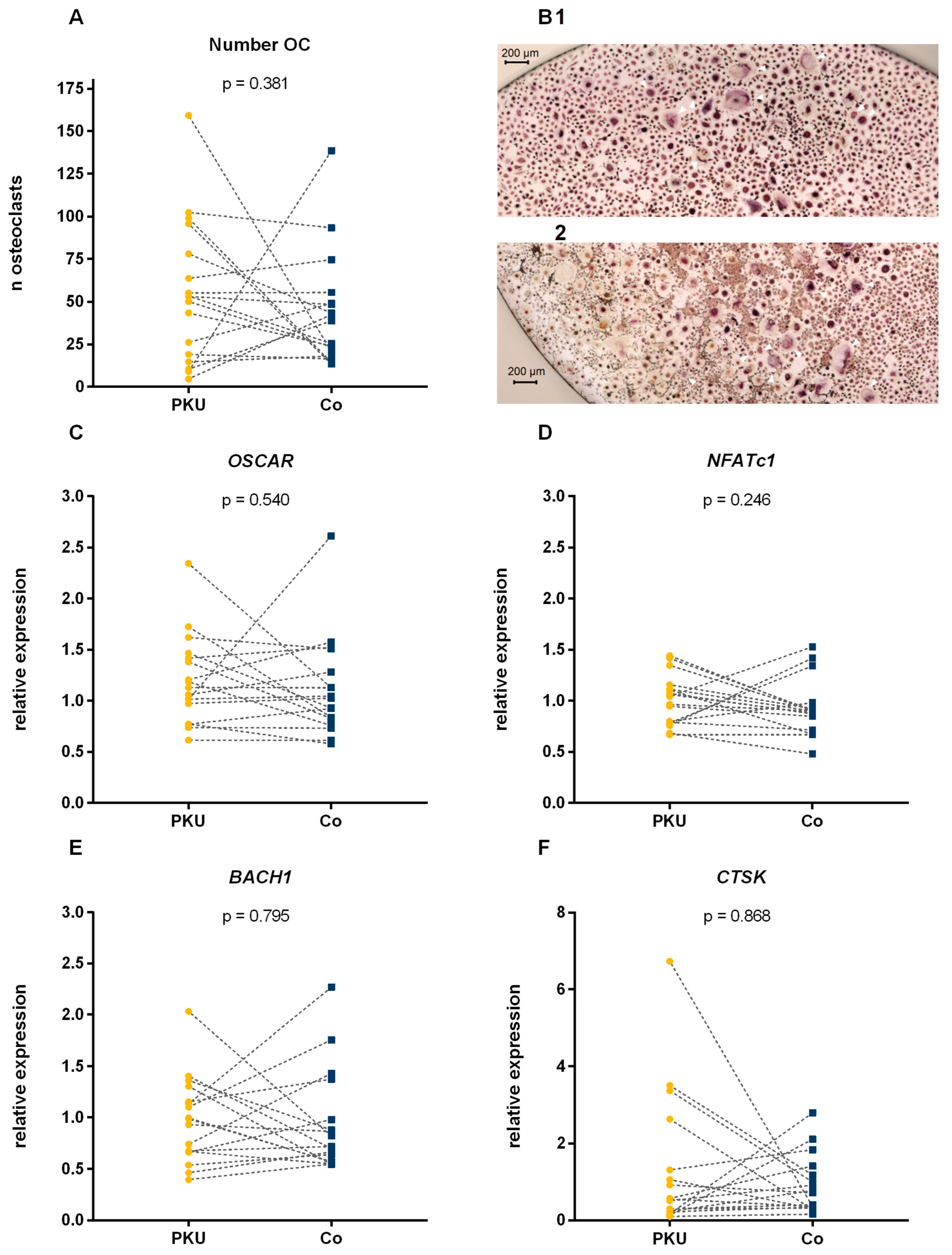
2.2. Number of Osteoclasts
2.3. Gene Expression
3. Discussion
Limitations
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric Data, Lifestyle, and Nutrition
4.3. Sampling and Biochemical Analyses
4.3.1. Osteoclast Differentiation
4.3.2. RNA Isolation, RT, and Real-Time PCR
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAM | amino acid mixtures |
| AAS | amino acid supplements |
| B2M | beta-2-microglobulin |
| BACH1 | BTB domain and CNC homolog 1 |
| BH4 | tetrahydrobiopterin |
| BMD | bone mineral density |
| Co | controls |
| CTSK | Cathepsin K |
| DXA | Dual X-ray Absorptiometry |
| EDTA | ethylenediaminetetraacetic acid |
| GMP | glycomacropeptides |
| LS | lumbar spine |
| MCSF | macrophage colony-stimulating factor |
| NFATc1 | Nuclear factor of activated T cells 1 |
| Nrf2 | nuclear factor E2-related factor 2 |
| OC | osteoclasts |
| OSCAR | Osteoclast-associated receptor |
| PAH | phenylalanine hydroxylase |
| PBMCs | peripheral blood mononucleated cells |
| Phe | phenylalanine |
| PKU | phenylketonuria |
| Prdm1 | PR domain zinc finger protein 1 |
| RANKL | receptor activator of NF-κB Ligand |
| ROS | reactive oxygen species |
| SD | standard deviation |
| Trap | tartrate-resistant acid phosphatase |
References
- Milstien, S.; Kaufman, S. Studies on the phenylalanine hydroxylase system in vivo. An in vivo assay based on the liberation of deuterium or tritium into the body water from ring-labeled L-phenylalanine. J. Biol. Chem. 1975, 250, 4782–4785. [Google Scholar] [CrossRef] [PubMed]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Brockow, I.; Blankenstein, O.; Ceglarek, U.; Ensenauer, R.; Fingerhut, R.; Gramer, G.; Hörster, F.; Janzen, N.; Klein, J.; Lankes, E.; et al. Nationaler Screeningreport Deutschland 2021; Deutsche Gesellschaft für Neugeborenenscreening e.V.: Oberschleißheim, Germany, 2024. [Google Scholar]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef]
- Becsei, D.; Hiripi, R.; Kiss, E.; Szatmári, I.; Arató, A.; Reusz, G.; Szabó, A.J.; Bókay, J.; Zsidegh, P. Quality of life in children living with PKU—A single-center, cross-sectional, observational study from Hungary. Mol. Genet. Metab. Rep. 2021, 29, 100823. [Google Scholar] [CrossRef]
- Charrière, S.; Maillot, F.; Bouée, S.; Douillard, C.; Jacob, C.; Schneider, K.M.; Theil, J.; Arnoux, J.-B. Health status and comorbidities of adult patients with phenylketonuria (PKU) in France with a focus on early-diagnosed patients—A nationwide study of health insurance claims data. Mol. Genet. Metab. 2023, 139, 107625. [Google Scholar] [CrossRef]
- Douillard, C.; Arnoux, J.-B.; Bouée, S.; Jacob, C.; Schneider, K.M.; Theil, J.; Charrière, S.; Maillot, F. Health status and comorbidities of adult patients with late-diagnosed phenylketonuria (PKU) born before the newborn screening in France—A nationwide study of health insurance claims data. Mol. Genet. Metab. 2023, 140, 107704. [Google Scholar] [CrossRef] [PubMed]
- Trefz, K.F.; Muntau, A.C.; Kohlscheen, K.M.; Altevers, J.; Jacob, C.; Braun, S.; Greiner, W.; Jha, A.; Jain, M.; Alvarez, I.; et al. Clinical burden of illness in patients with phenylketonuria (PKU) and associated comorbidities—A retrospective study of German health insurance claims data. Orphanet J. Rare Dis. 2019, 14, 181. [Google Scholar] [CrossRef]
- Doulgeraki, A.; Skarpalezou, A.; Theodosiadou, A.; Monopolis, I.; Schulpis, K. Body composition profile of young patients with phenylketonuria and mild hyperphenylalaninemia. Int. J. Endocrinol. Metab. 2014, 12, e16061. [Google Scholar] [CrossRef]
- Koura, H.M.; Abdallah Ismail, N.; Kamel, A.F.; Ahmed, A.M.; Saad-Hussein, A.; Effat, L.K. A long-term study of bone mineral density in patients with phenylketonuria under diet therapy. Arch. Med. Sci. 2011, 7, 493–500. [Google Scholar] [CrossRef]
- Lubout, C.M.A.; Arrieta Blanco, F.; Bartosiewicz, K.; Feillet, F.; Gizewska, M.; Hollak, C.; van der Lee, J.H.; Maillot, F.; Stepien, K.M.; Wagenmakers, M.A.E.M.; et al. Bone mineral density is within normal range in most adult phenylketonuria patients. J. Inherit. Metab. Dis. 2020, 43, 251–258. [Google Scholar] [CrossRef]
- Barat, P.; Barthe, N.; Redonnet-Vernhet, I.; Parrot, F. The impact of the control of serum phenylalanine levels on osteopenia in patients with phenylketonuria. Eur. J. Pediatr. 2002, 161, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Venegas, E.; Langeveld, S.; Ahring, K.; Benitez, R.; Desloovere, A.; Dios, E.; Gómez, E.; Hermida, A.; Marsaux, C.; Verloo, P.; et al. Nutrient Status and Intakes of Adults with Phenylketonuria. Nutrients 2024, 16, 2724. [Google Scholar] [CrossRef] [PubMed]
- Hanusch, B.; Falkenstein, M.; Volkenstein, S.; Dazert, S.; Lücke, T.; Sinningen, K. No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study. Nutrients 2024, 16, 2072. [Google Scholar] [CrossRef]
- Geiger, K.E.; Koeller, D.M.; Harding, C.O.; Huntington, K.L.; Gillingham, M.B. Normal vitamin D levels and bone mineral density among children with inborn errors of metabolism consuming medical food-based diets. Nutr. Res. 2016, 36, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Demirdas, S.; van Spronsen, F.J.; Hollak, C.E.; van der Lee, J.H.; Bisschop, P.H.; Vaz, F.M.; ter Horst, N.M.; Rubio-Gozalbo, M.E.; Bosch, A.M. Micronutrients, Essential Fatty Acids and Bone Health in Phenylketonuria. Ann. Nutr. Metab. 2017, 70, 111–121. [Google Scholar] [CrossRef]
- de Castro, M.J.; de Lamas, C.; Sánchez-Pintos, P.; González-Lamuño, D.; Couce, M.L. Bone Status in Patients with Phenylketonuria: A Systematic Review. Nutrients 2020, 12, 2154. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T. A study of bone turnover markers in prepubertal children with phenylketonuria. Eur. J. Pediatr. 2004, 163, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Choukair, D.; Kneppo, C.; Feneberg, R.; Schönau, E.; Lindner, M.; Kölker, S.; Hoffmann, G.F.; Tönshoff, B. Analysis of the functional muscle-bone unit of the forearm in patients with phenylketonuria by peripheral quantitative computed tomography. J. Inherit. Metab. Dis. 2017, 40, 219–226. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F.; Alexy, U.; Schoenau, E.; Wudy, S.A.; Shi, L. Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J. Clin. Endocrinol. Metab. 2011, 96, 2861–2868. [Google Scholar] [CrossRef]
- Zerjav Tansek, M.; Bertoncel, A.; Sebez, B.; Zibert, J.; Groselj, U.; Battelino, T.; Avbelj Stefanija, M. Anthropometry and bone mineral density in treated and untreated hyperphenylalaninemia. Endocr. Connect. 2020, 9, 649–657. [Google Scholar] [CrossRef]
- Stroup, B.M.; Sawin, E.A.; Murali, S.G.; Binkley, N.; Hansen, K.E.; Ney, D.M. Amino Acid Medical Foods Provide a High Dietary Acid Load and Increase Urinary Excretion of Renal Net Acid, Calcium, and Magnesium Compared with Glycomacropeptide Medical Foods in Phenylketonuria. J. Nutr. Metab. 2017, 2017, 1909101. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Ren, Y.; Yu, S.; Zheng, J.; Liu, L.; Sun, P.; Wu, J.; Yang, K. A Low-Phenylalanine-Containing Whey Protein Hydrolysate Stimulates Osteogenic Activity through the Activation of p38/Runx2 Signaling in Osteoblast Cells. Nutrients 2022, 14, 3135. [Google Scholar] [CrossRef]
- Bu, T.; Zhang, L.; Liu, L.; Yu, S.; Zheng, J.; Wu, J.; Yang, K. Evaluation of the anti-osteoporotic effect of a low-phenylalanine whey protein hydrolysate in an ovariectomized mice model. Food Funct. 2022, 13, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Roato, I.; Mussa, A.; Repici, M.; Gorassini, E.; Spada, M.; Ferracini, R. Increased spontaneous osteoclastogenesis from peripheral blood mononuclear cells in phenylketonuria. J. Inherit. Metab. Dis. 2008, 31 (Suppl. S2), S339–S342. [Google Scholar] [CrossRef]
- Roato, I.; Porta, F.; Mussa, A.; D’Amico, L.; Fiore, L.; Garelli, D.; Spada, M.; Ferracini, R. Bone impairment in phenylketonuria is characterized by circulating osteoclast precursors and activated T cell increase. PLoS ONE 2010, 5, e14167. [Google Scholar] [CrossRef]
- Millet, P.; Vilaseca, M.A.; Valls, C.; Pérez-Dueñas, B.; Artuch, R.; Gómez, L.; Lambruschini, N.; Campistol, J. Is deoxypyridinoline a good resorption marker to detect osteopenia in phenylketonuria? Clin. Biochem. 2005, 38, 1127–1132. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Tourkova, I.L.; Larrouture, Q.C.; Blair, H.C. Creatine energy substrate increases bone density in the Pahenu2 classical PKU mouse in the context of phenylalanine restriction. Mol. Genet. Metab. Rep. 2023, 36, 100996. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, S.F.; Phua, Y.L.; Tourkova, I.L.; Sudano, C.; Vockley, J.; Larrouture, Q.C.; Blair, H.C. Glutamine energy substrate anaplerosis increases bone density in the Pahenu2 classical PKU mouse in the absence of phenylalanine restriction. JIMD Rep. 2022, 63, 446–452. [Google Scholar] [CrossRef]
- Nagasaka, H.; Tsukahara, H.; Takatani, T.; Sanayama, Y.; Takayanagi, M.; Ohura, T.; Sakamoto, O.; Ito, T.; Wada, M.; Yoshino, M.; et al. Cross-sectional study of bone metabolism with nutrition in adult classical phenylketonuric patients diagnosed by neonatal screening. J. Bone Miner. Metab. 2011, 29, 737–743. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Wilson, S.R.; Peters, C.; Saftig, P.; Brömme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Hanusch, B.; Schlegtendal, A.; Grasemann, C.; Lücke, T.; Sinningen, K. Adults with Phenylketonuria have suboptimal bone mineral density apart from vitamin D and calcium sufficiency. Front. Endocrinol. 2025, 16, 1488215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, Y.; Li, X.-Y.; Kerk, S.A.; Lyssiotis, C.A.; Feng, W.; Sun, X.; Hespe, G.E.; Wang, Z.; Stemmler, M.P.; et al. A Zeb1/MtCK1 metabolic axis controls osteoclast activation and skeletal remodeling. EMBO J. 2023, 42, e111148. [Google Scholar] [CrossRef]
- Yang, K.; Cao, F.; Xue, Y.; Tao, L.; Zhu, Y. Three Classes of Antioxidant Defense Systems and the Development of Postmenopausal Osteoporosis. Front. Physiol. 2022, 13, 840293. [Google Scholar] [CrossRef]
- Fernandes, C.G.; Leipnitz, G.; Seminotti, B.; Amaral, A.U.; Zanatta, A.; Vargas, C.R.; Dutra Filho, C.S.; Wajner, M. Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell. Mol. Neurobiol. 2010, 30, 317–326. [Google Scholar] [CrossRef]
- Sitta, A.; Barschak, A.G.; Deon, M.; Terroso, T.; Pires, R.; Giugliani, R.; Dutra-Filho, C.S.; Wajner, M.; Vargas, C.R. Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab. Brain Dis. 2006, 21, 287–296. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Phua, Y.L.; Vockley, J.; Goetzman, E.; Blair, H.C. Phenylketonuria oxidative stress and energy dysregulation: Emerging pathophysiological elements provide interventional opportunity. Mol. Genet. Metab. 2022, 136, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Wei, X.; Niu, C.; Jia, M.; Li, Q.; Meng, D. Bach1: Function, Regulation, and Involvement in Disease. Oxid. Med. Cell. Longev. 2018, 2018, 1347969. [Google Scholar] [CrossRef]
- Hama, M.; Kirino, Y.; Takeno, M.; Takase, K.; Miyazaki, T.; Yoshimi, R.; Ueda, A.; Itoh-Nakadai, A.; Muto, A.; Igarashi, K.; et al. Bach1 regulates osteoclastogenesis in a mouse model via both heme oxygenase 1-dependent and heme oxygenase 1-independent pathways. Arthritis Rheum. 2012, 64, 1518–1528. [Google Scholar] [CrossRef]
- Kanzaki, H.; Shinohara, F.; Itohiya, K.; Yamaguchi, Y.; Katsumata, Y.; Matsuzawa, M.; Fukaya, S.; Miyamoto, Y.; Wada, S.; Nakamura, Y. RANKL induces Bach1 nuclear import and attenuates Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice. FASEB J. 2017, 31, 781–792. [Google Scholar] [CrossRef]
- Tao, H.; Li, X.; Wang, Q.; Yu, L.; Yang, P.; Chen, W.; Yang, X.; Zhou, J.; Geng, D. Redox signaling and antioxidant defense in osteoclasts. Free Radic. Biol. Med. 2024, 212, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Refaey, M.E.; Zhong, Q.; Ding, K.-H.; Shi, X.-M.; Xu, J.; Bollag, W.B.; Hill, W.D.; Chutkan, N.; Robbins, R.; Nadeau, H.; et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif. Tissue Int. 2014, 95, 174–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrmann, M.; Tami, A.; Wildemann, B.; Wolny, M.; Wagner, A.; Schorr, H.; Taban-Shomal, O.; Umanskaya, N.; Ross, S.; Garcia, P.; et al. Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone 2009, 44, 467–475. [Google Scholar] [CrossRef]
- Pawlak, R. Vitamin B12 status is a risk factor for bone fractures among vegans. Med. Hypotheses 2021, 153, 110625. [Google Scholar] [CrossRef] [PubMed]
- Stroup, B.M.; Hansen, K.E.; Krueger, D.; Binkley, N.; Ney, D.M. Sex differences in body composition and bone mineral density in phenylketonuria: A cross-sectional study. Mol. Genet. Metab. Rep. 2018, 15, 30–35. [Google Scholar] [CrossRef]
- Rovelli, V.; Ercoli, V.; Dionigi, A.R.; Paci, S.; Salvatici, E.; Zuvadelli, J.; Banderali, G. Low bone mineralization in phenylketonuria may be due to undiagnosed metabolic acidosis. Mol. Genet. Metab. Rep. 2023, 36, 100998. [Google Scholar] [CrossRef]
- Schwahn, B.; Mokov, E.; Scheidhauer, K.; Lettgen, B.; Schönau, E. Decreased trabecular bone mineral density in patients with phenylketonuria measured by peripheral quantitative computed tomography. Acta Paediatr. 1998, 87, 61–63. [Google Scholar] [CrossRef]
| Parameter | PKU | Co | p |
|---|---|---|---|
| n | 17 | 17 | - |
| Age (years) | 37.3 [23.4–44.5] | 38.3 [23.8–47.2] | 0.134 |
| Female n (%) | 10 (59%) | 9 (53%) | 0.500 a |
| GMP usage n (%) | 5 (41.7%) | - | - |
| Blood Phe 5 years (µmol/L) | 610.6 ± 344.7 | - | - |
| Blood Phe 5 years below 600 µmol/L n (%) | 7 (41.7%) | - | - |
| Blood Phe 12 months (µmol/L) | 756.7 ± 374.8 | - | - |
| Blood Phe 12 months below 600 µmol/L n (%) | 6 (35.3%) | - | - |
| T-Score in hip | −0.63 ± 1.07 | - | - |
| T-Score in LS | −0.74 ± 1.14 | - | - |
| Diet Compliance 12 Months | p | Diet Compliance 5 Years | p | |||
|---|---|---|---|---|---|---|
| Good | Poor | Good | Poor | |||
| Number of osteoclasts | 59.1 ± 27.1 | 52.9 ± 49.1 | 0.779 | 50.7 ± 31.3 | 58.0 ± 49.2 | 0.739 |
| CTSK | 0.37 [0.13–1.64] | 0.57 [0.30–3.37] | 0.256 | 0.22 [0.14–2.63] | 0.31 [0.74–1.86] | 0.133 |
| OSCAR | 1.30 ± 0.56 | 1.11 ± 0.37 | 0.486 | 1.30 ± 0.51 | 1.14 ± 0.38 | 0.463 |
| NFATc1 | 0.95 ± 0.18 | 1.04 ± 0.27 | 0.470 | 0.95 ± 0.19 | 1.05 ± 0.28 | 0.413 |
| BACH1 | 0.67 [0.50–1.06] | 1.10 [0.93–1.31] | 0.122 | 0.93 ± 0.55 | 1.00 ± 0.32 | 0.734 |
| Hips | p | LS | p | |||
|---|---|---|---|---|---|---|
| T-Score Above −1 | T-Score Below −1 | T-Score Above −1 | T-Score Below −1 | |||
| Number of osteoclasts | 51.0 ± 47.2 | 53.6 ± 35.8 | 0.903 | 50.7 ± 47.8 | 54.0 ± 34.7 | 0.882 |
| CTSK | 0.23 [0.15–0.79] | 0.92 [0.56–3.37] | 0.055 | 0.31 [0.20–2.04] | 0.91 [0.30–2.63] | 0.470 |
| OSCAR | 1.15 ± 0.34 | 1.24 ± 0.58 | 0.719 | 1.15 ± 0.34 | 1.24 ± 0.58 | 0.696 |
| NFATc1 | 0.96 ± 0.23 | 1.08 ± 0.28 | 0.380 | 0.92 ± 0.23 | 1.13 ± 0.25 | 0.103 |
| BACH1 | 0.74 [0.50–1.33] | 1.00 [0.93–1.15] | 0.536 | 0.92 ± 0.39 | 1.09 ± 0.47 | 0.457 |
| Protein | Gene | Sequence (5′ → 3′) | NCBI Reference Sequence | |
|---|---|---|---|---|
| beta-2-microglobulin | B2M | F: | TAGCTGTGCTCGCGCTACTCTCTC | NM_004048.4 |
| R: | AATGTCGGATGGATGAAACCCAGACAC | |||
| Cathepsin K | CTSK | F: | CCCGCAGTAATGACACCCTT | NC_000001.11 |
| R: | AAAGCCCAACAGGAACCACA | |||
| Osteoclast-associated receptor | OSCAR | F: | CACTCCGTCTGTGGCCATTA | NM_206818.4 |
| R: | AGGACACATCCCGGAAGAGA | |||
| Nuclear factor of activated T cells 1 | NFATc1 | F: | GTCCGTCTGTATGCGAGCAA | NM_172390.3 |
| R: | GGCTGCAACGGCGGAAGAAA | |||
| BTB domain and CNC homolog 1 | BACH1 | F: | GTTTGTGGCTGGGGAGAGAAGG | NM_206866.3 |
| R: | ATGTTGTCGGGAAGTTCAGTGG | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanusch, B.; Schlegtendal, A.; Lücke, T.; Sinningen, K. Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance. Int. J. Mol. Sci. 2025, 26, 5776. https://doi.org/10.3390/ijms26125776
Hanusch B, Schlegtendal A, Lücke T, Sinningen K. Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance. International Journal of Molecular Sciences. 2025; 26(12):5776. https://doi.org/10.3390/ijms26125776
Chicago/Turabian StyleHanusch, Beatrice, Anne Schlegtendal, Thomas Lücke, and Kathrin Sinningen. 2025. "Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance" International Journal of Molecular Sciences 26, no. 12: 5776. https://doi.org/10.3390/ijms26125776
APA StyleHanusch, B., Schlegtendal, A., Lücke, T., & Sinningen, K. (2025). Ex Vivo Osteoclastogenesis from Peripheral Blood Mononuclear Cells Is Unchanged in Adults with Phenylketonuria, Regardless of Dietary Compliance. International Journal of Molecular Sciences, 26(12), 5776. https://doi.org/10.3390/ijms26125776






