Three Dimensionally Printed Octacalcium Phosphate via Binder Jetting for Use in Bone Grafting Applications
Abstract
1. Introduction
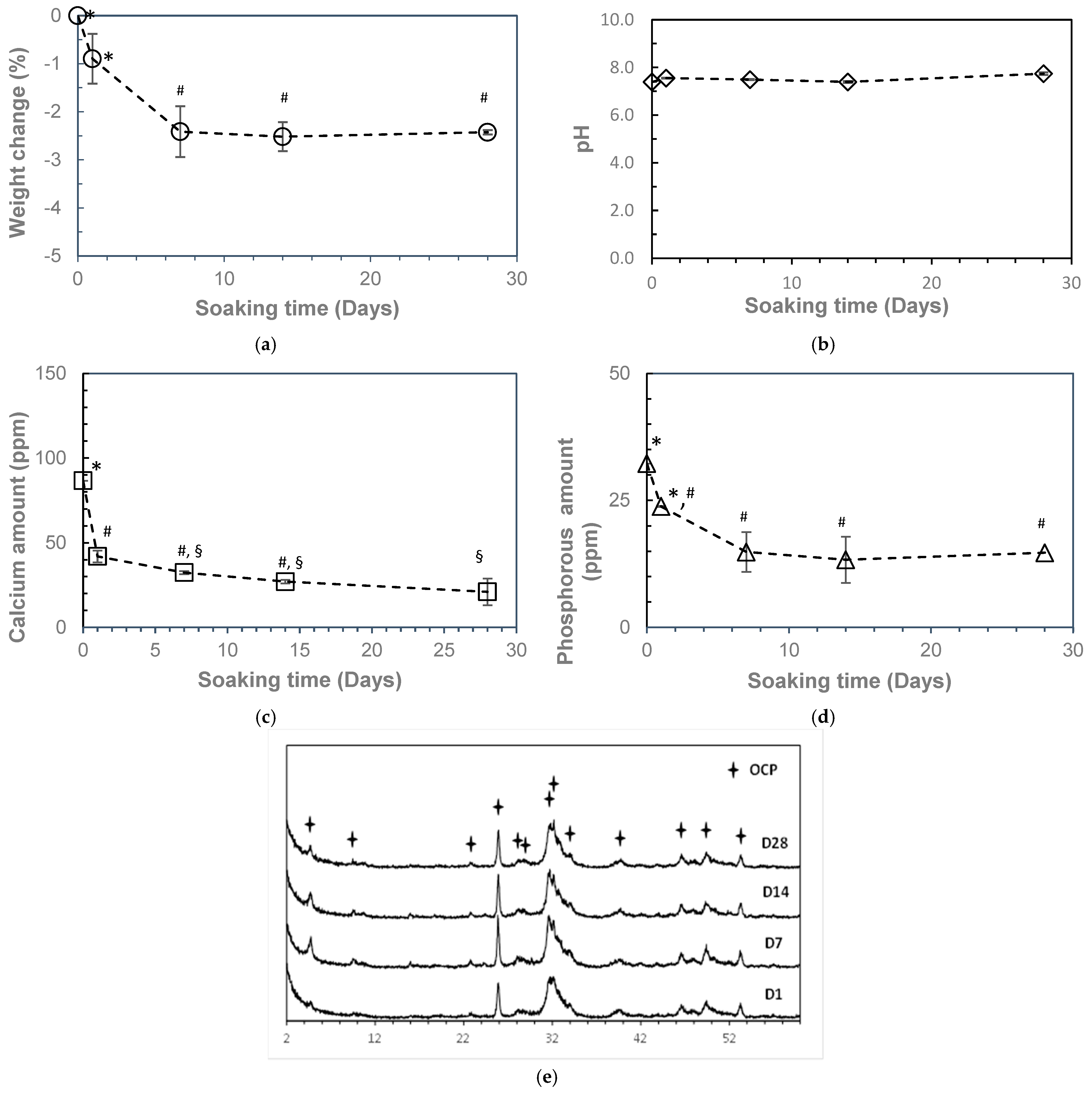
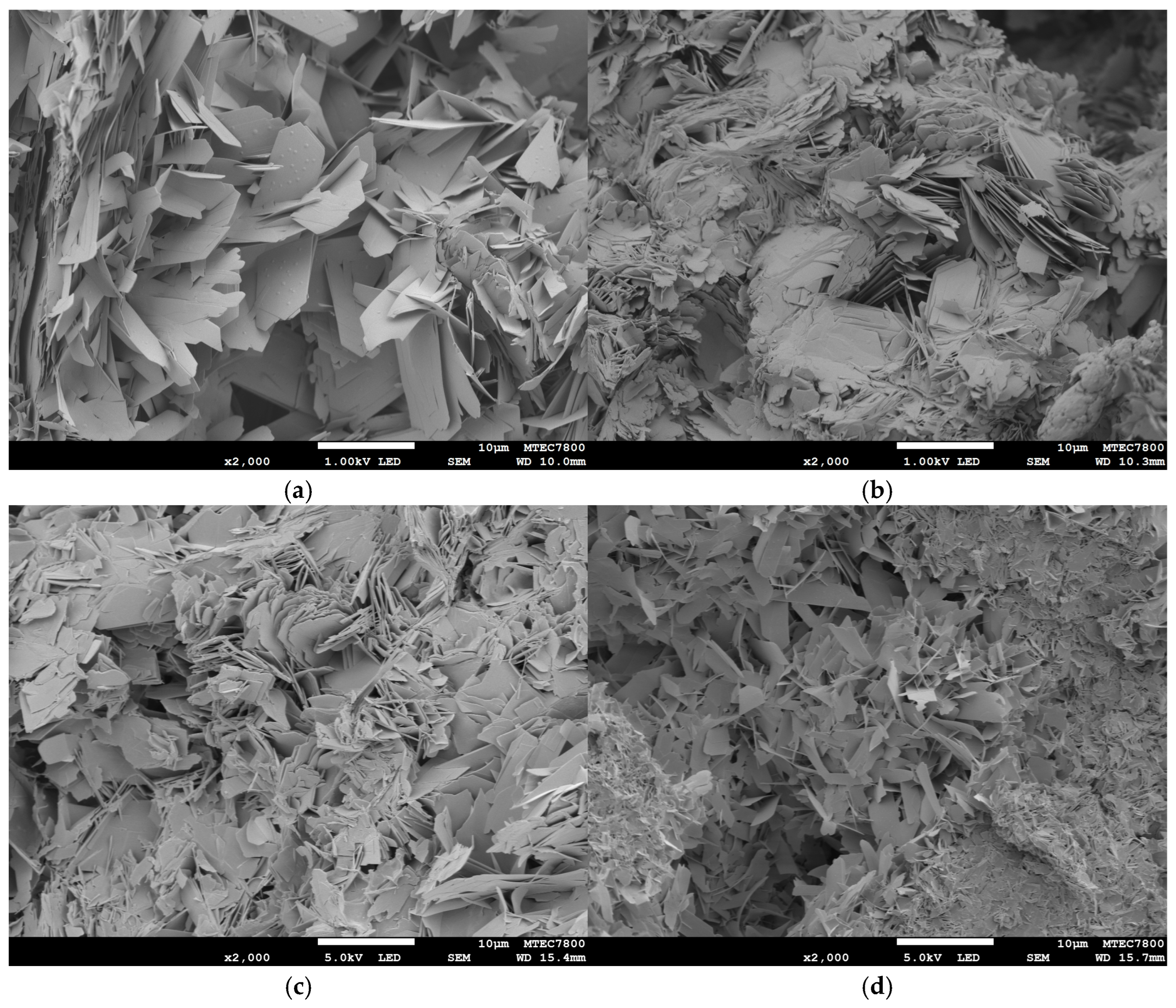
2. Results
3. Discussion
4. Materials and Methods
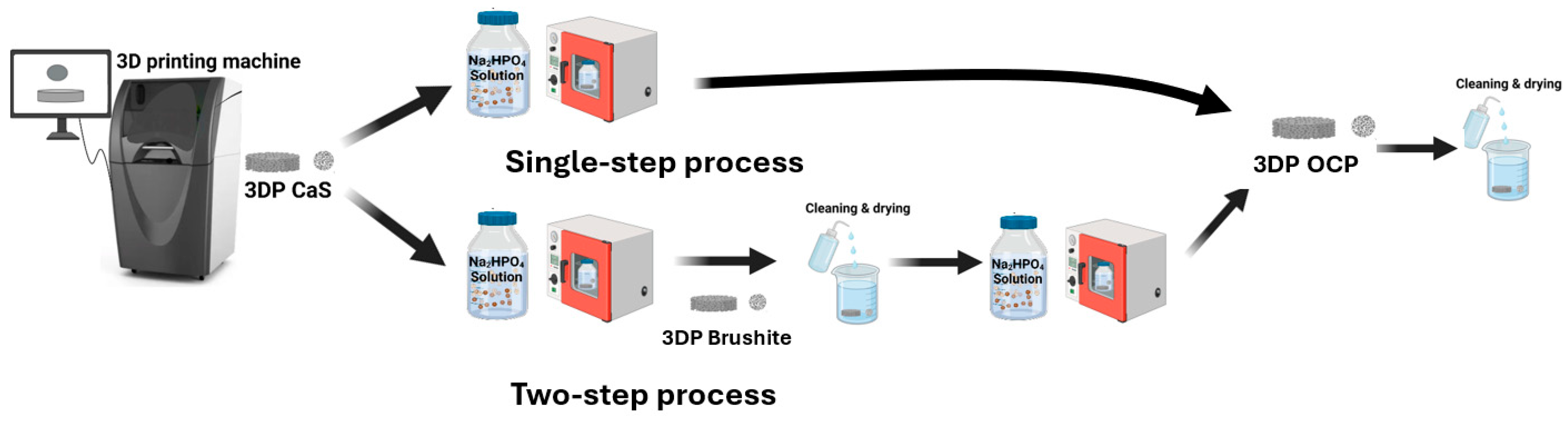
4.1. Raw Materials and Fabrication
4.2. Phase Transformation
4.3. Characterizations
4.4. Biocompatibility and Bioactivity Evaluation
4.4.1. Cytotoxicity
4.4.2. In Vitro MC3T3-E1 Cell Proliferation
4.4.3. ALP Activity Assay
4.5. In Vitro Resorbability
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OCP | Octacalcium Phosphate |
| ACP | Amorphous Calcium Phosphate |
| HA | Hydroxyapatite |
| XRD | X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| DNA | Deoxyribonucleic acid |
| VEGFA | Vascular Endothelial Growth Factor A |
| SBF | Simulated Body Fluid |
| DLP | Digital Light Processing |
| DIW | Direct Ink Writing |
| DTS | Diametral Tensile Strength |
| MEM | Minimum Essential Medium |
| α-MEM | Minimum Essential Medium Eagle-Alpha Modification |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| ALP | Alkaline Phosphatase |
| BM-MSCs | Bone Marrow-derived Mesenchymal Stem Cells |
| DMSO | Dimethyl Sulfoxide |
| PBS | Phosphate-Buffered Saline |
| OD | Optical Density |
| P38/MAPK | P38 Mitogen-activated Protein Kinases |
| AKT | Protein Kinase B or PKB |
| JNK | Jun N-terminal Kinases |
References
- Kovrlija, I.; Locs, J.; Loca, D. Octacalcium phosphate: Innovative vehicle for the local biologically active substance delivery in bone regeneration. Acta Biomater. 2021, 135, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Song, I. Octacalcium phosphate, a promising bone substitute material: A narrative review. J. Yeungnam Med. Sci. 2024, 41, 4–12. [Google Scholar] [CrossRef]
- Nakano, T.; Yamashita, Y. Octacalcium phosphate: A promising material for tissue engineering. Acta Biomater. 2020, 113, 84–96. [Google Scholar]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of bio-materials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenicity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Yokoi, T.; Goto, T.; Hara, M.; Sekino, T.; Seki, T.; Kamitakahara, M.; Ohtsuki, C.; Kitaoka, S.; Takahashi, S.; Kawashita, M. Incorporation of tetracarboxylate ions into octacalcium phosphate for the development of next-generation biofriendly materials. Commun. Chem. 2021, 4, 4. [Google Scholar] [CrossRef]
- Saito, S.; Hamai, R.; Shiwaku, Y.; Hasegawa, T.; Sakai, S.; Tsuchiya, K.; Sai, Y.; Iwama, R.; Amizuka, N.; Takahashi, T.; et al. Involvement of distant octacalcium phosphate scaffolds in enhancing early differentiation of osteocytes during bone regeneration. Acta Biomater. 2021, 129, 309–322. [Google Scholar] [CrossRef]
- Suzuki, O.; Imaizumi, H.; Kamakura, S.; Katagiri, T. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr. Med. Chem. 2008, 15, 305–313. [Google Scholar] [CrossRef]
- Komlev, V.S.; Fadeeva, I.V.; Shvorneva, L.I.; Fomin, A.S.; Barinov, S.M.; Ferro, D. Synthesis of octacalcium phosphate by precipitation from solution. Dokl. Chem. 2010, 432, 178–182. [Google Scholar] [CrossRef]
- Iijima, M.; Kamemizu, H.; Wakamatsu, N.; Goto, T.; Doi, Y.; Moriwaki, Y. Precipitation of octacalcium phosphate at 37 °C and at pH 7.4: In relation to enamel formation. J. Cryst. Growth 1991, 112, 467–473. [Google Scholar] [CrossRef]
- Tripathi, G.; Miyazaki, T. Spontaneous fabrication of octacalcium phosphate: Synthesis conditions and basic characterizations. Bull. Mater. Sci. 2021, 44, 163. [Google Scholar] [CrossRef]
- Kovrlija, I.; Menshikh, K.; Marsan, O.; Rey, C.; Combes, C.; Locs, J.; Loca, D. Exploring the formation kinetics of octacalcium phosphate from alpha-tricalcium phosphate: Synthesis scale-up, determination of transient phases, their morphology and biocompatibility. Biomolecules 2023, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.; Kelly, D. Synthesis methodologies options for large-scale manufacturer of octacalcium phosphate. In Octacalcium Phosphate Biomaterials: Understanding of Bioactive Properties and Application; Insley, G., Suzuki, O., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 147–176. [Google Scholar]
- Choudhary, R.; Indurkar, A.; Rubenis, K.; Grava, A.; Dubnika, A.; Hurle, K.; Locs, J. Ultrafast and reproducible synthesis of tailor-made octacalcium phosphate. ACS Omega 2024, 9, 36165–36176. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, A.Y.; Komlev, V.S. Review of Octacalcium Phosphate Materials for Bone Tissue Engineering. Inorg. Mater. Appl. Res. 2022, 13, 985–1004. [Google Scholar] [CrossRef]
- Zafar, M.J.; Zhu, D.; Zhang, Z. 3D Printing of Bioceramics for Bone Tissue Engineering. Materials 2019, 12, 3361. [Google Scholar] [CrossRef]
- Preobrazhenskiy, I.I.; Tikhonov, A.A.; Evdokimov, P.V.; Shibaev, A.V.; Putlyaev, V.I. DLP printing of hydrogel/calcium phosphate composites for the treatment of bone defects. J. Ceram. Sci. Technol. 2021, 6, 100115. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, B.; Zhao, C.; Lu, Y.; Huang, T.; Chen, Y.; Li, J.; Wu, R.; Liu, W.; Lin, J. Lanthanum doped octacalcium phosphate/polylactic acid scaffold fabricated by 3D printing for bone tissue engineering. J. Mater. Sci. Technol. 2022, 118, 229–242. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Lee, W.; Mertamani, R.N.; Yun, K.; Kim, S.; Kim, S.-J. 3D printed OCP bone scaffold with alginate enhancing osteogenic differentiation in MG-63 cells. MRS Commun. 2023, 13, 1433–1440. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–790. [Google Scholar] [CrossRef]
- Komlev, V.S.; Popov, V.K.; Mironov, A.V.; Fedotov, A.Y.; Teterina, A.Y.; Smirnov, I.V.; Bozo, I.Y.; Rybko, V.A.; Deev, R.V. 3D printing of octacalcium phosphate bone substitutes. Front. Bioeng. Biotechnol. 2015, 3, 81. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Deev, R.V.; Smirnov, I.V.; Fedotov, A.Y.; Popov, V.K.; Mironov, A.V.; Mironova, O.A.; Gerasimenko, A.Y.; Komlev, V.S. 3D printed gene-activated octacalcium phosphate implants for large bone defects engineering. Int. J. Bioprint. 2020, 6, 275. [Google Scholar] [CrossRef]
- Rahman, Z.; Charoo, N.A.; Kuttolamadom, M.; Asadi, A.; Khan, M.A. Printing of personalized medication using binder jetting 3D printer. In Precision Medicine for Investigators, Practitioners and Providers; Faintuch, J., Faintuch, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 473–481. [Google Scholar] [CrossRef]
- Clares, A.P.; Gao, Y.; Stebbins, R.; van Duin, A.C.T.; Manogharan, G. Increasing density and mechanical performance of binder jetting processing through bimodal particle size distribution. Mater. Sci. Addit. Manuf. 2022, 1, 20. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Suvannapruk, W.; Wasoontararat, K. Low temperature preparation of calcium phosphate structure via phosphorization of 3D-printed calcium sulfate hemihydrate based material. J. Mater. Sci. Mater. Med. 2010, 21, 419–429. [Google Scholar] [CrossRef]
- Srion, A.; Thammarakcharoen, F.; Suwanprateeb, J. Fabrication of Monetite by a controlled phase transformation of three dimensionally printed calcium sulfate construct. Chiang Mai J. Sci. 2022, 49, 131–144. [Google Scholar] [CrossRef]
- Srion, A.; Palanuruksa, P.; Thammarakcharoen, F.; Suwanprateeb, J. Low temperature fabrication of brushite by powder-based three dimensional printing coupled with phase transformation process. Chiang Mai J. Sci. 2020, 47, 738–751. [Google Scholar]
- Kijartorn, P.; Wongpairojpanich, J.; Thammarakcharoen, F.; Suwanprateeb, J.; Buranawat, B. Clinical evaluation of 3D printed nano-porous hydroxyapatite bone graft for alveolar ridge preservation: A randomized controlled trial. J. Dent. Sci. 2022, 17, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Mekcha, P.; Wongpairojpanich, J.; Thammarakcharoen, F.; Suwanprateeb, J.; Buranawat, B. Customized 3D printed nano-hydroxyapatite bone block grafts for implant sites: A case series. J. Prosthodont. Res. 2023, 67, 311–320. [Google Scholar] [CrossRef]
- Jiamton, C.; Apivatgaroon, A.; Aunaramwat, S.; Chawalitrujiwong, B.; Chuaychoosakoon, C.; Suwannaphisit, S.; Jirawison, C.; Iamsumang, C.; Kongmalai, P.; Sukvanich, P.; et al. Efficacy and safety of antibiotic impregnated microporous nanohydroxyapatite beads for chronic osteomyelitis treatment: A multicenter, open-label, prospective cohort study. Antibiotics 2023, 12, 1049. [Google Scholar] [CrossRef]
- Thammarakcharoen, F.; Srion, A.; Chokevivat, W.; Hemstapat, R.; Morales, N.P.; Suwanprateeb, J. Development of polycaprolactone infiltrated anti-tuberculosis drug-loaded 3D-printed hydroxyapatite for localized and sustained drug release in bone and joint tuberculosis treatment. Chiang Mai J. Sci. 2022, 49, 105–121. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Thammarakcharoen, F.; Phanphiriya, P.; Chokevivat, W.; Suvannapruk, W.; Chernchujit, B. Preparation and characterization of antibiotic impregnated microporous nano-hydroxyapatite for osteomyelitis treatment. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450041. [Google Scholar] [CrossRef]
- Mandel, S.; Tas, A.C. Brushite (CaHPO4·2H2O) to octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O) transformation in DMEM solutions at 36.5 °C. Mater. Sci. Eng. C Mater. Biol. Appl. 2010, 30, 245–254. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W.G.; Josan, M.S.; Nair, V.D. Inhibition of calcium phosphate precipitation under environmentally-relevant conditions. Sci. Total Environ. 2007, 383, 205–215. [Google Scholar] [CrossRef]
- Sugiura, Y.; Munar, M.L.; Ishikawa, K. Fabrication of octacalcium phosphate block through a dissolution-precipitation reaction using a calcium sulphate hemihydrate block as a precursor. J. Mater. Sci. Mater. Med. 2018, 29, 151. [Google Scholar] [CrossRef]
- Sugiura, Y.; Munar, M.L.; Ishikawa, K. Fabrication of octacalcium phosphate foam through phase conversion and its histological evaluation. Mater. Lett. 2018, 212, 28–31. [Google Scholar] [CrossRef]
- Sugiura, Y.; Ishikawa, K. Fabrication of pure octacalcium phosphate blocks from dicalcium hydrogen phosphate dihydrate blocks via a dissolution–precipitation reaction in a basic solution. Mater. Lett. 2019, 239, 143–146. [Google Scholar] [CrossRef]
- Sugiura, Y.; Ishikawa, K. Effect of Calcium and Phosphate on Compositional Conversion from Dicalcium Hydrogen Phosphate Dihydrate Blocks to Octacalcium Phosphate Blocks. Crystals 2018, 8, 222. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- He, W.; Ding, F.; Zhang, L.; Liu, W. In situ osteogenic activation of mesenchymal stem cells by the blood clot biomimetic mechanical microenvironment. Nat. Commun. 2025, 16, 1162. [Google Scholar] [CrossRef]
- Tran, P.A. Blood clots and tissue regeneration of 3D printed dual scale porous polymeric scaffolds. Mater. Lett. 2021, 285, 129184. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Takagi, S.; Ammon, H.L. Crystal structure of calcium adipate monohydrate. J. Crystallogr. Spectrosc. Res. 1993, 23, 617–621. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; McLean, J.D. High-resolution electron microscopy of octacalcium phosphate and its hydrolysis products. Calcif. Tissue Int. 1984, 36, 219–232. [Google Scholar] [CrossRef]
- Iijima, M.; Tohda, H.; Moriwaki, Y. Growth and structure of lamellar mixed crystals of octacalcium phosphate and apatite in a model system of enamel formation. J. Cryst. Growth 1992, 116, 319–326. [Google Scholar] [CrossRef]
- Horváthová, R.; Müller, L.; Helebrant, A.; Greil, P.; Müller, F.A. In vitro transformation of OCP into carbonated HA under physiological conditions. Mater. Sci. Eng. C 2008, 28, 1414–1419. [Google Scholar] [CrossRef]
- Rau, V.; Fosca, M.; Komlev, V.S.; Fadeeva, I.V.; Albertini, V.R.; Barinov, S.M. In Situ Time-Resolved Studies of Octacalcium Phosphate and Dicalcium Phosphate Dihydrate in Simulated Body Fluid: Cooperative Interactions and Nanoapatite Crystal Growth. Cryst. Growth Des. 2010, 10, 3824–3834. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef]
- Ban, S.; Jinde, T.; Hasegawa, J. Phase transformation of octacalcium phosphate in vivo and in vitro. Dent. Mater. J. 1992, 11, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Kim, I.Y.; Ohtsuki, C. Mineralization of calcium phosphate on octacalcium phosphate in a solution mimicking in vivo conditions. Phosphorus Res. Bull. 2012, 26, 71–76. [Google Scholar] [CrossRef][Green Version]
- Ito, N.; Kamitakahara, M.; Yoshimura, M.; Ioku, K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mater. Sci. Eng. C 2014, 40, 121–126. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; Salimi, H.; Nancollas, G.H. Octacalcium phosphate and apatite overgrowths: A crystallographic and kinetic study. J. Colloid Interface Sci. 1986, 110, 32–39. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; Barry, J.C. High-resolution electron microscopy of nonstoichiometric apatite crystals. Anat. Rec. 1989, 224, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Mathew, M.; Tung, M.S. Crystal chemistry of octacalcium phosphate. Prog. Cryst. Growth Charact. 1981, 4, 59–87. [Google Scholar] [CrossRef]
- Suzuki, O.; Yagishita, H.; Yamazaki, M.; Aoba, T. Adsorption of bovine serum albumin onto octacalcium phosphate and its hydrolyzates. Cells Mater. 1995, 5, 4. [Google Scholar]
- Petrakova, N.V.; Teterina, A.Y.; Mikheeva, P.V.; Akhmedova, S.A.; Kuvshinova, E.A.; Sviridova, I.K.; Sergeeva, N.S.; Smirnov, I.V.; Fedotov, A.Y.; Kargin, Y.F.; et al. In vitro study of octacalcium phosphate behavior in different model solutions. ACS Omega 2021, 6, 7487–7498. [Google Scholar] [CrossRef]
- Anada, T.; Sato, T.; Kamoya, T.; Shiwaku, Y.; Tsuchiya, K.; Takano-Yamamoto, T.; Sasaki, K.; Suzuki, O. Evaluation of bioactivity of octacalcium phosphate using osteoblastic cell aggregates on a spheroid culture device. Regen. Ther. 2016, 3, 58–62. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, J.; Kim, S.; Chung, S.H.; Wie, J. Multiple proliferation signaling pathways are modulated by octacalcium phosphate in osteoblasts. Int. J. Med. Sci. 2022, 19, 1724–1731. [Google Scholar] [CrossRef]
- Sugai, Y.; Hamai, R.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Kimura, T.; Tadano, M.; Yamauchi, K.; Takahashi, T.; Egusa, H.; et al. Effect of octacalcium phosphate on osteogenic differentiation of induced pluripotent stem cells in a 3D hybrid spheroid culture. Biomimetics 2025, 10, 205. [Google Scholar] [CrossRef] [PubMed]
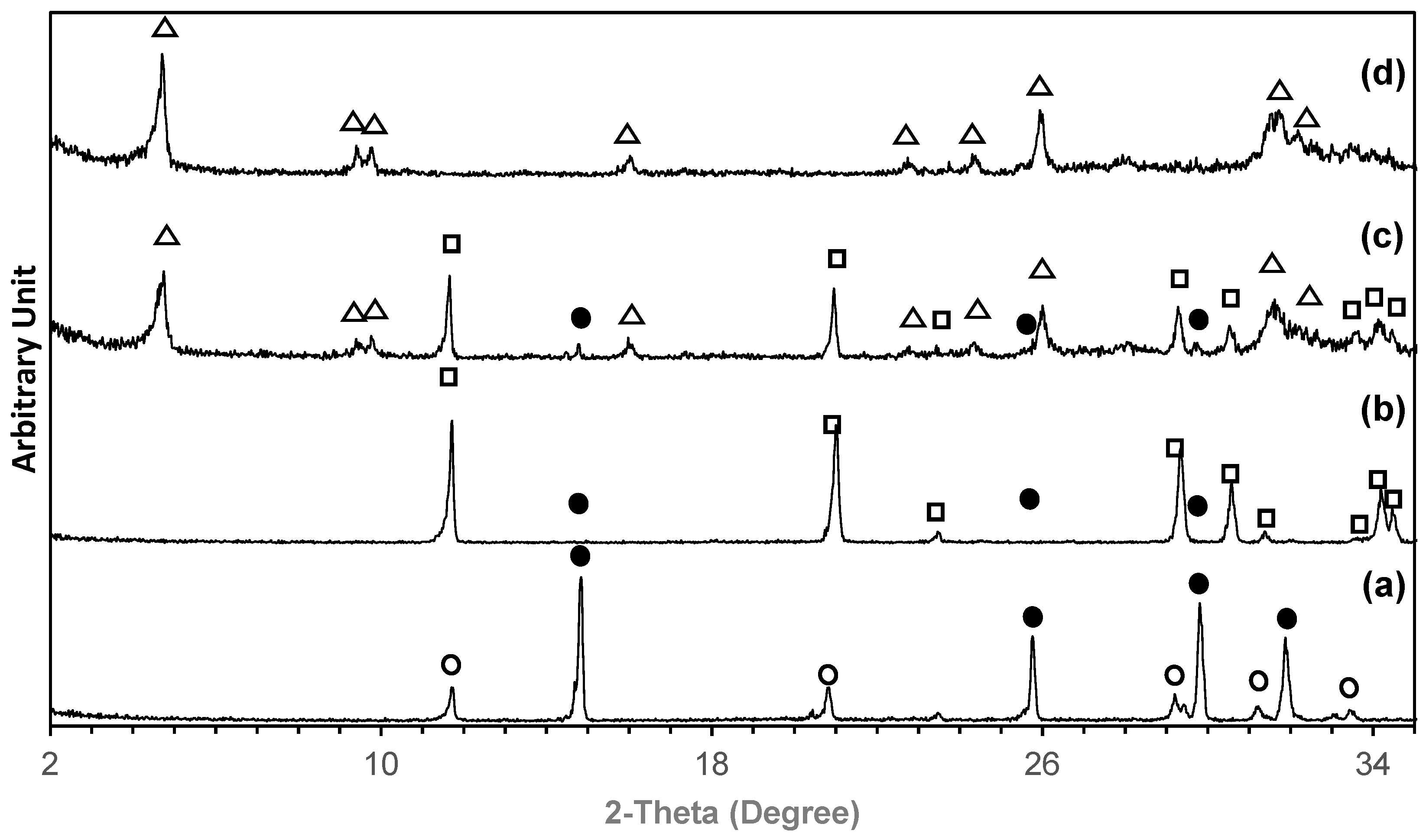
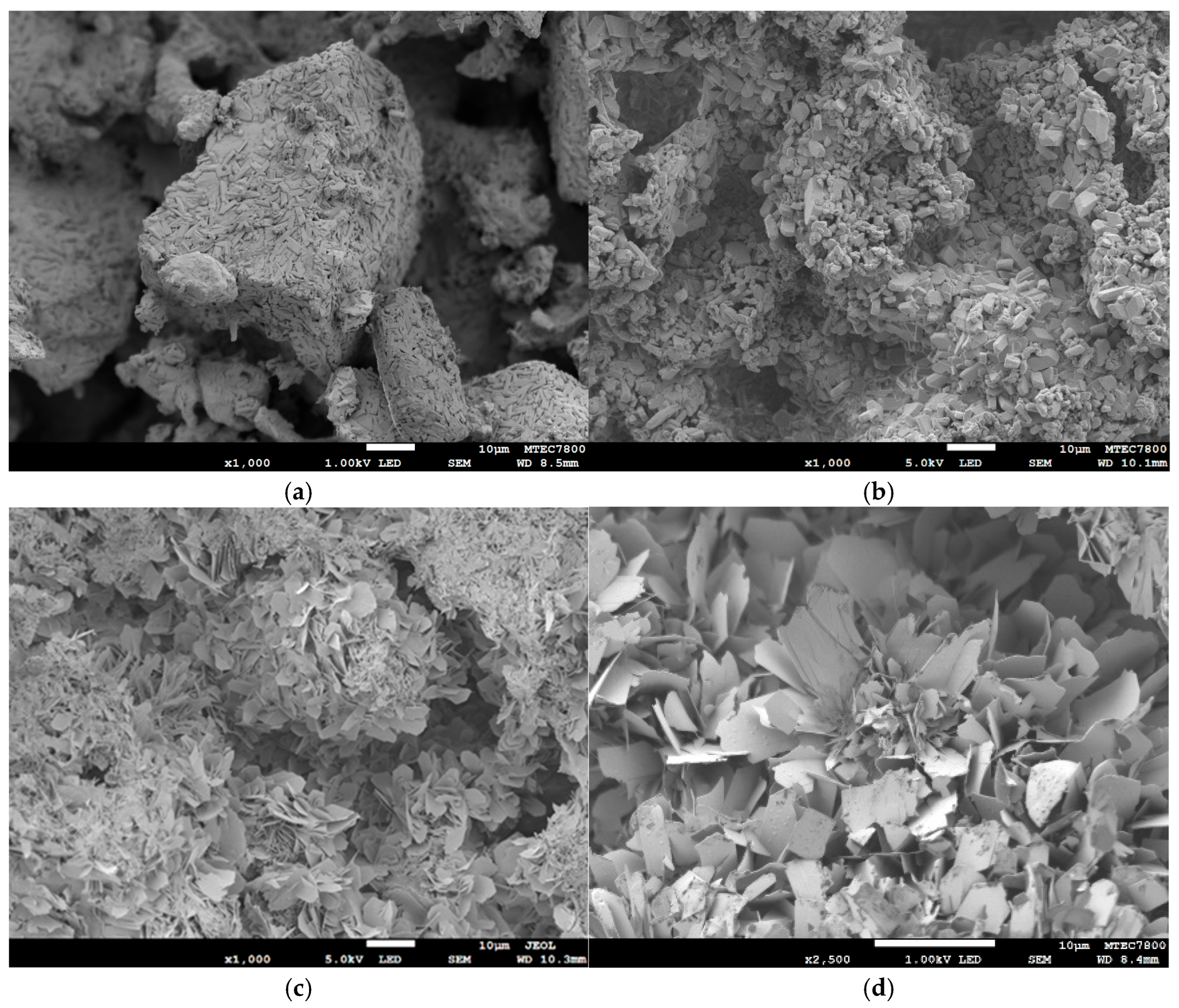
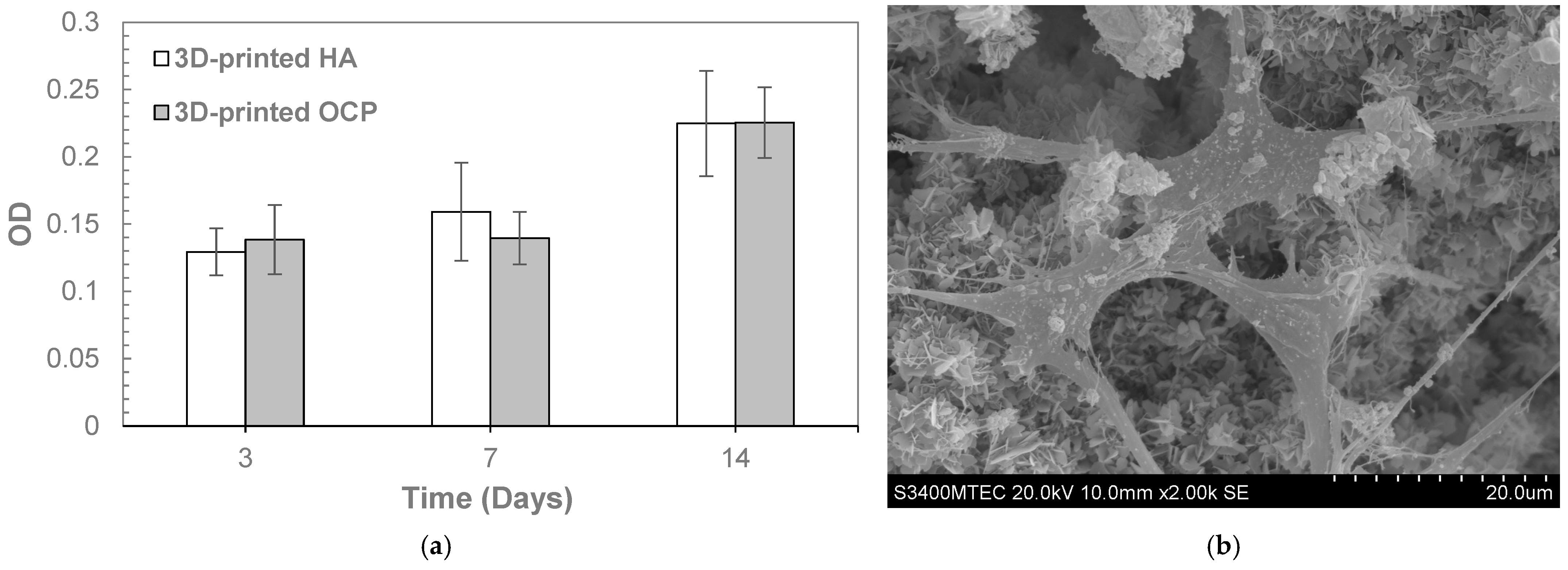
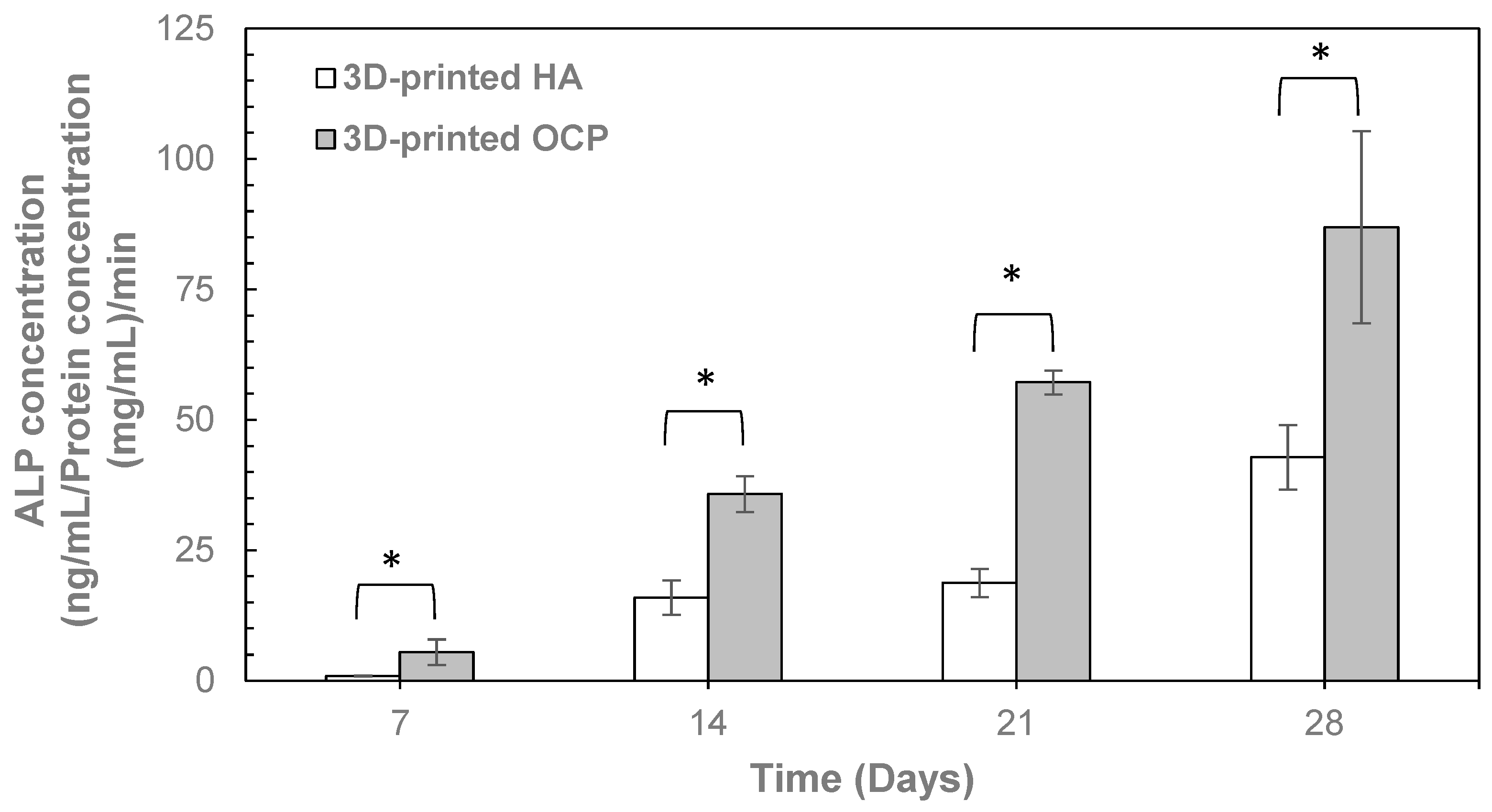

| Temperature (°C) | pH | Phase Composition (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial | After | Bassanite | Gypsum | Brushite | Monetite | OCP | HA | |
| 37 | 7.0 | 6.9 | 79.8 ± 1.6 | - | 20.2 ± 1.0 | - | - | - |
| 9.0 | 8.8 | 17.2 ± 0.9 | 26.2 ± 1.8 | - | - | 56.6 ± 4.7 | - | |
| 65 | 7.0 | 6.5 | 9.8 ± 0.4 | 1.3 ± 0.1 | - | 29.8 ± 1.1 | 59.1 ± 3.9 | - |
| 9.0 | 8.0 | 9.1 ± 0.5 | - | - | 1.4 ± 0.3 | 89.5 ± 6.5 | - | |
| 100 | 7.0 | 6.3 | - | - | - | 1.3 ± 0.1 | - | 98.7 ± 5.5 |
| 9.0 | 7.0 | - | - | - | - | - | 100 | |
| Times | pH | Phase Composition (%) | |||
|---|---|---|---|---|---|
| Initial | After | Bassanite | Brushite | OCP | |
| 24 | 8.0 | 6.5 | 0.8 | 8.9 | 90.4 ± 4.5 |
| 48 | 8.0 | 7.2 | - | - | 100 |
| Samples | Contact Angle (Degree) | Compressive Strength (MPa) |
|---|---|---|
| 3D-printed OCP | 0 ± 0.00 | 7.65 ± 0.46 |
| 3D-printed HA | 0 ± 0.00 | 7.32 ± 0.54 |
| Samples | % Viability |
|---|---|
| Blank | 100.0 ± 0.00 |
| Positive | 0.0 ± 0.00 |
| Negative | 100.1 ± 0.87 * |
| 3D-printed OCP | 96.7 ± 0.18 # |
| 3D-printed HA | 82.4 ± 1.91 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srion, A.; Thammarakcharoen, F.; Chokevivat, W.; Suvannapruk, W.; Suwanprateeb, J. Three Dimensionally Printed Octacalcium Phosphate via Binder Jetting for Use in Bone Grafting Applications. Int. J. Mol. Sci. 2025, 26, 5633. https://doi.org/10.3390/ijms26125633
Srion A, Thammarakcharoen F, Chokevivat W, Suvannapruk W, Suwanprateeb J. Three Dimensionally Printed Octacalcium Phosphate via Binder Jetting for Use in Bone Grafting Applications. International Journal of Molecular Sciences. 2025; 26(12):5633. https://doi.org/10.3390/ijms26125633
Chicago/Turabian StyleSrion, Autcharaporn, Faungchat Thammarakcharoen, Watchara Chokevivat, Waraporn Suvannapruk, and Jintamai Suwanprateeb. 2025. "Three Dimensionally Printed Octacalcium Phosphate via Binder Jetting for Use in Bone Grafting Applications" International Journal of Molecular Sciences 26, no. 12: 5633. https://doi.org/10.3390/ijms26125633
APA StyleSrion, A., Thammarakcharoen, F., Chokevivat, W., Suvannapruk, W., & Suwanprateeb, J. (2025). Three Dimensionally Printed Octacalcium Phosphate via Binder Jetting for Use in Bone Grafting Applications. International Journal of Molecular Sciences, 26(12), 5633. https://doi.org/10.3390/ijms26125633







