Intralesional Immunotherapy for Non-Genital Viral Warts: A Review of Current Evidence and Future Perspectives
Abstract
1. Introduction
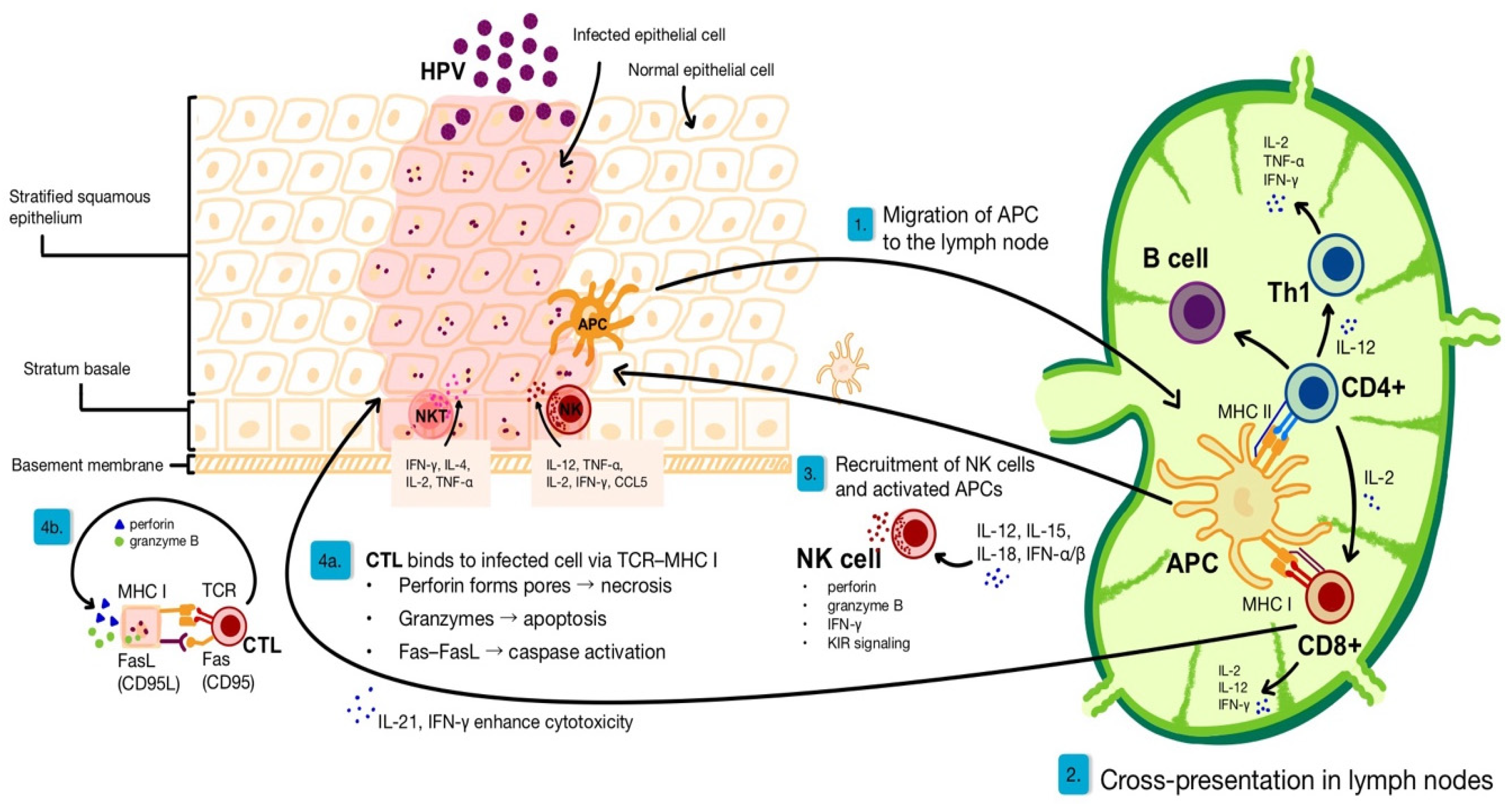
2. Immunological Basis of Anti-Viral Skin Response
2.1. Immune Responses for HPV Clearance
2.2. Immune Evasion Strategies of HPV
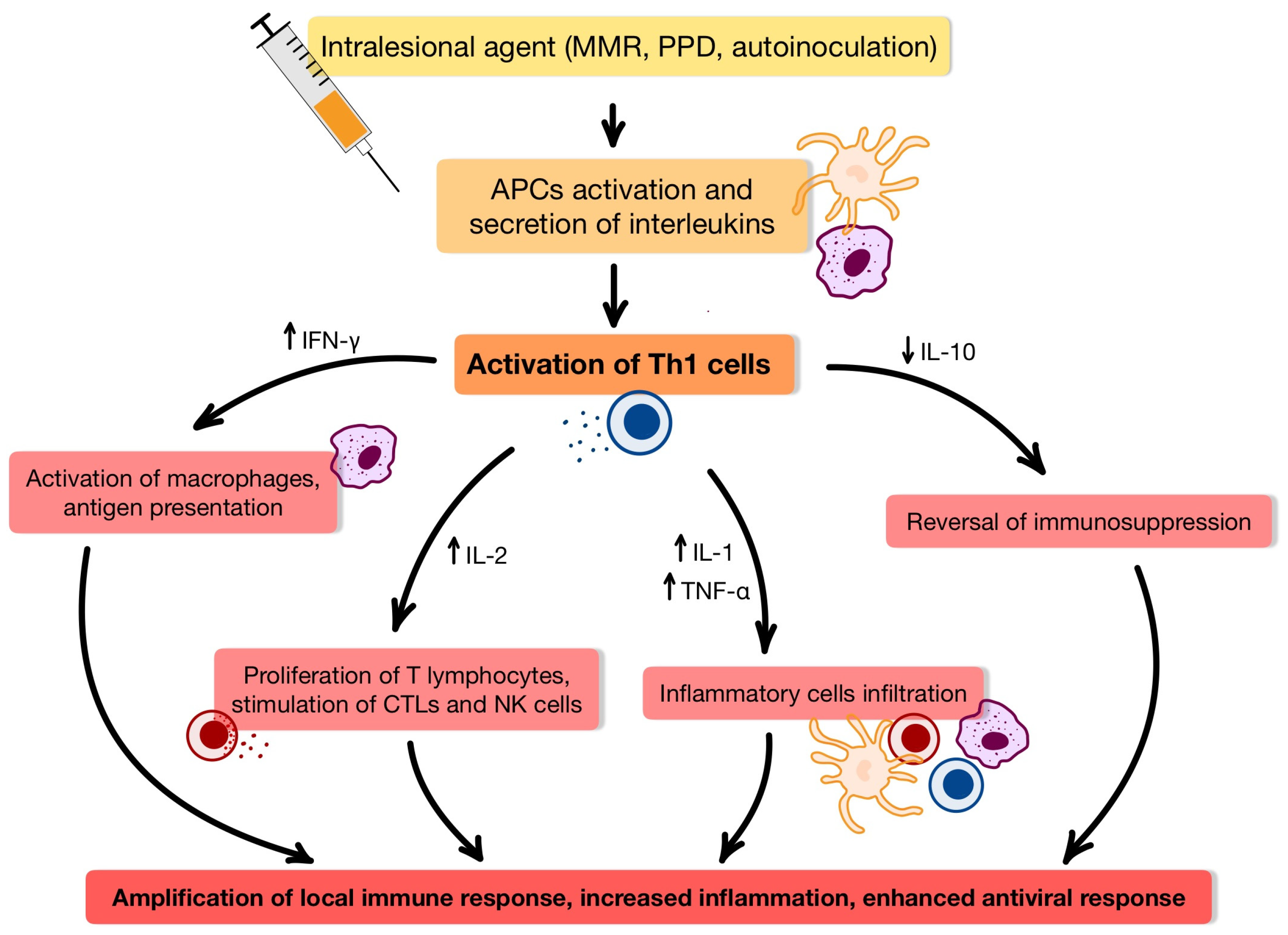
3. Intralesional Immunotherapy
3.1. Immunological Insights into Intralesional Therapy for HPV-Induced Wart
3.2. Measles-Mumps-Rubella (MMR) Vaccine
3.2.1. Characterization, Therapeutic Potential, and Clinical Considerations of the Investigated Agent
3.2.2. Dosage and Administration
3.3. Purified Protein Derivative (PPD), the Bacille Calmette–Guérin (BCG) VAccine and the Mycobacterium W Vaccine (MWV)
3.3.1. Characterization, Therapeutic Potential, and Clinical Considerations of the Investigated Agent
3.3.2. Dosage and Administration
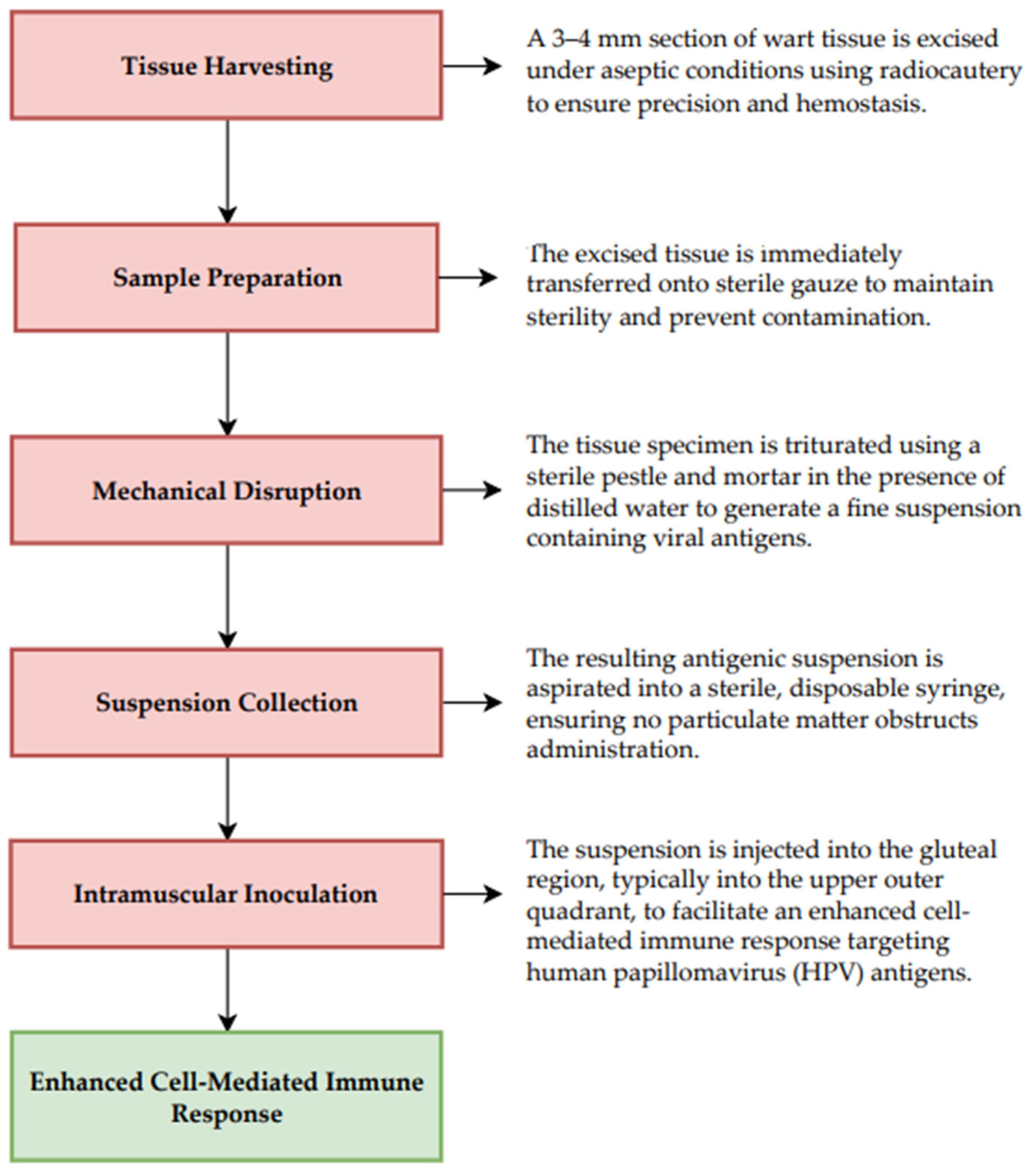
3.4. Autoinoculation Techniques
3.4.1. Characterization, Therapeutic Potential, and Clinical Considerations of the Investigated Agent
3.4.2. Dosage and Administration
3.5. Candida Antigen
3.5.1. Mechanism of Action, Advantages, and Limitations of the Applied Therapeutic Approach
3.5.2. Dosage and Administration
3.6. Trichophyton Antigen
3.6.1. Mechanism of Action, Advantages, and Limitations of the Applied Therapeutic Approach
3.6.2. Dosage and Administration
3.7. Interferons α-2B (IFNα-2B)
3.7.1. Mechanism of Action, Advantages, and Limitations of the Applied Therapeutic Approach
3.7.2. Dosage and Administration
3.8. Vitamin D3
3.8.1. Mechanism of Action, Advantages, and Limitations of the Applied Therapeutic Approach
3.8.2. Dosage and Administration
4. Conventional Intralesional Therapy
4.1. Bleomycin
4.2. Fluorouracil (5-FU)
4.3. Acyclovir
4.4. Cidofovir
4.5. Zinc Sulfate
4.6. Methotrexate
5. Comparison of Novel Immunomodulatory Intralesional Techniques
6. Future Perspectives and Research Directions
6.1. Cytokine Profiles
6.1.1. Interleukin-17A (IL-17A)
6.1.2. Interleukin-18 (IL-18)
6.1.3. Interleukin-4 (IL-4) and IFN-γ
6.1.4. Interleukin-10 (IL-10)
6.2. Combination Therapies with Intralesional Immunotherapy
6.2.1. PPD + Cryotherapy
6.2.2. Candida + Cryotherapy
6.2.3. Intralesional Candida + Oral Isotretinoin
6.2.4. Intralesional Candida + Oral Acitretin
6.2.5. PPD + Candida Combination Therapy for Plane Warts
6.2.6. IFN-α2b + Pulsed Dye Laser for Periungual Warts
6.2.7. IFN-α2b + Candida/Mumps/Trichophyton Antigens
6.2.8. PPD + MMR + Candida Antigen
6.3. Intralesional HPV Vaccination in Recalcitrant Wart Immunotherapy
6.4. Clinical Trials
6.4.1. Efficacy of Cryotherapy Combined with Intralesional Hepatitis B Virus Vaccine
6.4.2. Sclerotherapy and Candida Antigen in Treatment of Common Warts
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molla, A.; Tobaiqi, M.; Elbadawy, H.; Jannadi, R.; Eltahir, H.; Albadawi, E.; Alzaman, N.; Aloufi, M.; Abouzied, M.; Albadrani, M. Comparative Analysis of Intralesional Immunotherapy and Conventional Treatments for Non-Genital Warts: A Systematic Review and Network Meta-Analysis. Dermatol. Pract. Concept. 2024, 14, e2024215. [Google Scholar] [CrossRef] [PubMed]
- Cubie, H.A. Diseases Associated with Human Papillomavirus Infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Luria, L.; Cardoza-Favarato, G. Human Papillomavirus. In Encyclopedia of Child and Adolescent Health, 1st ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 1, pp. 953–964. [Google Scholar] [CrossRef]
- Al Aboud, A.M.; Nigam, P.K. Wart. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Loo, S.K.F.; Tang, W.Y.M. Warts (Non-Genital). BMJ Clin. Evid. 2009, 2009, 1710. [Google Scholar]
- Bacaj, P.; Burch, D. Human Papillomavirus Infection of the Skin. Arch. Pathol. Lab. Med. 2018, 142, 700–705. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Xu, S. Successful Treatment of Multiple Plantar Warts Complicated by Systemic Lupus Erythematosus with ALA-PDT, a Case Report. Photodiagn. Photodyn. Ther. 2025, 53, 104583. [Google Scholar] [CrossRef]
- Van Haalen, F.M.; Bruggink, S.C.; Gussekloo, J.; Assendelft, W.J.J.; Eekhof, J.A.H. Warts in Primary Schoolchildren: Prevalence and Relation with Environmental Factors. Br. J. Dermatol. 2009, 161, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, M.; Merlin, K.; Young, R.; Marks, R. The Prevalence of Common Skin Conditions in Australian School Students: 1. Common, Plane and Plantar Viral Warts. Br. J. Dermatol. 1998, 138, 840–845. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yang, F.; Ren, Y.; Xia, T.; Zhao, Z.; Cao, X.; Wang, Z.; Yin, M.; Lu, S. Epidemiology and Clinical Profile of Cutaneous Warts in Chinese College Students: A Cross-Sectional and Follow-Up Study. Sci. Rep. 2018, 8, 15450. [Google Scholar] [CrossRef]
- Kwok, C.S.; Gibbs, S.; Bennett, C.; Holland, R.; Abbott, R. Topical Treatments for Cutaneous Warts. Cochrane Database Syst. Rev. 2012, 2012, CD001781. [Google Scholar] [CrossRef]
- Singh, Y.; Rajamohanan, R.R.; Vasudevan, S.; Kuruvila, S. Effectiveness of Intralesional Bleomycin in the Management of Difficult-to-Treat and Resistant Cutaneous Warts in a Tertiary Care Teaching Hospital in Puducherry: A Quasi-Experimental Study. J. Cutan. Aesthet. Surg. 2025, 18, 108. [Google Scholar] [CrossRef]
- Friedman, P.C. Management of Difficult-to-Treat Warts: Traditional and New Approaches. Am. J. Clin. Dermatol. 2021, 22, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Khozeimeh, F.; Jabbari Azad, F.; Mahboubi Oskouei, Y.; Jafari, M.; Tehranian, S.; Alizadehsani, R.; Layegh, P. Intralesional Immunotherapy Compared to Cryotherapy in the Treatment of Warts. Int. J. Dermatol. 2017, 56, 474–478. [Google Scholar] [CrossRef]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and Intrinsic Antiviral Immunity in Skin. J. Dermatol. Sci. 2014, 75, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, A.; Riemer, A.B. Immune Evasion Mechanisms of Human Papillomavirus: An Update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lei, V.; Petty, A.J.; Atwater, A.R.; Wolfe, S.A.; MacLeod, A.S. Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Front. Immunol. 2020, 11, 593901. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, C.; Sun, Q.; Wang, Y.; Yu, W.; Wei, F.; Ren, X. Trained Immunity of IL-12-, IL-15-, and IL-18-Induced CD3+CD56+ NKT-Like Cells. J. Oncol. 2022, 2022, 8724933. [Google Scholar] [CrossRef]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Pre-Activation with IL-12, IL-15, and IL-18 Induces CD25 and a Functional High Affinity IL-2 Receptor on Human Cytokine-Induced Memory-like NK Cells. Biol. Blood Marrow Transplant. 2014, 20, 463–473. [Google Scholar] [CrossRef]
- Coquet, J.M.; Kyparissoudis, K.; Pellicci, D.G.; Besra, G.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. IL-21 Is Produced by NKT Cells and Modulates NKT Cell Activation and Cytokine Production. J. Immunol. 2007, 178, 2827–2834. [Google Scholar] [CrossRef]
- Yi, J.S.; Du, M.; Zajac, A.J. A Vital Role for Interleukin-21 in the Control of a Chronic Viral Infection. Science 2009, 324, 1572–1576. [Google Scholar] [CrossRef]
- Gusho, E.; Laimins, L.A. Human Papillomaviruses Sensitize Cells to DNA Damage Induced Apoptosis by Targeting the Innate Immune Sensor CGAS. PLoS Pathog. 2022, 18, e1010725. [Google Scholar] [CrossRef]
- Beglin, M.; Melar-New, M.; Laimins, L. Human Papillomaviruses and the Interferon Response. J. Interferon Cytokine Res. 2009, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Shirakara, Y.; Yamasaki, K.; Sayama, K.; Hashimoto, K. Differentiated Keratinocytes Are Responsible for TNF-Alpha Regulated Production of Macrophage Inflammatory Protein 3alpha/CCL20, a Potent Chemokine for Langerhans Cells. J. Dermatol. Sci. 2001, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Asagoe, K.; Yamauchi, A.; Yamamoto, T. Dendritic Cell Subsets and Immunological Milieu in Inflammatory Human Papilloma Virus-Related Skin Lesions. J. Dermatol. Sci. 2011, 63, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Shimazaki, K.; Kume, T.; Suzumiya, J.; Kanda, M.; Kikuchi, M. Perforin and Fas Pathways of Cytotoxic T-Cells in Histiocytic Necrotizing Lymphadenitis. Histopathology 1998, 33, 471–478. [Google Scholar] [CrossRef]
- Zhou, F. Perforin: More than Just a Pore-Forming Protein. Int. Rev. Immunol. 2010, 29, 56–76. [Google Scholar] [CrossRef]
- Grabowska, A.K. The Invisible Enemy—How Human Papillomaviruses Avoid Recognition and Clearance by the Host Immune System. Open Virol. J. 2013, 6, 249–256. [Google Scholar] [CrossRef]
- Stanley, M. Immunology of HPV Infection. Curr. Obstet. Gynecol. Rep. 2015, 4, 195–200. [Google Scholar] [CrossRef]
- Nunes, R.A.L.; Morale, M.G.; Silva, G.Á.F.; Villa, L.L.; Termini, L. Innate Immunity and HPV: Friends or Foes. Clinics 2018, 73, e549s. [Google Scholar] [CrossRef]
- Scott, M.E.; Ma, Y.; Farhat, S.; Moscicki, A.B. Expression of Nucleic Acid-Sensing Toll-like Receptors Predicts HPV16 Clearance Associated with an E6-Directed Cell-Mediated Response. Int. J. Cancer 2015, 136, 2402–2408. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Rocha-Zavaleta, L.; Lizano, M.; Ramírez-Alcántara, K.M.; Madrid-Marina, V.; Manzo-Merino, J. Alteration of the IFN-Pathway by Human Papillomavirus Proteins: Antiviral Immune Response Evasion Mechanism. Biomedicines 2022, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kim, S.S.; Jones, R.N.; Zhang, L.; Guram, K.; Sharma, S.; Schoenberger, S.P.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. Human Papillomavirus E5 Suppresses Immunity via Inhibition of the Immunoproteasome and STING Pathway. Cell Rep. 2023, 42, 112508. [Google Scholar] [CrossRef] [PubMed]
- Cac, N.N.; Ballas, Z.K. Recalcitrant Warts, Associated with Natural Killer Cell Dysfunction, Treated with Systemic IFN-α. J. Allergy Clin. Immunol. 2006, 118, 526–528. [Google Scholar] [CrossRef]
- Janssen, E.; Tohme, M.; Butts, J.; Giguere, S.; Sage, P.T.; Velázquez, F.E.; Kam, C.; Milin, E.; Das, M.; Sobh, A.; et al. DOCK8 Is Essential for LFA-1-Dependent Positioning of T Follicular Helper Cells in Germinal Centers. JCI Insight 2020, 5, e134508. [Google Scholar] [CrossRef]
- Ju, H.J.; Park, H.R.; Kim, J.Y.; Kim, G.M.; Bae, J.M.; Lee, J.H. Intralesional Immunotherapy for Non-Genital Warts: A Systematic Review and Meta-Analysis. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Abeck, D.; Tetsch, L.; Lüftl, M.; Biedermann, T. Extragenital Cutaneous Warts—Clinical Presentation, Diagnosis and Treatment. JDDG J. Der Dtsch. Dermatol. Gesellschaft 2019, 17, 613–634. [Google Scholar] [CrossRef]
- Achdiat, P.A.; Yunitasari; Usman, H.A.; Maharani, R.H. A Case of Genital and Extragenital Warts Unresponsive to Immunotherapy Using Measles, Mumps, Rubella Vaccine. Int. Med. Case Rep. J. 2023, 16, 739–746. [Google Scholar] [CrossRef]
- Shahid, M.W.; Iftikhar, N.; Irshad, M.; Akhtar, A.; Hafeez, J.; Ali, U.A. Efficacy of Autoinoculation in Treatment of Multiple Viral Warts—A Single Arm Study. J. Coll. Physicians Surg. Pak. 2023, 33, 141–144. [Google Scholar] [CrossRef]
- Ashraf, U.; Ahmed, N.; Tahir, M.; Hasan, F.; Shah, A.H.; Farooq, O. Effectiveness of Autoinoculation in Patients with Multiple Warts Presenting at a Tertiary Care Hospital. J. Coll. Physicians Surg. Pak. 2023, 33, 16–19. [Google Scholar] [CrossRef]
- Azab, M.; El-Shabrawy, M.M.; Nafea, E.R.A.; Nada, H.A. Measurement of Serum Interleukin 17 Level in Patients with Genital Warts before and after Intralesional Tuberculin Injection. J. Mens. Health 2021, 18, 1–7. [Google Scholar] [CrossRef]
- Nakagawa, M.; Coleman, H.N.; Wang, X.; Daniels, J.; Sikes, J.; Nagarajan, U.M. IL-12 Secretion by Langerhans Cells Stimulated with Candida Skin Test Reagent Is Mediated by Dectin-1 in Some Healthy Individuals. Cytokine 2014, 65, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.; Nofal, E. Intralesional Immunotherapy of Common Warts: Successful Treatment with Mumps, Measles and Rubella Vaccine. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1166–1170. [Google Scholar] [CrossRef]
- Varghese, A.; George, N.M.; Wadhwa, S. Delayed Complete Clearance of Recalcitrant Warts Due to Intralesional Measles, Mumps, and Rubella: Case Report and Review. Cureus 2024, 16, e67225. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.R.; Saikaly, S.K.; Schoch, J.J. Intralesional Immunotherapy for Pediatric Warts: A Review. Pediatr. Dermatol. 2020, 37, 265–271. [Google Scholar] [CrossRef]
- Johnson, S.M.; Roberson, P.K.; Horn, T.D. Intralesional Injection of Mumps or Candida Skin Test Antigens: A Novel Immunotherapy for Warts. Arch. Dermatol. 2001, 137, 451–455. [Google Scholar]
- Na, C.H.; Choi, H.; Song, S.H.; Kim, M.S.; Shin, B.S. Two-Year Experience of Using the Measles, Mumps and Rubella Vaccine as Intralesional Immunotherapy for Warts. Clin. Exp. Dermatol. 2014, 39, 583–589. [Google Scholar] [CrossRef]
- Al-Qassabi, A.M.; Al Kindi, A. Intralesional Immunotherapy with Measles-Mumps-Rubella Vaccine for Recalcitrant Facial Warts: A Report of Two Cases. Oman Med. J. 2022, 37, e353. [Google Scholar] [CrossRef] [PubMed]
- Al-Qassabi, A.M.; Al-Farsi, F. Intralesional Measles-Mumps-Rubella Vaccine for Genital Warts Report of Two Cases with a Review of Literature. Sultan Qaboos Univ. Med. J. 2022, 22, 413–416. [Google Scholar] [CrossRef]
- Kaur, A.; Brar, B.; Kumar, S.; Brar, S.; Boparai, A.; Puri, N. A Randomized Comparative Study of MIP and MMR Vaccine for the Treatment of Cutaneous Warts. Indian J. Dermatol. 2021, 66, 151–158. [Google Scholar] [CrossRef]
- Shaker, E.S.E.; Doghim, N.N.; Hassan, A.M.; Musafa, S.S.; Fawzy, M.M. Immunotherapy in Cutaneous Warts: Comparative Clinical Study between MMR Vaccine, Tuberculin, and BCG Vaccine. J. Cosmet. Dermatol. 2021, 20, 2657–2666. [Google Scholar] [CrossRef]
- Lahoria, U.; Singh, S.; Bhardwaj, A.; Budania, A.; Chhajed, N.; Rajagopal, S.V.; Singh, S. A Prospective Randomized Controlled Study of Mycobacterium Indicus Pranii Vaccine, Measles Mumps Rubella Vaccine and Vitamin D3 in Extragenital Cutaneous Warts. J. Cosmet. Dermatol. 2023, 22, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Awal, G.; Kaur, S. Therapeutic Outcome of Intralesional Immunotherapy in Cutaneous Warts Using the Mumps, Measles, and Rubella Vaccine: A Randomized, Placebo-Controlled Trial. J. Clin. Aesthetic Dermatol. 2018, 11, 15–20. [Google Scholar]
- Sobhy Mohamad, N.; Badran, F.; Yakout, E. Evaluation of the Efficacy of a Combination—Measles, Mumps and Rubella Vaccine in the Treatment of Plantar Warts. Our Dermatol. Online 2013, 4, 463–467. [Google Scholar] [CrossRef]
- Rageh, R.M.; Hewedy, E.S.S.; Hegab, D.S. Intralesional Injection of Candida Albicans Antigen versus Measles, Mumps, and Rubella Vaccine for Treatment of Plantar Warts. Acta Dermatovenerol. Alp. Pannonica Adriat. 2021, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- El-Khalawany, M.; Shaaban, D.; Aboeldahab, S. Immunotherapy of Viral Warts: Myth and Reality. Egypt. J. Dermatol. Venerol. 2015, 35, 1. [Google Scholar] [CrossRef]
- Abd-Elazeim, F.M.A.; Mohammed, G.F.A.; Fathy, A.; Mohamed, R.W. Evaluation of IL-12 Serum Level in Patients with Recalcitrant Multiple Common Warts, Treated by Intralesional Tuberculin Antigen. J. Dermatol. Treat. 2014, 25, 264–267. [Google Scholar] [CrossRef]
- Shaheen, M.A.; Salem, S.A.M.; Fouad, D.A.; El-Fatah, A.A.A. Intralesional Tuberculin (PPD) versus Measles, Mumps, Rubella (MMR) Vaccine in Treatment of Multiple Warts: A Comparative Clinical and Immunological Study. Dermatol. Ther. 2015, 28, 194–200. [Google Scholar] [CrossRef]
- Abou-Taleb, D.A.E.; Abou-Taleb, H.A.; El-Badawy, O.; Ahmed, A.O.; Thabiet Hassan, A.E.; Awad, S.M. Intralesional Vitamin D3 versus Intralesional Purified Protein Derivative in Treatment of Multiple Warts: A Comparative Clinical and Immunological Study. Dermatol. Ther. 2019, 32, e13034. [Google Scholar] [CrossRef]
- Sil, A.; Dasgupta, S.; Chandra, S.; Datta, A.; Banerjee, A.; Das, N. Changes in Cytokine Profile with Immunotherapy in Viral Warts Using Purified Protein Derivative, Mumps Measles Rubella Vaccine, and Mycobacterium w Vaccine. Indian J. Dermatol. 2021, 66, 67–73. [Google Scholar] [CrossRef]
- Aldahan, A.S.; Mlacker, S.; Shah, V.V.; Kamath, P.; Alsaidan, M.; Samarkandy, S.; Nouri, K. Efficacy of Intralesional Immunotherapy for the Treatment of Warts: A Review of the Literature. Dermatol. Ther. 2016, 29, 197–207. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Rawi, J.R.; Al-Nuaimy, A.A.; Radhy, S.H. Bacille Calmette-Guerin Immunotherapy of Viral Warts. Saudi Med. J. 2008, 29, 589–593. [Google Scholar] [PubMed]
- Mohta, A.; Kushwaha, R.K.; Agrawal, A.; Sharma, M.K.; Gautam, U.; Jain, S.K. Evaluation of the Efficacy of Intralesional Measles, Mumps, and Rubella Vaccine with Intralesional Vitamin D3 as Immunotherapies in the Treatment of Recalcitrant Cutaneous Warts in Adult- A Randomized Placebo-Controlled Study. Indian Dermatol. Online J. 2021, 12, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Baveja, S. Intralesional Immunotherapy for Difficult to Treat Warts with Mycobacterium w Vaccine. J. Cutan. Aesthet. Surg. 2014, 7, 203–208. [Google Scholar] [CrossRef]
- Meena, J.K.; Malhotra, A.K.; Mathur, D.K.; Mathur, D.C. Intralesional Immunotherapy with Mycobacterium w Vaccine in Patients with Multiple Cutaneous Warts: Uncontrolled Open Study. JAMA Dermatol. 2013, 149, 237–239. [Google Scholar] [CrossRef]
- Thappa, D.; Chiramel, M. Evolving Role of Immunotherapy in the Treatment of Refractory Warts. Indian Dermatol. Online J. 2016, 7, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.M.; Nofal, A.; Alakad, R. Intralesional Antigen Immunotherapy for the Treatment of Plane Warts: A Comparative Study. Dermatol. Ther. 2020, 33, e13807. [Google Scholar] [CrossRef]
- Elsayed Ghaly, N.; El-Ashmawy, A.A.; Abou Zeid, M.; Engi, E.S. Efficacy and Safety of Intralesional Injection of Vitamin D3 versus Tuberculin PPD in the Treatment of Plantar Warts: A Comparative Controlled Study. J. Cosmet. Dermatol. 2021, 20, 1231–1240. [Google Scholar] [CrossRef]
- Nofal, A.; Alakad, R.; Fouda, I.; Fawzy, M.M. Intralesional Antigen Immunotherapy in the Treatment of Periungual Warts. J. Cutan. Med. Surg. 2021, 25, 286–292. [Google Scholar] [CrossRef]
- Rutnin, S.; Namasondhi, A.; Pomsoong, C.; Kositkuljorn, C.; Anuntrangsee, T.; Thadanipon, K. Intralesional Measles, Mumps, Rubella Vaccine versus Tuberculin Purified Protein Derivative Injections in the Treatment of Palmoplantar and Periungual Warts: A Double-Blind Randomized Controlled Trial. Dermatology 2023, 239, 109–115. [Google Scholar] [CrossRef]
- Ebrahim, H.M.; Asaad, A.M.; El Desoky, F.; Morsi, H.M. Bacillus Calmette-Guerin Polysaccharide Nucleic Acid vs Bacillus Calmette-Guerin Vaccine in the Treatment of Warts: A Comparative, Double-Blind, Controlled Study. Dermatol. Ther. 2021, 34, e14549. [Google Scholar] [CrossRef]
- Eldahshan, R.M.; Ashry, W.M.O.; Elsaie, M.L. Comparative Study between Intralesional Injection of MMR, BCG, and Candida Albicans Antigen in Treatment of Multiple Recalcitrant Warts. J. Cosmet. Dermatol. 2022, 21, 1120–1126. [Google Scholar] [CrossRef]
- Dhakar, A.K.; Dogra, S.; Vinay, K.; Sarangal, R.; Kanwar, A.J.; Singh, M.P. Intralesional Mycobacterium w Vaccine versus Cryotherapy in Treatment of Refractory Extragenital Warts: A Randomized, Open-Label, Comparative Study. J. Cutan. Med. Surg. 2016, 20, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Nischal, K.; Sowmya, C.; Swaroop, M.; Agrawal, D.; Basavaraj, H.; Sathyanarayana, B. A Novel Modification of the Autoimplantation Therapy for the Treatment of Multiple, Recurrent and Palmoplantar Warts. J. Cutan. Aesthet. Surg. 2012, 5, 26–29. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Bajaj, A.K. Autowart Injection Therapy for Recalcitrant Warts. Indian J. Dermatol. 2010, 55, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, K.N.; Shukla, P. Role of Autoinoculation in the Management of Cutaneous Warts: A Comparison Study with 100% Tricholoroacetic Acid Application. Int. J. Res. Dermatol. 2020, 6, 537–543. [Google Scholar] [CrossRef]
- Shivakumar, V.; Okade, R.; Rajkumar, V. Autoimplantation Therapy for Multiple Warts. Indian J Dermatol Venereol Leprol 2009, 75, 593. [Google Scholar] [CrossRef]
- Taneja, G.; Hazarika, N.; Bhatia, R. Effectiveness of Autoinoculation in Viral Warts: A Single Arm, Open-Label, and Clinical Trial. Dermatol. Ther. 2020, 33, e14122. [Google Scholar] [CrossRef]
- Abdelmonaem, N.A.; Shaheen, M.A.; Mohsen Mohamed Foad, T.; El-Husseiny, R. Efficacy and Safety of Homologous Autoinoculation in Treatment of Multiple Recalcitrant Warts of Different Types. J. Cosmet. Dermatol. 2021, 20, 2240–2246. [Google Scholar] [CrossRef]
- Hammad, N.M.; Marei, A.; El-Didamony, G.; Mortada, Z.; Elradi, M.; Afifi, A.H.M.; Kadry, H.M. Predictors of the Therapeutic Response to Intralesional Bivalent Hpv Vaccine in Wart Immunotherapy. Vaccines 2021, 9, 1280. [Google Scholar] [CrossRef]
- Acharya, R.; Bush, R.; Johns, F.; Upadhyay, K. Efficacy and Safety of Local Candida Immunotherapy in Recalcitrant Warts in Pediatric Kidney Transplantation: A Case Report. World J. Transplant. 2023, 13, 201–207. [Google Scholar] [CrossRef]
- Phillips, R.; TS, R.; JL, P.; MR, G. Treatment of Warts with Candida Antigen Injection. Arch Dermatol 2000, 136. [Google Scholar]
- Majid, I.; Imran, S. Immunotherapy with Intralesional Candida Albicans Antigen in Resistant or Recurrent Warts: A Study. Indian J. Dermatol. 2013, 58, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Griffin, J.R.; Newman, C.C. Use of Candida Antigen Injections for the Treatment of Verruca Vulgaris: A Two-Year Mayo Clinic Experience. J. Dermatol. Treat. 2016, 27, 355–358. [Google Scholar] [CrossRef]
- Kawashima, S.; Joachim, K.; Abdelrahim, M.; Abudayyeh, A.; Jhaveri, K.D.; Murakami, N. Immune Checkpoint Inhibitors for Solid Organ Transplant Recipients: Clinical Updates. Korean J. Transplant. 2022, 36, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Guo, K.; Heilman, R.L.; Poggio, E.D.; Taber, D.J.; Marsh, C.L.; Kurian, S.M.; Kleiboeker, S.; Weems, J.; Holman, J.; et al. Combining Blood Gene Expression and Cellfree Dna to Diagnose Subclinical Rejection in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2021, 16, 1539–1551. [Google Scholar] [CrossRef]
- Dandamudi, R.; Gu, H.; Goss, C.W.; Walther, L.; Dharnidharka, V.R. Longitudinal Evaluation of Donor-Derived Cellfree DNA in Pediatric Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2022, 17, 1646–1655. [Google Scholar] [CrossRef]
- Marei, A.; Nofal, A.; Alakad, R.; Abdel-Hady, A. Combined Bivalent Human Papillomavirus Vaccine and Candida Antigen versus Candida Antigen Alone in the Treatment of Recalcitrant Warts. J. Cosmet. Dermatol. 2020, 19, 758–762. [Google Scholar] [CrossRef]
- Abdelaal, M.A.; Abdelaziz, H.M.; Ahmed, K.A.; Elsaie, M.L. Comparative Study of Intralesional Vitamin D3 Injection and Candida Albicans Antigen in Treating Plantar Warts. J. Drugs Dermatol. 2021, 20, 546–549. [Google Scholar] [CrossRef]
- Nofal, A.; El-arab, R.E.; Nasr, M.; Alakad, R. Intralesional Measles, Mumps, and Rubella Vaccine Versus Intralesional Candida Antigen in the Treatment of Common and Plantar Warts. J. Cutan. Med. Surg. 2021, 25, 377–383. [Google Scholar] [CrossRef]
- Nofal, A.; Khedr, A.; Fathy, M. Combined Oral Isotretinoin and Candida Antigen versus Either Agent Alone in the Treatment of Plane Warts. J. Dermatol. Treat. 2022, 33, 342–347. [Google Scholar] [CrossRef]
- Nasr, M.; Abdelaty, S.; Elkholy, B.M. A Comparative Clinico-Dermoscopic Study of Intralesional Injection of Combined Digoxin and Furosemide, Candida Antigen, and Vitamin D3 for Multiple Warts. J. Cosmet. Dermatol. 2023, 22, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Youssef, E.M.K.; Eissa, M.A.A.; Bakr, R.M. Intralesional Candida Albicans Antigen versus Intralesional Zinc Sulfate in Treatment of Cutaneous Warts. Arch. Dermatol. Res. 2023, 315, 1305–1314. [Google Scholar] [CrossRef]
- Hodeib, A.A.E.; Al-Sharkawy, B.G.; Hegab, D.S.; Talaat, R.A.Z. A Comparative Study of Intralesional Injection of Candida Albicans Antigen, Bleomycin and 5-Fluorouracil for Treatment of Plane Warts. J. Dermatolog Treat. 2021, 32, 663–668. [Google Scholar] [CrossRef]
- Aly, R. Ecology and Epidemiology of Dermatophyte Infections. J. Am. Acad. Dermatol. 1994, 31, S21–S25. [Google Scholar] [CrossRef] [PubMed]
- Woodfolk, J.A.; Wheatley, L.M.; Piyasena, R.V.; Benjamin, D.C.; Platts-Mills, T.A.E. Trichophyton Antigens Associated with IgE Antibodies and Delayed Type Hypersensitivity. Sequence Homology to Two Families of Serine Proteinases. J. Biol. Chem. 1998, 273, 29489–29496. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.D.; Johnson, S.M.; Helm, R.M.; Roberson, P.K. Intralesional Immunotherapy of Warts with Mumps, Candida, and Trichophyton Skin Test Antigens: A Single-Blinded, Randomized, and Controlled Trial. Arch. Dermatol. 2005, 141, 589–594. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.G.; Zeng, Z.M.; Yu, Z.J.; Huang, N.; Deng, Q. wen Interferon for the Treatment of Genital Warts: A Systematic Review. BMC Infect. Dis. 2009, 9, 156. [Google Scholar] [CrossRef]
- Varnavides, C.K.; Henderson, C.A.; Cunliffe, W.J. Intralesional Interferon: Ineffective in Common Viral Warts. J. Dermatol. Treat. 1997, 8, 169–172. [Google Scholar] [CrossRef]
- Lee, S.W.; Houh, D.; Kim, H.O.; Kim, C.W.; Kim, T.Y. Clinical Trials of Interferon-Gamma in Treating Warts. Ann. Dermatol. 1990, 2, 77–82. [Google Scholar] [CrossRef]
- Vance, J.C.; Bart, B.J.; Hansen, R.C.; Reichman, R.C.; McEwen, C.; Hatch, K.D.; Berman, B.; Tanner, D.J. Intralesional Recombinant Alpha-2 Interferon for the Treatment of Patients with Condyloma Acuminatum or Verruca Plantaris. Arch. Dermatol. 1986, 122, 272–277. [Google Scholar] [CrossRef]
- Al-Sabak, H.; Al-Hattab, M.; Al-Rammahi, M.; Al-Dhalimi, M. The Efficacy of Intralesional Vitamin D3 Injection in the Treatment of Cutaneous Warts: A Clinical Therapeutic Trial Study. Skin Res. Technol. 2023, 29, e13442. [Google Scholar] [CrossRef] [PubMed]
- Prathibha, J.P.; Varghese, N.; Aithal, V.V. Intralesional Vitamin D3 versus Bleomycin for Difficult-to-Heal Palmoplantar Warts: A Comparative Study. J. Cutan. Aesthet. Surg. 2023, 16, 114–120. [Google Scholar] [CrossRef]
- Chia-Han Yeh, M.; Tsai, T.Y.; Huang, Y.C. Intralesional Vitamin D3 Injection in the Treatment of Warts: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2020, 82, 1013–1015. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdel Fadeel, D.A.; Sadek, A.; Fadel, M.; Tawfik, A. Intralesional Vitamin D3 versus New Topical Photodynamic Therapy in Recalcitrant Palmoplanter Warts Randomized Comparative Controlled Study. Photodiagn. Photodyn. Ther. 2020, 32, 101979. [Google Scholar] [CrossRef]
- Zainab, Z.; Malik, N.A.; Obaid, S.; Mumtaz, M.; Aftab, K.; Malik, S. Role of Intralesional Vitamin-D in Viral Warts. J. Ayub Med. Coll. Abbottabad 2021, 33, 598–601. [Google Scholar]
- Abdel Razik, L.H.; Obaid, Z.M.; Fouda, I. Intralesional Candida Antigen versus Intralesional Vitamin D3 in the Treatment of Recalcitrant Multiple Common Warts. J. Cosmet. Dermatol. 2021, 20, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Almuhyi, R.A.; Alhamdi, K.I.; Alhamdi, D.K. Topical Vitamin D3 Derivative (Calcipotriol) Versus Intralesional Vitamin D3 in the Treatment of Cutaneous Warts: A Clinical Therapeutic Comparative Trial. Dermatol. Res. Pract. 2024, 2024, 5236290. [Google Scholar] [CrossRef]
- Bacelieri, R.; Johnson, S.M. Cutaneous Warts: An Evidence-Based Approach to Therapy. Am. Fam. Physician 2005, 72, 647–652. [Google Scholar] [PubMed]
- Mullen, S.A.; Myers, E.L.; Brenner, R.L.; Nguyen, K.T.; Harper, T.A.; Welsh, D.; Keffer, S.; Mueller, J.; Whitley, M.J. Systematic Review of Intralesional Therapies for Cutaneous Warts. JID Innov. Ski. Sci. Mol. Popul. Health 2024, 4, 100264. [Google Scholar] [CrossRef]
- Martin, A.; Thatiparthi, A.; Nourmohammadi, N.; Nguyen, C.; Sung, C.T.; Mesinkovska, N.A. Emerging Intralesional Treatments for Plantar Warts: A Systematic Review. J. Drugs Dermatol. 2022, 21, 1322–1329. [Google Scholar] [CrossRef]
- Al-Naggar, M.R.; Al-Adl, A.S.; Rabie, A.R.; Abdelkhalk, M.R.; Elsaie, M.L. Intralesional Bleomycin Injection vs Microneedling-Assisted Topical Bleomycin Spraying in Treatment of Plantar Warts. J. Cosmet. Dermatol. 2019, 18, 124–128. [Google Scholar] [CrossRef]
- Yazdanfar, A.; Farshchian, M.; Fereydoonnejad, M.; Farshchian, M. Treatment of Common Warts with an Intralesional Mixture of 5-Fluorouracil, Lidocaine, and Epinephrine: A Prospective Placebo-Controlled, Double-Blind Randomized Trial. Dermatol. Surg. 2008, 34, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Sepaskhah, M.; Sarani, M.B.; Bagheri, Z. Comparison of the Efficacy of Intralesional 5-Fluorouracil/Lidocaine/Epinephrine Injection with Cryotherapy to Treat Common and Palmoplantar Warts: A Randomized, Controlled Trial. Dermatol. Ther. 2022, 35, e15726. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Gerriets, V. Acyclovir. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Elion, G.B. Mechanism of Action and Selectivity of Acyclovir. Am. J. Med. 1982, 73, 7–13. [Google Scholar] [CrossRef]
- Agarwal, A.; Bansal, P.; Madhual, S.; Panda, M. Modified Intralesional Aciclovir Treatment in the Management of Recalcitrant Palmoplantar and Ungual Warts: A Case Series of 14 Patients. Clin. Exp. Dermatol. 2025, 50, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Meghana Reddy, E.; Rajashekar, T.S.; Suresh Kumar, K. A Comparative Study of Intralesional Acyclovir vs Immunotherapy for Treatment of Viral Warts. Cureus 2023, 15, e38781. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Zhou, C.; Cao, X. A Real-World Disproportionality Analysis of Cidofovir from the FDA Adverse Event Reporting System (FAERS) Database. Expert. Opin. Drug Saf. 2025, 1–9. [Google Scholar] [CrossRef]
- Cook, M.K.; Hagen, E.M.; Feldman, S.R. Cidofovir in the Management of Non-Genital Warts:A Review. J. Drugs Dermatol. 2023, 22, 1009–1016. [Google Scholar] [CrossRef]
- Washif, M.; Kawasumi, R.; Hirota, K. PrimPol-Mediated Repriming Elicits Gap-Filling by Template Switching and Promotes Cellular Tolerance to Cidofovir. DNA Repair 2025, 145, 103787. [Google Scholar] [CrossRef]
- Poppens, M.; Davis, J. Intralesional Cidofovir for the Treatment of Recalcitrant Periungual Warts. J. Dermatolog Treat. 2023, 34, 2154569. [Google Scholar] [CrossRef]
- Anshelevich, E.E.; Barbieri, J.S.; Kovarik, C.L. Intralesional Cidofovir for Treatment of Recalcitrant Warts in Both Immunocompetent and Immunocompromised Patients: A Retrospective Analysis of 58 Patients. J. Am. Acad. Dermatol. 2021, 84, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.H.; Kim, S.H.; Jung, D.S.; Ko, H.C.; Kim, B.S.; Kwon, K.S.; Kim, M.B. Oral Zinc Sulfate Treatment for Viral Warts: An Open-Label Study. J. Dermatol. 2011, 38, 541–545. [Google Scholar] [CrossRef] [PubMed]
- El-Haggar, S.M.; Attalla, D.S.; Elhelbawy, M.; El-Afify, D.R. A Randomized Clinical Study to Evaluate the Possible Antifibrotic Effect of Zinc Sulfate in Chronic HCV Patient Receiving Direct-Acting Anti-Viral Therapy. Inflammopharmacology 2025, 33, 329. [Google Scholar] [CrossRef]
- El Taweel, A.A.; Salem, R.; Allam, A. Intralesional 2% Zinc Sulfate Solution for Plane Warts: A Case Report. Dermatol. Ther. 2019, 32, e12761. [Google Scholar] [CrossRef]
- El Sayed, M.H.; Sayed, F.S.; Afify, A.A. Intralesional Zinc Sulfate 2% vs Intralesional Vitamin D in Plantar Warts: A Clinicodermoscopic Study. Dermatol. Ther. 2020, 33, e13308. [Google Scholar] [CrossRef]
- Mohamed, E.E.M.; Tawfik, K.M.; Mahmoud, A.M. The Clinical Effectiveness of Intralesional Injection of 2% Zinc Sulfate Solution in the Treatment of Common Warts. Scientifica 2016, 2016, 1082979. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Intralesional Methotrexate in Dermatology: Diverse Indications and Practical Considerations. Dermatol. Ther. 2021, 34, e14404. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Abdo, H.M.; Elrewiny, E.M.; Elkholy, M.S.; Ibrahim, S.M. Efficacy of Intralesional Methotrexate in the Treatment of Plantar Warts. Dermatol. Ther. 2020, 33, e13228. [Google Scholar] [CrossRef]
- Zoheir, M.G.T.; Almohsen, A.M.; Amer, M.A.E.M.; Abdel-Hameed, A.K.S. Intralesional Full-Concentration (25 Mg/Ml) Methotrexate in the Treatment of Plantar Warts: A Pilot Study. Dermatol. Ther. 2022, 35, e15815. [Google Scholar] [CrossRef]
- Salman, S.; Ahmed, M.S.; Ibrahim, A.M.; Mattar, O.M.; El-Shirbiny, H.; Sarsik, S.; Afifi, A.M.; Anis, R.M.; Yakoub Agha, N.A.; Abushouk, A.I. Intralesional Immunotherapy for the Treatment of Warts: A Network Meta-Analysis. J. Am. Acad. Dermatol. 2019, 80, 922–930.e4. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Nofal, A.; Bakr, N.M.; Essam, R.; Alakad, R. Correlation of Serum Interleukin 17 and Macrophage Migration Inhibitory Factor Levels with Clinical Response to Intralesional Candida Antigen and Their Potential Use as Predictors of Clinical Outcome in Patients with Multiple Common Warts. J. Cosmet. Dermatol. 2022, 21, 3970–3978. [Google Scholar] [CrossRef]
- Korsa, H.H.A.; Nashaat, H.A.H.; Halim, H.M.; Atwa, M.A.; Mahmoud Marie, R.E.S. Interleukin-18 Serum Level before and after Intralesional Immunotherapy with Tuberculin Purified Protein Derivative in Patients with Cutaneous and Genital Warts. J. Cosmet. Dermatol. 2022, 21, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; El Taieb, M.; Nada, E.; Kamal, E.; Hegazy, E. Combined Intralesional Injection of Tuberculin Purified Protein Derivative plus Cryotherapy versus Each Alone in the Treatment of Multiple Common Warts. Dermatol. Ther. 2022, 35, e15350. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.; Gomaa, A.S.; Hassan, H.A.; Tawfik, Y.M. Efficacy of Cryotherapy Combined with Intralesional Purified Protein Derivative (PPD) Versus Intralesional PPD Monotherapy in the Treatment of Multiple Common Warts. J. Cutan. Med. Surg. 2023, 27, 117–125. [Google Scholar] [CrossRef]
- Attwa, E.; Elawady, R.; Salah, E. ‘Cryo-Immuno-Therapy’ Is Superior to Intralesional Candida Antigen Monotherapy in the Treatment of Multiple Common Warts. J. Dermatol. Treat. 2021, 32, 1018–1025. [Google Scholar] [CrossRef]
- Nofal, A.; Khattab, F.; Nofal, E.; Elgohary, A. Combined Acitretin and Candida Antigen versus Either Agent Alone in the Treatment of Recalcitrant Warts. J. Am. Acad. Dermatol. 2018, 79, 377–378. [Google Scholar] [CrossRef]
- Nofal, A.; Yehia, E.; Khater, E.; Bessar, H. Alternating Intralesional Purified Protein Derivative and Candida Antigen versus Either Agent Alone in the Treatment of Multiple Common Warts. J. Am. Acad. Dermatol. 2020, 83, 208–210. [Google Scholar] [CrossRef]
- Choi, G.S.; Park, J.H.; Kim, Y.K.; Choi, G.S. Combination Therapy with Intralesional Interferon α-2b and Pulsed Dye Laser for the Treatment of Periungual Warts. Ann. Dermatol. 2002, 14, 82–87. [Google Scholar] [CrossRef]
- Nofal, A.A.; Elkholy, B.M.; Abd-Elmonsef, E.R.; Nofal, H.O. Triple Intralesional Antigen Immunotherapy versus Monoantigen in the Treatment of Multiple Recalcitrant Warts. Dermatol. Ther. 2022, 12, 1225–1237. [Google Scholar] [CrossRef]
- Ferguson, S.B.; Gallo, E.S. Nonavalent Human Papillomavirus Vaccination as a Treatment for Warts in an Immunosuppressed Adult. JAAD Case Rep. 2017, 3, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Wyant, W.A.; Burke, G.W.; Ioannides, T.; Nichols, A.J. Systemic 9-Valent Human Papillomavirus Vaccine for Recalcitrant Common Cutaneous Warts in Preparation for Renal Transplant. JAAD Case Rep. 2022, 22, 62–63. [Google Scholar] [CrossRef]
- Nofal, A.; Marei, A.; Ibrahim, A.-S.M.; Nofal, E.; Nabil, M. Intralesional versus Intramuscular Bivalent Human Papillomavirus Vaccine in the Treatment of Recalcitrant Common Warts. J. Am. Acad. Dermatol. 2020, 82, 94–100. [Google Scholar] [CrossRef]
- Fouda, I.; Mohammed, H.A.K.; Mohammed, G.M.Y. Intralesional Quadrivalent Human Papilloma Virus Vaccine Versus Candida Antigen in the Treatment of Multiple Recalcitrant Non-Genital Warts. Dermatol. Pract. Concept. 2024, 14, e2024066. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, M.I.; Obaid, Z.M.; Fouda, I. Efficacy of Intralesional Acyclovir versus Quadrivalent Human Papillomavirus Vaccine for Treatment of Recalcitrant Cutaneous Warts: A Clinical Trial. Arch. Dermatol. Res. 2025, 317, 540. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, E.; Bar, J.; Baniel, A.; Slodownik, D.; Artzi, O.; Samuelov, L.; Sprecher, E.; Mashiah, J. Intralesional Human Papillomavirus Vaccine for the Treatment of Recalcitrant Cutaneous Warts. J. Dermatol. 2023, 50, 1373–1380. [Google Scholar] [CrossRef]
- Nofal, A.; Elaraby, A.; Elkholy, B.M. Intralesional Versus Intramuscular Hepatitis B Virus Vaccine in the Treatment of Multiple Common Warts. Dermatol. Surg. 2022, 48, 1178–1184. [Google Scholar] [CrossRef]
| IFN Type | Produced by | Key Immune Functions | HPV Evasion Strategy |
|---|---|---|---|
| IFN-α/β [23,32,33] | Keratinocytes, DCs |
|
|
| IFN-γ [32,33] | NK cells, NKT cells, activated CD4+ and CD8+ T cells |
|
|
| IFN-λ [32,33] | Epithelial cells, DCs at mucosal/skin surfaces |
|
|
| Author (Year) | Type of Warts | Injection Protocol | Follow-Up | Complete Clearance Rate |
|---|---|---|---|---|
| Shaker ESE (2021) [51] | Single or multiple cutaneous | Up to 3, every 3 weeks | 6 months | 30% |
| Kaur A (2021) [50] | Cutaneous | 3 sessions, every 3 weeks | Every 4 weeks for 24 weeks | 76.67% |
| Lahoria U. (2023) [52] | Extragenital cutaneous | Every 2 weeks, up to 7 or until clearance | Not specified | 58% |
| Study | Cytokines Affected | Direction of Change Post-PPD |
|---|---|---|
| Abd-Elazeim et al. (2014) [57] | IL-12 | ↑ |
| Shaheen et al. (2015) [58] | IL-12 | ↑ |
| Abou-Taleb et al. (2019) [59] | IFN-γ, IL-12 | ↑ |
| Sil et al. (2021) [60] | IL-10 | ↓ |
| Author (Year) | Type of Warts | Agent & Dose | Injection Protocol | Complete Clearance Rate |
|---|---|---|---|---|
| Fawzy et al. (2020) [67] | Plane | PPD 0.1 mL | Every 2 weeks, max 5 sessions | 55% |
| Ghaly et al. (2021) [68] | Plantar | PPD 0.1 mL | Every 2 weeks, max 3 sessions | 50% |
| Nofal et al. (2021) [69] | Periungual | PPD 0.1 mL | Every 2 weeks, max 5 sessions | 70% |
| Rutnin et al. (2023) [70] | Palmoplantar & periungual | PPD 0.3 mL (20 IU/mL) | Every 2 weeks, max 5 sessions | 80% |
| Ebrahim et al. (2021) [71] | Multiple | BCG 0.1 mL | Up to 5 sessions, 3 weeks apart | 63.8% |
| Eldahshan et al. (2022) [72] | Recalcitrant extragenital | BCG 0.1 mL | Every 2 weeks, up to 5 sessions | 70% |
| Dhakar et al. (2016) [73] | Refractory extragenital | Mw 0.1 mL | Into largest/3 lesions; weekly, max 12 or until clearance | 66.7% |
| Author (Year) | Type of Warts | Injection Protocol | Follow-Up | Clearance Rate |
|---|---|---|---|---|
| Taneja et al. (2020) [78] | Multiple viral | 3 sessions (months 0, 1, 2); fewer if cleared | Final check at 3 months | 67% |
| Abdelmonaem et al. (2021) [79] | Multiple recalcitrant | 1 session | Weeks 1, 2, 12, 16; based on size/number | 66% |
| Ashraf et al. (2023) [40] | Multiple | Up to 3, at 1-month intervals | 3 months after last session | 91.66% |
| Shahid et al. (2023) [39] | Multiple viral | Max 3, monthly | Months 1, 2, 3; based on count reduction | 77.1% |
| First Author (Year) | Type of Warts | Candida Solution Dose Per Session | Injection Protocol | Complete Clearance Rate |
|---|---|---|---|---|
| Fawzy MM (2020) [67] | Plane warts | 0.3 mL of 1/100 solution | Every 2 weeks, max 5 sessions | 76.7% |
| Marei A (2020) [88] | Recalcitrant warts | 0.2 mL of 1/1000 solution | Every 2 weeks, 5 sessions | 40% |
| Rageh RM (2021) [55] | Plantar warts | 0.3 mL of 1:100 solution | Every 3 weeks, max 5 sessions | 80% |
| Abdelaal MA (2021) [89] | Plantar warts | 0.1 mL into largest wart | Every 3 weeks, up to 3 sessions | 40% |
| Hodeib AAE (2021) [94] | Plane warts | 0.3 mL | Every 2 weeks, up to 4 sessions | 60% |
| Nofal A (2021) [90] | Common & plantar warts | 0.2 mL into largest wart | Every 2 weeks, max 5 sessions | 73.5% |
| Nofal A (2022) [91] | Plane warts | 0.1 mL of 1/1000 solution | Every 2 weeks, max 5 sessions | 55.6% |
| Nasr M (2023) [92] | Multiple warts | Not specified | Every 2 weeks, up to 5 sessions | 60% |
| Youssef EMK (2023) [93] | Common, plantar, plane | 0.1 mL of 1/100 and 1/1000 solutions | Not clearly defined | 94.3% (1/100); 77.1% (1/1000) |
| First Author (Year) | Type of Warts | Vitamin D3 Dose Per Session | Injection Protocol | Clearance Rate |
|---|---|---|---|---|
| Ibrahim NA et al. (2020) [105] | Recalcitrant palmoplantar | 0.2 mL (300,000 IU/mL) | Up to 5 warts/session, monthly, max 4 sessions | 88.89% |
| Zainab Z et al. (2021) [106] | Cutaneous | 0.2 mL (15 mg/mL) | Every 2 weeks, 4 sessions | 57.9% |
| Abdel Razik LH et al. (2021) [107] | Recalcitrant multiple common | 0.6 mL (60,000 IU/wart) | Every 3 weeks until clearance | 20% |
| Lahoria U et al. (2023) [52] | Extragenital cutaneous | 0.2 mL (IU not specified) | Every 2 weeks, response monitored | 64% |
| Nasr et al. (2023) [92] | Multiple | 0.3 mL (100,000 IU/mL) | Every 2 weeks, up to 5 sessions | 48% |
| Prathibha et al. (2023) [103] | Palmoplantar | 0.2–0.5 mL (~15 mg/mL) | Every 2 weeks, up to 4 sessions | 88.5% |
| Al-Sabak et al. (2023) [102] | Cutaneous | 0.2 mL (600,000 IU/ampoule) | 4 sessions every 2 weeks | 81.9% |
| Almuhyi et al. (2024) [108] | Cutaneous | 0.2–0.3 mL (300,000 IU/ampoule) | 4 sessions every 2 weeks | 59% |
| First Author (Year) | Compared Interventions | Categories of Treated Cutaneous Warts | Dosage | Regimen | Study Population | Complete Clearence Rate | Additional Remarks |
|---|---|---|---|---|---|---|---|
| Abdel Razik L, (2021) [107] | Candida antigen and vitamin D3 | Recalcitrant multiple common warts | Group I: 0.3 mL of 1/100 Candida antigen solution Group II: 0.6 mL of cholecalciferol aqueous solution Group III: 0.3 mL of normal saline. | Solution injected into largest wart at 3-week intervals until complete clearance or for maximum 4 treatment sessions | 80 patients with 30 patients assigned to group I and group II and 20 to group III | 76.7% in Candida antigen group; 20% in vitamin D3 group | |
| Fawzy MM, (2020) [67] | PPD and Candida antigen and MMR vaccine | Plane warts | Group I: 0.1 mL of PPD Group II: 0.1 mL of 1/1000 solution of Candida antigen Group III: 0.1 mL of MMR vaccine | Patients were injected directly, without pre- sensitization. Injections were administered at 2-week intervals until complete clearance or for a maximum of 5 treatment sessions | 120 patients, with 40 patients assigned to each treatment group | 55% in the PPD group; 70% in the Candida antigen group 62.5% in the MMR group | Recurrence of warts was observed in 3 patients of the PPD group, while Candida antigen and MMR groups showed no recurrence of the lesions after the 6 month follow-up period |
| Kaur A, 2021 [50] | MIP and MMR vaccine | Cutaneous warts | Group A: 0.1 mL of MIP vaccine Group B: 0.5 mL of MMR vaccine | Injections without pre- sensitization. were repeated at intervals of 3 weeks, or until complete clearance or maximum of 3 treatment sessions | 60 patients, with 30 patients assigned to each treatment group | 90% in the MIP group; 76.67% in the MMR group | MIP showed an early response compared to MMR (9.41 vs. 11.71 weeks) |
| Nasr M, (2023) [92] | Candida antigen and vitamin D3 | Multiple warts | Group A: 0.3 mL of 1/100 solution of Candida antigen Group B: 0.3 mL of cholecalciferol aqueous solution | Solution of Candida antigen was injected into the base of largest wart; cholecalciferol aqueous was injected into the base of each wart with maximum of 5 warts; Injections were administered at 2-weeks intervals until full clearance, or for a total of five sessions | 50 patients with 25 patients assigned to each treatment group | 60% in the Candida antigen group; 48% in vitamin D3 group | Candida antigen is superior in common warts, palmoplantar warts and subungual warts |
| Rageh RM, (2021) [55] | Candida albicans antigen and MMR vaccine | Plantar warts | Group A: 0.3 mL of 1/100 solution of Candida antigen Group B: 0.3 mL of MMR vaccine | Solution injected into largest wart at 3-week intervals until complete clearance or for maximum 5 treatment sessions | 60 patients, with 30 patients assigned to each treatment group | 80% in the Candida antigen group; 26.7% in the MMR group | 41.7% patients with concomitant distant warts in Group A and 12.5% in Group B showed resolution of their distant un-injected warts |
| Rutnin S, (2023) [70] | MMR vaccine and PPD | Palmoplantar and Periungual Warts | Group A: 0.3 mL of MMR vaccine Group B: 0.3 mL of PPD 20 iu/mL | Injection to the wart or largest wart in patients with multiple lesions; reinjection every 2 weeks until complete clearance or a maximum of 5 injections | 40 patients, with 20 patients assigned to each treatment group | 90% in the MMR treated group; 80% in PPD treated group | MMR showing trend toward faster clearance |
| Treatment Group | Complete Response | Partial Response | No Response | Distant Response (Complete) | Distant Response (Partial) |
|---|---|---|---|---|---|
| HPV Vaccine | 75% | 0% | 25% | 72.7% | 27.3% |
| Candida Antigen | 40% | 15% | 45% | 33.3% | 50% |
| Placebo (Saline) | 5% | 15% | 80% | 11.1% | 22.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharczyk, E.; Pawłuszkiewicz, K.; Biliński, K.; Maj, J.; Ponikowska, M. Intralesional Immunotherapy for Non-Genital Viral Warts: A Review of Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2025, 26, 5644. https://doi.org/10.3390/ijms26125644
Kucharczyk E, Pawłuszkiewicz K, Biliński K, Maj J, Ponikowska M. Intralesional Immunotherapy for Non-Genital Viral Warts: A Review of Current Evidence and Future Perspectives. International Journal of Molecular Sciences. 2025; 26(12):5644. https://doi.org/10.3390/ijms26125644
Chicago/Turabian StyleKucharczyk, Emilia, Karolina Pawłuszkiewicz, Karol Biliński, Joanna Maj, and Małgorzata Ponikowska. 2025. "Intralesional Immunotherapy for Non-Genital Viral Warts: A Review of Current Evidence and Future Perspectives" International Journal of Molecular Sciences 26, no. 12: 5644. https://doi.org/10.3390/ijms26125644
APA StyleKucharczyk, E., Pawłuszkiewicz, K., Biliński, K., Maj, J., & Ponikowska, M. (2025). Intralesional Immunotherapy for Non-Genital Viral Warts: A Review of Current Evidence and Future Perspectives. International Journal of Molecular Sciences, 26(12), 5644. https://doi.org/10.3390/ijms26125644