A Comprehensive Review of Phenolic Compounds in Horticultural Plants
Abstract
1. Introduction
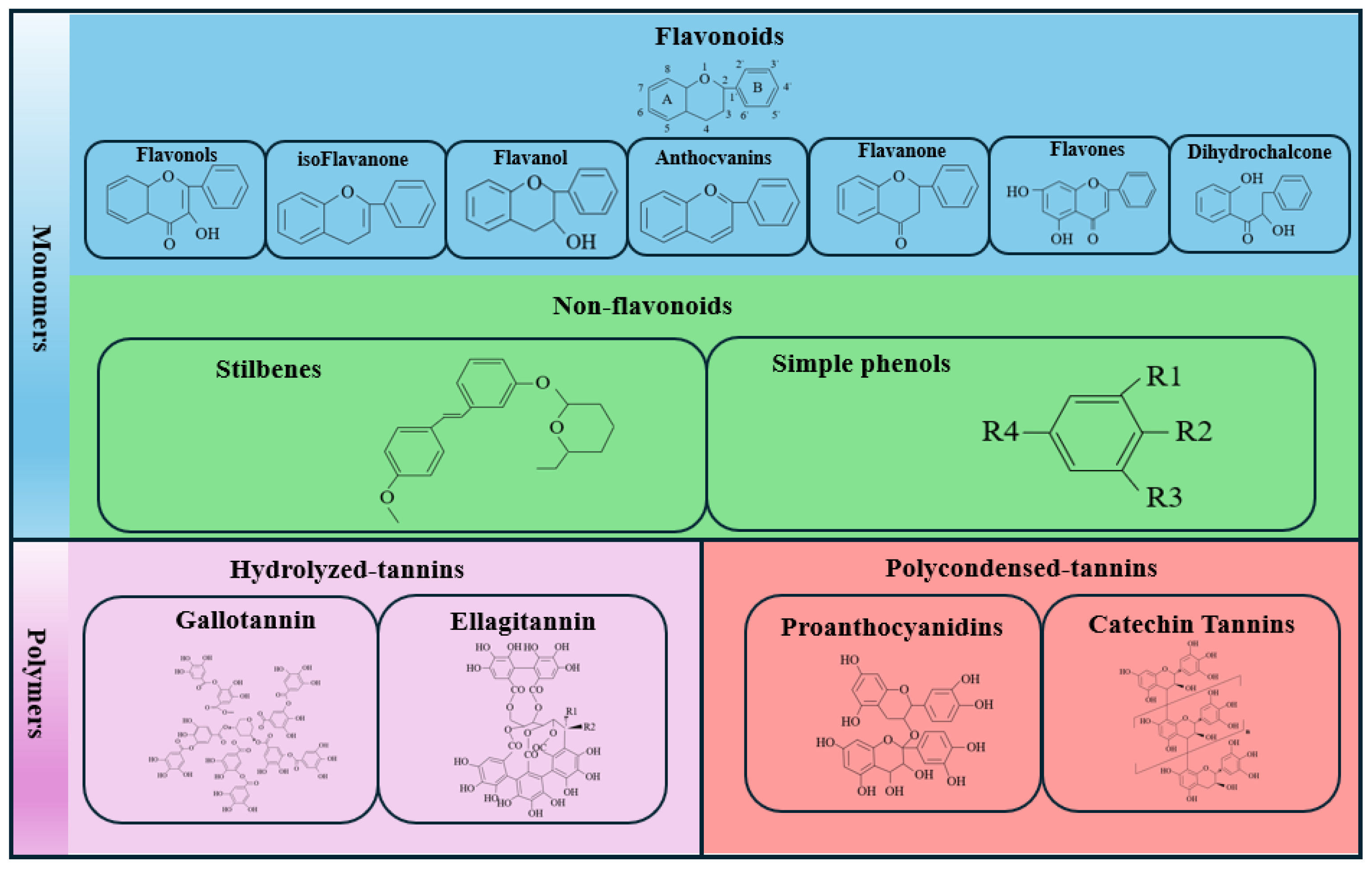
2. Classification of PCs
3. Metabolic Pathways of PCs in Plants
3.1. Biosynthetic Pathway Centered on Phenylpropanoid Metabolism
3.2. Enzymatic and Non-Enzymatic Synergistic Degradation Pathways
4. Extraction and Separation of PCs in Plants
4.1. Extraction of PCs
4.2. Separation of PCs
5. Biological Functions of PCs
5.1. Growth and Development
5.2. Antioxidant Capacity
5.2.1. Abiotic Stress
5.2.2. Biotic Stress
6. Factors Affecting the Accumulation of PCs
6.1. Genetic Factors
6.2. External Factors
7. Regulatory Networks of PCs
8. Discussion
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Nagendran, B.; Kalyana, S.; Samir, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2021, 66, 3270–3289. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; Barbosa, R. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Wang, J.; Yang, B.; Chen, J.; Wu, H.; Zhang, Z.; Lu, C.; Lin, W.; Wu, L. Linking Short-Chain N-Acyl Homoserine Lactone-Mediated Quorum Sensing and Replant Disease: A Case Study of Rehmannia glutinosa. Front. Plant Sci. 2020, 11, 787–799. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Puglia, D.; Kenny, J.M.; Liu, T.; Guo, J.; Wang, Q.; Qu, R.; Xu, P.; Ma, P.; et al. Bio-renewable polymers based on lignin-derived phenol monomers: Synthesis, applications, and perspectives. SusMat 2022, 2, 34. [Google Scholar] [CrossRef]
- Gao, S.; Wang, F.; Zhang, X.; Li, B.; Yao, Y. Characterization of anthocyanin and nonanthocyanidin phenolic compounds and/or their biosynthesis pathway in red-fleshed ‘Kanghong’ grape berries and their wine. Food Res. Int. 2022, 161, 111789. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Gang, D.R.; Costa, M.A.; Fujita, M.; Dinkova-Kostova, A.T.; Wang, H.B.; Burlat, V.; Martin, W.; Sarkanen, S.; Davin, L.B.; Lewis, N.G. Regiochemical control of monolignol radical coupling: A new paradigm for lignin and lignan biosynthesis. Chem. Biol. 1999, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Liu, Y.; Zhuang, J.; Zhao, Y.; Dai, X.; Jiang, C.; Wang, Z.; Jiang, X.; Zhang, S.; Qian, Y.; et al. Insights into Acylation Mechanisms: Co-expression of Serine Carboxypeptidase-like Acyltransferases and Their Non-catalytic Companion Paralogs. Plant J. 2022, 111, 117–133. [Google Scholar] [CrossRef]
- Bustamante, M.; Gil-Cortiella, M.; Peña-Neira, Á.; Gombau, J.; García-Roldán, A.; Cisterna, M.; Montané, X.; Fort, F.; Rozès, N.; Canals, J.M.; et al. Oxygen-induced enzymatic and chemical degradation kinetics in wine model solution of selected phenolic compounds involved in browning. Food Chem. 2025, 144421, 0308–8146. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zhang, Z.; Wang, W.; Xu, S.; He, X. Isolation, identification and characterization of phenolic acid-degrading bacteria from soil. J. Appl. Microbiol. 2021, 131, 208–220. [Google Scholar] [CrossRef]
- Wei, X.; Shu, J.; Fahad, S.; Tao, K.; Zhang, J.; Chen, G.; Liang, Y.; Wang, M.; Chen, S.; Liao, J. Polyphenol oxidases regulate pollen development through modulating flavonoids homeostasis in tobacco. Plant Physiol. Biochem. 2023, 198, 107702. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B. Glycosyltransferases: Key players involved in the modification of plant secondary metabolites. Front. Biol. China 2009, 4, 39–46. [Google Scholar] [CrossRef]
- Rai, N.; Neugart, S.; Schröter, D.; Lindfors, A.V.; Aphalo, P.J. Responses of flavonoids to solar UV radiation and gradual soil drying in two Medicago truncatula accessions. Photochem. Photobiol. Sci. 2023, 22, 1637–1654. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules 2023, 28, 3610. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Pressurized GRAS solvents for the green extraction of phenolic compounds from hibiscus sabdariffa calyces. Food Res. Int. 2020, 137, 109466. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.H.; Yu, Z.L.; Lu, Y.A.; Liu, S.J.; Li, Y.M.; Xie, M.X.; Lin, L.M. Green and Efficient Extraction of Phenolic Components from Plants with Supramolecular Solvents: Experimental and Theoretical Studies. Molecules 2024, 29, 2067. [Google Scholar] [CrossRef]
- Ratananikom, K.; Premprayoon, K. Ultrasonic-Assisted Extraction of Phenolic Compounds, Flavonoids, and Antioxidants from Dill (Anethum graveolens L.). Scientifica 2022, 2022, 3848261. [Google Scholar] [CrossRef]
- Chisha, G.; Li, C.; Cui, C.Z. Multiscale mechanism exploration and experimental optimization for rosmarinic acid extraction from Rosmarinus officinalis using natural deep eutectic solvents. Ind. Crops Prod. 2022, 188, 115637. [Google Scholar] [CrossRef]
- Vargas-Serna, C.L.; Ochoa-Martínez, C.I.; Vélez-Pasos, C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae 2022, 8, 791. [Google Scholar] [CrossRef]
- Hong, N.; Yaylayan, V.A.; Raghavan, G.S.; Paré, J.R.; Bélanger, J.M. Microwave-assisted Extraction of Phenolic Compounds from Grape Seed. Nat. Prod. Lett. 2001, 15, 197–204. [Google Scholar] [CrossRef]
- Tsiaka, T.; Lantzouraki, D.Z.; Polychronaki, G.; Sotiroudis, G.; Kritsi, E.; Sinanoglou, V.J.; Kalogianni, D.P.; Zoumpoulakis, P. Optimization of Ultrasound- and Microwave-Assisted Extraction for the Determination of Phenolic Compounds in Peach Byproducts Using Experimental Design and Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2023, 28, 518. [Google Scholar] [CrossRef]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef]
- de Miera, B.S.; Cañadas, R.; González-Miquel, M.; González, E.J. Recovery of Phenolic Compounds from Orange Peel Waste by Conventional and Assisted Extraction Techniques Using Sustainable Solvents. Front. Biosci. 2023, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Visentín, A.; Cismondi, M.; Maestri, D. Supercritical CO2 fractionation of rosemary ethanolic oleoresins as a method to improve carnosic acid recovery. Innov. Food Sci. Emerg. 2011, 12, 142–145. [Google Scholar] [CrossRef]
- Natolino, A.; da Porto, C.; Rodríguez-Rojo, S.; Moreno, T.; Cocero, M.J. Supercritical antisolvent precipitation of polyphenols from grape marc extract. J. Supercrit. Fluids 2016, 118, 54–63. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluids extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef]
- Prado, I.M.; Prado, G.H.C.; Prado, J.M.; Meireles, A.A. Supercritical CO2 and low-pressure solvent extraction of mango (Mangifera indica) leaves: Global yield, extraction kinetics, chemical composition and cost of manufacturing. Food Bioprod. Process. 2013, 91, 656–664. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Rafińska, K.; Walczak-Skierska, J.; Buszewski, B. The Influence of Plant Material Enzymatic Hydrolysis and Extraction Conditions on the Polyphenolic Profiles and Antioxidant Activity of Extracts: A Green and Efficient Approach. Molecules 2020, 25, 2074. [Google Scholar] [CrossRef]
- Barbosa, P.P.M.; Ruviaro, A.R.; Macedo, G.A. Conditions of enzyme-assisted extraction to increase the recovery of flavanone aglycones from pectin waste. J. Food Sci. Technol. 2021, 58, 4303–4312. [Google Scholar] [CrossRef]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Pulsed electric fields-assisted extraction of valuable compounds from red grape pomace: Process optimization using response surface methodology. Front. Nutr. 2023, 10, 1158019. [Google Scholar] [CrossRef]
- Tzani, A.; Lymperopoulou, T.; Pitterou, I.; Karetta, I.; Belfquih, F.; Detsi, A. Development and optimization of green extraction process of spent coffee grounds using natural deep eutectic solvents. SCAP 2023, 34, 101144. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication−synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.d.S.; Koblitz, M.G.B. Extraction of phenolic compounds from agro−industrial by−products using natural deep eutectic solvents: A review of green and advanced techniques. Separations 2025, 12, 150. [Google Scholar] [CrossRef]
- Wen, C.; Chen, X.; Lai, L. Identifying metabolic biomarkers and pathways in pulpitis: A metabolomic study using ultra-high-performance liquid chromatography/orbitrap mass spectrometry. J. Appl. Oral Sci. 2025, 33, e20240428. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, J.; Shen, C.; Liang, Q.; An, Y.; Zhou, C.; Liu, K.; Xia, Q.; He, Q.; Zhang, H. Study of the Chemical Composition and Anti-Inflammatory Mechanism of Shiyiwei Golden Pill Based on UPLC-Q-TOF/MS and Network Pharmacology. Drug Des. Dev. Ther. 2025, 19, 3159–3177. [Google Scholar] [CrossRef]
- Mitić, S.S.; Mitić, M.N.; Nikolić, J.S.; Stojanović, B.T.; Mitić, V.D.; Marković, M.S.; Stankov, J.V.P. Influence of location and season on phenolic composition and antioxidant activity of Sempervivum tectorum. Nat. Prod. Res. 2025, 28, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Meng, J.; Du, J.; Liu, X.; Pu, Q.; Di, D.; Chen, C. Preparative Separation of Flavonoids from Goji Berries by Mixed-Mode Macroporous Adsorption Resins and Effect on Aβ-Expressing and Anti-Aging Genes. Molecules 2020, 31, 3511. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, R.T.; Zhao, Y.Y.; Zhang, J.; Zhang, B.Y.; Chen, Z.C.; Liu, P.; Chen, Z.B.; Liu, C.L.; Li, X.M. Separation and purification of flavonoids from polygonum cuspidatum using macroporous adsorption resin. Pigm. Resin. Technol. 2021, 50, 574–584. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef]
- Berli, F.; D’Angelo, J.; Cavagnaro, B.; Bottini, R.; Wuilloud, R.; Silva, M.F. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. J. Agric. Food Chem. 2008, 56, 2892. [Google Scholar] [CrossRef]
- Ji, M.; Li, C.; Li, Q. Rapid separation and identification of phenolics in crude red grape skin extracts by high performance liquid chromatography coupled to diode array detection and tandem mass spectrometry. J. Chromatogr. A 2015, 1414, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Jeong, H.; Choi, Y.D.; Jang, G. Auxin controls the division of root endodermal cells. Plant Physiol. 2021, 187, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.G.; Park, C.M. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010, 188, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Balarynová, J.; Klčová, B.; Sekaninová, J.; Kobrlová, L.; Cechová, M.Z.; Krejčí, P.; Leonova, T.; Gorbach, D.; Ihling, C.; Smržová, L.; et al. The loss of polyphenol oxidase function is associated with hilum pigmentation and has been selected during pea domestication. New Phytol. 2022, 235, 1807–1821. [Google Scholar] [CrossRef]
- Teale, W.D.; Pasternak, T.; Dal Bosco, C.; Dovzhenko, A.; Kratzat, K.; Bildl, W.; Schwörer, M.; Falk, T.; Ruperti, B.; Schaefer, J.V.; et al. Flavonol-mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO J. 2021, 40, e104416. [Google Scholar] [CrossRef]
- Huang, G.; Hu, H.; van de Meene, A.; Zhang, J.; Dong, L.; Zheng, S.; Zhang, F.; Betts, N.S.; Liang, W.; Bennett, M.J.; et al. AUXIN RESPONSE FACTORS 6 and 17 control the flag leaf angle in rice by regulating secondary cell wall biosynthesis of lamina joints. Plant Cell 2021, 33, 3120–3133. [Google Scholar] [CrossRef]
- Corcoran, M.R.; Geissman, T.A.; Phinney, B.O. Tannins as gibberellin antagonists. Plant Physiol. 1972, 49, 323–330. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.T.; Seo, H.S. Ammonium-mediated reduction in salicylic acid content and recovery of plant growth in Arabidopsis siz1 mutants is modulated by NDR1 and NPR1. Plant Signal. Behav. 2021, 16, 1928819. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, P.; Tang, Y.; Liu, J.; Tang, Y.; Zhuang, Y.; Li, X.; Xu, K.; Zhou, Z.; Li, J.; et al. NPR1 promotes blue light-induced plant photomorphogenesis by ubiquitinating and degrading PIF4. Proc. Natl. Acad. Sci. USA 2024, 121, e2412755121. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Li, K.; Gao, Y.; Li, H.; Long, L.; Chu, Z.; Du, Y.; Zhao, X.; Zhao, B.; et al. Regulation of lignin biosynthesis by GhCAD37 affects fiber quality and anther vitality in upland cotton. Plant J. 2024, 120, 2846–2860. [Google Scholar] [CrossRef]
- Pei, L.; Gao, Y.; Feng, L.; Zhang, Z.; Liu, N.; Yang, B.; Zhao, N. Phenolic Acids and Flavonoids Play Important Roles in Flower Bud Differentiation in Mikania micrantha: Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2023, 24, 16550. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Lakshmanan, M.; Lim, S.H.; Kim, J.K.; Ha, S.H.; Lee, D.Y. Light-specific transcriptional regulation of the accumulation of carotenoids and phenolic compounds in rice leaves. Plant Signal. Behav. 2016, 11, e1184808. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Shulaev, V.; Yalpani, N.; Lawton, M.A.; Raskin, I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 10413–10417. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Magwanga, R.O.; Lu, H.; Kirungu, J.N.; Wei, Y.; Dong, Q.; Wang, X.; Cai, X.; Zhou, Z.; Wang, K.; et al. A novel G-protein-coupled receptors gene from upland cotton enhances salt stress tolerance in transgenic Arabidopsis. Genes 2018, 9, 209. [Google Scholar] [CrossRef]
- Xi, Y.; Cheng, D.; Zeng, X.; Cao, J.; Jiang, W. Evidences for Chlorogenic Acid--A Major Endogenous Polyphenol Involved in Regulation of Ripening and Senescence of Apple Fruit. PLoS ONE 2016, 11, e0146940. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Palumbo, M.; Laurino, C.; Picariello, G.; Siano, F.; De Giulio, B.; Pelosi, S.; Cefola, M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods 2021, 10, 3102. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Q.; Yang, Y.; Jiang, Q.; Cao, H.; Zhang, Z. Light influences the effect of exogenous ethylene on the phenolic composition of Cabernet Sauvignon grapes. Front. Plant Sci. 2024, 15, 1356257. [Google Scholar] [CrossRef]
- Changwal, C.; Shukla, T.; Hussain, Z.; Singh, N.; Kar, A.; Singh, V.P.; Abdin, M.Z.; Arora, A. Regulation of Postharvest Tomato Fruit Ripening by Endogenous Salicylic Acid. Front. Plant Sci. 2021, 12, 663943. [Google Scholar] [CrossRef]
- Cui, M.; Mo, R.; Li, Q.; Wang, R.; Shen, D.; Tang, F.; Liu, Y. Maturation-induced changes in phenolic forms and their antioxidant activities of walnuts: A dual view from kernel and pellicle. Food Chem. X 2024, 23, 101792. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, L.; Yao, J.L.; Zhao, P.; Jiang, S.; Sun, X.; Dong, C.; Jiang, H.; Xu, X.; Zhang, Y. Hypermethylation in the promoter regions of flavonoid pathway genes is associated with skin color fading during ‘Daihong’ apple fruit development. Hortic. Res. 2024, 11, uhae031. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Massonnet, M.; Fasoli, M.; Tornielli, G.B.; Altieri, M.; Sandri, M.; Zuccolotto, P.; Paci, P.; Gardiman, M.; Zenoni, S.; Pezzotti, M. Ripening Transcriptomic Program in Red and White Grapevine Varieties Correlates with Berry Skin Anthocyanin Accumulation. Plant Physiol. 2017, 174, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Zhang, M.; Wu, T.; Song, T.; Yao, Y.; Zhang, J.; Tian, J. MdWER interacts with MdERF109 and MdJAZ2 to mediate methyl jasmonate- and light-induced anthocyanin biosynthesis in apple fruit. Plant J. 2024, 118, 1327–1342. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, B.; Li, T.; Liu, R.H. Black goji berry anthocyanins extend lifespan and enhance the antioxidant defenses in Caenorhabditis elegans via the JNK-1 and DAF-16/FOXO pathways. J. Sci. Food Agric. 2025, 105, 2282–2293. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate−Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, X.; Zhang, A.; Wang, P.; Chen, Q.; Ma, T.; Li, W.; Liang, Y.; Sun, X.; Fang, Y. Foliar Phenylalanine Application Promoted Antioxidant Activities in Cabernet Sauvignon by Regulating Phenolic Biosynthesis. J. Agric. Food Chem. 2020, 68, 15390–15402. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Luo, W.; Tang, J.; Tuo, Y.; Liao, N.; Zhuang, D.; Yang, K.; Lin, J.; Zhang, Y.; et al. Study on metabolic variation reveals metabolites important for flavor development and antioxidant property of Hainan Dayezhong black tea. Food Res. Int. 2024, 196, 115112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, R.; Guo, R.; Nong, H.; Qin, Y.; Dong, N. Integrative Analysis of Metabolome and Transcriptome Reveals Molecular Insight into Metabolomic Variations during Hawthorn Fruit Development. Metabolites 2023, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1073–1088. [Google Scholar] [CrossRef]
- Naranjo, R.A.; Moreta, J.G.P.; Albuja, G.G.; Iturralde, G.; Paramás, A.M.G.; Suarez, J.M.A. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Ray, A.; Kundu, S.; Mohapatra, S.S.; Sinha, S.; Khoshru, B.; Keswani, C.; Debasis Mitra, D. An Insight into the Role of Phenolics in Abiotic Stress Tolerance in Plants: Current Perspective for Sustainable Environment. J. Pure Appl. Microbiol. 2024, 18, 8964. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Nanoscale structure, mechanics and growth of epidermal cell walls. Curr. Opin. Plant Biol. 2018, 46, 77–86. [Google Scholar] [CrossRef]
- Ren, H.; Yang, W.; Jing, W.; Shahid, M.O.; Liu, Y.; Qiu, X.; Choisy, P.; Xu, T.; Ma, N.; Gao, J.; et al. Multi-omics analysis reveals key regulatory defense pathways and genes involved in salt tolerance of rose plants. Hortic. Res. 2024, 11, uhae068. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; You, X.; Gao, L.; Zhou, P.; Shi, F.; An, Y. MsMYB206-MsMYB450-MsHY5 complex regulates alfalfa tolerance to salt stress via regulating flavonoid biosynthesis during the day and night cycles. Plant J. 2025, 121, e17216. [Google Scholar] [CrossRef]
- Li, S.; Chang, L.; Sun, R.; Dong, J.; Zhong, C.; Gao, Y.; Zhang, H.; Wei, L.; Wei, Y.; Zhang, Y.; et al. Combined transcriptomic and metabolomic analysis reveals a role for adenosine triphosphate-binding cassette transporters and cell wall remodeling in response to salt stress in strawberry. Front. Plant Sci. 2022, 13, 996765. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.R.; Yang, K.; Wang, X.; Lin, X.L.; Rui, L.; Liu, H.F.; Liu, D.D.; You, C.X. Overexpression of MdZAT5, an C2H2-Type Zinc Finger Protein, Regulates Anthocyanin Accumulation and Salt Stress Response in Apple Calli and Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1897. [Google Scholar] [CrossRef] [PubMed]
- Daldoul, S.; Hanzouli, F.; Boubakri, H.; Nick, P.; Mliki, A.; Gargouri, M. Deciphering the regulatory networks involved in mild and severe salt stress responses in the roots of wild grapevine Vitis vinifera spp. sylvestris. Protoplasma 2024, 261, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, J.; Zhu, C.; Jing, B.; Shi, K.; Yu, J.; Hu, Z. Exogenous Rosmarinic Acid Application Enhances Thermo tolerance in Tomatoes. Plants 2022, 11, 1172. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef]
- Magri, A.; Rega, P.; Capriolo, G.; Petriccione, M. Impact of Novel Active Layer-by-Layer Edible Coating on the Qualitative and Biochemical Traits of Minimally Processed ‘Annurca Rossa del Sud’ Apple Fruit. Int. J. Mol. Sci. 2023, 24, 8315. [Google Scholar] [CrossRef]
- Zheng, R.; Xiong, X.; Li, X.; Wang, D.; Xu, Z.; Li, X.; Yang, M.; Ren, X.; Kong, Q. Changes in Polyphenolic Compounds of Hutai No. 8 Grapes during Low-Temperature Storage and Their Shelf-Life Prediction by Identifying Biomarkers. J. Agric. Food Chem. 2022, 70, 15818–15829. [Google Scholar] [CrossRef]
- Abidi, W.; Akrimi, R. Phenotypic diversity of nutritional quality attributes and chilling injury symptoms in four early peach [Prunus persica (L.) Batsch] cultivars grown in west central Tunisia. J. Food Sci. Technol. 2022, 59, 3938–3950. [Google Scholar] [CrossRef]
- Li, S.; He, L.; Yang, Y.; Zhang, Y.; Han, X.; Hu, Y.; Jiang, Y. INDUCER OF CBF EXPRESSION 1 promotes cold-enhanced immunity by directly activating salicylic acid signaling. Plant Cell 2024, 36, 2587–2606. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef]
- Mihai, R.A.; Acurio Criollo, O.S.; Quishpe Nasimba, J.P.; Melo Heras, E.J.; Galván Acaro, D.K.; Landazuri Abarca, P.A.; Florescu, L.I.; Catana, R.D. Influence of Soil Nutrient Toxicity and Deficiency from Three Ecuadorian Climatic Regions on the Variation of Biological, Metabolic, and Nutritional Properties of Moringa oleifera Lam. Toxics 2022, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; et al. Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.D.S.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar]
- Soriano, G.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic compounds from different bryophyte species and cell compartments respond specifically to ultraviolet radiation, but not particularly quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef]
- Valenta, K.; Dimac-Stohl, K.; Baines, F.; Smith, T.; Piotrowski, G.; Hill, N.; Kuppler, J.; Nevo, O. Ultraviolet radiation changes plant color. BMC Plant Biol. 2020, 20, 253. [Google Scholar] [CrossRef]
- Jensen, K.; Koide, R.T. The protection of Salicornia rubra from ultraviolet radiation by betacyanins and phenolic compounds. Plant Environ. Interact. 2021, 2, 229–234. [Google Scholar] [CrossRef]
- González Moreno, A.; de Cózar, A.; Prieto, P.; Domínguez, E.; Heredia, A. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nat. Commun. 2022, 13, 1786. [Google Scholar] [CrossRef]
- Field, B.; Jordon, F.; Osbourn, A. First encounters—Deployment of defence related natural products by plants. New Phytol. 2006, 172, 193–207. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant−microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Ndakidemi, P.A.; Dakora, F.D. Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Rev. Funct. Plant Biol. 2003, 30, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, B.; Bai, Y.; Chen, Y.; Li, F.; Du, J.; Wu, X.; Yan, L.; Bai, Y.; Chai, G. Saline-alkali Stress Affects the Accumulation of Proanthocyanidins and Sesquiterpenoids via the MYB5-ANR/TPS31 Cascades in the Rose Petals. Hortic. Res. 2024, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Phil Morris, J.; Hooker, J.E. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol. 2006, 8, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Kumaki, T.; Hashimoto, S.; Saeki, K.; Ayabe, S.I.; Higashitani, A.; Akashi, T.; Sato, S.; Aoki, T. Phenolic Acids Induce Nod Factor Production in Lotus japonicus-Mesorhizobium Symbiosis. Microbes Environ. 2022, 37, ME21094. [Google Scholar] [CrossRef]
- Bao, L.; Liu, Y.; Ding, Y.; Shang, J.; Wei, Y.; Tan, Y.; Zi, F. Interactions Between Phenolic Acids and Microorganisms in Rhizospheric Soil from Continuous Cropping of Panax notoginseng. Front. Microbiol. 2022, 13, 791603. [Google Scholar] [CrossRef]
- Mobin, L.; Saeed, S.A.; Ali, R.; Saeed, S.G.; Ahmed, R. Antibacterial and antifungal activities of the polyphenolic fractions isolated from the seed coat of Abrus precatorius and Caesalpinia crista. Nat. Prod. Res. 2018, 32, 2835–2839. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Z.Q.; Liu, Y.S.; Wang, H.M.; Qiu, M.; Qu, Y.H.; Yuan, W.P. The optimization of extraction process, antioxidant, whitening and antibacterial effects of fengdan peony flavonoids. Molecules 2022, 27, 506. [Google Scholar] [CrossRef]
- Ghouila, Z.; Laurent, S.; Boutry, S.; Vander, E.L.; Nateche, F.; Muller, R.; Baaliouamer, A. Antioxidant, antibacterial and cell toxicity effects of polyphenols from ahm eur bouamer grape seed extracts. J. Fundam. Appl. Sci. 2017, 9, 392–420. [Google Scholar] [CrossRef]
- Wang, H.X.; Xu, F.; Zhang, X.; Shao, X.F.; Wei, Y.Y.; Wang, H.F. Transcriptomic analysis reveals antibacterial mechanism of flavonoids from Sedum aizoon L. against Pseudomonas fragi. Food Control 2022, 134, 108755. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R. Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: A Meta-Analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Zhao, J.; Chen, M. Effect of Tannic Acid on Nutrition and Activities of Detoxification Enzymes and Acetylcholinesterase of the Fall Webworm (Lepidoptera: Arctiidae). J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.M.; Bender, C.L.; Kunkel, B.N. The Pseudomonas syringae phyto toxin coronatine promotes virulence by overcoming salicylic acid- dependent defences in Arabidopsis thaliana. Mol. Plant Pathol. 2005, 6, 629–639. [Google Scholar] [CrossRef]
- Koornneef, A.; Leon-Reyes, A.; Ritsema, T.; Verhage, A.; Den Otter, F.C.; Van Loon, L.C.; Pieterse, C.M.J. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 2008, 147, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shen, J.; Li, H.; Sun, Y.; Jiang, T.; Kong, X.; Han, R.; Zhao, C.; Zhang, X.; Zhao, X. Physiological and molecular mechanism of Populus pseudo-cathayana × Populus deltoides response to Hyphantria cunea. Pestic. Biochem. Physiol. 2024, 202, 105969. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; He, L.; Huang, F.; Zhang, D.; Wang, Y.; Wei, X.; Han, M.; Deng, H.; Luo, L.; et al. Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 2023, 622, 139–148. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S.; Ereifej, K.; Johargy, A.; Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]
- Lee, C.D.; Cho, H.; Shim, J.; Tran, G.H.; Lee, H.D.; Ahn, K.H.; Yoo, E.; Chung, M.J.; Lee, S. Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors. Molecules 2023, 28, 6266. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Zhang, M.; Hu, Y.; Ye, Z. Profiling of naturally occurring proanthocyanidins and other phenolic compounds in a diverse peach germplasm by LC-MS/MS. Food Chem. 2023, 403, 134471. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules 2019, 24, 4310. [Google Scholar] [CrossRef]
- Busatto, N.; Matsumoto, D.; Tadiello, A.; Vrhovsek, U.; Costa, F. Multifaceted analyses disclose the role of fruit size and skin-russeting in the accumulation pattern of phenolic compounds in apple. PLoS ONE 2019, 14, e0219354. [Google Scholar] [CrossRef]
- Herniter, I.A.; Kim, Y.; Wang, Y.; Havill, J.S.; Johnson-Cicalese, J.; Muehlbauer, G.J.; Iorizzo, M.; Vorsa, N. Trait Mapping of Phenolic Acids in an Interspecific (Vaccinium corymbosum var. caesariense × V. darrowii) Diploid Blueberry Population. Plants 2023, 12, 1346. [Google Scholar]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. [Google Scholar] [CrossRef]
- Savitha, S.; Bhatkar, N.; Chakraborty, S.; Thorat, B.N. Onion quercetin: As immune boosters, extraction, and effect of dehydration. Food Biosci. 2021, 44, 101457. [Google Scholar] [CrossRef]
- Krzymińska, A.; Gąsecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules 2020, 25, 5627. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, D.Y.; Park, K.I.; Kim, C.K. Differential expression of anthocyanin structural genes and transcription factors determines coloration patterns in gerbera flowers. 3 Biotech 2018, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Wei, K.; Cheng, H.; Wang, L.Y.; Zhang, C.C. Accumulation of catechins and expression of catechin synthetic genes in Camellia sinensis at different developmental stages. Bot. Stud. 2016, 57, 31. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortiz, J.J.; Morales-Ventura, J.M.; Colín-Chávez, C.; Esquivel-Chávez, F.; Vargas-Arispuro, I.; Aispuro-Hernández, E.; Martínez-Téllez, M.A. Postharvest Application of Pectic-Oligosaccharides on Quality Attributes, Activities of Defense-Related Enzymes, and Anthocyanin Accumulation in Strawberry. J. Sci. Food Agric. 2020, 100, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium barbarum) and Black Goji Berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Tomaz, I.; Marchal, C.; Kontić, J.K.; Lacombe, T.; Maletić, E.; Preiner, D. Discrimination of Genetic and Geographical Groups of Grape Varieties (Vitis vinifera L.) Based on Their Volatile Organic Compounds. Front. Plant Sci. 2022, 13, 942148. [Google Scholar] [CrossRef]
- Crozier, A.; Burns, J.; Aziz, A.A.; Stewart, A.J.; Rabiasz, H.S.; Jenkins, G.I.; Edwards, C.A.; Lean, M.E. Antioxidant Flavonols from Fruits, Vegetables and Beverages: Measurements and Bioavailability. Biol. Res. 2000, 33, 79–88. [Google Scholar] [CrossRef]
- Lin, L.Z.; Lu, S.; Harnly, J.M. Detection and Quantification of Glycosylated Flavonoid Malonates in Celery, Chinese Celery, and Celery Seed by LC-DAD-ESI/MS. J. Agric. Food Chem. 2007, 55, 1321–1326. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary Flavonols: Chemistry, Food Content, and Metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant Capacity, Anticancer Ability and Flavonoids Composition of 35 Citrus (Citrus reticulata Blanco) Varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Di Lecce, G.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Screening of the Polyphenol Content of Tomato-Based Products Through Accurate-Mass Spectrometry (HPLC-ESI-QTOF). Food Chem. 2011, 129, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guan, L.; Cao, Y.; Li, C.; Chen, J.; Li, J.; Liu, G.; Li, S.; Wu, B. Diversity of Polyphenols in the Peel of Apple (Malus sp.) Germplasm from Different Countries of Origin. Int. J. Food Sci. Technol. 2016, 51, 222–230. [Google Scholar] [CrossRef]
- Maronpot, R.R. Toxicological assessment of Ashitaba Chalcone. Food Chem. Toxicol. 2015, 77, 111–119. [Google Scholar] [CrossRef]
- Yan, Z.; Alimu, R.; Wan, J.; Liao, X.; Lin, S.; Dai, S.; Chen, F.; Zhang, S.; Tong, Y.; Liu, H.; et al. Composition of Major Quinochalcone Hydroxysafflor Yellow A and Anhydrosafflor Yellow B Is Associated with Colour of Safflower (Carthamus tinctorius) during Colour-Transition but Not with Overall Antioxidant Capacity: A Study on 144 Cultivars. Food Res. Int. 2022, 162, 112098. [Google Scholar] [CrossRef]
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding Vegetables with Increased Content in Bioactive Phenolic Acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable Amounts of trans-Resveratrol in Seed and Berry Skin in Vitis Evaluated at the Germplasm Level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, W.J.; Wu, C.S.; Wang, S.C.; Chang, W.C.; Hung, M.C. Tannic Acids and Proanthocyanidins in Tea Inhibit SARS-CoV-2 Variants Infection. Am. J. Cancer Res. 2024, 14, 2555–2569. [Google Scholar] [CrossRef]
- Das, P.R.; Eun, J.B. Removal of Astringency in Persimmon Fruits (Diospyros kaki) Subjected to Different Freezing Temperature Treatments. J. Food Sci. Technol. 2021, 58, 3154–3163. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Wang, J.; Qu, J.; Dai, S. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, W.; Cheng, L.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Nat. Prod. Bioprospect. 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yi, L.; Ren, D.; Li, B. Mass defect filtering combined with molecular networking to profile flavonoids in citrus fruit based on liquid chromatography-high resolution mass spectrometry platform: Citrus sinensis (L.) Osbeck as a case study. J. Chromatogr. A 2022, 1685, 463640. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S.; Mendke, S. Quantification of Flavonoids, Phenols and Antioxidant Potential from Dropped Citrus reticulata Blanco Fruits Influenced by Drying Techniques. Molecules 2021, 26, 4159. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, X.; Shen, Y.; Guo, Y.; Wang, T.; Wang, J.; Lin, L.; Deng, H.; Deng, Q.; Xu, K.; et al. Comparative analysis of flavonoids in white and red table grape cultivars during ripening by widely targeted metabolome and transcript levels. J. Food Sci. 2022, 87, 1650–1661. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Davies, T.; Watts, S.; McClure, K.; Migicovsky, Z.; Myles, S. Phenotypic divergence between the cultivated apple (Malus domestica) and its primary wild progenitor (Malus sieversii). PLoS ONE 2022, 17, e0250751. [Google Scholar] [CrossRef]
- Kaur, K.; Grewal, S.K.; Gill, P.S.; Singh, S. Comparison of cultivated and wild chickpea genotypes for nutritional quality and antioxidant potential. J. Food Sci. Technol. 2019, 56, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wu, D.; Guo, R.; Li, H.; Wei, R.; Zhang, J.; Wei, Z.; Meng, X.; Yu, H.; Xie, L.; et al. Chromosome-scale Genomics, Metabolomics, and Transcriptomics Provide Insight into the Synthesis and Regulation of Phenols in Vitis adenoclada Grapes. Front. Plant Sci. 2023, 14, 1124046. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Lai, Y.C.; Hsu, Y.S.; Ho, C.T. Phenolic content, antioxidant activity and effective compounds of kumquat extracted by different solvents. Food Chem. 2016, 197, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhao, A.; Wang, Y.; Ren, H.; Du, J.; Li, D.; Li, Y. Composition and content of phenolic acids and flavonoids among the different varieties, development stages, and tissues of Chinese Jujube (Ziziphus jujuba Mill.). PLoS ONE 2021, 16, e0254058. [Google Scholar] [CrossRef]
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest China. Molecules 2009, 14, 4922–4935. [Google Scholar] [CrossRef]
- Savina, T.; Lisun, V.; Feduraev, P.; Skrypnik, L. Variation in Phenolic Compounds, Antioxidant and Antibacterial Activities of Extracts from Different Plant Organs of Meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules 2023, 28, 3512. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.Y.; Li, J.Y.; Yuan, L.; Zhang, L.L.; Wang, Z.Y.; Xu, X.; Davis, S.J.; Liu, J.X. A Competition-attenuation Mechanism Modulates Thermoresponsive Growth at Warm Temperatures in Plants. New Phytol. 2023, 237, 177–191. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Zhang, X.; Chen, M.; Wu, T.; Zhang, J.; Xing, Y.; Tian, J.; Yao, Y. ROS1 Promotes Low Temperature-induced Anthocyanin Accumulation in Apple by Demethylating the Promoter of Anthocyanin-associated Genes. Hortic. Res. 2022, 9, uhac007. [Google Scholar] [CrossRef]
- Angmo, P.; Phuntsog, N.; Namgail, D.; Chaurasia, O.P.; Stobdan, T. Effect of shading and high temperature amplitude in greenhouse on growth, photosynthesis, yield and phenolic contents of tomato (Lycopersicum esculentum Mill.). Physiol. Mol. Biol. Plants 2021, 27, 1539–1546. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef]
- Gajewska, E.; Witusińska, A.; Kornaś, A.; Wielanek, M. Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress. Int. J. Mol. Sci. 2025, 26, 1237. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.F.F.; Castro-Alves, V.; Morales, L.O.; Rosenqvist, E.; Ottosen, C.O.; Hyötyläinen, T.; Strid, Å. Metabolic Changes in Cucumber Leaves are Enhanced by Blue Light but Differentially Affected by UV Interactions with Light Signalling Pathways in the Visible Spectrum. Plant Sci. 2022, 321, 111326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liang, X.; Zhang, Y.; Dai, P.; Liang, B.; Li, J.; Sun, C.; Lin, X. Role of Sucrose in Modulating the Low-nitrogen-induced Accumulation of Phenolic Compounds in Lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2020, 100, 5412–5421. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Ortiz, V.; Gruz, J.; Casas, J.L. Changes in the Free Phenolic Acid Composition of Pepper (Capsicum annuum L.) Leaves in Response to Green Peach Aphid (Myzus persicae Sulzer) Infestation. J. Plant Interact. 2021, 16, 329–336. [Google Scholar] [CrossRef]
- Lan, H.; Lai, B.; Zhao, P.; Dong, X.; Wei, W.; Ye, Y.; Wu, Z. Cucumber Mosaic Virus Infection Modulated the Phytochemical Contents of Passiflora edulis. Microb. Pathog. 2020, 138, 103828. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Wei, R.; Cheng, G.; Zhou, Y.; Liu, J.; Xie, T.; Guo, R.; Zhou, S. Widely Targeted Metabolomics Profiling Reveals the Effect of Powdery Mildew on Wine Grape Varieties with Different Levels of Tolerance to the Disease. Foods 2022, 11, 2461. [Google Scholar] [CrossRef]
- Radl, V.; Winkler, J.B.; Kublik, S.; Yang, L.; Winkelmann, T.; Vestergaard, G.; Schröder, P.; Schloter, M. Reduced Microbial Potential for the Degradation of Phenolic Compounds in the Rhizosphere of Apple Plantlets Grown in Soils Affected by Replant Disease. Environ. Microbiome 2019, 14, 8. [Google Scholar]
- Koichi, S.; Zager, J.J.; St, A.B.; Lange, B.M.; Howe, G.A. Flavonoid deficiency disrupts redox homeostasis and terpenoid biosynthesis in glandular trichomes of tomato. Plant Physiol. 2022, 188, 1450–1468. [Google Scholar]
- Kolo, Z.; Majola, A.; Phillips, K.; Ali, A.E.E.; Sharp, R.E.; Ludidi, N. Water Deficit-Induced Changes in Phenolic Acid Content in Maize Leaves Is Associated with Altered Expression of Cinnamate 4-Hydroxylase and p-Coumaric Acid 3-Hydroxylase. Plants 2022, 12, 101. [Google Scholar] [CrossRef]
- Park, Y.J.; Kwon, D.Y.; Koo, S.Y.; Truong, T.Q.; Hong, S.C.; Choi, J.; Moon, J.; Kim, S.M. Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligularia fischeri. Front. Plant Sci. 2023, 14, 1140509. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.M.; Zhang, W.D.; Li, S.H.; Gao, Y.; Ai, X.Z.; Zhang, D.L.; Liu, B.B.; Li, Q.M. Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.J.; Yu, L.; Guo, Z.W.; Chen, Z.J.; Jiang, S.H.; Xu, H.F.; Fang, H.C.; Wang, Y.C.; Zhang, Z.Y.; et al. HEAT SHOCK FACTOR A8a Modulates Flavonoid Synthesis and Drought Tolerance. Plant Physiol. 2020, 184, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, X.; Gao, Q.; Huang, C.; Luo, Z.; Liu, P.; Wu, M.; Huang, H.; Yang, J.; Zeng, J.; et al. NtERF4 promotes the biosynthesis of chlorogenic acid and flavonoids by targeting PAL genes in Nicotiana tabacum. Planta 2023, 259, 31. [Google Scholar] [CrossRef]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants 2021, 10, 2346. [Google Scholar] [CrossRef]
- Li, H.H.; Flachowsky, H.; Fischer, T.C.; Hanke, M.-V.; Forkmann, G.; Treutter, D.; Schwab, W.; Hoffmann, T.; Szankowski, I. Maize Lc transcription factor enhances biosynthesis of anthocyanins, distinct proanthocyanidins and phenylpropanoids in apple (Malus domestica Borkh.). Planta 2007, 226, 1243–1254. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Ray, H.; Yu, M.; Auser, P.; Blahut-Beatty, L.; McKersie, B.; Bowley, S.; Westcott, N.; Coulman, B.; Lloyd, A.; Gruber, M.Y. Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol. 2003, 132, 1448–1463. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Wahab, P.E.; Halim, M.R. Effect of different light intensities on total phenolics and flavonoids synthesis and antioxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar] [CrossRef]
- Lai, C.C.; Pan, H.; Zhang, J.; Wang, Q.; Que, Q.X.; Pan, R.; Lai, Z.X.; Lai, G.T. Light Quality Modulates Growth, Triggers Differential Accumulation of Phenolic Compounds, and Changes the Total Antioxidant Capacity in the Red Callus of Vitis davidii. J. Agric. Food Chem. 2022, 70, 13264–13278. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in Achillea millefolium. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Merging Heat Stress Tolerance and Health-Promoting Properties: The Effects of Exogenous Arginine in Cauliflower (Brassica oleracea var. botrytis L.). Foods 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low Temperature Promotes Anthocyanin Biosynthesis and Related Gene Expression in the Seedlings of Purple Head Chinese Cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. Low Temperature Promotes Anthocyanin Biosynthesis but Does Not Accelerate Endogenous Abscisic Acid Accumulation in Red-Skinned Grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef]
- Xue, X.; Duan, Y.; Wang, J.; Ma, F.; Li, P. Nighttime Temperatures and Sunlight Intensities Interact to Influence Anthocyanin Biosynthesis and Photooxidative Sunburn in “Fuji” Apple. Front. Plant Sci. 2021, 12, 694954. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Ma, Y.; Wang, F.; Wang, J.; Zhang, Y.; Hu, X. The bZIP transcription factor SlAREB1 regulates anthocyanin biosynthesis in response to low temperature in tomato. Plant J. 2023, 115, 205–219. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.H.; Lin, C.Y.; Zhao, P.J.; Wang, B.; Zou, L.P.; Li, S.X.; Yu, X.L.; Chen, Y.H.; Zhang, P.; et al. Down-regulation of MeMYB2 leads to anthocyanin accumulation and increases chilling tolerance in cassava (Manihot esculenta Crantz). J. Integr. Agric. 2023, 11, 1181–1191. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Shi, Y.; Lv, S.; Zhu, C.; Xu, C.; Zhang, B.; Allan, A.C.; Grierson, D.; Chen, K. The R2R3 MYB Ruby1 is activated by two cold-responsive ethylene response factors, via the retrotransposon in its promoter, to positively regulate anthocyanin biosynthesis in citrus. Plant J. 2024, 119, 1433–1448. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Niu, Z.; Qiao, X.; Yang, B. Influence of genetic background, growth latitude and bagging treatment on phenolic compounds in fruits of commercial cultivars and wild types of apples (Malus sp.). Eur. Food Res. Technol. 2021, 247, 1149–1165. [Google Scholar] [CrossRef]
- Mao, W.; Han, Y.; Chen, Y.; Sun, M.; Feng, Q.; Li, L.; Liu, L.; Zhang, K.; Wei, L.; Han, Z.; et al. Low Temperature Inhibits Anthocyanin Accumulation in Strawberry Fruit by Activating FvMAPK3-induced Phosphorylation of FvMYB10 and Degradation of Chalcone Synthase 1. Plant Cell 2022, 34, 1226–1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Zhang, Q.; Li, B.; Zhang, M.; Qin, J.; Shi, W.; Jia, C. SlNAC12, a novel NAC-type transcription factor, confers salt stress tolerance in tomato. Plant Cell Rep. 2024, 44, 5. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wu, M.; Wang, H.; Shu, P.; Song, H.; Deng, H.; Yu, S.; Garcia-Caparros, P.; Bouzayen, M.; Zhang, Y.; et al. Bi-functional Transcription Factor SlbHLH95 Regulates Fruit Flavonoid Metabolism and Grey Mould Resistance in Tomato. Plant Biotechnol. J. 2025, 23, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, M.; Deng, C.P.; Lu, S.J.; Huang, F.F.; Wang, Y.; Kai, G.Y. The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2021, 10, 8. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Zhang, Y.; Pan, Z.; Xie, X.; Zhang, L.; Sun, P.; Feng, H.; Xue, H.; Fang, C.; et al. The R2R3-MYB transcription factor FaMYB63 participates in regulation of eugenol production in strawberry. Plant Physiol. 2022, 188, 2146–2165. [Google Scholar] [CrossRef]
- Wang, L.; Tang, X.; Zhang, S.; Xie, X.; Li, M.; Liu, Y.; Wang, S. Tea GOLDEN2-LIKE genes enhance catechin biosynthesis through activating R2R3-MYB transcription factor. Hortic. Res. 2022, 9, uhac117. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Meng, Z.; Jia, Z.; Fu, F.; Jin, B.; Cao, F.; Wang, L. The LncNAT11-MYB11-F3’H/FLS module mediates flavonol biosynthesis to regulate salt stress tolerance in Ginkgo biloba. J. Exp. Bot. 2025, 76, 1179–1201. [Google Scholar] [CrossRef]
- An, J.P.; Qu, F.J.; Yao, J.F.; Wang, X.N.; You, C.X.; Wang, X.F.; Hao, Y.J. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Hortic. Res. 2017, 4, 17023. [Google Scholar] [CrossRef]
- An, J.P.; Yao, J.F.; Xu, R.R.; You, C.X.; Wang, X.F.; Hao, Y.J. Apple bZIP transcription factor MdbZIP44 regulates abscisic acid-promoted anthocyanin accumulation. Plant Cell Environ. 2018, 41, 2678–2692. [Google Scholar] [CrossRef]
- Tu, M.; Fang, J.; Zhao, R.; Liu, X.; Yin, W.; Wang, Y.; Wang, X.; Wang, X.; Fang, Y. CRISPR/Cas9-mediated mutagenesis of VvbZIP36 promotes anthocyanin accumulation in grapevine (Vitis vinifera). Hortic. Res. 2022, 9, uhac022. [Google Scholar] [CrossRef]
- Sun, M.H.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.P.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G.Y. The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 2019, 70, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, Y.; Wang, T.; Jiang, P.; Wen, W.; Nie, J. Combined transcriptomic and metabolomic analyses identifies CsERF003, a citrus ERF transcription factor, as flavonoid activator. Plant Sci. 2023, 334, 111762. [Google Scholar] [CrossRef] [PubMed]
- Badim, H.; Vale, M.; Coelho, M.; Granell, A.; Gerós, H.; Conde, A. Constitutive expression of VviNAC17 transcription factor significantly induces the synthesis of flavonoids and other phenolics in transgenic grape berry cells. Front. Plant Sci. 2022, 13, 964621. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.L.; Wang, W.N.; Nan, Q.; Liu, J.W.; Ju, Y.L.; Fang, Y.L. VvNAC17, a grape NAC transcription factor, regulates plant response to drought-tolerance and anthocyanin synthesis. Plant Physiol. Biochem. 2025, 219, 109379. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, S.; Zhang, T.; Xu, H.; Fang, H.; Zhang, J.; Su, M.; Wang, Y.; Zhang, Z.; Wang, N.; et al. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019, 289, 110286. [Google Scholar] [CrossRef]
- Wang, R.; Liu, K.; Tang, B.; Su, D.; He, X.; Deng, H.; Wu, M.; Bouzayen, M.; Grierson, D.; Liu, M. The MADS-box protein SlTAGL1 regulates a ripening-associated SlDQD/SDH2 involved in flavonoid biosynthesis and resistance against Botrytis cinerea in post-harvest tomato fruit. Plant J. 2023, 115, 1746–1757. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, S.; Chen, B.; Bai, X.; Li, Y.; Yang, J.; Wang, W.; Li, C.; Li, Y.; Li, Z. The MADS-box transcription factor GlMADS1 regulates secondary metabolism in Ganoderma lucidum. Mycologia 2021, 113, 12–19. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Zhang, L.; Zhu, S. AcbHLH144 transcription factor negatively regulates phenolic biosynthesis to modulate pineapple internal browning. Hortic. Res. 2023, 10, uhad185. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Shi, M.; Maoz, I.; Gao, X.; Sun, M.; Yuan, T.; Li, K.; Zhou, W.; Guo, X.; et al. SmbHLH60 and SmMYC2 Antagonistically Regulate Phenolic Acids and Anthocyanins Biosynthesis in Salvia miltiorrhiza. J. Adv. Res. 2022, 42, 205–219. [Google Scholar] [CrossRef]
- Matousek, J.; Kocábek, T.; Patzak, J.; Stehlík, J.; Füssy, Z.; Krofta, K.; Heyerick, A.; Roldán-Ruiz, I.; Maloukh, L.; De Keukeleire, D. Cloning and molecular analysis of HlbZIP1 and HlbZIP2 transcription factors putatively involved in the regulation of the lupulin metabolome in hop (Humulus lupulus L.). J. Agric. Food Chem. 2010, 58, 902–912. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.H.; Fang, J.H.; Yin, W.C.; Yan, X.X.; Tu, M.X.; Liu, H.; Zhang, Z.; Li, Z.; Gao, M.; et al. VqWRKY56 interacts with VqbZIPC22 in grapevine to promote proanthocyanidin biosynthesis and increase resistance to powdery mildew. New Phytol. 2023, 237, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Ordoñez-Trejo, E.J.; Rasori, A.; Varotto, S.; Ruperti, B.; Bonghi, C. Dissecting Postharvest Chilling Injuries in Pome and Stone Fruit Through Integrated Omics. Front. Plant Sci. 2024, 14, 1272986. [Google Scholar] [CrossRef] [PubMed]
- Makowski, W.; Królicka, A.; Hinc, K.; Szopa, A.; Kubica, P.; Sroka, J.; Tokarz, B.; Tokarz, K.M. Reynoutria japonica Houtt. Transformed Hairy Root Cultures as an Effective Platform for Producing Phenolic Compounds with Strong Bactericidal Properties. Int. J. Mol. Sci. 2025, 26, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhou, Z.; Yin, Z.; Zhang, L.; Zhang, Q.; Xie, Y.; Chen, J. Effect of Different Agrobacterium rhizogenes Strains on Hairy Root Induction and Analysis of Metabolites in Physalis peruviana L. J. Plant Physiol. 2025, 305, 154431. [Google Scholar] [CrossRef]
- Mackon, E.; Jeazet Dongho Epse Mackon, G.C.; Guo, Y.; Ma, Y.; Yao, Y.; Liu, P. Development and Application of CRISPR/Cas9 to Improve Anthocyanin Pigmentation in Plants: Opportunities and Perspectives. Plant Sci. 2023, 333, 111746. [Google Scholar] [CrossRef]
- Lai, G.; Fu, P.; He, L.; Che, J.; Wang, Q.; Lai, P.; Lu, J.; Lai, C. CRISPR/Cas9-mediated CHS2 Mutation Provides a New Insight into Resveratrol Biosynthesis by Causing a Metabolic Pathway Shift from Flavonoids to Stilbenoids in Vitis davidii Cells. Hortic. Res. 2024, 12, uhae268. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, B.; Li, Q.; Hui, W.; Yang, L.; Wang, Z.; Zhang, W.; Yue, F.; Liu, N.; Li, H.; et al. CRISPR/Cas9 Mutated p-coumaroyl Shikimate 3’-hydroxylase 3 Gene in Populus tomentosa Reveals Lignin Functioning on Supporting Tree Upright. Int. J. Biol. Macromol. 2023, 253, 126762. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Constabel, C.P. CRISPR/Cas9 Disruption of MYB134 and MYB115 in Transgenic Poplar Leads to Differential Reduction of Proanthocyanidin Synthesis in Roots and Leaves. Plant Cell Physiol. 2023, 64, 1189–1203. [Google Scholar] [CrossRef]

| Classification | Species | Content Range (g/Kg Fresh Weight) | References |
|---|---|---|---|
| Anthocyanin | Strawberry | 0.15–0.60 | [143] |
| Goji | 0.24–72.86 | [144] | |
| Blueberry | 1.00–2.00 | [145] | |
| Grape | 0.43–4.99 | [146] | |
| Flavone/Flavonol | Onion | 0.10–13.59 | [147] |
| Mexican oregano | 9.01–11.37 | [148] | |
| Chamomile | 3.00–5.00 | [149] | |
| Peas | 0.98–1.45 | [147] | |
| Cranberry | 1.49 | [150] | |
| Flavanone/Flavanonol | Citrus | 0.10–6.30 | [151] |
| Tomato | 1.3–22.2 × 10−3 | [152] | |
| Flavanols | Apple | 0.032–1.66 | [153] |
| Chalcone | Ashitaba | 2.45–266.70 × 10−3 | [154] |
| safflower | 55.00 | [155] | |
| Phenolic acid | Eggplant | 3.20 × 10−3 | [156] |
| Carrot | 2.95 × 10−3 | [156] | |
| Stilbenes | Grape | 0.05–0.1 | [157] |
| Tannin | Tea | 6.00–14.00 | [158] |
| Persimmon | 0.27–1.65 | [159] |
| Classification | Stressors | Content Change (Fold/Increased) | Species | PCs | References |
|---|---|---|---|---|---|
| Abiotic stress | High temperature | 1–1.80 | Yarrow | Tannins | [177]; |
| 0–0.65 | Tomato | Flavonols | [104] | ||
| Low temperature | - | Strawberry | Anthocyanin | [112]; | |
| 1.00–2.00 | Apple | [178]; | |||
| 42.39–158.31 mg/kg | Cabbage | [179] | |||
| Salinity | ≥2.00 | Rose | Flavonoids | [177] | |
| Drought | 0.30–2.88 | Tomato | Flavonoids | [180] | |
| Metal toxicity | 2.60–5.00 | Cucumber | Epicatechin and flavone | [181] | |
| UV radiation | - | Grape | Flavonol | [104]; | |
| - | Cucumber | phenolic acids | [182] | ||
| Nutrient deficiency | 1.00–3.00 | Lettuce | Flavonoids and phenolic acids | [183] | |
| Biotic stress | Aphids | 1–2.82 | Pepper | Cinnamic acids | [184] |
| Viruses | 1.26–1.58 | Cucumber | Flavonoids | [185] | |
| Powdery Mildew | 1–17.00 | Grape | Stilbenes; flavonoids | [186] | |
| Bacteria | - | Apple | Phenolic acids | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, X. A Comprehensive Review of Phenolic Compounds in Horticultural Plants. Int. J. Mol. Sci. 2025, 26, 5767. https://doi.org/10.3390/ijms26125767
Xu L, Wang X. A Comprehensive Review of Phenolic Compounds in Horticultural Plants. International Journal of Molecular Sciences. 2025; 26(12):5767. https://doi.org/10.3390/ijms26125767
Chicago/Turabian StyleXu, Lili, and Xianpu Wang. 2025. "A Comprehensive Review of Phenolic Compounds in Horticultural Plants" International Journal of Molecular Sciences 26, no. 12: 5767. https://doi.org/10.3390/ijms26125767
APA StyleXu, L., & Wang, X. (2025). A Comprehensive Review of Phenolic Compounds in Horticultural Plants. International Journal of Molecular Sciences, 26(12), 5767. https://doi.org/10.3390/ijms26125767





