Molecular Basis of Chronic Intestinal Wall Fibrosis in Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Inflammatory Bowel Disease (IBD)
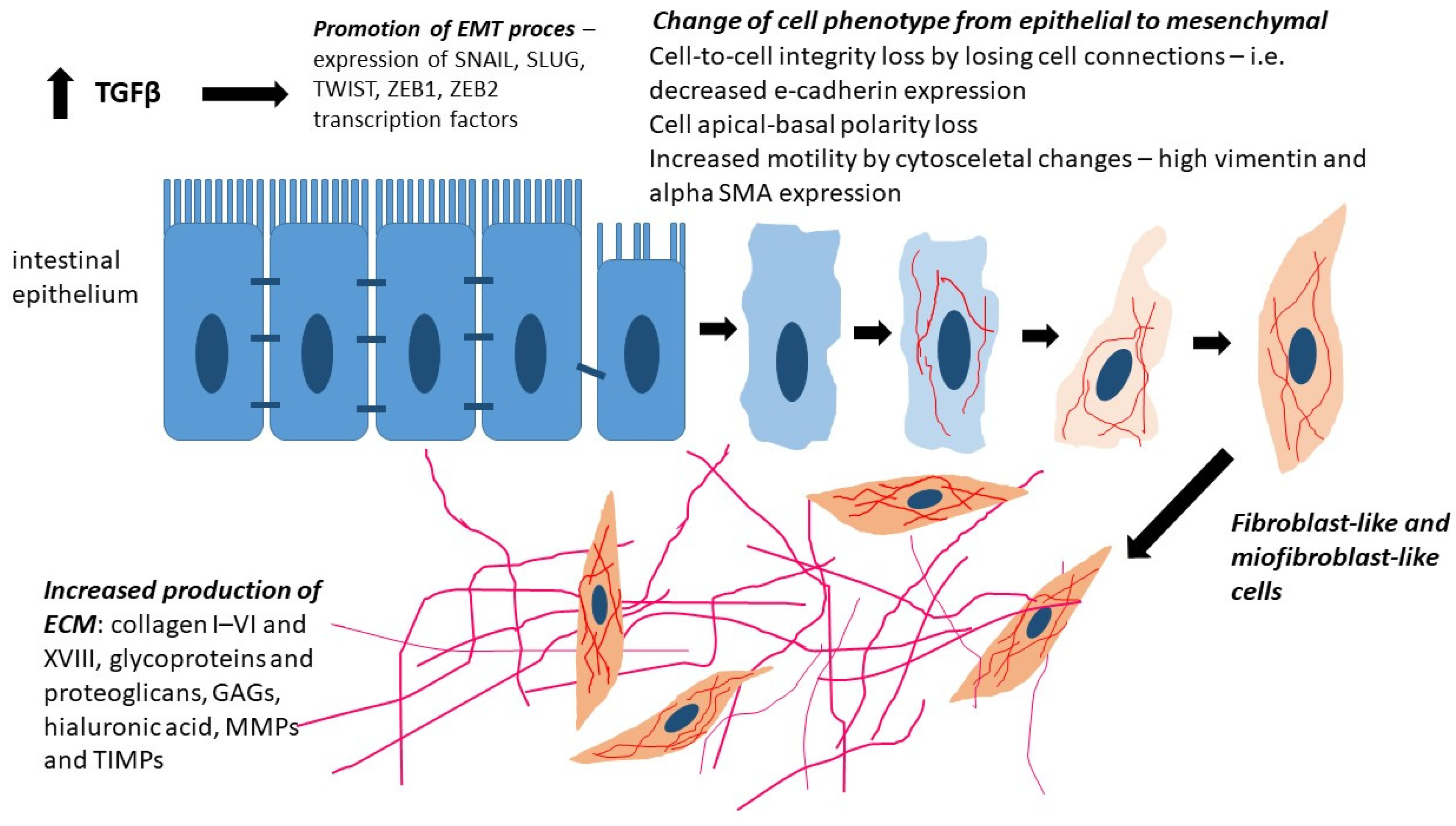
3. Epithelial–Mesenchymal Transition (EMT)
4. Type 2 EMT
5. Cytokines and Growth Factors Contributing to Intestinal Fibrosis
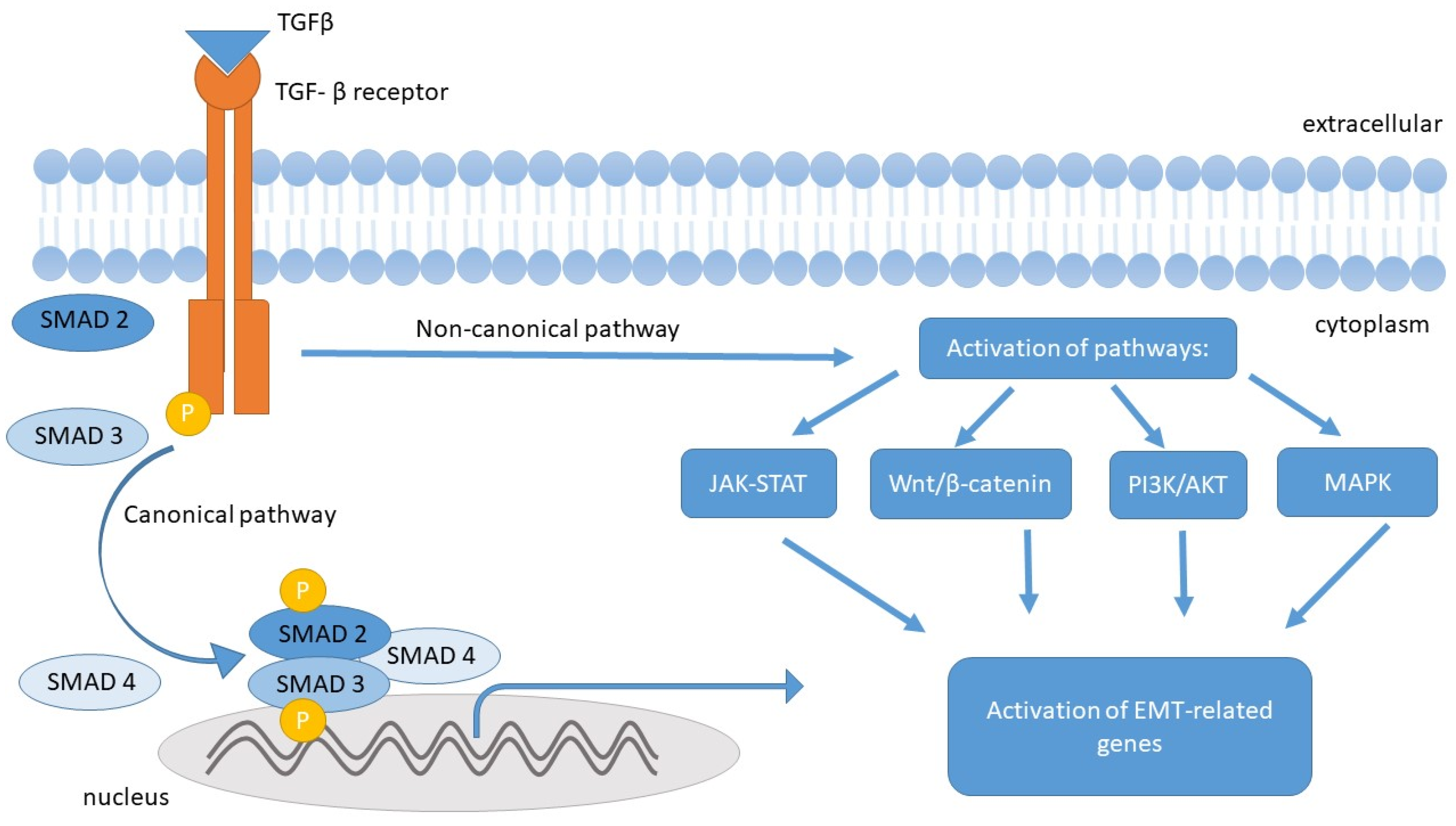
5.1. Transforming Growth Factor β (TGF-β)
5.2. Platelet-Derived Growth Factor (PDGF)
5.3. Pro-Inflammatory Interleukins IL-1 β, IL-17, and IL-33
5.4. Tumor Necrosis Factor-Alpha (TNF-α)
6. The Fibrosis Process in IBD
7. The Role of Gut Microbiota in the Pathogenesis of Intestinal Fibrosis in IBD
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| IBD-U | IBD-unclassified |
| TGF-β | transforming growth factor β |
| TNF-α | tumor necrosis factor-α |
| EMT | epithelial–mesenchymal transition |
| VEO-IBD | very early-onset IBD |
| ECM | extracellular matrix |
| MET | mesenchymal–epithelial transition |
| FSP1 | ferroptosis suppressor protein 1 |
| α-SMA | alpha-smooth muscle actin |
| TGF-α | transforming growth factor α |
| EGF | epidermal growth factor |
| FGF | fibroblast growth factor |
| PDGF | platelet-derived growth factor |
| VEGF | vascular endothelial growth factor |
| TIEMTA | the EMT International Association |
| ZO-1 | zonula occludens protein 1 |
| Zeb-1 | zinc-finger E-box binding protein 1 |
| Zeb-2 | zinc-finger E-box binding protein 2 |
| GAGs | Glycosaminoglycans |
| CTGF | connective tissue growth factor |
| ROS | reactive oxygen species |
| MAPK | Mitogen-activated protein kinase |
| JAK-STAT | Janus kinase—Signal transducer and activator of transcription signaling pathway |
| PI3K/AKT | Phosphoinositide 3/serine/threonine-specific protein kinases signaling pathway |
| SMADs | Sma- and Mad-Related Proteins |
| MMPs | matrix metalloproteinases |
| TIMPs | tissue inhibitors of metalloproteinases |
| FSP1 | fibroblast-specific protein 1 |
| TKIs | tyrosine kinase inhibitors |
| PDGFR | platelet-derived growth factor |
| ARBs | angiotensin II receptor blockers |
| EIEC | enteroinvasive Escherichia coli |
| CTGF | connective tissue growth factor |
| IGF | insulin-like growth factor |
| IL-1 | interleukin 1 |
| IL-1aIL-1bIL-1β | Interleukin 1ainterleukin 1binterleukin 1β |
| IL-2 | interleukin 2 |
| IL-4 | interleukin 4 |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-10 | interleukin 10 |
| IL-12 | interleukin 12 |
| IL-13 | interleukin 13 |
| IL-15 | interleukin 15 |
| IL-17 | interleukin 17 |
| IL-17A | interleukin 17A |
| IL-17F | interleukin 17F |
| IL-18 | interleukin 18 |
| IL-21 | interleukin 21 |
| IL-22 | interleukin 22 |
| IL-23 | interleukin 23 |
| IL-33 | interleukin 33 |
References
- Sarter, H.; Crétin, T.; Savoye, G.; Fumery, M.; Leroyer, A.; Dauchet, L.; Paupard, T.; Coevoet, H.; Wils, P.; Richard, N.; et al. EPIMAD study Group. Incidence, prevalence and clinical presentation of inflammatory bowel diseases in Northern France: A 30-year population-based study. Lancet Reg. Health Eur. 2024, 47, 101097. [Google Scholar] [CrossRef] [PubMed]
- Jarmakiewicz-Czaja, S.; Sokal, A.; Ferenc, K.; Motyka, E.; Helma, K.; Filip, R. The Role of Genetic and Epigenetic Regulation in Intestinal Fibrosis in Inflammatory Bowel Disease: A Descending Process or a Programmed Consequence? Genes 2023, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Krzesiek, E.; Kofla-Dlubacz, A.; Akutko, K.; Stawarski, A. The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. J. Clin. Med. 2021, 10, 3994. [Google Scholar] [CrossRef]
- Peña-Sánchez, J.N.; Osei, J.A.; Marques Santos, J.D.; Jennings, D.; Andkhoie, M.; Brass, C.; Bukassa-Kazadi, G.; Lu, X.; Johnson-Jennings, M.; Porter, L.; et al. Increasing Prevalence and Stable Incidence Rates of Inflammatory Bowel Disease Among First Nations: Population-Based Evidence from a Western Canadian Province. Inflamm. Bowel Dis. 2022, 28, 514–522. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD-Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Day, A.S. Crohn’s and colitis in children and adolescents. World J. Gastroenterol. 2012, 18, 5862–5869. [Google Scholar] [CrossRef]
- Nahid, K.L.; Rukunuzzaman, M.; Fathema, K. Very Early Onset Inflammatory Bowel Disease: Diagnostic and Therapeutic Challenges for Pediatric Gastroenterologists. Mymensingh Med. J. 2024, 33, 944–951. [Google Scholar]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. COLORS in IBD Study Group and NEOPICS. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e3. [Google Scholar] [CrossRef]
- Mrowicki, J.; Mrowicka, M.; Majsterek, I. Czynniki środowiskowe zwiększające ryzyko aktywacji i rozwoju chorób zapalnych jelit. Postępy Biochem. 2020, 66, 167–175. [Google Scholar] [CrossRef]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Socha-Banasiak, A.; Sputa-Grzegrzółka, P.; Grzegrzółka, J.; Pacześ, K.; Dzięgiel, P.; Sordyl, B.; Romanowicz, H.; Czkwianianc, E. Metallothioneins in Inflammatory Bowel Diseases: Importance in Pathogenesis and Potential Therapy Target. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6665697. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Raj, J.P. Role of biologics and biosimilars in inflammatory bowel disease: Current trends and future perspectives. J. Inflamm. Res. 2018, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Fina, D.; Caruso, R.; Pallone, F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2006, 22, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; De Salvo, C.; Pizarro, T.T. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr. Opin. Gastroenterol. 2014, 30, 531–538. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Colombel, J.F.; Sandborn, W.J. The natural history of adult Crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 2010, 105, 289–297. [Google Scholar] [CrossRef]
- Ishige, T. Growth failure in pediatric onset inflammatory bowel disease: Mechanisms, epidemiology, and management. Transl. Pediatr. 2019, 8, 16–22. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Sarrand, J.; Soyfoo, M.S. Involvement of Epithelial-Mesenchymal Transition (EMT) in Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 14481. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Hay, E.D. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef]
- Samavarchi-Tehrani, P.; Golipour, A.; David, L.; Sung, H.K.; Beyer, T.A.; Datti, A.; Woltjen, K.; Nagy, A.; Wrana, J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 2010, 7, 64–77. [Google Scholar] [CrossRef]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef]
- Nakaya, Y.; Sheng, G. Epithelial to mesenchymal transition during gastrulation: An embryological view. Dev. Growth Differ. 2008, 50, 755–766. [Google Scholar] [CrossRef]
- Mutsaers, H.A.M.; Merrild, C.; Nørregaard, R.; Plana-Ripoll, O. The impact of fibrotic diseases on global mortality from 1990 to 2019. J. Transl. Med. 2023, 21, 818. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-mesenchymal transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Okajčeková, T.; Strnádel, J.; Vidomanová, E.; Halašová, E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review). Int. J. Mol. Med. 2018, 41, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Moulin, V.; Castilloux, G.; Auger, F.A.; Garrel, D.; O’Connor-McCourt, M.D.; Germain, L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp. Cell Res. 1998, 238, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Yang, M.; Mao, D.; Zhu, M.; Che, Y.; Kong, D.; Li, C. Co-Transplantation of Skin-Derived Precursors and Collagen Sponge Facilitates Diabetic Wound Healing by Promoting Local Vascular Regeneration. Cell. Physiol. Biochem. 2015, 37, 1725–1737. [Google Scholar] [CrossRef]
- Xu, R.; Won, J.Y.; Kim, C.H.; Kim, D.E.; Yim, H. Roles of the Phosphorylation of Transcriptional Factors in Epithelial-Mesenchymal Transition. J. Oncol. 2019, 2019, 5810465. [Google Scholar] [CrossRef]
- Ambrozkiewicz, F.; Karczmarski, J.; Kulecka, M.; Paziewska, A.; Niemira, M.; Zeber-Lubecka, N.; Zagorowicz, E.; Kretowski, A.; Ostrowski, J. In search for interplay between stool microRNAs, microbiota and short chain fatty acids in Crohn’s disease—A preliminary study. BMC Gastroenterol. 2020, 20, 307. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Creber, K.; Hamilton, D.W. Wound healing and fibrosis: A contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 2020, 318, C1065–C1077. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.; Kim, B.G.; Lee, K.L.; Kim, D.W.; Kim, J.H.; Kim, J.W.; Kim, J.S. Matricellular Protein Periostin Mediates Intestinal Inflammation through the Activation of Nuclear Factor κB Signaling. PLoS ONE 2016, 11, e0149652. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A. Molecular Basis of Intestinal Fibrosis in Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2022, 7, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Speca, S.; Giusti, I.; Rieder, F.; Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef] [PubMed]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kuemmerle, J.F. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1250–1258. [Google Scholar] [CrossRef]
- McKaig, B.C.; Hughes, K.; Tighe, P.J.; Mahida, Y.R. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am. J. Physiol. Cell Physiol. 2002, 282, C172–C182. [Google Scholar] [CrossRef]
- Shah, M.; Foreman, D.M.; Ferguson, M.J. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [CrossRef]
- Shah, M.; Foreman, D.M.; Ferguson, M.J. Neutralising antibody to TGF-β1, 2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994, 107, 1137–1157. [Google Scholar] [CrossRef]
- Yun, S.M.; Kim, S.H.; Kim, E.H. The Molecular Mechanism of Transforming Growth Factor-β Signaling for Intestinal Fibrosis: A Mini-Review. Front. Pharmacol. 2019, 10, 162. [Google Scholar] [CrossRef]
- Ghorbaninejad, M.; Abdollahpour-Alitappeh, M.; Shahrokh, S.; Fayazzadeh, S.; Asadzadeh-Aghdaei, H.; Meyfour, A. TGF-β receptor I inhibitor may restrict the induction of EMT in inflamed intestinal epithelial cells. Exp. Biol. Med. 2023, 248, 665–676. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- McKaig, B.C.; McWilliams, D.; Watson, S.A.; Mahida, Y.R. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am. J. Pathol. 2003, 162, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Leeb, S.N.; Vogl, D.; Grossmann, J.; Falk, W.; Schölmerich, J.; Rogler, G.; Gelbmann, C.M. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am. J. Gastroenterol. 2004, 99, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.S.; Murthy, K.S.; Grider, J.R.; Kellum, J.M.; Kuemmerle, J.F. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn’s disease. Gastroenterology 2010, 138, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Schuppan, D.; Riese, H.H.; Matthes, H.; Riecken, E.O. Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease. Gastroenterology 1992, 102, 1920–1929. [Google Scholar] [CrossRef]
- di Mola, F.F.; Friess, H.; Scheuren, A.; Di Sebastiano, P.; Graber, H.; Egger, B.; Zimmermann, A.; Korc, M.; Büchler, M.W. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn’s disease. Ann. Surg. 1999, 229, 67–75. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Maxwell, L.; Doe, W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm. Bowel Dis. 2001, 7, 16–26. [Google Scholar] [CrossRef]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Hata, K.; Andoh, A.; Shimada, M.; Fujino, S.; Bamba, S.; Araki, Y.; Okuno, T.; Fujiyama, Y.; Bamba, T. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, 1035–1044. [Google Scholar] [CrossRef]
- Sponheim, J.; Pollheimer, J.; Olsen, T.; Balogh, J.; Hammarström, C.; Loos, T.; Kasprzycka, M.; Sørensen, D.R.; Nilsen, H.R.; Küchler, A.M.; et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 2010, 177, 2804–2815. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Duk Ye, B.; Cross, R.K.; Danese, S.; D’Haens, G.; Jung, J. The position of anti-Tumor Necrosis Factor agents for the treatment of adult patients with Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stidham, R.W.; Lee, T.C.; Higgins, P.D.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Elmunzer, B.J.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic review with network meta-analysis: The efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sandborn, W.J.; Khan, K.J.; Hanauer, S.B.; Talley, N.J.; Moayyedi, P. Efficacy of biological therapies in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 644–659. [Google Scholar] [CrossRef]
- Wong, U.; Cross, R.K. Primary and secondary nonresponse to infliximab: Mechanisms and countermeasures. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1039–1046. [Google Scholar] [CrossRef]
- Manrai, M.; Jha, A.A.; Dawra, S.; Pachisia, A.V. Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future. Future Pharmacol. 2024, 4, 279–316. [Google Scholar] [CrossRef]
- Tavares de Sousa, H.; Magro, F. How to Evaluate Fibrosis in IBD? Diagnostics 2023, 13, 2188. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef]
- D’Haens, G.; Rieder, F.; Feagan, B.G.; Higgins, P.D.R.; Panés, J.; Maaser, C.; Rogler, G.; Löwenberg, M.; van der Voort, R.; Pinzani, M.; et al. Challenges in the Pathophysiology, Diagnosis, and Management of Intestinal Fibrosis in Inflammatory Bowel Disease. Gastroenterology 2022, 162, 26–31. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar] [CrossRef]
- Goulston, S.J.M.; McGovern, V.J. The Nature of Benign Strictures in Ulcerative Colitis. N. Engl. J. Med. 1969, 281, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Achrafie, L.; Kodjamanova, P.; Tencer, T.; Kumar, J. Endoscopic mucosal healing and histologic remission in ulcerative colitis: A systematic literature review of clinical, quality-of-life and economic outcomes. Curr. Med. Res. Opin. 2022, 38, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Sousa, H.T. Editorial: Ulcerative colitis submucosal fibrosis and inflammation: More than just strictures. Aliment. Pharmacol. Ther. 2018, 47, 1033–1034. [Google Scholar] [CrossRef]
- Andoh, A.; Bamba, S.; Brittan, M.; Fujiyama, Y.; Wright, N.A. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol. Ther. 2007, 114, 94–106. [Google Scholar] [CrossRef]
- Greenstein, A.J.; Lachman, P.; Sachar, D.B.; Springhorn, J.; Heimann, T.; Janowitz, H.D.; Aufses, A.H., Jr. Perforating and non-perforating indications for repeated operations in Crohn’s disease: Evidence for two clinical forms. Gut 1988, 29, 588–592. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef]
- Louis, E.; Collard, A.; Oger, A.F.; Degroote, E.; El Yafi, F.A.A.N.; Belaiche, J. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef]
- Yoo, J.H.; Holubar, S.; Rieder, F. Fibrostenotic strictures in Crohn’s disease. Intest. Res. 2020, 18, 379–401. [Google Scholar] [CrossRef]
- Rimola, J.; Capozzi, N. Differentiation of fibrotic and inflammatory component of Crohn’s disease-associated strictures. Intest. Res. 2020, 18, 144–150. [Google Scholar] [CrossRef]
- Laudadio, I.; Carissimi, C.; Scafa, N.; Bastianelli, A.; Fulci, V.; Renzini, A.; Russo, G.; Oliva, S.; Vitali, R.; Palone, F.; et al. Characterization of patient-derived intestinal organoids for modelling fibrosis in Inflammatory Bowel Disease. Inflamm. Res. 2024, 73, 1359–1370. [Google Scholar] [CrossRef]
- Solitano, V.; Dal Buono, A.; Gabbiadini, R.; Wozny, M.; Repici, A.; Spinelli, A.; Vetrano, S.; Armuzzi, A. Fibro-Stenosing Crohn’s Disease: What Is New and What Is Next? J. Clin. Med. 2023, 22, 3052. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Fujii, T.; Okamoto, R.; Yamada, A.; Kunisaki, R.; Matsuura, M. Characteristics of adult patients newly diagnosed with Crohn’s disease: Interim analysis of the nation-wide inception cohort registry study of patients with Crohn’s disease in Japan (iCREST-CD). J. Gastroenterol. 2022, 57, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, X.; Tan, J.; Liu, Z.; He, J.; Hu, F.; Wang, Y.; Chen, M.; Liu, F.; Mao, R. Cellular and Molecular Mechanisms of Intestinal Fibrosis. Gut Liver 2023, 15, 360–374. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakase, H. The molecular mechanisms of intestinal inflammation and fibrosis in Crohn’s Disease. Front. Physiol. 2022, 13, 845078. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Brown, J.M.; van Wagoner, D.; Fiocchi, C.; Rieder, F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol. Rev. 2021, 302, 211–227. [Google Scholar] [CrossRef]
- Lenti, M.V.; Santacroce, G.; Broglio, G.; Rossi, C.M.; Di Sabatino, A. Recent advances in intestinal fibrosis. Mol. Aspects Med. 2024, 96, 101251. [Google Scholar] [CrossRef]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef]
- Lovisa, S.; Genovese, G.; Danese, S. Role of epithelial-to-mesenchymal transition in inflammatory Bowel disease. J. Crohn’s Colitis 2019, 13, 659–668. [Google Scholar] [CrossRef]
- Sandbo, N.; Mantle, S.; McKean, D.; Wermers, J.D.; Bonnes, S.L.; Jensen, M.D.; Tchkonia, T.; Kirkland, J.L. The Role of Interleukin-33 in Organ Fibrosis. Discov. Immunol. 2022, 1, kyac006. [Google Scholar] [CrossRef]
- Nijhuis, A.; Curciarello, R.; Mehta, S.; Feakins, R.; Bloom, S.; Andrews, C.; Travis, S.P.; Maher, J.J.; Mortensen, N.J.; Orchard, T.; et al. MCL-1 Is Modulated in Crohn’s Disease Fibrosis by miR-29b via IL-6 and IL-8. Cell Tissue Res. 2017, 368, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Wei, X.; Chen, J.; Wang, R.; Wang, Y.; Fan, X.; Zhou, J. MicroRNA Signatures in Digestive Pathologies: Diagnostic and Functional Roles of miR-21, miR-155 and Others in IBD. J. Clin. Med. 2022, 14, 2054. [Google Scholar] [CrossRef]
- Scholten, D.; Hausmann, M.; Wu, X.; Buning, C.; Büning, J.; Giese, T.; Büttner, C.; Kucharzik, T.; Schreiber, S.; Stange, E.F.; et al. Intestinal Fibrosis in Inflammatory Bowel Disease and the Role of MMPs/TIMPs. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in IBD—A dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 228–235. [Google Scholar] [CrossRef]
- Rieder, F.; Latella, G.; Magro, F.; Armuzzi, A.; Papa, A.; Rogler, G.; Šeruga, B.; Louis, E.; Feakins, R.; de Bruyn, J.R.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Ciccocioppo, R.; Luinetti, O.; Ricevuti, L.; Cazzola, P.; Bianchi, C.G.; Corazza, G.R. Role of IL-33 and miRNAs in intestinal fibrosis. Clin. Exp. Immunol. 2016, 185, 87–95. [Google Scholar] [CrossRef][Green Version]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, a TNF-α inhibitor, reduces inflammatory activity in Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Rieder, F.; Rogler, G. Pathogenesis and clinical consequences of intestinal fibrosis in IBD. Curr. Opin. Gastroenterol. 2020, 36, 265–272. [Google Scholar] [CrossRef]
- Biancheri, P.; Di Sabatino, A.; Corazza, G.R. Transforming growth factor-β signaling in intestinal fibrosis. Front. Med. 2017, 4, 60. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Pan, K.; Wei, H. Mechanisms and therapeutic research progress in intestinal fibrosis. Front. Med. 2024, 11, 1368977. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor-β in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.E.; Lasky, J.A. Imatinib treatment for fibrosis: Mechanistic and clinical perspectives. Chest 2011, 140, 1395–1402. [Google Scholar] [CrossRef]
- Kagami, S.; Border, W.A.; Miller, D.E.; Noble, N.A. Angiotensin II stimulates extracellular matrix protein synthesis via TGF-β in glomerular cells. Am. J. Pathol. 1994, 145, 207–217. [Google Scholar]
- Vermeire, S.; Loftus, E.V.; Colombel, J.F.; Feagan, B.G.; Sandborn, W.J.; Sands, B.E.; Danese, S.; Panaccione, R.; D’Haens, G.; Schreiber, S.; et al. Long-term safety of vedolizumab for ulcerative colitis and Crohn’s disease. Aliment. Pharmacol. Ther. 2019, 50, 587–594. [Google Scholar] [CrossRef]
- Medina, C.; Radomski, M.W. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006, 318, 933–938. [Google Scholar] [CrossRef]
- Wollin, L.; Distler, J.H.W.; Redente, E.F.; Riches, D.W.H.; Stowasser, S.; Schlenker-Herceg, R.; Maher, T.M.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Chau, B.N.; Xin, C.; Hartner, J.; Ren, S.; Castano, A.P.; Linn, G.; Li, J.; Tran, P.T.; Kaimal, V.; Huang, X.; et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012, 4, 121ra18. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Anderson, P.; Gonzalez, M.A.; Rico, L.; Büscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Gutiérrez Casbas, A.; Yilmaz, B.; Francés, R. Dysbiotic Microbiota Interactions in Crohn’s Disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef]
- Yilmaz, B.; Juillerat, P.; Øyås, Ø.; Ramon, C.; Bravo, F.D.; Franc, Y.; Fournier, N.; Michetti, P.; Mueller, C.; Geuking, M.B.; et al. Microbial Network Disturbances in Relapsing Refractory Crohn’s Disease. Nat. Med. 2019, 25, 323–336. [Google Scholar] [CrossRef]
- Imai, J.; Kitamoto, S.; Sugihara, K.; Nagao-Kitamoto, H.; Kuffa, P.; Hill, D.A.; Kamada, N.; Núñez, G. Flagellin-Mediated Activation of IL-33-ST2 Signaling by a Pathobiont Promotes Intestinal Fibrosis. Mucosal Immunol. 2019, 12, 632–643. [Google Scholar] [CrossRef]
- Grassl, G.A.; Valdez, Y.; Bergstrom, K.S.B.; Vallance, B.A.; Finlay, B.B. Chronic Enteric Salmonella Infection in Mice Leads to Severe and Persistent Intestinal Fibrosis. Gastroenterology 2008, 134, 768–780.e2. [Google Scholar] [CrossRef]
| Characteristic | Crohn’s Disease | Ulcerative Colitis |
|---|---|---|
| Site of lesions | Segmental changes throughout the gastrointestinal tract, most commonly at the ileocecal junction. | Continuous changes localized in the rectum and extending proximally towards the colon. |
| Extent of affected structures in the intestinal wall | Changes begin in the mucosa and gradually involve all layers of the intestinal wall. | Changes only in the mucosa and submucosa of the intestine. |
| Clinical features | Abdominal pain (usually in the right lower quadrant) and bloody diarrhea | Rectal bleeding, rectal urgency, and a feeling of incomplete bowel movement. |
| Intestinal complications | Fistulas, abscesses, strictures, perforations, obstruction, cachexia, and malabsorption syndrome. | Gastrointestinal bleeding, toxic megacolon, and increased risk of malignancy. |
| Immunological basis | Stimulation of Th1 cells is promoted by IL-12, IL-15, IL-18, IL-21, and IL-23. | Excessive secretion of IL-4, which stimulates CD4+ lymphocytes to differentiate towards Th2 and leads to an increased production of IL-13. |
| Type of Study | Key Findings | Conclusions on Intestinal Fibrosis | Reference |
|---|---|---|---|
| In vitro studies on human intestinal fibroblasts | Overexpression of TGF-β1/β2, decreased TGF-β3; increased proliferation and type I collagen production | TGF-β1 and TGF-β2 promote fibrosis; TGF-β3 may have anti-fibrotic effects | McKaig et al. [45]., Flynn et al. [53] |
| In vitro: fibroblasts exposed to PDGF | Dose-dependent effect on type III collagen production | PDGF can either stimulate or limit fibrosis depending on context | Stallmach et al. [54], Lawrance et al. [56] |
| In vitro: IL-17A stimulation of fibroblasts | Increased collagen production and TGF-β1-dependent EMT induction | IL-17A enhances EMT and fibrotic responses | Hata et al. [58] |
| In vivo: resected bowel segments from CD patients (strictured vs. non-strictured areas) | Higher expression of TGF-β1 and TGF-β3 in fibroblasts, smooth muscle cells, and myofibroblasts | Confirms in vivo role of TGF-β1 in promoting fibrosis | Letterio et al. [43]., Li et al. [44] |
| Animal model (cutaneous wounds in rats) | TGF-β1/2 promote scarring; TGF-β3 reduces fibrosis | Different TGF-β isoforms have opposing roles—therapeutic potential of TGF-β3 | Shah et al. [46] |
| In vivo: human IBD biopsies | Upregulation of IL-33 in UC lesions, absent in CD | IL-33 may serve as a biomarker distinguishing fibrotic phenotypes in IBD | Sponheim et al. [59] |
| Clinical observations in CD patients | Most patients develop strictures or fistulas within 40 years of disease | Chronic inflammation drives irreversible fibrosis | Cosnes et al. [76]., Li et al. [44] |
| In vivo: analysis of SMAD2/3/4 and α-SMA expression | TGF-β activates SMAD and PI3K/AKT pathways; increased α-SMA and type I collagen | TGF-β promotes EMT and myofibroblast proliferation | Yun et al. [48], Ghorbaninejad et al. [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sputa-Grzegrzolka, P.; Socha-Banasiak, A.; Dziegiel, P.; Kempisty, B. Molecular Basis of Chronic Intestinal Wall Fibrosis in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2025, 26, 5754. https://doi.org/10.3390/ijms26125754
Sputa-Grzegrzolka P, Socha-Banasiak A, Dziegiel P, Kempisty B. Molecular Basis of Chronic Intestinal Wall Fibrosis in Inflammatory Bowel Diseases. International Journal of Molecular Sciences. 2025; 26(12):5754. https://doi.org/10.3390/ijms26125754
Chicago/Turabian StyleSputa-Grzegrzolka, Patrycja, Anna Socha-Banasiak, Piotr Dziegiel, and Bartosz Kempisty. 2025. "Molecular Basis of Chronic Intestinal Wall Fibrosis in Inflammatory Bowel Diseases" International Journal of Molecular Sciences 26, no. 12: 5754. https://doi.org/10.3390/ijms26125754
APA StyleSputa-Grzegrzolka, P., Socha-Banasiak, A., Dziegiel, P., & Kempisty, B. (2025). Molecular Basis of Chronic Intestinal Wall Fibrosis in Inflammatory Bowel Diseases. International Journal of Molecular Sciences, 26(12), 5754. https://doi.org/10.3390/ijms26125754





