Apocynin Mitigates Diabetic Muscle Atrophy by Lowering Muscle Triglycerides and Oxidative Stress
Abstract
1. Introduction
2. Results
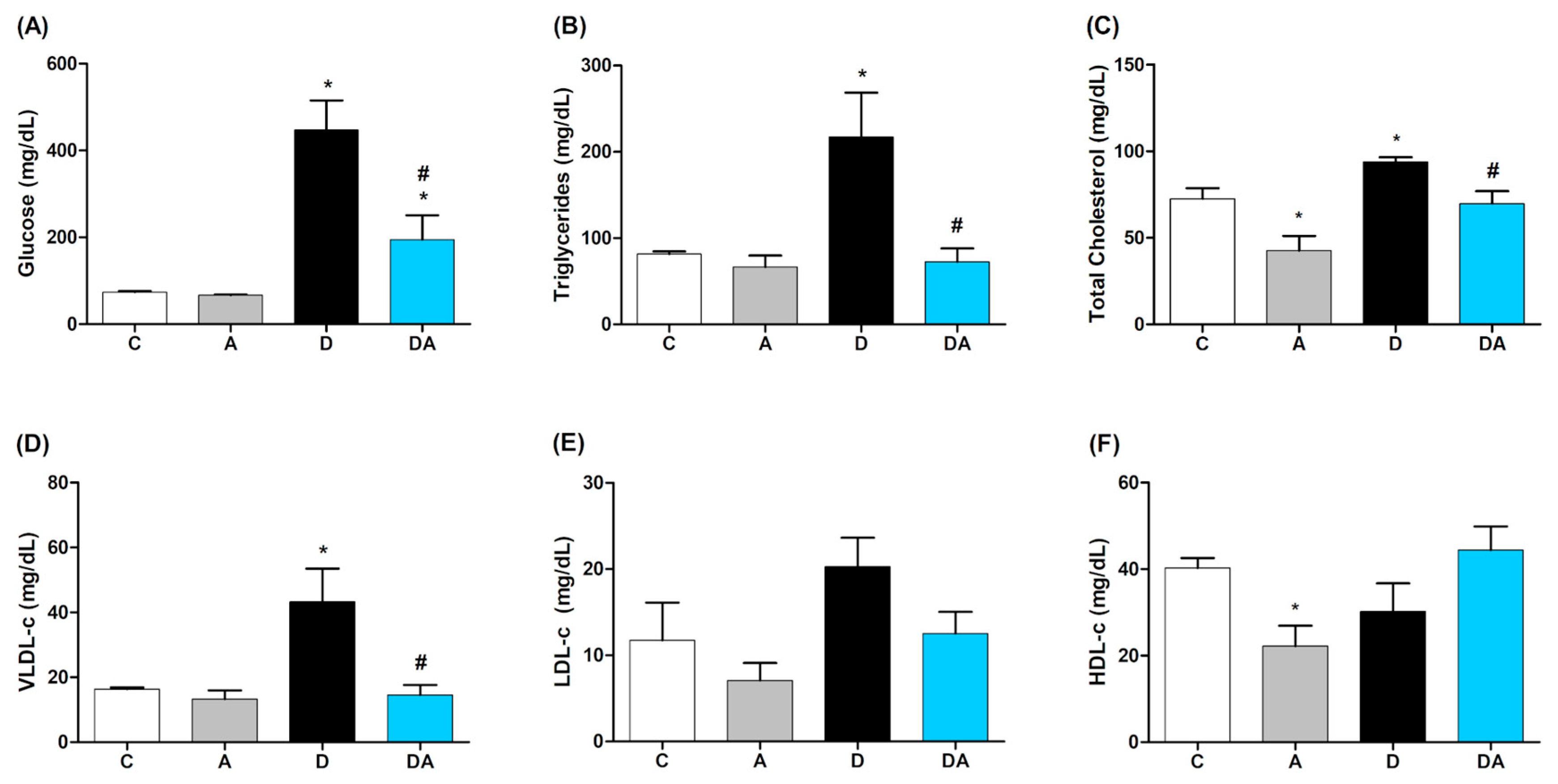
2.1. Effect of Apocynin on Blood Glucose and Lipid Profile
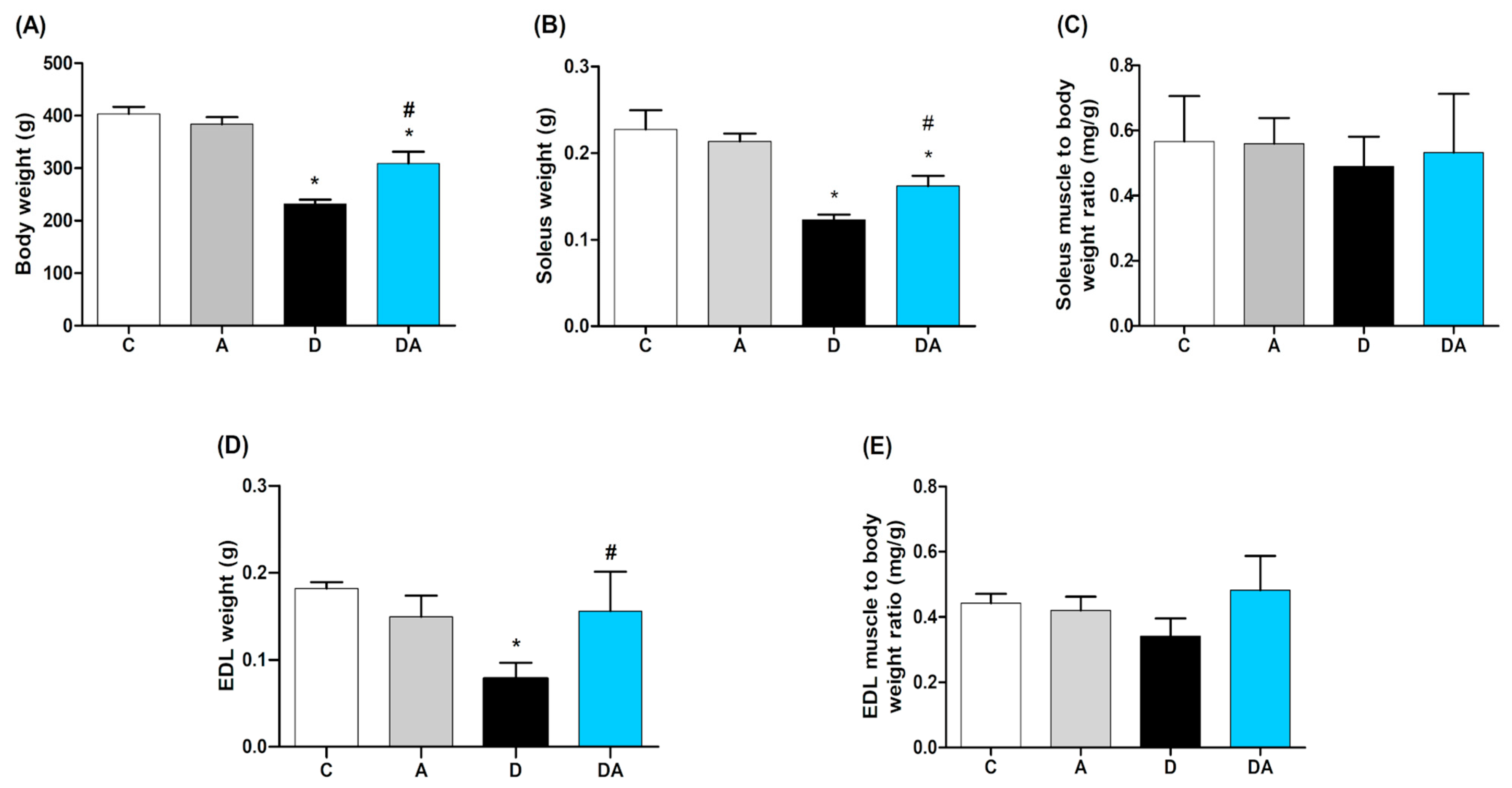
2.2. Effect of Apocynin on Body Weight and Muscle Mass
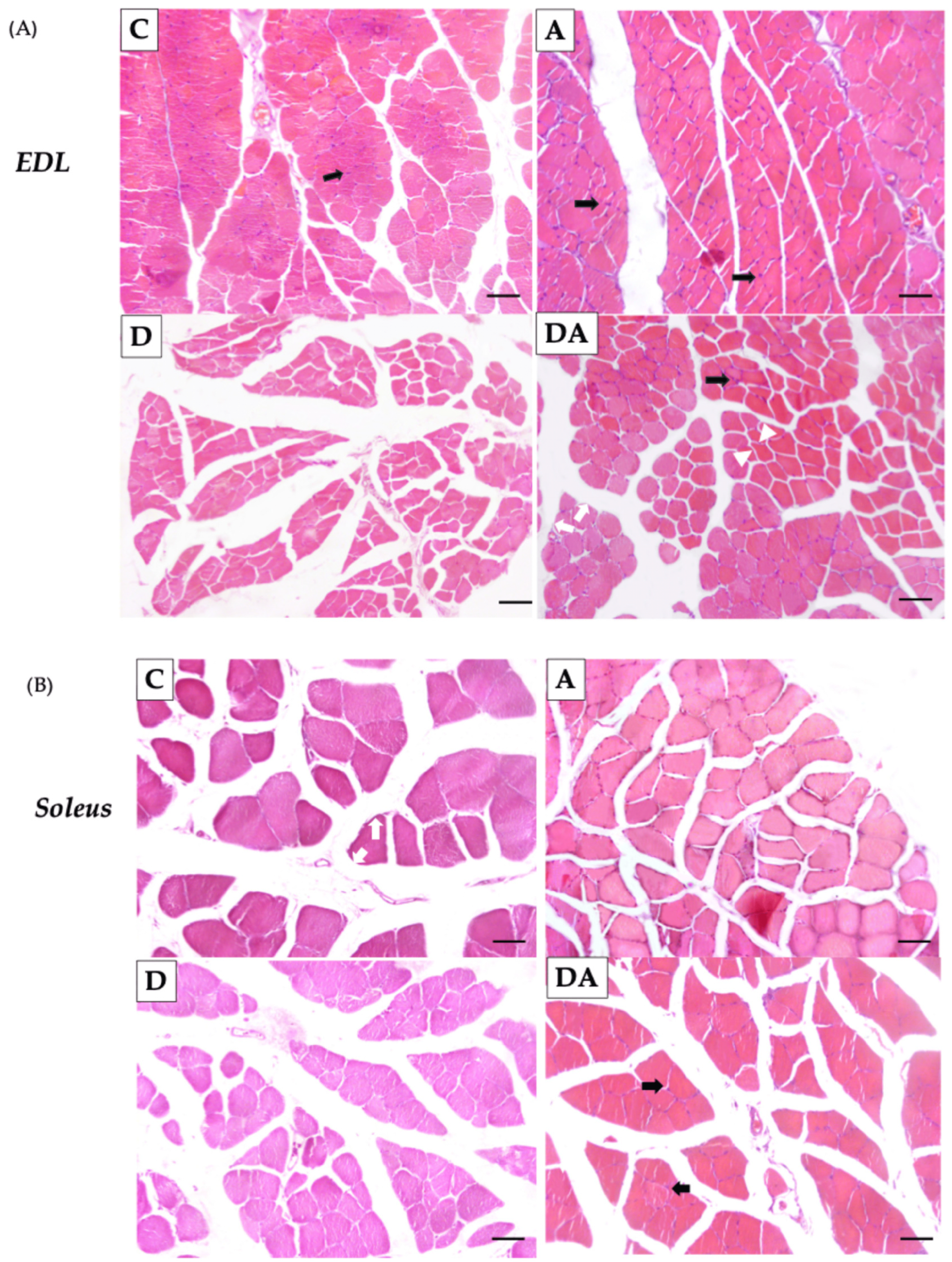
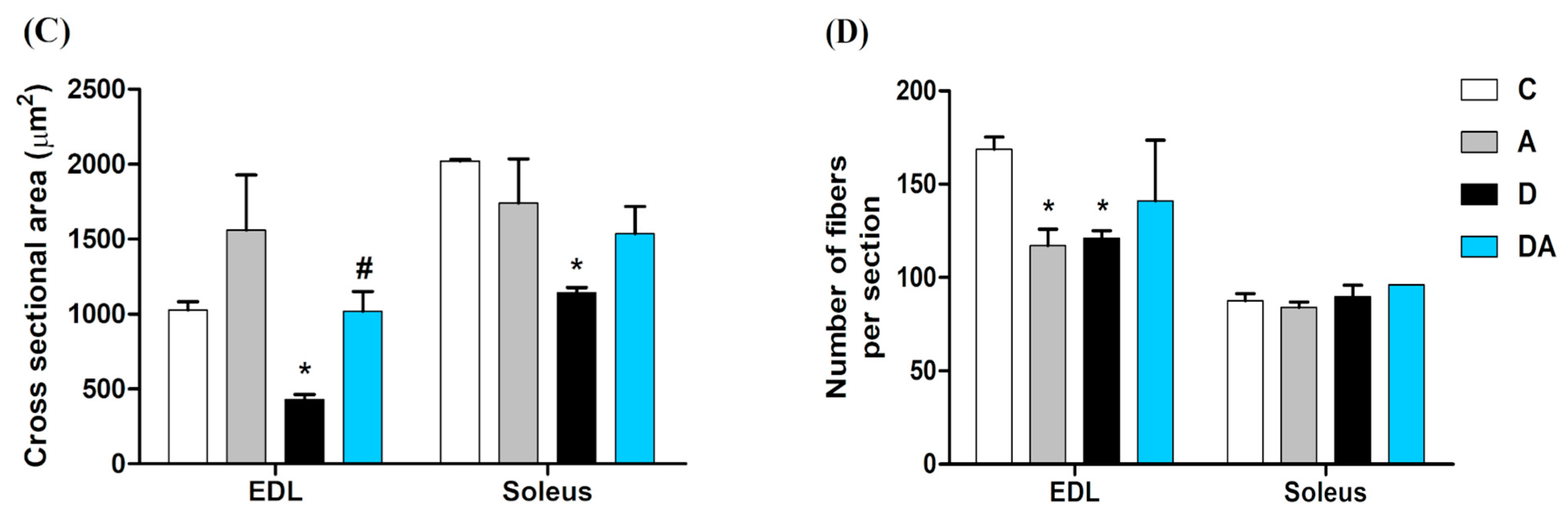
2.3. Effect of Apocynin on the Size and Number of Skeletal Muscle Fibers in Diabetic Rats
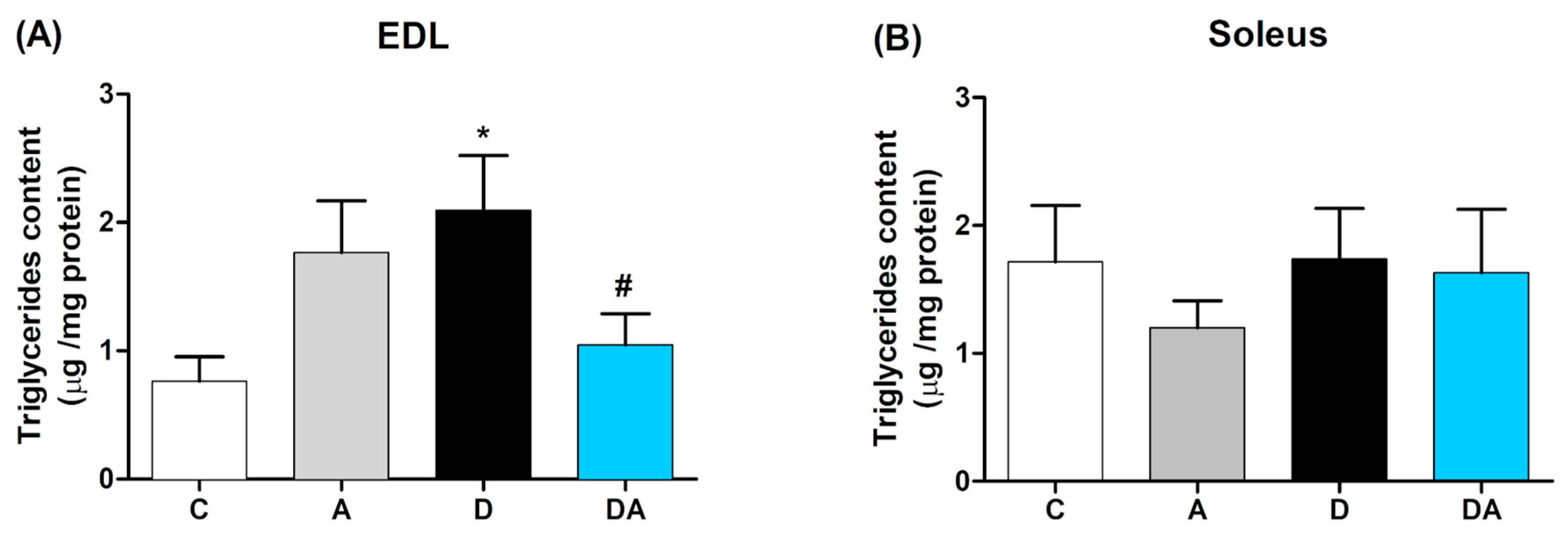
2.4. Inhibition of Nox by Apocynin Reduces Muscle Triglycerides Content in Diabetic Rats
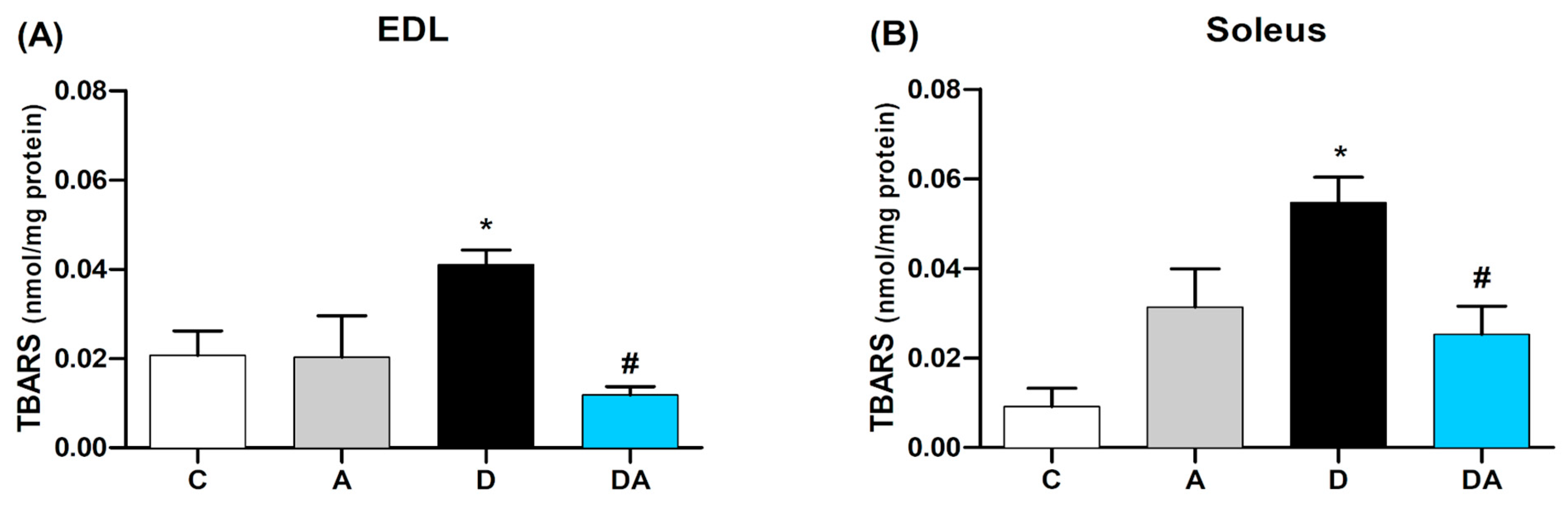
2.5. Apocynin Reduces Lipid Peroxidation in Fast and Slow Diabetic Skeletal Muscles
2.6. Effect of Apocynin on the Gene Expression of Muscle Mass Regulators and Oxidative Capacity in the Skeletal Muscle of Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Animal Experiment and Ethical Approval
4.2. Induction of Diabetes and Experimental Design
4.3. Lipid Profile
4.4. Muscle Triglycerides Content
4.5. Muscle Histological Analysis
4.6. Determination of Lipid Peroxidation
4.7. RNA Isolation and RT-qPCR for mRNA Expression Analyses
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMCL | Intramyocellular lipids |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| DAGs | Diacylglycerols |
| ROS | Reactive oxygen species |
| EDL | Extensor digitorum longus |
| STZ | Streptozotocin |
| FA | Fatty acids |
| TG | Triglycerides |
| NOX | NADPH oxidase |
| SOD | superoxide dismutase |
| CSA | cross-sectional area |
| TBARS | Thiobarbituric acid reactive substances |
| COPD | chronic obstructive pulmonary disease |
| NF-κB | Nuclear factor-kappa B |
| TGF-β | Transforming growth factor-beta |
| p47phox | Neutrophil cytosolic factor 1 |
| cDNA | complementary DNA |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
References
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dhirani, D.; Shahid, A.; Mumtaz, H. A New Kind of Diabetes Medication Approved by the FDA: Is There Hope for Obesity? Int. J. Surg. 2023, 109, 81–82. [Google Scholar] [CrossRef]
- Fritzsche, K.; Blüher, M.; Schering, S.; Buchwalow, I.B.; Kern, M.; Linke, A.; Oberbach, A.; Adams, V.; Punkt, K. Metabolic Profile and Nitric Oxide Synthase Expression of Skeletal Muscle Fibers Are Altered in Patients with Type 1 Diabetes. Exp. Clin. Endocrinol. Diabetes 2008, 116, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Gaur, K.; Mohapatra, L.; Wal, P.; Parveen, A.; Kumar, S.; Gupta, V. Deciphering the Mechanisms and Effects of Hyperglycemia on Skeletal Muscle Atrophy. Metab. Open 2024, 24, 100332. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Zheng, C. Type 2 Diabetes Mellitus-Related Sarcopenia: A Type of Muscle Loss Distinct from Sarcopenia and Disuse Muscle Atrophy. Front. Endocrinol. 2024, 15, 1375610. [Google Scholar] [CrossRef]
- Fujimaki, S.; Kuwabara, T. Diabetes-Induced Dysfunction of Mitochondria and Stem Cells in Skeletal Muscle and the Nervous System. Int. J. Mol. Sci. 2017, 18, 2147. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, M.; Deng, C.; Qiu, J.; Wang, K.; Chang, M.; Zhou, S.; Gu, Y.; Shen, Y.; Wang, W.; et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants 2022, 12, 44. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y.; et al. Biogenesis and Function of Extracellular Vesicles in Pathophysiological Processes of Skeletal Muscle Atrophy. Biochem. Pharmacol. 2022, 198, 114954. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef]
- Dotzert, M.S.; Murray, M.R.; McDonald, M.W.; Olver, T.D.; Velenosi, T.J.; Hennop, A.; Noble, E.G.; Urquhart, B.L.; Melling, C.W. Metabolomic Response of Skeletal Muscle to Aerobic Exercise Training in Insulin Resistant Type 1 Diabetic Rats. Sci. Rep. 2016, 6, 26379. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Blaak, E.E.; van Loon, L.J.C. Lipotoxicity Plays a Key Role in the Development of Both Insulin Resistance and Muscle Atrophy in Patients with Type 2 Diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.S.P.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 Mediates Skeletal Muscle Insulin Resistance Induced by a High Fat Diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Matsushima, S.; Inoue, N.; Ohta, Y.; Hamaguchi, S.; Sobirin, M.A.; Ono, T.; Suga, T.; et al. Oxidative Stress in Skeletal Muscle Impairs Mitochondrial Respiration and Limits Exercise Capacity in Type 2 Diabetic Mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1069–H1077. [Google Scholar] [CrossRef]
- Espino-Gonzalez, E.; Dalbram, E.; Mounier, R.; Gondin, J.; Farup, J.; Jessen, N.; Treebak, J.T. Impaired Skeletal Muscle Regeneration in Diabetes: From Cellular and Molecular Mechanisms to Novel Treatments. Cell Metab. 2024, 36, 1204–1236. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of Mitochondrial and Non-Mitochondrial Sources Implicate NADPH Oxidase in the Increased Generation of Superoxide in Skeletal Muscle During Contractile Activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH Oxidases in Skeletal Muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.S.; Aasum, E.; Hafstad, A.D. The role of NADPH oxidases in diabetic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1908–1913. [Google Scholar] [CrossRef]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol. Ther. 2020, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Luo, G.; Zhou, J.; Wang, N.; Wang, S.; Zhao, R.; Cao, X.; Ma, Y.; Liu, G.; et al. Nox4 as a novel therapeutic target for diabetic vascular complications. Redox Biol. 2023, 64, 102781. [Google Scholar] [CrossRef]
- Shieh, P.B. Muscular Dystrophies and Other Genetic Myopathies. Neurol. Clin. 2013, 31, 1009–1029. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Takada, S.; Yokota, T.; Furihata, T.; Matsumoto, J.; Tsuda, M.; Mizushima, W.; Fukushima, A.; Okita, K.; Kinugawa, S. Deletion of NAD(P)H Oxidase 2 Prevents Angiotensin II-Induced Skeletal Muscle Atrophy. BioMed Res. Int. 2018, 2018, 3194917. [Google Scholar] [CrossRef] [PubMed]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of Apocynin: A Natural Acetophenone. Metabol. Drug. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, O.J.; Bae, J.; Chung, T.N. Effect of Pre-Treatment with NADPH Oxidase Inhibitor Apocynin on the Therapeutic Efficacy of Human Placenta-Derived Mesenchymal Stem Cells in Intracerebral Hemorrhage. Int. J. Mol. Sci. 2018, 19, 3679. [Google Scholar] [CrossRef]
- Du, Z.D.; Yu, S.; Qi, Y.; Qu, T.F.; He, L.; Wei, W.; Liu, K.; Gong, S.S. The NADPH Oxidase Inhibitor Apocynin Decreases Mitochondrial Dysfunction and Apoptosis in the Ventral Cochlear Nucleus of a D-Galactose-Induced Aging Model in Rats. Neurochem. Int. 2019, 124, 31–40. [Google Scholar] [CrossRef]
- Bravo-Sánchez, E.; Peña-Montes, D.; Sánchez-Duarte, S.; Saavedra-Molina, A.; Sánchez-Duarte, E.; Montoya-Pérez, R. Effects of Apocynin on Oxidative Stress in the Cardiac Muscle of Rats with Experimental Diabetes: Implications for Mitochondria. Antioxidants 2021, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Antioxidant Effect of Apocynin on Insulin Resistance in Fat-Rich Diet Mice. Ann. Clin. Lab. Sci. 2011, 41, 236–243. [Google Scholar] [PubMed]
- Gimenes, R.; Gimenes, C.; Rosa, C.M.; Xavier, N.P.; Campos, D.H.S.; Fernandes, A.A.H.; Cezar, M.D.M.; Guirado, G.N.; Pagan, L.U.; Chaer, I.D.; et al. Influence of Apocynin on Cardiac Remodeling in Rats with Streptozotocin-Induced Diabetes Mellitus. Cardiovasc. Diabetol. 2018, 17, 15. [Google Scholar] [CrossRef]
- Sánchez-Duarte, S.; Montoya-Pérez, R.; Márquez-Gamiño, S.; Vera-Delgado, K.S.; Caudillo-Cisneros, C.; Sotelo-Barroso, F.; Sánchez-Briones, L.A.; Sánchez-Duarte, E. Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles. Life 2022, 12, 674. [Google Scholar] [CrossRef]
- Das, A.K.; Kalra, S.; Punyani, H.; Deshmukh, S.; Taur, S. ‘Oxidative Stress’—A New Target in the Management of Diabetes Mellitus. J. Fam. Med. Prim. Care 2023, 12, 2552–2557. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative Stress in Diabetes Mellitus and Its Complications: From Pathophysiology to Therapeutic Strategies. Chin. Med. J. 2025, 138, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.J. Streptozotocin-Induced Type 1 Diabetes in Rodents as a Model for Studying Mitochondrial Mechanisms of Diabetic β-Cell Glucotoxicity. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 181–188. [Google Scholar] [CrossRef]
- Winiarska, K.; Grabowski, M.; Rogacki, M.K. The Inhibition of Renal Gluconeogenesis Contributes to the Hypoglycemic Action of Apocynin, an NADPH Oxidase Inhibitor. Chem.-Biol. Interact. 2011, 189, 119–124. [Google Scholar] [CrossRef]
- Coleman, S.K.; Rebalka, I.A.; D’Souza, D.M.; Hawke, T.J. Skeletal Muscle as a Therapeutic Target to Delay Type 1 Diabetes Complications. World J. Diabetes 2015, 6, 1323–1336. [Google Scholar] [CrossRef]
- Liu, J.; Liang, X.; Zhou, D.; Lai, L.; Xiao, L.; Liu, L.; Fu, T.; Kong, Y.; Zhou, Q.; Vega, R.B.; et al. Coupling of Mitochondrial Function and Skeletal Muscle Fiber Type by a miR-499/Fnip1/AMPK Circuit. EMBO Mol. Med. 2016, 8, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Albadrani, H.; Ammar, T.; Bader, M.; Renaud, J.M. Angiotensin 1-7 prevents the excessive force loss resulting from 14- and 28-day denervation in mouse EDL and soleus muscle. J. Gen. Physiol. 2021, 153, e201912556. [Google Scholar] [CrossRef]
- Chan, S.M.H.; Bernardo, I.; Mastronardo, C.; Mou, K.; De Luca, S.N.; Seow, H.J.; Dobric, A.; Brassington, K.; Selemidis, S.; Bozinovski, S.; et al. Apocynin Prevents Cigarette Smoking-Induced Loss of Skeletal Muscle Mass and Function in Mice by Preserving Proteostatic Signaling. Br. J. Pharmacol. 2021, 178, 3049–3066. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Perry, C.G.R.; Hawke, T.J. Diabetic Myopathy: Current Molecular Understanding of This Novel Neuromuscular Disorder. Curr. Opin. Neurol. 2017, 30, 545–552. [Google Scholar] [CrossRef]
- Vergès, B. Dyslipidemia in Type 1 Diabetes: A Masked Danger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Nwosu, B.U. The Theory of Hyperlipidemic Memory of Type 1 Diabetes. Front. Endocrinol. 2022, 13, 819544. [Google Scholar] [CrossRef]
- Park, Y.J.; Gil, T.Y.; Jin, B.R.; Cha, Y.Y.; An, H.J. Apocynin Alleviates Weight Gain and Obesity-Induced Adipose Tissue Inflammation in High-Fat Diet-Fed C57BL/6 Mice. Phytother. Res. 2023, 37, 3481–3494. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muse, A.Y.; Khan, D.M.I.O.; Ahmed, I.H.; Subhan, N.; Reza, H.M.; Alam, M.A.; Nahar, L.; Sarker, S.D. Apocynin Prevented Inflammation and Oxidative Stress in Carbon Tetrachloride Induced Hepatic Dysfunction in Rats. Biomed. Pharmacother. 2017, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.P.; Temmel, H.; Das, S.K.; Al Zoughbi, W.; Schauer, S.P.; Vesely, P.W.; Hoefler, G. Skeletal Muscle Damage and Impaired Regeneration Due to LPL-Mediated Lipotoxicity. Cell Death Dis. 2012, 3, e354. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E. Skeletal Muscle Triglycerides: An Aspect of Regional Adiposity and Insulin Resistance. Ann. N. Y. Acad. Sci. 2002, 967, 135–145. [Google Scholar] [CrossRef]
- Phillips, D.I.; Caddy, S.; Ilic, V.; Fielding, B.A.; Frayn, K.N.; Borthwick, A.C.; Taylor, R. Intramuscular Triglyceride and Muscle Insulin Sensitivity: Evidence for a Relationship in Nondiabetic Subjects. Metabolism 1996, 45, 947–950. [Google Scholar] [CrossRef]
- Gilbert, M. Role of Skeletal Muscle Lipids in the Pathogenesis of Insulin Resistance of Obesity and Type 2 Diabetes. J. Diabetes Investig. 2021, 12, 1934–1941. [Google Scholar] [CrossRef]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice. Bosn J. Basic Med. Sci. 2021, 21, 730–738. [Google Scholar] [CrossRef]
- Wang, L.; Valencak, T.G.; Shan, T. Fat Infiltration in Skeletal Muscle: Influential Triggers and Regulatory Mechanism. Iscience 2024, 27, 109221. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The Role of Oxidative Stress in Skeletal Muscle Injury and Regeneration: Focus on Antioxidant Enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–399. [Google Scholar] [CrossRef]
- Diniz Vilela, D.; Gomes Peixoto, L.; Teixeira, R.R.; Belele Baptista, N.; Carvalho Caixeta, D.; de Souza, A.V.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxidative Med. Cell. Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Duarte, S.; Márquez-Gamiño, S.; Montoya-Pérez, R.; Villicaña-Gómez, E.A.; Vera-Delgado, K.S.; Caudillo-Cisneros, C.; Sotelo-Barroso, F.; Melchor-Moreno, M.T.; Sánchez-Duarte, E. Nicorandil Decreases Oxidative Stress in Slow- and Fast-Twitch Muscle Fibers of Diabetic Rats by Improving the Glutathione System Functioning. J. Diabetes Investig. 2021, 12, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sun, B.; Wang, M.; Li, T.; Li, H.; Han, Q.; Liao, J.; Tang, Z. N-Acetylcysteine Alleviates Oxidative Stress and Apoptosis and Prevents Skeletal Muscle Atrophy in Type 1 Diabetes Mellitus Through the NRF2/HO-1 Pathway. Life Sci. 2023, 329, 121975. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic Myopathy: Impact of Diabetes Mellitus on Skeletal Muscle Progenitor Cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [CrossRef]
- Agrawal, S.; Chakole, S.; Shetty, N.; Prasad, R.; Lohakare, T.; Wanjari, M. Exploring the Role of Oxidative Stress in Skeletal Muscle Atrophy: Mechanisms and Implications. Cureus 2023, 15, e42178. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.C.; do Rêgo-Monteiro, I.C.; Louzada, R.A.; Ortenzi, V.H.; de Aguiar, A.P.; de Abreu, E.S.; Cavalcanti-de-Albuquerque, J.P.A.; Hecht, F.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Differential Expression of NADPH Oxidases Depends on the Fiber Type of Skeletal Muscle in Rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Bechara, L.R.; Moreira, J.B.; Jannig, P.R.; Voltarelli, V.A.; Dourado, P.M.; Vasconcelos, A.R.; Scavone, C.; Ramires, P.R.; Brum, P.C. NADPH Oxidase Hyperactivity Induces Plantaris Atrophy in Heart Failure Rats. Int. J. Cardiol. 2014, 175, 499–507. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Deng, B.; Liu, M. Ellagic Acid Attenuates Lipid Accumulation and Dysfunction of Skeletal Muscle in High-Fat Diet-Induced Obese Mice. J. Nutr. Biochem. 2023, 111, 109253. [Google Scholar]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin is Not an Inhibitor of Vascular NADPH Oxidases but an Antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef]
- Lawler, J.M.; Hord, J.M.; Ryan, P.; Holly, D.; Janini Gomes, M.; Rodriguez, D.; Guzzoni, V.; Garcia-Villatoro, E.; Green, C.; Lee, Y.; et al. Nox2 Inhibition Regulates Stress Response and Mitigates Skeletal Muscle Fiber Atrophy during Simulated Microgravity. Int. J. Mol. Sci. 2021, 22, 3252. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The Physiopathologic Role of Oxidative Stress in Skeletal Muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Pohl, C.; Dikic, I. Cellular Quality Control by the Ubiquitin-Proteasome System and Autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef]
- Al Mamun, A.; Rahman, M.M.; Zaman, S.; Munira, M.S.; Uddin, M.S.; Rauf, A.; Banu, N.; Ashraf, G.M. Molecular Perspectives on the Interaction Between UPS Components and Alzheimer’s Disease. Curr. Protein Pept. Sci. 2020, 21, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Chen, P.J.; Xiao, W.H. Signaling Pathways That Control Skeletal Muscle Mass. Acta Physiol. Sin. 2019, 71, 671–679. [Google Scholar] [CrossRef]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological Inhibition of Myostatin Suppresses Systemic Inflammation and Muscle Atrophy in Mice with Chronic Kidney Disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscle. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Tammam, A.A.-E.; Rizg, W.Y.; Boushra, A.F.; Alhelf, M.; Alissa, M.; Soliman, G.F.; Ouais, G.N.; Hosny, K.M.; Alkhalidi, H.M.; Elebiary, A.M. Telmisartan Versus Metformin in Downregulating Myostatin Gene Expression and Enhancing Insulin Sensitivity in the Skeletal Muscles of Type 2 Diabetic Rat Model. Front. Pharmacol. 2023, 14, 1228525. [Google Scholar] [CrossRef]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging Role of Myostatin and Its Inhibition in the Setting of Chronic Kidney Disease. Kidney Int. 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Wang, Y.; Pessin, J.E. Mechanisms for Fiber-Type Specificity of Skeletal Muscle Atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef]
- Kang, C.; Li, J. Role of PGC-1α Signaling in Skeletal Muscle Health and Disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wicks, S.E.; Vandanmagsar, B.; Haynie, K.R.; Fuller, S.E.; Warfel, J.D.; Stephens, J.M.; Wang, M.; Han, X.; Zhang, J.; Noland, R.C.; et al. Impaired Mitochondrial Fat Oxidation Induces Adaptive Remodeling of Muscle Metabolism. Proc. Natl. Acad. Sci. USA. 2015, 112, E3300–E3309. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kanatous, S.B.; Thurmond, F.A.; Gallardo, T.; Isotani, E.; Bassel-Duby, R.; Williams, R.S. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002, 296, 349–352. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, A.L.; Pérez, S.; Sandhu, M.A. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View of Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1α Protects Skeletal Muscle from Atrophy by Suppressing FoxO3 Action and Atrophy-Specific Gene Transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Morales, M.G.; Abrigo, J.; Acuña, M.J.; Santos, R.A.; Bader, M.; Brandan, E.; Simon, F.; Olguin, H.; Cabrera, D.; Cabello-Verrugio, C. Angiotensin-(1-7) Attenuates Disuse Skeletal Muscle Atrophy in Mice via Its Receptor, Mas. Dis. Models Mech. 2016, 9, 441–449. [Google Scholar] [CrossRef]
- Semprun-Prieto, L.C.; Sukhanov, S.; Yoshida, T.; Rezk, B.M.; Gonzalez-Villalobos, R.A.; Vaughn, C.; Tabony, A.M.; Delafontaine, P. Angiotensin II-Induced Catabolic Effect and Muscle Atrophy Are Redox-Dependent. Biochem. Biophys. Res. Commun. 2011, 409, 217–221. [Google Scholar] [CrossRef]
- Islam, S.M.T.; Osa-Andrews, B.; Jones, P.M.; Muthukumar, A.R.; Hashim, I.; Cao, J. Methods of Low-Density Lipoprotein-Cholesterol Measurement: Analytical and Clinical Applications. Ejifcc 2022, 33, 282–294. [Google Scholar]
- Aguilera-Méndez, A.; Fernández-Mejía, C. The Hypotriglyceridemic Effect of Biotin Supplementation Involves Increased Levels of cGMP and AMPK Activation. Biofactors 2012, 38, 387–394. [Google Scholar] [CrossRef]
- Villa-Villaseñor, I.M.; Yañez-Rivera, B.; Rueda-Jasso, R.A.; Herrera-Vargas, M.A.; Hernandez-Morales, R.; Melendez-Herrera, E.; Domínguez-Domínguez, O. Differential Sensitivity of Offspring from Four Species of Goodeine Freshwater Fish to Acute Exposure to Nitrates. Front. Ecol. Evol. 2022, 10, 1014814. [Google Scholar] [CrossRef]
- Sánchez-Duarte, E.; Trujillo, X.; Cortés-Rojo, C.; Saavedra-Molina, A.; Camargo, G.; Hernández, L.; Huerta, M.; Montoya-Pérez, R. Nicorandil Improves Post-Fatigue Tension in Slow Skeletal Muscle Fibers by Modulating Glutathione Redox State. J. Bioenerg. Biomembr. 2017, 49, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction: Twenty-Something Years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences |
|---|---|
| MuRF-1 | Forward: 5′-GAAGTGATCATGGACCGGCA-3′ |
| Reverse: 5′-ACAAGGAGCAAGTAGGCACC-3′ | |
| Atrogin-1 | Forward: 5′-AGCTTGTGCGATGTTACCCA-3′ |
| Reverse: 5′-GGTGAAAGTGAGACGGAGCA-3′ | |
| Myostatin | Forward: 5′-ATCACGCTACCACGGAAACA-3′ |
| Reverse: 5′-AGCTGGGCCTTTACCACTTT-3′ | |
| Follistatin | Forward: 5′-TGTGAAGACATCCAGTGCGG-3′ |
| Reverse: 5′-TCCGAGATGGAGTTGCAAGA-3′ | |
| PGC-1α | Forward: 5′-GAGGACACGAGGAAAGGAAGACT-3′ |
| Reverse: 5′-ACTGGCTTGAATCTGTGGAAGAAC-3′ | |
| p47phox | Forward: 5′-CTTCATTCGCCACATCGCCCTCC-3′ |
| Reverse: 5′-CACCTTCTCCGACAGGTCCTGCC-3′ | |
| 18s | Forward: 5′-GCAAATTACCCACTCCCGAC-3′ |
| Reverse: 5′-CCGCTCCCAAGA TCCAACTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Duarte, S.; Sánchez-Duarte, E.; Sánchez-Briones, L.A.; Meléndez-Herrera, E.; Herrera-Vargas, M.A.; Márquez-Gamiño, S.; Vera-Delgado, K.S.; Montoya-Pérez, R. Apocynin Mitigates Diabetic Muscle Atrophy by Lowering Muscle Triglycerides and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 5636. https://doi.org/10.3390/ijms26125636
Sánchez-Duarte S, Sánchez-Duarte E, Sánchez-Briones LA, Meléndez-Herrera E, Herrera-Vargas MA, Márquez-Gamiño S, Vera-Delgado KS, Montoya-Pérez R. Apocynin Mitigates Diabetic Muscle Atrophy by Lowering Muscle Triglycerides and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(12):5636. https://doi.org/10.3390/ijms26125636
Chicago/Turabian StyleSánchez-Duarte, Sarai, Elizabeth Sánchez-Duarte, Luis A. Sánchez-Briones, Esperanza Meléndez-Herrera, Ma. Antonia Herrera-Vargas, Sergio Márquez-Gamiño, Karla S. Vera-Delgado, and Rocío Montoya-Pérez. 2025. "Apocynin Mitigates Diabetic Muscle Atrophy by Lowering Muscle Triglycerides and Oxidative Stress" International Journal of Molecular Sciences 26, no. 12: 5636. https://doi.org/10.3390/ijms26125636
APA StyleSánchez-Duarte, S., Sánchez-Duarte, E., Sánchez-Briones, L. A., Meléndez-Herrera, E., Herrera-Vargas, M. A., Márquez-Gamiño, S., Vera-Delgado, K. S., & Montoya-Pérez, R. (2025). Apocynin Mitigates Diabetic Muscle Atrophy by Lowering Muscle Triglycerides and Oxidative Stress. International Journal of Molecular Sciences, 26(12), 5636. https://doi.org/10.3390/ijms26125636






