Abstract
HSD3B1 encodes an enzyme that catalyzes the conversion of adrenal precursors into potent sex steroids. A common germline variant (c.1100C) enhances this effect and is linked to breast cancer (BC) progression. As the HSD3B1 genotypes contribute to differences in local and adrenal steroid production, their transcriptional and phenotypic effects on cancers influenced by hormonal signaling such as BC and endometrial cancer (EC)—particularly in relation to menopausal status—remain unclear. We analyzed BC and EC sequenced from patients that received diagnostic tests in oncology clinics, and we determined the germline HSD3B1 c.1100 genotype (AA, AC, CC) from tumor DNA sequencing by using variant allele frequency, with inferred menopausal status assumed by age at molecular profiling. Whole-transcriptome RNA sequencing and gene set enrichment analysis showed that adrenal-permissive homozygous (CC) tumors in premenopausal ER + BC were enriched for hormone-related pathways, including Estrogen Response Early (NES ≈ +1.8). In premenopausal triple-negative BC, adrenal-restrictive homozygous (AA) tumors exhibited the elevated expression of immune and epithelial genes and the increased prevalence of MED12 alterations (AA 0.25% vs. CC 8%, p < 0.01). In endometrioid EC, CC tumors demonstrated the suppression of immune and proliferative pathways. Postmenopausal cases had higher progesterone receptor IHC positivity (AA 75% vs. CC 83%, p < 0.05) and numerically more frequent ESR1 copy number gains (AA 2.0% vs. CC 4.0%). Results highlight context-specific associations between germline HSD3B1 genotypes and tumor biology in BC and EC.
1. Introduction
The subsets of breast and endometrial cancers express the estrogen receptor (ER) and can be driven by receptor signaling activity. Therefore, studying genes and pathways that regulate estrogen or progesterone receptor-associated pathways may provide information regarding the mechanisms of disease pathogenesis. The majority of these malignancies fall under the estrogen-driven category, where carcinogenesis is primarily driven by pathways influenced by estrogen and progesterone receptors signaling. In premenopausal women, the primary source of estrogen is derived from ovarian production, with a smaller contribution from extragonadal sources. In postmenopausal women, the predominant source of estrogen shifts to extragonadal conversion, through the peripheral conversion of adrenal-derived androgens into estrone and estradiol by aromatase, mainly in adipose tissue. Recent evidence has implicated the enzyme 3β-hydroxysteroid dehydrogenase-1 (3βHSD1), encoded by the hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 gene (HSD3B1), in the synthesis of androgens from adrenal precursors [1]. Circulating dehydroepiandrosterone (DHEA) is a steroid primarily produced by the adrenal glands. 3βHSD1 acts as a rate-limiting step for the conversion of DHEA into androgens and then estrogens [2]. A germline polymorphism in the HSD3B1 gene (c.1100C) yields two protein products of 3βHSD1. The adrenal-restrictive HSD3B1 genotype (AA) remains a rate-limiting step for sex hormone production. In contrast, the adrenal permissive HSD3B1 genotype (CC) yields a stabilized protein product [3], which in turn enhances this DHEA conversion to bolster the local levels of potent androgens and estrogens [4]. Importantly, in multiple clinical studies of prostate cancer patients, the HSD3B1 CC genotype predicts poor responses to hormone therapies and increased prostate cancer-specific mortality [2,5,6].
Estrogen signaling through the ER promotes the growth and progression of subsets of breast and endometrial cancers [7]. As the ER signaling is a primary therapeutic axis, the processes that regulate estrogen production and the ligand activation of the ER also have important implications in disease progression. In hormone receptor-positive breast cancers (BC) and endometrial cancers (EC), premenopausal and postmenopausal women exhibit distinct hormonal and biological environments that may influence clinical outcomes and treatment responses. One key distinction is the shift of the major estrogen source following menopause. The HSD3B1 genotype, which regulates adrenal-derived androgen production and its subsequent peripheral conversion to estrogen, may have particular relevance in postmenopausal settings. Related to this study, the adrenal-permissive genotype (CC) of HSD3B1 has been preliminarily associated with poor outcomes in hormone receptor-positive BC and EC. For example, ER-positive (ER+) BC harboring the CC genotype has been shown to exhibit a greater propensity for distant metastases in postmenopausal women compared to those with the AA genotype [8]. These hormonal distinctions underscore the biological rationale for stratifying analyses by menopausal status and highlight the need to explore genotype-driven differences. Conversely, recent studies have shown that BCs and ECs harboring the adrenal-restrictive AA HSD3B1 genotype are instead enriched in basal-like BC, as well as ECs that harbor high copy number amplifications (CNAs) and/or non-endometrioid subtypes [9]. While these findings support a role for germline HSD3B1 at the population level, the functional consequences of germline HSD3B1 genotypes in human tumors remain largely uncharacterized in the literature, with limited mechanistic insights into tumor-specific effects.
Our hypothesis is that HSD3B1 genotypes could correlate with distinct tumor characteristics and clinical outcomes in pre- and postmenopausal BC and EC subtypes. Further, these genotypes may be differentially relevant in the setting of pre- and postmenopausal cancers, in which patients may have distinct sources and levels of circulating hormones. To comprehensively evaluate the role of HSD3B1 genotypes, our study integrates germline genotype inference with transcriptomic and genomic profiling to analyze its influence on tumor biology and clinical outcomes in BC and EC, stratified by inferred menopausal status and cancer subtype.
2. Results
In this study, we classified samples based on the HSD3B1 genotype as either adrenal-permissive or -restrictive, using our recently developed approach to infer germline HSD3B1 status using the variant allele frequency (VAF) obtained from tumor DNA sequencing [10]. Distinct from prior studies, our access to paired whole exomes and transcriptomes of these samples permitted the examination of the interactions of germline genotypes with the somatic tumoral features, serving as the largest cohort used to study HSD3B1 in BC and EC to date. Our study demonstrates that HSD3B1 genotypes distinctly impact tumor transcriptional profiles, genomic alterations, and the expression of immune and non-immune pathways across BC and EC subtypes, in both pre- and postmenopausal tumors.
2.1. HSD3B1 Genotypes and Demographics Data
We examined tumoral characteristics and clinical outcomes among pre- and postmenopausal patients with BC (n = 1628 and 3457, respectively), stratified into ER+/HER2 negative (HER2-) (n = 3333) and TNBC (n = 1752) subtypes across HSD3B1 genotypes. Similarly, for EC, we analyzed pre- and postmenopausal patients (n = 771 and 5000, respectively), further categorized into endometrioid (n = 3303) and non-endometrioid subtypes (serous, clear cell, and carcinosarcoma; n = 2468). Patient demographics and clinical characteristics stratified by the HSD3B1 genotype and menopausal status for BC and EC are summarized in Table 1 and Table 2, respectively. The prevalence of the CC genotype ranged from 7.5% to 9.4% across BC subtypes based on the menopausal status. In EC, the CC genotype prevalence varied from 3.45% to 6.11% across subtypes, though interpretation is limited by small sample sizes in non-endometrioid EC.

Table 1.
Baseline characteristics of breast cancer cohort (n = 5085), including HSD3B1 genotype, race, inferred menopausal status, molecular subtype, and TMB/MSI status.

Table 2.
Baseline characteristics of endometrial cancer cohort (n = 5771), including HSD3B1 genotype, race, inferred menopausal status, molecular subtype, estrogen receptor status, and TMB/MSI status.
2.2. HSD3B1 Variants Have Limited Impact on Survival Outcomes in BC and EC
We evaluated the overall survival (OS) across ER + BC, triple-negative BC (TNBC), and endometrioid EC, stratified by the HSD3B1 genotype (AA vs. AC vs. CC) and inferred menopausal status. The median OS (in months) was compared using the Kaplan–Meier analysis. Survival curves for non-endometrioid EC could not be generated due to an insufficient sample size.
The comparisons of OS based on tumors stratified by HSD3B1 genotypes were not statistically significant in any of the analyses (Figure 1). In ER + BC, the median OS ranged from 30.5 to 96.2 months, with survival rates appearing similar across pre- and postmenopausal patients. Among the premenopausal cohort, the CC and AA survival curves trended lower than AC up to approximately 75–100 months, after which curves overlapped. However, interpretation beyond this point is limited by small sample sizes (single-digit patient numbers). Notably, the wide confidence interval for the CC group (30.5–96.2 months) reflects variability and uncertainty, likely due to the smaller sample size (n = 86 for CC). In postmenopausal women with the CC genotype, our data demonstrated overlapping survival curves and confidence intervals, suggesting no conclusive survival differences between HSD3B1 genotypes in this cohort (p > 0.05) (Figure 1). This may reflect the tumor heterogeneity and the advanced/metastatic nature of the ER + BC population represented in the dataset, when the molecular testing is typically pursued. In TNBC, the median OS ranged from 13.3 to 38.1 months. Survival rates were relatively lower in premenopausal patients compared to postmenopausal women. Although emerging evidence suggests that the AA genotype may be associated with poorer prognosis in hormone-independent BC [9], no statistically significant differences in OS were observed across HSD3B1 genotypes in our TNBC cohort (Figure 1).
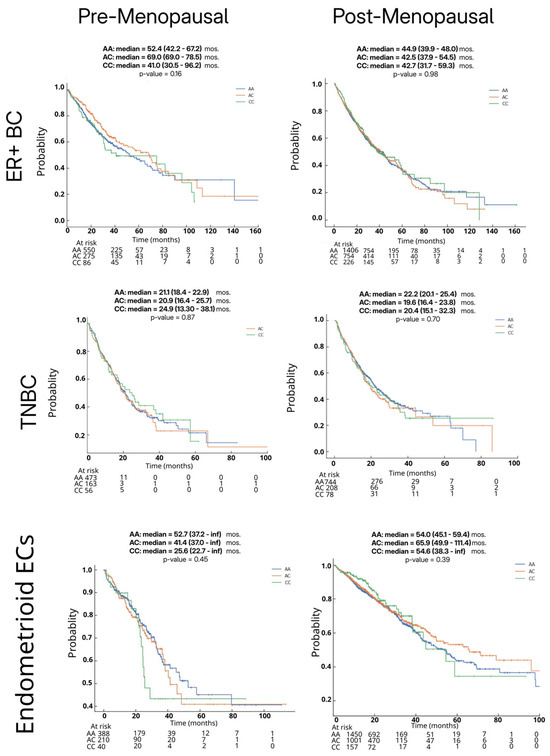
Figure 1.
Survival outcomes by germline HSD3B1 genotype across pre- and postmenopausal ER + BC, TNBC, and endometrioid EC patients. Kaplan–Meier overall survival curves are shown for AA, AC, and CC genotypes in each cohort. Statistical significance was assessed using Cox proportional hazards regression analysis.
As a sufficient number of each HSD3B1 genotype was only found in the 3303 ECs annotated as the endometrioid subtype (Table 2), the subsequent analyses of ECs focused on examining these tumors. In endometrioid ECs, there was a non-significant trend toward the worse median OS in premenopausal patients with the CC genotype compared to those with AC or AA genotypes (Figure 1). Among postmenopausal patients, no statistically significant survival differences were noted between genotypes. Overall, in this analysis across ER + BC, TNBC, and endometrioid EC, we found no statistically significant association between HSD3B1 genotypes and OS. These findings suggest that additional clinical or molecular factors may influence survival outcomes in patients that received multiple lines of therapies.
2.3. HSD3B1 Genotypes Exhibit Different Distributions by Race
When examining patients by self-reported race, White patients had the highest proportion of the adrenal-permissive CC genotype across cancer types: approximately 10% in ER + BC, 11% in TNBC, and 7% in endometrioid EC (Figure 2A). Prior studies using aggregate global allele frequencies from dbGaP have indicated that, in a non-cancerous general population, the CC genotype frequencies are approximately 10%, 1%, and 1% for Whites, African Americans, and Asian Americans, respectively [11]. We observed CC genotype frequencies among White patients (7–11.3%) mirrored the non-cancerous general population-based expectations (Figure 2A). In contrast, CC genotype frequencies among Black and Asian/PI patients were more variable, particularly when further stratified by inferred menopausal status (Figure 2B,C). Most strikingly, the CC genotype was observed in 14.6% of Asian/PI premenopausal ER+ breast cancer patients (Figure 2B), higher than the frequency among overall Asian cohort (5.9%) (see Supplementary Table S1). This trend was also observed for premenopausal Asian patients in TNBCs (6.1% vs. 2.1%). Furthermore, significant differences in the HSD3B1 genotype distribution were found when comparing Asian/PI and White patients in premenopausal ER + BC (p = 0.039) and postmenopausal TNBC (p = 0.001) (See Supplementary Table S2). These findings suggest a potential association between genotype variation and tumor predisposition or progression among Asian/PI patients. However, similar enrichment was not observed in Asian postmenopausal ER + BC or EC (Figure 2C). Some results may be skewed by the small sample size, particularly among Asian participants (Table 1), and warrant confirmation in future studies. Overall, these racial differences in the HSD3B1 genotype distribution may have important clinical implications, where 3βHSD1-mediated adrenal androgen metabolism could influence tumor biology, disease progression, and treatment outcomes.
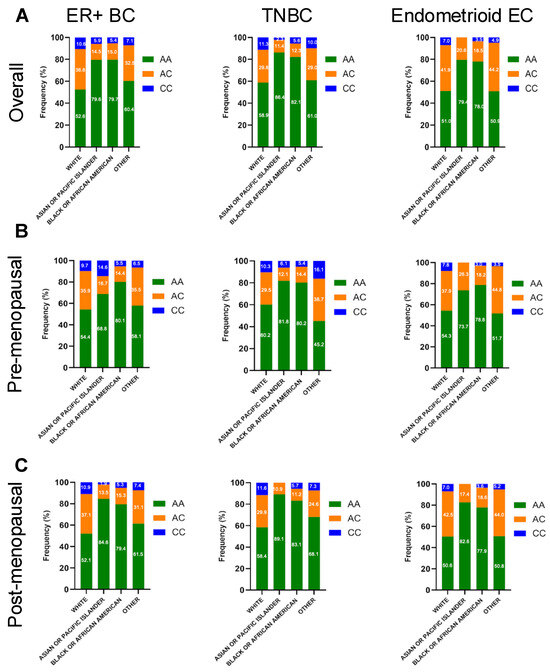
Figure 2.
Frequency of genomic alterations in germline HSD3B1 AA vs. CC tumors distributed by race across pre- and postmenopausal ER + BC, TNBC, and endometrioid EC cases. (A) Distribution across overall population, (B) Distribution across pre-menopausal population, (C) Distribution across post-menopausal population. Bar plots display percentage of tumors with specific alterations across genotype. See more details in Supplementary Tables S1 and S2.
2.4. HSD3B1 Variants Are Associated with Somatic Alterations in BC and EC
To assess whether the HSD3B1 c.1100 genotype is associated with specific somatic alterations, we compared the frequency of key genomic mutations, CNAs, and gene fusions between AA and CC across BC and EC, stratified by inferred menopausal status. Tumors with the heterozygous AC genotype were excluded to focus on genotype extremes (i.e., to compare the two homozygous groups). In ER + BC, significant differences in genomic alterations were observed across HSD3B1 homozygous genotypes in each of the cancer subtypes (Figure 3 and Supplementary Table S3). Further, the differential rates of alterations were dependent on the inferred menopausal status of the patients. Among premenopausal cases, ER + BC that harbored the CC genotype exhibited the elevated rates of CTCF and HOXB13 alterations (p < 0.05 and p < 0.01, respectively), as well as CNAs in H3F3B, JAK3, and XPC (all p < 0.05; ** p < 0.001 for H3F3B and XPC) (Figure 3A and Supplementary Table S3). In postmenopausal ER + BC, the CC genotype more frequently harbored alterations in APC (p < 0.01), CNAs in FGFR3, FOXA1, and MAP2K2, and fusion events involving PDCDC1 (the gene encoding PD-1) and BRAF (all p < 0.05 to ** p < 0.001) (Figure 3A). These findings indicate that the inferred menopausal status and the HSD3B1 genotypes are factors that can lead to distinct somatic features, suggestive of the tumors progressing through divergent pathways.
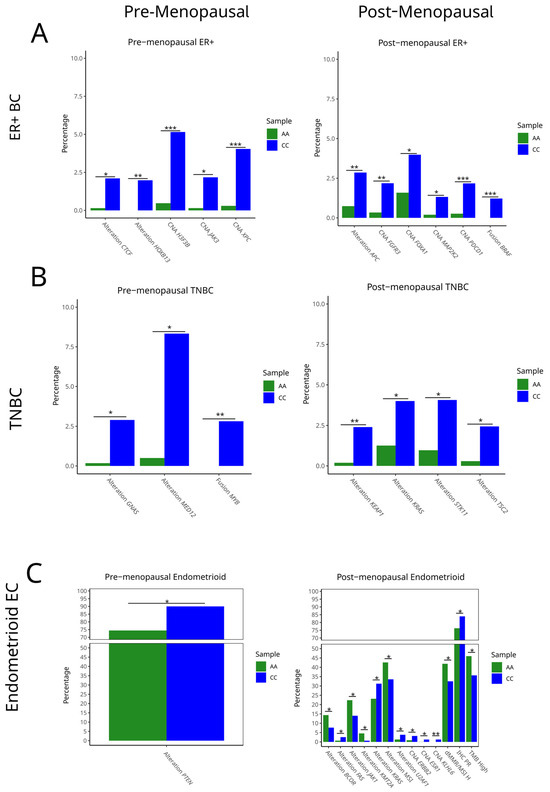
Figure 3.
Genomic alterations by germline HSD3B1 genotype across (A) pre- and post-menopausal ER + BC, (B) pre- and post-menopausal TNBC, and (C) pre- and post-menopausal endometrioid EC cases. Bar plots display frequency of select pathogenic mutations, copy number amplifications (CNAs), and gene fusions in adrenal-permissive (CC) and adrenal-restrictive (AA) tumors, stratified by the cancer type and inferred menopausal status. Tumors with the heterozygous AC genotype were excluded. Differences in alteration frequencies were evaluated using Fisher’s exact test (* p < 0.05, ** p < 0.01, *** p < 0.001). See Supplementary Table S3 for further details.
In TNBC, genotype-associated differences were also evident. Among premenopausal tumors, CC cases showed significantly higher rates of GNAS and MED12 alterations (p < 0.05 and p < 0.01), as well as MYB fusions (p < 0.01). In postmenopausal TNBC, CC tumors demonstrated increased mutations in KEAP1, KRAS, STK11, and TSC2 (p < 0.05 to * p < 0.01) (Figure 3B and Supplementary Table S3). As these genes are involved in oxidative stress responses, RAS signaling, and mTOR regulation, this suggests that the CC genotype in TNBC may be associated with a more proliferative and signal-enriched tumor phenotype in postmenopausal BC samples. KEAP1, a tumor suppressor gene that promotes antitumor immunity, is frequently mutated in non-small cell lung cancer, leading to increased NRF2 activity and poorer clinical outcomes. These mutations are associated with elevated PD-L1 expression and altered responses to immune checkpoint inhibitors in NSCLC [12]. While the relevance of KEAP1 in TNBC is still evolving, emerging evidence suggests it may also be linked to poorer outcomes [13].
In endometrioid EC, somatic profiles also differed by genotype and the inferred menopausal status. The PTEN function is essential toward endometrioid tumorigenesis [1]. While PTEN alteration events were overall prevalent, in premenopausal EC, CC tumors harbored an increased frequency of PTEN alterations compared to AA tumors (p < 0.05) (Figure 3C and Supplementary Table S3). Increased PTEN alterations may have contributed to the poorer survival trends observed among the CC genotypes in the premenopausal cohort (Figure 1). In postmenopausal EC, AA tumors were enriched for mutations in BCOR, JAK1, and ZFHX3 (p < 0.05), while CC tumors had higher frequencies of KRAS, MTMR2, and MTA2 mutations and CNAs in ESR1, ARID2, and KLF6 (p < 0.05). Immunohistochemistry (IHC) measurements of PR positivity were also significantly elevated in CC tumors (p < 0.05), consistent with a more hormone-responsive phenotype (Figure 3C and Supplementary Table S3). These findings indicate that the HSD3B1 genotype and inferred menopausal status together define endometrioid EC with divergent molecular features.
2.5. Differences in Gene Expression Across HSD3B1 Genotypes Is Observed in BC and EC
To investigate the potential tumor-intrinsic effects of the HSD3B1 genotype, we performed differential gene expression analyses comparing BC and EC tumor subtypes that harbor either the CC or AA genotypes. Similar to our prior genomic analysis, tumors with the heterogeneous AC genotype were excluded. In ER + BC tumors, across HSD3B1 genotypes in both pre- and postmenopausal tumors, there was minimal variation in the transcriptome, including in the expression of canonical hormone receptor genes (ESR1, ESR2, PGR) (Figure 4, and Supplementary Table S4). In TNBC, transcriptional differences between HSD3B1 genotypes were overall more pronounced. In premenopausal TNBC, tumors harboring the CC genotype had reduced expression of inflammatory genes, like S100A7 and LY6D [14,15] (p-values = 0.0006 and = 0.0052, respectively), and basal-like genes, such as SPRR2G [16] (p-value = 0.0039). Furthermore, in both pre- and postmenopausal TNBC, we noted that SERF1A, a gene with no known function and part of a gene signature used to estimate the likelihood of BC recurrence [17], was upregulated in the CC genotype (p-value = 0.0004 and 0.0083, respectively). Hormone-related genes, like ESR2 and NR3C2 (encoding the mineralocorticoid receptor), showed no significant differential expression between genotypes across inferred menopausal statuses in TNBC (Figure 4B and Supplementary Table S4). In endometrioid ECs, HSD3B1 genotypes differed in expression in only a few genes. Across both menopausal statuses, there were no significant differences in canonical hormone receptor genes. In pre- but and not postmenopausal tumors, the CC genotype harbored the increased expression of CSNS1, in which high expression has been associated with worse survival outcomes in BC [18] and SERPINA11, which has been implicated in tumor suppression in hepatocellular carcinoma [19] (Figure 4C and Supplementary Table S5). Overall, transcriptional variation dependent on HSD3B1 genotypes differed by the cancer type, with the majority of differences seen in TNBC and less variation observed in ER + BC and endometrioid EC.
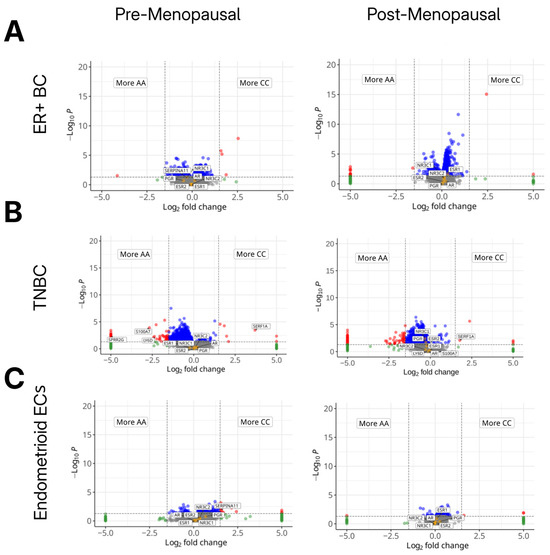
Figure 4.
Differences in gene expression across germline HSD3B1 genotypes. Volcano plots showing differential gene expression between adrenal-permissive (CC) and adrenal-restrictive (AA) tumors across cancer types and inferred menopausal states. (A) ER+ BC, (B) TNBC, and (C) endometrioid EC, each stratified by inferred menopausal status. Log2 fold change reflects gene expression in CC relative to AA (positive = up in CC; negative = up in AA). AC tumors were included in cohort but not directly compared in these analyses. Red and blue points indicate significantly upregulated genes in CC or AA tumors, respectively (FDR < 0.05, |log2FC| > 1). Orange dots reflect genes that are hormone receptors (ESR1/2, PGR, AR, NR3C1/2). Transcriptional differences were minimal in ER + BC but more pronounced in premenopausal TNBC and EC. See further details in Supplementary Tables S4 and S5.
2.6. HSD3B1 Regulates Hallmark Signaling Pathways and Immune-Related Genes
We then explored HSD3B1 genotype-associated transcriptional programs at the pathway level by conducting gene set enrichment analysis (GSEA) [20] across BC and EC subtypes. ER + BC harboring the CC genotype exhibited the enrichment of pancreatic beta cells, the only significantly upregulated hallmark pathway. In contrast, multiple immune-related pathways, including interferon gamma response and inflammatory response, were significantly downregulated in CC tumors compared to AA, indicating a reduction in immune activation within the tumor microenvironment (Figure 5A and Supplementary Table S6). In TNBC, CC tumors—especially in premenopausal patients—showed the significant enrichment of proliferative and metabolic pathways (Figure 5A and Supplementary Table S6). These included MYC targets V1, E2F targets, G2M checkpoint, DNA repair, and MTORC1 signaling, suggesting that the CC genotype may be linked to enhanced cell cycle progression and stress-response signaling. This pattern was not accompanied by the significant enrichment of immune-related pathways, reinforcing a proliferation-dominant transcriptional phenotype.
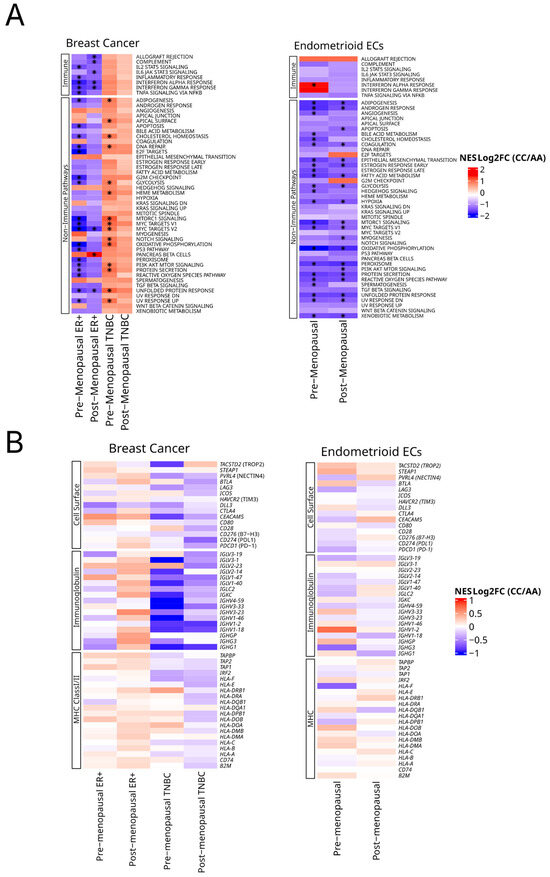
Figure 5.
Pathway and gene expression analysis across germline HSD3B1 genotypes. (A) Gene set enrichment analysis (GSEA) of 50 hallmark pathways reflecting enrichment in CC vs. AA. This is stratified by tumor type and inferred menopausal status. Hallmark pathways are grouped by immune and non-immune signaling in BC and EC. Heatmaps represent normalized enrichment scores (NES) with asterisks indicating FDR of <0.05. See Supplementary Tables S6 and S7 for further details. (B) Relative expression of cell surface, immunoglobin and MHC class I/II gene pathways as grouped by BCs and ECs.
In endometrioid EC, CC tumors exhibited the broad depletion of transcriptional programs across both inferred menopausal groups. However, in premenopausal CC tumors, the interferon alpha response was the only hallmark pathway significantly enriched, indicating selective immune engagement despite overall transcriptional suppression (Figure 5A and Supplementary Table S6). These findings suggest that the HSD3B1 CC genotype is associated with distinct transcriptional states: selective immune suppression in ER + BC, proliferative signaling in TNBC, and widespread downregulation in EC—with limited immune compensation in premenopausal tumors.
To examine if HSD3B1 genotypes influence the expression of individual cell surface genes that are currently drug targets or ones that are associated with immunity, we also performed the focused analysis of a collection of tumor-associated cell surface and immune markers [10] across both BC and EC subtypes and menopausal status (Figure 5B and Supplementary Table S7). Here, based on (log2) fold changes in expression between CC and AA tumors, while we observed trends of the up- or downregulation of these genes, none of these genes exhibited significant differences based on an FDR of < 0.05.
3. Discussion
While prior studies have primarily examined HSD3B1 c.1100 genotypes in prostate cancer outcomes [2,5,6] and their impact on androgen deprivation therapy response [5], emerging evidence has extended their relevance to other hormone-driven cancers such as BC and EC. Specifically, the adrenal-permissive CC genotype has been linked to disease progression in postmenopausal ER + BC via enhanced intratumoral estrogen synthesis [21,22], while emerging evidence links AA genotypes to non-hormone-driven BC and EC [9]. These genotype-driven differences in steroid metabolism may influence tumor behavior, prognosis, and treatment response. We analyzed the clinical, genomic, and transcriptomic landscape of HSD3B1 genotypes across BC and EC subtypes, stratified by inferred menopausal status—representing the largest cohort to date exploring these associations.
Contrary to early reports, we did not observe statistically significant differences in OS based on the HSD3B1 genotype in any cancer subtype or menopausal group. Unlike prior studies showing worse outcomes—such as increased distant metastases and reduced survival—in postmenopausal women with the CC genotype [21], our data revealed overlapping survival curves and confidence intervals, indicating no definitive survival differences between HSD3B1 genotypes in this cohort. Poorer non-significant survival trends were noted among CC genotypes compared to AA or AC among premenopausal EC patients. Our cohort, selected from a genomically tested population likely enriched for advanced or metastatic disease, may reflect a clinical context where the influence of adrenal-derived steroid metabolism is diminished due to tumor burden or prior therapies. In TNBC and EC, where hormonal dependence is variable, the absence of genotype-associated survival differences may similarly reflect tumor heterogeneity or the limited role of HSD3B1 in late-stage disease. These findings suggest that, while the HSD3B1 genotype may have prognostic relevance in the early-stage, treatment-naive settings, its role in advanced cancers may be less clear and potentially overshadowed by additional clinical and molecular drivers. Further studies in more homogeneous, early-stage populations are needed to clarify the potential clinical utility of HSD3B1 genotyping for prognostication and/or treatment selection. An ongoing clinical trial (NCT05183828) is evaluating whether the HSD3B1 germline variant influences tumor proliferation markers, hormone receptor expression, and letrozole response in postmenopausal women with ER + HER2- BC [23].
This study is the first to highlight racial disparities in the HSD3B1 genotype distribution across all tumor subtypes. White patients generally exhibited the highest proportion of the homozygous adrenal-permissive CC genotype (10%), consistent with known population data, while the CC genotype is markedly less common (~1%) in Black and East Asian non-cancerous populations [11]. We observed notable differences in the HSD3B1 genotype distribution when stratified by the cancer subtype and inferred menopausal status. Most prominently, CC genotype enrichment was seen in Asian/PI premenopausal ER + BC (14.6%) and TNBC (6.1%) subgroups, exceeding population-based expectations and suggesting a potential link to tumor predisposition or progression. Cross-race comparisons between Asian/PI and White patients revealed significant differences in the HSD3B1 genotype distribution in premenopausal ER + BC and postmenopausal TNBC. These patterns were not observed in postmenopausal ER + BC and EC, and findings should be interpreted cautiously due to limited sample sizes. Supporting the broader relevance of this observation, our previously published prostate cancer cohort also showed CC genotype frequencies of 9–10% among Black and Asian patients [10], again supporting the potential role of HSD3B1 in hormone-driven cancer biology and cancer predisposition. Prior studies, such as those by Kruse et al., focused exclusively on White populations and did not address racial variability [22]. Our findings raise the possibility that inherited differences in adrenal androgen metabolism may contribute to racial disparities in cancer predisposition and progression, particularly in hormonally influenced cancers. While these patterns were not directly tied to survival in our cohort and results could be skewed due to a lower sample size among non-White population specifically Asian patients in our cohort, they warrant further investigation in racially diverse, early-stage populations to better understand the interplay between the HSD3B1 genotype, genetic ancestry, and clinical outcomes.
Our findings in which tumors harbored context-dependent signaling pathways based on the HSD3B1 adrenal-permissive and -restrictive genotypes drives our understanding of both proliferative and metabolic activity in the clinical subtypes of BC. The role of HSD3B1 genotypes in extragonadal androgen metabolism and steroidogenesis has been well established [2], but we present here, to our knowledge, the first consortium study that deploys the RNA-based measurements of oncogenic and immune regulatory pathways in BC and EC specimens derived from human tumors. Through the GSEA studies, we uncovered that CC tumors in premenopausal patients, particularly those with TNBC, show significant enrichment in MTORC1 signaling, MYC target gene sets, and DNA repair pathways. These findings altogether implicate that the HSD3B1 genotypes may stratify tumors with divergent metabolic and proliferative functions. In these tumors, the enrichment of DNA repair pathways may further suggest the potential for the adrenal permissive genotype to regulate genomic stability or respond to DNA-damaging therapies, which is consistent with previous reports linking steroidogenic enzymes to DNA repair modulation. Independent of HSD3B1 genotypes, MYC and MTORC1 are critical regulatory pathways in aggressive BC [24,25]. Therefore, it is critical to examine mechanisms related to how the permissive genotype contributes to the enhancement of these signaling pathways, and why this is unique to premenopausal TNBCs. In the case that 3βHSD1 acts as an upstream regulator for these processes, the emergence of experimental agents that target 3βHSD1 function may be worth exploring in these clinical settings [26]. It is also curious why the adrenal-permissive HSD3B1 genotypes were associated with these pathways in TNBC patients, as one may surmise that these hormone-receptor-negative tumors may have little dependency on enzymes that regulate this process. Through the analysis of NGS data, it remains unclear if 3βHSD1 has direct or indirect functions beyond regulating estrogen production.
Unlike TNBCs, in ER + BCs, the CC genotype exhibited the depletion of immune-related hallmark pathways, which is suggestive of immune suppression. While these findings are specific to BC, prior studies on prostate cancer indicate that steroid hormone signaling can suppress antitumor immunity. In prostate cancer, androgen receptor (AR) signaling has been shown to downregulate MHC class I (HLA) expression, thereby reducing tumor immunogenicity, a process that is reversed by AR inhibition [27]. AR blockade has also been shown to promote antitumor immunity and increases in interferon signaling, consistent with the broader literature linking hormone signaling to the repression of immune pathways [28,29]. These observations suggest that hormone-driven immune suppression, which is well characterized in prostate cancer, may also play a role in the immune-depleted phenotype we observe in ER + BC. Interestingly, in postmenopausal ER+ tumors that harbor the permissive HSD3B1 genotype, the only upregulated pathway in this context was the pancreatic beta cell signature, a pathway previously associated with both insulin signaling and metabolic stress in tumors [30,31]. This again suggests that 3βHSD1 may have non-canonical functions. Collectively, our observations in BC indicate that HSD3B1 genotypes are potential regulators of oncogenic signaling and metabolic plasticity across BC subtypes, and this is dependent on the inferred menopause/hormonal status. Therefore, we propose that future studies should consider investigating if tumors that express the protein products of adrenal-permissive or -restrictive HSD3B1 in fact yield specific therapeutic vulnerabilities, such as sensitivity to mTOR pathway inhibitors or agents that target tumors with dependency on DNA repair signaling. Importantly, this would support the use of stratifying BC patients by HSD3B1 genotypes and reporting this information in the clinical setting.
In endometrioid ECs, we found that independent of the inferred menopausal status, tumors harboring the CC genotype generally exhibit reduced oncogenic signaling pathways. The consistent negative enrichment of these pathways is perhaps indicative of a transcriptionally quiescent phenotype in the CC tumors. This may be aligned with prior studies that noted reduced proliferative signaling in hormone-sensitive tumors with high PR expression [32]. In premenopausal ECs, the only pathway that was significant was the interferon alpha response pathway, which in the setting of tumor indicates an association with antitumor immunity in such tumors [33]. Additionally, in premenopausal EC, CC genotype tumors showed a higher frequency of PTEN alterations compared to AA, which may have contributed to the poorer survival trends observed in the CC group. In postmenopausal tumors, genomic analyses also revealed that adrenal-permissive patients were more likely to exhibit positivity of PR via IHC, as well as CNAs in ESR1 and ERBB2. While the magnitude of differences in prevalence may be subtle, the consistency of genes associated with dysregulated hormone receptor signaling indicate a potential interaction with the HSD3B1-permissive genotype in postmenopausal patients. While our data do not support a universally pro-oncogenic role for the CC genotype in ECs, they instead suggest a genotype that could be associated with transcriptional quiescence and hormone receptor preservation, similar to what we find in BC subtypes, and the further mechanistic investigations of HSD3B1-permissive and -restrictive protein functions could contribute to novel insights in patients with EC.
For over twelve years, HSD3B1 has been studied in prostate cancer, where the adrenal-permissive allele is associated with enhanced extragonadal and intratumoral androgen synthesis, resistance to androgen deprivation therapies, and poor outcomes [2,4]. Despite this, its functions in oncogenesis in other hormonally driven cancers were relatively unexplored, especially in human cancer specimens. Regardless of its established role in androgen regulation and androgen signaling in prostate cancer, our findings suggest that tumors harboring the adrenal-permissive HSD3B1 exhibit profound differences in biological pathways. In prostate cancer, our recent study conducted in over 5000 tumor specimens, based on the identical method of patient stratification and using the same Caris diagnostic tests, indicated that the permissive genotype drives did not promote significant differences in the expression of the same genes or the activity of the oncogenic pathways we found in these BCs and ECs [10]. However, as the diagnostic testing of tumors is biased toward late-stage disease, the role of HSD3B1 in the early stages of cancer remains to be determined in human tumors. Regardless, these cross-cancer differences accentuate the need for a more expansive examination with regard to the clinical relevance of HSD3B1, in not just BC and EC, but likely in other cancer types as well. As of now, our findings support the potential of germline HSD3B1 genotype as a clinically relevant genetic marker that could be incorporated into the existing diagnostic workflows for these endocrine-driven cancers.
Limitations of the Study
This study has several inherent limitations. It is retrospective in design, and detailed clinical information, such as menopausal status, tumor grade, disease stage, and treatments administered, was therefore not available. Immune cell profiling was based on bulk RNA sequencing rather than single-cell resolution, which may limit interpretability. The actual menopausal status was not available; therefore, the key conclusions derived based on the inferred annotation must be further explored through a study with the actual menopausal status at the time of cancer diagnosis. Here, the menopausal status was approximated using the age at the time of molecular profiling, in which samples from patients older than 55 years were annotated as menopausal. This is based on prior meta-analyses and population studies in BC patients [21,22,34,35]. Additionally, patient data were categorized by self-reported race, leading to the exclusion of individuals with unreported race classifications.
Among endometrioid EC cases, the ER status was determined at the level of demographic descriptive analysis (Table 2) that showed approximately 85–92% of endometrioid cases to be ER-positive across HSD3B1 genotypes. However, endometrioid EC cases in this study were analyzed as a single group without further stratification by the ER status or histologic grade. Given that approximately 10–15% of endometrioid tumors are ER-negative and that grade 3 tumors are biologically distinct—often associated with TP53 mutations and worse outcomes—this grouping may have introduced heterogeneity. Future analyses should consider restricting comparisons to ER-positive, grade 1–2 endometrioid tumors to reflect estrogen-driven biology more accurately. Finally, the classifications of ECs in this study are based on histology; therefore, the findings may differ if these samples were re-classified based on molecular classification schemes, which are now being adapted in oncology clinics [36].
4. Materials and Methods
4.1. Patient Specimens
Formalin-fixed, paraffin-embedded (FFPE) specimens from BC and EC patients were manually microdissected to enrich tumor tissue and underwent molecular testing at Caris Life Sciences (Phoenix, AZ, USA), a College of American Pathologists/Clinical Laboratory Improvement Amendments (CAP/CLIA)-certified laboratory. Hematoxylin and eosin (H&E)-stained, formalin-fixed, paraffin-embedded (FFPE) slides of the patient’s tumor underwent review by a board-certified pathologist or trained pathologist assistant. Tumor enrichment was achieved by harvesting targeted tissue using manual microdissection techniques.
4.2. Patient Cohort and HSD3B1 Genotype
We included ER + BC and TNBC patients (n = 5085) and EC patients (n = 5700) queried from the dataset, including samples that had undergone NextGen Sequencing of DNA and RNA whole-transcriptome data. Patients’ subtype was stratified via variant allele frequency, and IHC detection was used to determine the ER/TNBC status in the BC cohort. HER2 positive BC cases were excluded to maintain the study’s focus on evaluating the impact of HSD3B1 genotypes within hormone-driven (ER-active) and non-hormone-driven (TNBC/basal-like) subtypes. Inferred menopausal status was assumed based on age at the time of molecular profiling, with <55 years classified as premenopausal and ≥55 years as postmenopausal. EC cases were classified by their histological subtype—endometrioid, serous, clear cell, and carcinosarcoma—and also categorized by their ER status (see Table 2). Baseline demographic and clinical characteristics for the BC and EC cohorts—stratified by the HSD3B1 genotype, cancer subtype, inferred menopausal status, self-reported race, tumor mutational burden (TMB), and microsatellite instability (MSI)—are summarized in Table 1 and Table 2. Endometrioid EC cases were further analyzed as a single group (includes both type I and II endometrioid EC) for survival outcomes and tumor profiling and were not further stratified by the ER status due to sample size limitations. Supplementary Table S8 shows annotations used by Caris to group endometrioid EC subtypes. Based on this cohort, we focused on analyzing endometrioid ECs and excluded other EC Subtypes beyond descriptive demographic analysis due to the limited samples in the premenopausal setting (n = 4), which limits the statistical power for any analysis. Analysis based on the histologic grades and molecular classification of EC is not included as this is not the primary study focus. The Kaplan–Meier overall survival analysis curves were generated and are presented in Figure 1.
The germline variant of HSD3B1 was determined from evaluating the VAF of HSD3B1 at position c.1100. Tumors with 0% c.1100A VAF character were considered adrenal-permissive (CC), 40–60% for c.1100A were considered heterozygous (AC), and those with 100% VAF for c.1100A were considered adrenal-restrictive (AA) (Table 1 and Table 2). Any VAF outside of these ranges was considered uncategorized.
4.3. Whole-Exome Sequencing and NGS
NGS was performed on genomic DNA isolated from formalin-fixed, paraffin-embedded (FFPE) tumor samples using the NovaSeq 6000 and the NextSeq sequencing platforms (Illumina, Inc., San Diego, CA, USA). Caris tests are certified by Clinical Laboratory Improvement Amendments (CLIA). Samples in this study were not prospectively sequenced and were processed similar to prior studies [10]. Caris does not process samples more than five years old, which is in part due to the known effects of degradation. Internal standards are applied to evaluate RNA integrity and sequencing, and results are only reported if they pass these quality controls.
Following DNA extraction, a custom-designed SureSelect biotinylated baits panel (Agilent Technologies, Santa Clara, CA, USA) was used to enrich 592 whole-gene targets that recurrently altered in cancer. Library preparation was performed using SureSelectXT reagents, followed by normalization and pooling. Demultiplexed FASTQ files underwent bioinformatic processing for sequence alignment, variant detection, quality-based filtering, and annotation of the sequencing data.
A combination of bait panels was used for WES analysis, including the SureSelect Human All Exon v7 panel (Agilent Technologies, Santa Clara, CA, USA) covering 99% of coding regions, a hybrid pull-down panel of baits designed to enrich 720 genes recurrently altered in cancer, an intronic fusion panel, a pathogenic baits panel, and a 250K SNP backbone panel to assist with gene amplification/deletion measurements and other analyses (Agilent Technologies, Santa Clara, CA, USA). Library preparation, quantification, normalization, and pooling were performed using the Bravo Automated Liquid Handling Platform and the Agilent Tape Station 4200 (Agilent Technologies). Whole-exome sequencing was performed on the NovaSeq 6000 platform (Illumina, Inc., San Diego, CA, USA) (RRID:SCR_016387). The average sequencing depth of coverage was >500×.
Genetic variants were categorized as “pathogenic”, “likely pathogenic”, “variant of unknown significance”, “likely benign”, or “benign” according to the ACMG standards.
4.4. Whole-Transcriptome Sequencing
RNA was extracted using an RNA FFPE Extraction Kit (Qiagen, Hilden, Germany), and RNA quality and quantity were determined using the Agilent TapeStation (Agilent Technologies, Santa Clara, CA, USA). A SureSelect Human All Exon v7 panel of biotinylated RNA baits (Agilent Technologies) were hybridized to the synthesized and purified cDNA targets, and the bait–target complexes were amplified in a post-capture PCR reaction. The resultant libraries were quantified and normalized, and the pooled libraries were denatured and diluted. Library preparation, quantification, normalization, and pooling were performed using the Bravo Automated Liquid Handling Platform and the Agilent Tape Station 4200 (Agilent Technologies). WTS was performed on the Novaseq 6000 System (Illumina, San Diego, CA, USA) (RRID:SCR_016387).
4.5. Immune Signatures
The relative abundance of immune cell infiltrates in the tumor microenvironment were calculated from the WTS data using quanTIseq (RRID:SCR_022993) [37].
4.6. Statistical Analysis
For statistical analysis, Kruskal–Wallis, Chi-square, and Fisher’s exact tests were used. For the demographic tables, FDR q-values of <0.05 (Benjamini–Hochberg procedures) were considered significant. For the GSEA ranked analysis test, statistical significance was assessed with an NES score and a calculated FDR value. The GSEA analysis was performed using the GSEApy python package v1.1.6.
4.7. TMB and MSI/dMMR
TMB was calculated by the addition of all non-synonymous missense, nonsense, inframe insertion/deletion, and frameshift mutations found per tumor. A cutoff value of ≥10 mutations per MB was used. The MSI/MMR status was inferred using a combination of NGS and IHC markers compared to the hg19 human genome.
5. Conclusions
Our work presents a novel analysis of the association between inferred germline HSD3B1 c.1100 genotypes and the somatic landscape in BC and EC. This study indicates that across the subtypes of BC and EC, in both pre- and postmenopausal tumors, HSD3B1 genotypes distinctly influence transcriptional profiles, genomic alterations, and differences in the expression of immune and non-immune pathways. While our findings are hypothesis-generating and require further validation, the influence of HSD3B1 genotypes in BC and EC appears substantial and warrants continued investigation for potential relevance to treatment decision making.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26125720/s1.
Author Contributions
Conceptualization, N.V., A.M., S.K. and J.H.; formal analysis, S.K., S.S.N. and A.E.; supervision, E.S.A. and J.H.; visualization, S.K., S.S.N. and A.E.; writing—original draft, N.V., A.M., S.K., D.S., A.D. and J.H.; writing—review and editing, N.V., A.M., S.K., S.S.N., A.E., D.S., A.D., G.v.D., E.J., P.C.B., N.A., M.A.G., B.K.E., G.S., J.H.O., R.R.M., C.J.R., N.S., E.S.A. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute of General Medical Sciences and National Institutes of Health and National Cancer Institute, through the grants 5R01CA249279 and 5R01CA172382 (to N.S.), R01 CA249279 (to C.J.R.), P30 CA077598 (to E.S.A.), R01 CA236948 and NIH/NCI and W81XWH2210716- DoD BCRP (to J.H.O), and R37 CA288972 (to J.H.). E.S.A. is also partially supported by the DOD grant W81XWH-22-2-0025.
Institutional Review Board Statement
This retrospective study was conducted under Caris Life Sciences’ Research Data Banking protocol, which was reviewed and granted IRB exemption by the WCG IRB. The study adhered to the ethical guidelines of the Declaration of Helsinki, the Belmont Report, and the U.S. Common Rule.
Informed Consent Statement
Patient consent was waived since this retrospective study was conducted under Caris Life Sciences’ Research Data Banking protocol, which was reviewed, and the IRB determined that the proposed activity is not research involving human subjects as defined by the DHHS and FDA regulations. To arrive at this inference, the IRB used “WORKSHEET: Human Research (HRP-310).” This study was granted IRB exemption by the WCG IRB. The study adhered to the ethical guidelines of the Declaration of Helsinki, the Belmont Report, and the U.S. Common Rule.
Data Availability Statement
This study did not generate any new raw DNA/RNA sequencing data but rather used the existing data from the database through a formal letter of intent and a subsequent data use agreement. Here, we researched the de-identified data collected in a real-world health care setting, and this is subject to controlled access for privacy and proprietary reasons. When possible, derived data supporting the findings of this study have been made available within the paper and its Supplementary Figures/Tables. Other data can be acquired through a letter of intent to Caris Life Sciences (https://www.carislifesciences.com/letter-of-intent/ accessed on 12 June 2025). Additional inquiries can be sent to Andrew Elliott at aelliott@carisls.com.
Conflicts of Interest
S.S.N., A.E., and G.S. are employees of Caris Life Sciences. J.H.O reports the following grants: R01 CA236948, NIH/NCI and W81XWH2210716, DoD BCRP. P.C.B. reports grants and personal fees from Astellas, AstraZeneca, Bayer, Eisai, ESSA Pharma, Ipsen, Caris Life Sciences, Exelixis, Janssen, EMD Serono, Dendreon, Pfizer, Seattle Genetics, Merck, Merus, BMS, Bayer, Guardant Health, Myovant, UroToday, OncLive, and Targeted Oncology. C.J.R. consulted for Oric and Bayer and has received fees from Pfizer. N.S. is a co-inventor on patents related to HSD3B1 filed by his former employer, Cleveland Clinic, and received funding from 5R01CA249279 and 5R01CA172382. E.S.A. reports grants and personal fees from Janssen, Sanofi, Bayer, Bristol Myers Squibb, Curium, MacroGenics, Merck, Pfizer, AstraZeneca, and Clovis; personal fees from Aadi Bioscience, Aikido Pharma, Astellas, Amgen, Blue Earth, Corcept Therapeutics, Exact Sciences, Hookipa Pharma, Invitae, Eli Lilly, Foundation Medicine, Menarini-Silicon Biosystems, Tango Therapeutics, Tempus, and Z-alpha; grants from Novartis, Celgene, and Orion; and has a patent for an AR-V7 biomarker technology that has been licensed to Qiagen. J.H. consults for Tempus and is a co-founder of EMRGNSE LLC., and received funding from R37 CA288972. B.K.E. reports personal fees from GSK, Merck, Gilead, and Immunogen. M.A.G. consulting fees from GSK and Astra Zeneca.
Abbreviations
The following abbreviations are used in this manuscript:
| 3βHSD1 | 3β-Hydroxysteroid Dehydrogenase Type 1 |
| AR | Androgen Receptor |
| BC | Breast Cancer |
| CAN | Copy Number Amplification |
| DHEA | Dehydroepiandrosterone |
| DHT | Dihydrotestosterone |
| EC | Endometrial Cancer |
| ER | Estrogen Receptor |
| GSEA | Gene Set Enrichment Analysis |
| HSD3B1 | Hydroxy-delta-5-steroid Dehydrogenase, 3 Beta-and Steroid Delta-Isomerase 1 |
| IHC | Immunohistochemistry |
| MMR | Mismatch Repair |
| MSI | Microsatellite Instability |
| OS | Overall Survival |
| PI | Pacific Islanders |
| PR | Progesterone Receptor |
| TMB | Tumor Mutational Burden |
| TNBC | Triple-negative Breast Cancer |
References
- Naelitz, B.D.; Sharifi, N. Through the Looking-Glass: Reevaluating DHEA Metabolism Through HSD3B1 Genetics. Trends Endocrinol. Metab. 2020, 31, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.F.S.; Abdshah, A.; McKay, R.R.; Sharifi, N. HSD3B1, Prostate Cancer Mortality and Modifiable Outcomes. Nat. Rev. Urol. 2024, 22, 313–320. [Google Scholar] [CrossRef]
- Thomas, L.; Sharifi, N. Germline HSD3B1 Genetics and Prostate Cancer Outcomes. Urology 2020, 145, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Li, R.; Kuri, B.; Lotan, Y.; Roehrborn, C.G.; Liu, J.; Vessella, R.; Nelson, P.S.; Kapur, P.; Guo, X.; et al. A Gain-of-Function Mutation in DHT Synthesis in Castration-Resistant Prostate Cancer. Cell 2013, 154, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.W.D.; AbuAli, G.; Reichard, C.A.; Reddy, C.A.; Magi-Galluzzi, C.; Chang, K.-H.; Carlson, R.; Rangel, L.; Reagan, K.; Davis, B.J.; et al. HSD3B1 and Resistance to Androgen-Deprivation Therapy in Prostate Cancer: A Retrospective, Multicohort Study. Lancet Oncol. 2016, 17, 1435–1444. [Google Scholar] [CrossRef]
- McKay, R.R.; Nelson, T.J.; Pagadala, M.S.; Teerlink, C.C.; Gao, A.; Bryant, A.K.; Agiri, F.Y.; Guram, K.; Thompson, R.F.; Pridgen, K.M.; et al. Adrenal-Permissive Germline HSD3B1 Allele and Prostate Cancer Outcomes. JAMA Netw. Open 2024, 7, e242976. [Google Scholar] [CrossRef]
- Saha Roy, S.; Vadlamudi, R.K. Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef]
- Flanagan, M.R.; Sharifi, N.; Gadi, V.K. ASO Author Reflections: Inheritance of Adrenal Permissive HSD3B1 Genotype Negatively Impacts Outcomes in Hormone Receptor-Positive Postmenopausal Breast Cancer. Ann. Surg. Oncol. 2022, 29, 7202–7203. [Google Scholar] [CrossRef]
- McManus, J.M.; Vargas, R.; Bazeley, P.S.; Schumacher, F.R.; Sharifi, N. Association Between Adrenal-Restrictive HSD3B1 Inheritance and Hormone-Independent Subtypes of Endometrial and Breast Cancer. JNCI Cancer Spectr. 2022, 6, pkac061. [Google Scholar] [CrossRef]
- Kellen, S.; Makovec, A.; Miller, C.D.; Nazari, S.S.; Elliott, A.; Deacon, A.; John, E.; Vobugari, N.; Agarwal, N.; McKay, R.R.; et al. The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer. Cancers 2025, 17, 1270. [Google Scholar] [CrossRef]
- Rs1047303 RefSNP Report-dbSNP-NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1047303?horizontal_tab=true#frequency_tab (accessed on 23 April 2025).
- Li, J.; Shi, D.; Li, S.; Shi, X.; Liu, Y.; Zhang, Y.; Wang, G.; Zhang, C.; Xia, T.; Piao, H.; et al. KEAP1 Promotes Anti-Tumor Immunity by Inhibiting PD-L1 Expression in NSCLC. Cell Death Dis. 2024, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, J.; Jung, E.S.; Lee, A. High Expression of NRF2 and Low Expression of KEAP1 Predict Worse Survival in Patients With Operable Triple-Negative Breast Cancer. J. Breast Cancer 2023, 26, 461–478. [Google Scholar] [CrossRef]
- West, N.R.; Watson, P.H. S100A7 (Psoriasin) is Induced by the Proinflammatory Cytokines Oncostatin-M and Interleukin-6 in Human Breast Cancer. Oncogene 2010, 29, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.; Kang, Y.-W.; Kim, Y.; Park, M.-Y.; Song, J.-H.; Jo, Y.; Dao, T.; Ryu, D.; Lee, J.; et al. LY6D Is Crucial for Lipid Accumulation and Inflammation in Nonalcoholic Fatty Liver Disease. Exp. Mol. Med. 2023, 55, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lv, X.; Li, Y.; Li, M. Attention-Based GCN Integrates Multi-Omics Data for Breast Cancer Subtype Classification and Patient-Specific Gene Marker Identification. Brief. Funct. Genom. 2023, 22, 463–474. [Google Scholar] [CrossRef]
- Mustacchi, G.; Sormani, M.P.; Bruzzi, P.; Gennari, A.; Zanconati, F.; Bonifacio, D.; Monzoni, A.; Morandi, L. Identification and Validation of a New Set of Five Genes for Prediction of Risk in Early Breast Cancer. Int. J. Mol. Sci. 2013, 14, 9686–9702. [Google Scholar] [CrossRef]
- Mou, M.A.; Keya, N.A.; Islam, M.; Hossain, M.J.; Al Habib, M.S.; Alam, R.; Rana, S.; Samad, A.; Ahammad, F. Validation of CSN1S1 Transcriptional Expression, Promoter Methylation, and Prognostic Power in Breast Cancer Using Independent Datasets. Biochem. Biophys. Rep. 2020, 24, 100867. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Li, L.; Zhou, H.; Zeng, T.-T.; Jin, C.; Lin, J.-R.; Gao, S.; Li, Y.; Guan, X.-Y.; et al. SERPINA11 Inhibits Metastasis in Hepatocellular Carcinoma by Suppressing MEK/ERK Signaling Pathway. J. Hepatocell. Carcinoma 2021, 8, 759–771. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Flanagan, M.R.; Doody, D.R.; Voutsinas, J.; Wu, Q.; Banda, K.; Sharifi, N.; Li, C.I.; Gadi, V.K. Association of HSD3B1 Genotype and Clinical Outcomes in Postmenopausal Estrogen-Receptor-Positive Breast Cancer. Ann. Surg. Oncol. 2022, 29, 7194–7201. [Google Scholar] [CrossRef]
- Kruse, M.L.; Patel, M.; McManus, J.; Chung, Y.-M.; Li, X.; Wei, W.; Bazeley, P.S.; Nakamura, F.; Hardaway, A.; Downs, E.; et al. Adrenal-Permissive HSD3B1 Genetic Inheritance and Risk of Estrogen-Driven Postmenopausal Breast Cancer. JCI Insight 2021, 6, e150403. [Google Scholar] [CrossRef]
- University of Washington, Association of HSD3B1 Genotype with Response to Preoperative Letrozole Therapy Among Postmenopausal Women with Estrogen-Receptor Positive (ER+) HER2/Neu-Negative (HER2-) Invasive Carcinomas of the Breast; clinicaltrials.gov. 2025. Available online: https://clinicaltrials.gov/study/NCT05183828?term=hsd3b1&cond=breast%20cancer&rank=1 (accessed on 12 June 2025).
- Gabay, M.; Li, Y.; Felsher, D.W. MYC Activation Is a Hallmark of Cancer Initiation and Maintenance. Cold Spring Harb. Perspect. Med. 2014, 4, a014241. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Hou, Z.; Huang, S.; Mei, Z.; Chen, L.; Guo, J.; Gao, Y.; Zhuang, Q.; Zhang, X.; Tan, Q.; Yang, T.; et al. Inhibiting 3βHSD1 to Eliminate the Oncogenic Effects of Progesterone in Prostate Cancer. Cell Rep. Med. 2022, 3, 100561. [Google Scholar] [CrossRef]
- Chesner, L.N.; Polesso, F.; Graff, J.N.; Hawley, J.E.; Smith, A.K.; Lundberg, A.; Das, R.; Shenoy, T.; Sjöström, M.; Zhao, F.; et al. Androgen Receptor Inhibition Increases MHC Class I Expression and Improves Immune Response in Prostate Cancer. Cancer Discov. 2025, 15, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen Receptor Activity in T Cells Limits Checkpoint Blockade Efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, J.C.; Chen, B.; Nip, C.; Van Dyke, J.E.; Zhang, X.; Chen, H.-W.; Evans, C.P.; Murphy, W.J.; Liu, C. Androgen Receptor Blockade Resistance with Enzalutamide in Prostate Cancer Results in Immunosuppressive Alterations in the Tumor Immune Microenvironment. J. Immunother. Cancer 2023, 11, e006581. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel Excess and Beta-Cell Dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef]
- Faustin, B.; Lartigue, L.; Bruey, J.-M.; Luciano, F.; Sergienko, E.; Bailly-Maitre, B.; Volkmann, N.; Hanein, D.; Rouiller, I.; Reed, J.C. Reconstituted NALP1 Inflammasome Reveals Two-Step Mechanism of Caspase-1 Activation. Mol. Cell 2007, 25, 713–724. [Google Scholar] [CrossRef]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Dev. Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef]
- Rothlin, C.V.; Ghosh, S. Lifting the Innate Immune Barriers to Antitumor Immunity. J. Immunother. Cancer 2020, 8, e000695. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.K.; Burkman, R.T.; Cushing-Haugen, K.L.; Voigt, L.F.; Simon, M.S.; Daling, J.R.; Norman, S.A.; Bernstein, L.; Ursin, G.; Marchbanks, P.A.; et al. Hormone Replacement Therapy Regimens and Breast Cancer Risk. Obstet. Gynecol. 2002, 100, 1148. [Google Scholar] [PubMed]
- Chang, C.-H.; Lee, Y.-C.; Huang, C.-W.; Lu, Y.-M. Is Tamoxifen Good Enough for the Asian Population in ER+ HER2- Post-Menopausal Women with Early Breast Cancer? A Nationwide Population-Based Cohort Study. PLoS ONE 2024, 19, e0313120. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Finotello, F.; Mayer, C.; Plattner, C.; Laschober, G.; Rieder, D.; Hackl, H.; Krogsdam, A.; Loncova, Z.; Posch, W.; Wilflingseder, D.; et al. Molecular and Pharmacological Modulators of the Tumor Immune Contexture Revealed by Deconvolution of RNA-Seq Data. Genome Med. 2019, 11, 34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).