Antimicrobial Efficacy of Nd:YAG Laser in Polymicrobial Root Canal Infections: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Study Selection Process
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment
2.6. Data Extraction
3. Results
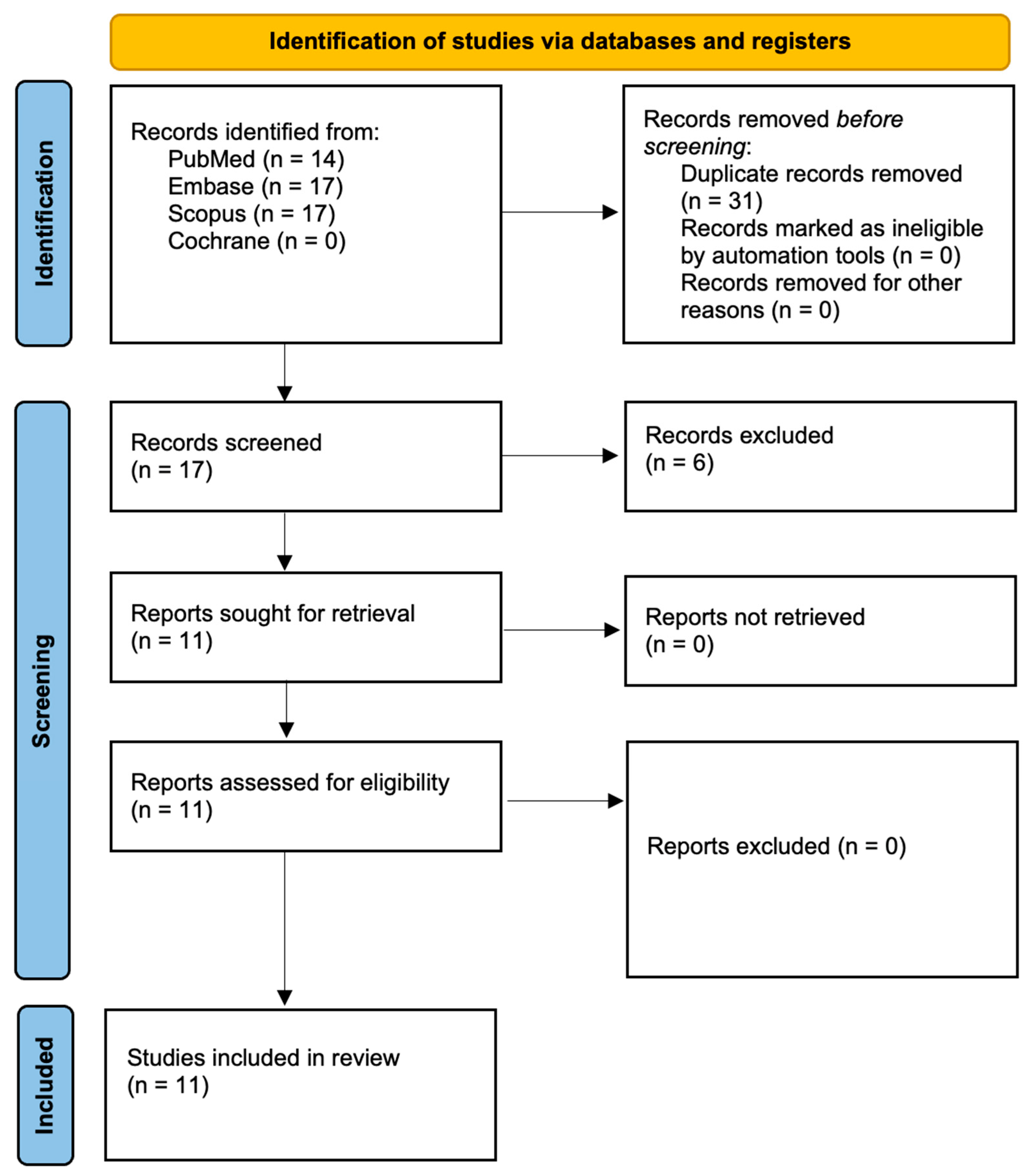
3.1. Study Selection
3.2. Data Presentation
3.3. Overview of Study Characteristics
| Study | Country |
|---|---|
| Bergmans et al., 2006 [38] | Belgium |
| Cheng et al., 2012 [39] | China |
| Jurić et al., 2016 [40] | Croatia |
| Kasić et al., 2017 [41] | Croatia and France |
| Katalinić et al., 2014 [42] | Croatia and Austria |
| Meire et al., 2012 [43] | Belgium |
| Moshonov et al., 1995 [44] | USA |
| Pirnat et al., 2011 [45] | Slovenia |
| Schoop et al., 2004 [46] | Austria |
| Vatkar et al., 2016 [47] | India |
| Wang et al., 2018 [48] | China |
3.4. Main Study Outcomes
| Reference Number | Author and Year | Microorganisms Tested | Study Groups | Outcomes |
|---|---|---|---|---|
| [38] | Bergmans et al., 2006 | Enterococcus faecalis (LMG 7937), Streptococcus anginosus, and Actinomyces naeslundii | The experimental design included three main models. In the microbiological analysis, six root canals were inoculated with E. faecalis and divided into laser-treated and control groups, using a cross-over approach. For CSEM, dentine discs were inoculated with S. anginosus and A. naeslundii, then treated with one, two, or three cycles of laser irradiation through 1 mm thick dentine. ESEM was employed to assess the effect of direct laser irradiation on biofilms formed by E. faecalis or micro-colonies of S. anginosus, with specimens undergoing either two or three irradiation cycles and being analyzed in situ. |
|
| [39] | Cheng et al., 2012 | Enterococcus faecalis (ATCC 4083) | The study comprised seven groups: five experimental groups—(1) Nd:YAG laser, (2) Er:YAG/NaClO/NS/DW, (3) Er:YAG/NS/DW, (4) Er,Cr:YSGG laser, and (5) aPDT—and two control groups: 5.25% NaClO as the positive control and 0.9% normal saline as the negative control. Each group included 20 specimens for bacteriological analysis and 10 for SEM imaging. Laser settings and procedural specifics followed manufacturer guidelines. |
|
| [40] | Jurić et al., 2016 | Enterococcus faecalis (ATCC 29212) | A total of 65 dentine samples were randomly assigned to five groups: aPDT using phenothiazinium chloride and 660 nm laser; Nd:YAG laser irradiation at 2 W, 15 Hz; QMiX solution (a mix of CHX, EDTA, and detergent); 5.25% NaOCl as the negative control (gold standard disinfectant); and a positive control group with no disinfection. Each group included 15 samples for quantitative and microscopic analysis. |
|
| [41] | Kasić et al., 2017 | Enterococcus faecalis and Candida albicans | Thirty extracted single-rooted human teeth were divided into three groups (n = 10), each disinfected with a different laser system plus saline irrigation. Group 1 received Er:YAG laser using the PIPS technique, Group 2 underwent Nd:YAG laser treatment with spiral fiber motion, and Group 3 was treated with Er,Cr:YSGG laser using a radially firing tip. CFUs were measured before and after treatment to evaluate antimicrobial effectiveness. |
|
| [42] | Katalinić et al., 2014 | * | Sixty extracted single-rooted anterior teeth were divided into four groups (n = 15) based on the final disinfection method: (1) 2.5% NaOCl, (2) 0.2% CHX, (3) gaseous ozone, and (4) Nd:YAG laser. After disinfection, canals were filled, post spaces prepared, and fiber-reinforced composite posts cemented with a self-etch adhesive. Push-out bond strength was measured using a universal testing machine. |
|
| [43] | Meire et al., 2012 | Enterococcus faecalis (ATCC 10541), Candida albicans (ATCC 10231), and Propionibacterium acnes (LMG 16711) | Two laser systems were tested in vitro: Er:YAG (2940 nm) in single-pulse mode with energies from 40–400 mJ and pulse durations of 100–1000 μs, and Nd:YAG (1064 nm) in pulse train mode at 15 W, 100 Hz, applied for 5–120 s. Microbe-inoculated agar plates were irradiated through a 5 mm spot; for Nd:YAG, spot size was varied to assess irradiance effects. Antimicrobial efficacy was evaluated by measuring inhibition or clear zone diameters post-irradiation. |
|
| [44] | Moshonov et al., 1995 | Enterococcus faecalis | Seventy-five teeth were assigned to six groups: (1) non-infected control, (2) infected control, (3) infected + Nd:YAG laser with nigrosin dye, (4) infected + dye only, (5) infected + dye and air-water spray (no laser), and (6) infected + 1% NaOCl (positive control). In Group 3, laser irradiation was applied using a 400 µm fiber at 4.5 W, alternating between apical and coronal directions. Post-treatment, all teeth were sampled for bacterial growth, and selected specimens underwent SEM analysis. |
|
| [45] | Pirnat et al., 2011 | Enterococcus faecalis (ATCC 29212) | The experiment tested two Nd:YAG laser heating protocols: (1) single 25 ms pulses (60–100 J/cm2) and (2) pulse trains of ten 1 ms pulses with 30 ms intervals (total fluence 100–260 J/cm2). Bacterial viability was assessed via culture plates and flow cytometry. Thermal behavior and disinfection efficacy were also compared between healthy and carious dentin using modeled heat pulses with fluences from 30 to 300 J/cm2. |
|
| [46] | Schoop et al., 2004 | Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 29212) | Four dental lasers were tested: Nd:YAG (1064 nm), diode (810 nm), Er:YAG (2940 nm), and Er,Cr:YSGG (2780 nm), each at 1 W and 1.5 W, forming eight test groups plus controls. Each group included 20 dentin slices. Laser irradiation was applied indirectly through the non-inoculated side to simulate clinical deep-layer disinfection. Control groups received no irradiation. Temperature measurements were also recorded to evaluate thermal effects. |
|
| [47] | Vatkar et al., 2016 | Enterococcus faecalis (ATCC 29212) | Group I (control) received no disinfection, allowing assessment of natural bacterial colonization. Group II was irrigated with 0.9% saline, Group III with 5.25% NaOCl, and Group IV with 2% CHX. Groups V and VI underwent laser disinfection using Nd:YAG and diode lasers, respectively, with specified power settings and fiber-optic delivery. |
|
| [48] | Wang et al., 2018 | Enterococcus faecalis (ATCC 29212) | Specimens were randomly divided into six groups, each tested at 1 and 3 min intervals: (A) 5.25% NaOCl, (B) Nd:YAG laser, (C) diode laser, (D) Nd:YAP laser, (E) Er,Cr:YSGG + NaOCl, and (F) Er:YAG + NaOCl. Bactericidal effects were evaluated using CLSM with LIVE/DEAD staining, comparing outcomes across laser types and exposure times. |
|
3.5. Characteristics of Light Sources Used in PDT
| Property | Description/Significance |
|---|---|
| Wavelength | 1064 nm (near-infrared). |
| Chromophore Absorption | Highly absorbed by bacterial pigments (melanin, dark pigments); poor absorption in water and hydroxyapatite. |
| Mode of Action | Primarily photothermal; energy absorbed by chromophores within bacteria leads to localized heating and bacterial destruction. |
| Penetration Depth | Effective bactericidal penetration up to 1 mm into dentinal tubules, deeper than many other lasers. |
| Antibacterial Mechanism | Thermal denaturation of proteins, disruption of bacterial cell membranes, and direct bactericidal effects due to intracellular heating. |
| Effect on Smear Layer | Causes evaporation, melting, contraction, and recrystallization of smear layer; at higher energies, complete removal and structural changes occur. |
| Morphological Effects | Melting, glazing, recrystallization, and partial or complete occlusion of dentinal tubules observed. |
| Thermal Effects | Risk of thermal damage and carbonization at higher energy levels (>3 W); optimal bactericidal effects achieved at controlled parameters (15 Hz, 100 mJ, 1.5 W). |
| Clinical Usage Recommendation | Used as adjunct to conventional chemical irrigation (NaOCl); does not fully replace chemical disinfection methods. |
| Author and Year | Light Source | Operating Mode | Power Output (mW) | Irradiation Time (s) |
| Bergmans et al., 2006 [38] | Nd:YAG laser (Smarty A10; DEKA, Firenze, Italy) | 15 Hz, short-pulsed mode | 1500 | 4 × 5 |
| Cheng et al., 2012 [39] | Nd:YAG laser (Fontona Lasers, Stegne-7-1210, Ljubljana, Slovenia) | 15 Hz, pulsed | 1500 | 4 × 4 |
| Jurić et al., 2016 [40] | Nd:YAG (Fotona, Ljubljana, Slovenia) | 15 Hz, pulsed | 2000 | 4 × 5 |
| Kasić et al., 2017 [41] | Nd:YAG (LightWalker, Fotona, Ljubljana, Slovenia) | 15 Hz, pulsed | 1500 | Not specified |
| Katalinić et al., 2014 [42] | Nd:YAG (Fotona, Ljubljana, Slovenia) | 10 Hz, very short-pulsed mode | 4000 | 10 |
| Meire et al., 2012 [43] | Nd:YAG (AT Fidelis, Fotona) | 100 Hz, pulsed | 15,000 | 10–120 |
| Moshonov et al., 1995 [44] | Nd:YAG | Not reported | 4500 | 4 × 15 |
| Pirnat et al., 2011 [45] | Nd:YAG (XP-2, Fotona d.d., Slovenia) | Pulsed | Not reported | Sub-second, ms pulses |
| Schoop et al., 2004 [46] | Nd:YAG (American Dental Technologies, Texas, USA) | 10–200 Hz, pulsed | 200–5000 | 0.1 |
| Vatkar et al., 2016 [47] | Nd:YAG (Fotona Fidelis III, Slovenia, Europe) | Continuous mode | 1500 | 5 |
| Wang et al., 2018 [48] | Nd:YAG (Fotona, Ljubljana, Slovenia) | 15 Hz, pulsed | 1500 | 15 × 4 |
4. Discussion
4.1. Results in the Context of Other Evidence
4.2. Limitations of the Evidence
4.3. Limitations of the Review Process
4.4. Implications for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gomes, B.P.F.A.; Aveiro, E.; Kishen, A. Irrigants and irrigation activation systems in Endodontics. Braz. Dent. J. 2023, 34, 1–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iqbal, A. Antimicrobial irrigants in the endodontic therapy. Int. J. Health Sci. 2012, 6, 186–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Association of Endodontists. Treatment Standards; American Association of Endodontists: Chicago, IL, USA, 2018; Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2018/04/TreatmentStandards_Whitepaper.pdf (accessed on 21 April 2025).
- Al Ajmi, N.F.; Alshenaifi, M.K.; Binsalem, M.M.; Alahmary, K.A.; Al Shahrani, N.A.; Alasim, A.M.; Alotaibi, F.R.; Ali, A.S.; Attar, S.M.; Al Shahrani, N.M. Microbial challenges and solutions in root canal therapy. Int. J. Community Med. Public Health 2024, 11, 3672–3676. [Google Scholar] [CrossRef]
- Saklar, F.; Öncü, A.; Sevgi, S.; Çelikten, B. Endodontic Treatment of Premolar Teeth with Different Root Canal Anatomy: Two Case Reports and Literature Review. Cyprus J. Med. Sci. 2023, 8, 453–456. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Mamat, R.; Nik Abdul Ghani, N.R. The Complexity of the Root Canal Anatomy and Its Influence on Root Canal Debridement in the Apical Region: A Review. Cureus 2023, 15, e49024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal Infection—Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shanker, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Xiao, S.; Sun, G.; Huang, S.; Lin, C.; Li, Y. Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections. Pharmaceutics 2024, 16, 759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleh, I.M.; Ruyter, I.E.; Haapasalo, M.; Ørstavik, D. Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int. Endod. J. 2004, 37, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Coleman, A.; Lessani, M. Success and failure of endodontic treatment: Predictability, complications, challenges and maintenance. Br. Dent. J. 2025, 238, 527–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief Overview of Approaches and Challenges in New Antibiotic Development: A Focus on Drug Repurposing. Front. Cell Infect. Microbiol. 2021, 11, 684515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borkenstein, A.F.; Borkenstein, E.M. Neodymium-doped yttrium aluminum garnet (Nd: YAG) laser treatment in ophthalmology: A review of the most common procedures Capsulotomy and Iridotomy. Lasers Med. Sci. 2024, 39, 167. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, W.T.; Vorwaller, C.E. Utilization of the 1064 nm Wavelength in Photobiomodulation: A Systematic Review and Meta-Analysis. J. Lasers Med. Sci. 2021, 12, e86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grönqvist, A.; Wiström, J.; Axner, O.; Monsen, T.J. Bactericidal effect of pulsed 1,064 nm Nd:YAG laser light on Staphylococcus epidermidis is of photothermal origin: An in vitro study. Lasers Surg. Med. 2000, 27, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, A.; Aminsobhani, M.; Sohrabi, K.; Chiniforush, N.; Ghafari, S.; Shamshiri, A.R.; Noroozi, N. Penetration Depth of Sodium Hypochlorite in Dentinal Tubules after Conventional Irrigation, Passive Ultrasonic Agitation and Nd:YAG Laser Activated Irrigation. J. Lasers Med. Sci. 2016, 7, 105–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dembicka-Mączka, D.; Gryka-Deszczyńska, M.; Sitkiewicz, J.; Makara, A.; Fiegler-Rudol, J.; Wiench, R. Evaluation of the Disinfection Efficacy of Er-YAG Laser Light on Single-Species Candida Biofilms: Systematic Review. Microorganisms 2025, 13, 942. [Google Scholar] [CrossRef]
- Granevik Lindström, M.; Wolf, E.; Fransson, H. The Antibacterial Effect of Nd:YAG Laser Treatment of Teeth with Apical Periodontitis: A Randomized Controlled Trial. J. Endod. 2017, 43, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Tkaczyk, M.; Grzech-Leśniak, K.; Zawilska, A.; Wiench, R. Enhancing Root Canal Disinfection with Er:YAG Laser: A Systematic Review. Dent. J. 2025, 13, 101. [Google Scholar] [CrossRef]
- Kurt, S.; Kırtıloğlu, T.; Yılmaz, N.A.; Ertaş, E.; Oruçoğlu, H. Evaluation of the effects of Er:YAG laser, Nd:YAG laser, and two different desensitizers on dentin permeability: In Vitro study. Lasers Med. Sci. 2018, 33, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Z.; Lyu, P.; Zhou, X.; Fan, Y. Current Applications and Future Directions of Lasers in Endodontics: A Narrative Review. Bioengineering 2023, 10, 296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarano, A.; Lorusso, F.; Inchingolo, F.; Postiglione, F.; Petrini, M. The Effects of Erbium-Doped Yttrium Aluminum Garnet Laser (Er: YAG) Irradiation on Sandblasted and Acid-Etched (SLA) Titanium, an In Vitro Study. Materials. 2020, 13, 4174. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Huang, F.; Wang, S.; Li, M.; Lu, Y.; Pei, D.; Li, A. Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode. Materials 2021, 14, 4746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ateş, M.O.; Uğur Aydın, Z. Evaluation of the effectiveness of different treatment approaches in preventing coronal discoloration caused by regenerative endodontic treatment. Clin. Oral Investig. 2023, 27, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018, 210, 2–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linden, A.H.; Hönekopp, J. Heterogeneity of Research Results: A New Perspective from Which to Assess and Promote Progress in Psychological Science. Perspect. Psychol. Sci. 2021, 16, 358–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wassenberg, M.W.M.; Kluytmans, J.A.J.W.; Box, A.T.A.; Bosboom, R.W.; Buiting, A.G.M.; Van Elzakker, E.P.M.; Melchers, W.J.G.; Van Rijen, M.M.L.; Thijsen, S.F.T.; Troelstra, A.; et al. Rapid screening of methicillin-resistant Staphylococcus aureus using PCR and chromogenic agar: A prospective study to evaluate costs and effects. Clin. Microbiol. Infect. 2010, 16, 1754–1761. [Google Scholar] [CrossRef]
- van Belkum, A.; Durand, G.; Peyret, M.; Chatellier, S.; Zambardi, G.; Schrenzel, J.; Shortridge, D.; Engelhardt, A.; Dunne, W.M., Jr. Rapid clinical bacteriology and its future impact. Ann. Lab. Med. 2010, 33, 14–27. [Google Scholar] [CrossRef]
- He, Z.; Tang, X.; Yang, X.; Guo, Y.; George, T.J.; Charness, N.; Quan Hem, K.B.; Hogan, W.; Bian, J. Clinical Trial Generalizability Assessment in the Big Data Era: A Review. Clin. Transl. Sci. 2020, 13, 675–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024. [Google Scholar]
- Bergmans, L.; Moisiadis, P.; Teughels, W.; Van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Bactericidal effect of Nd:YAG laser irradiation on some endodontic pathogens ex vivo. Int. Endod. J. 2006, 39, 547–557. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, S.; Lu, H.; Zhao, C.; Chen, X.; Li, N.; Bai, Q.; Tian, Y.; Yu, Q. Evaluation of the bactericidal effect of Nd:YAG, Er:YAG, Er,Cr:YSGG laser radiation, and antimicrobial photodynamic therapy (aPDT) in experimentally infected root canals. Lasers Surg. Med. 2012, 44, 824–831. [Google Scholar] [CrossRef]
- Juric, I.B.; Plecko, V.; Anic, I.; Plesko, S.; Jakovljevic, S.; Rocca, J.P.; Medioni, E. Antimicrobial efficacy of photodynamic therapy, Nd:YAG laser and QMiX solution against Enterococcus faecalis biofilm. Photodiagnosis Photodyn. Ther. 2016, 13, 238–243. [Google Scholar] [CrossRef]
- Kasic, S.; Knezovic, M.; Beader, N.; Gabric, D.; Ivanisevic Malcic, A.; Baraba, A. Efficacy of three different lasers on eradication of Enterococcus faecalis and Candida albicans biofilms in root canal system. Photomed. Laser Surg. 2017, 35, 372–377. [Google Scholar] [CrossRef]
- Katalinić, I.; Glockner, K.; Anić, I. Influence of several root canal disinfection methods on pushout bond strength of self-etch post and core systems. Int. Endod. J. 2014, 47, 140–146. [Google Scholar] [CrossRef]
- Meire, M.A.; Coenye, T.; Nelis, H.J.; De Moor, R.J.G. In vitro inactivation of endodontic pathogens with Nd:YAG and Er:YAG lasers. Lasers Med. Sci. 2012, 27, 695–701. [Google Scholar] [CrossRef]
- Moshonov, J.; Ørstavik, D.; Yamauchi, S.; Pettiette, M.; Trope, M. Nd:YAG laser irradiation in root canal disinfection. Endod. Dent. Traumatol. 1995, 11, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Pirnat, S.; Lukac, M.; Ihan, A. Thermal tolerance of E. faecalis to pulsed heating in the millisecond range. Lasers Med. Sci. 2011, 26, 229–237. [Google Scholar] [CrossRef]
- Schoop, U.; Kluger, W.; Moritz, A.; Nedjelik, N.; Georgopoulos, A.; Sperr, W. Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg. Med. 2004, 35, 111–116. [Google Scholar] [CrossRef]
- Vatkar, N.A.; Hegde, V.; Sathe, S. Vitality of Enterococcus faecalis inside dentinal tubules after five root canal disinfection methods. J. Conserv. Dent. 2016, 19, 445–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Cheng, X.; Liu, X.; Wang, Z.; Wang, J.; Guo, C.; Zhang, Y.; He, W. Bactericidal effect of various laser irradiation systems on Enterococcus faecalis biofilms in dentinal tubules: A confocal laser scanning microscopy study. Photomed. Laser Surg. 2018, 36, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef]
- Golob Deeb, J.; Reddy, N.; Kitten, T.; Carrico, C.K.; Grzech-Leśniak, K. Viability of bacteria associated with root caries after Nd:YAG laser application in combination with various antimicrobial agents: An in vitro study. Dent. Med. Probl. 2023, 60, 649–655. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Combination Strategies of Different Antimicrobials: An Efficient and Alternative Tool for Pathogen Inactivation. Biomedicines 2022, 10, 2219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moritz, A.; Schoop, U.; Goharkhay, K.; Schauer, P.; Doertbudak, O.; Sperr, W. The bactericidal effect of Nd:YAG laser irradiation within root canal dentin. J. Clin. Laser Med. Surg. 1997, 15, 29–31. [Google Scholar]
- Gutknecht, N.; Moritz, A.; Conrads, G.; Sievert, T.; Lampert, F. Bactericidal effect of the Nd:YAG laser in in vitro root canals. J. Clin. Laser Med. Surg. 1996, 14, 77–80. [Google Scholar] [CrossRef]
- Klinke, T.; Klimm, W.; Gutknecht, N. Antibacterial effects of Nd:YAG laser irradiation within root canal dentine. J. Clin. Laser Med. Surg. 1997, 15, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Ozses Ozkaya, B.; Gulsahi, K.; Ungor, M.; Gocmen, J.S. A Comparison of Er:YAG Laser with Photon-Initiated Photoacoustic Streaming, Nd:YAG Laser, and Conventional Irrigation on the Eradication of Root Dentinal Tubule Infection by Enterococcus faecalis Biofilms: A Scanning Electron Microscopy Study. Scanning 2017, 2017, 6215482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutknecht, N. Lasers in Endodontics. Laser Health Acad. Rev. 2008, 4, 1–10. [Google Scholar]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Abe, T.; Medina, C.C.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Examination of the Effect of the Combined Use of Nd: YAG Laser Irradiation and Mechanical Force Loading on Bone Metabolism Using Cultured Human Osteoblasts. J. Lasers Med. Sci. 2020, 11, 138–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dilber, E.; Malkoc, M.A.; Ozturk, A.N.; Ozturk, F. Effect of various laser irradiations on the mineral content of dentin. Eur. J. Dent. 2013, 7, 74–80. [Google Scholar] [PubMed] [PubMed Central]
- Hazrati, P.; Azadi, A.; Tizno, A.; Asnaashari, M. The Effect of Lasers on the Healing of Periapical Lesion: A Systematic Review. J. Lasers Med. Sci. 2024, 15, e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahimi, S.; Shahi, S.; Gholizadeh, S.; Shakouie, S.; Rikhtegaran, S.; Soroush Barhaghi, M.H.; Ghojazadeh, M.; Froughreyhani, M.; Abdolrahimi, M. Bactericidal effects of Nd:YAG laser irradiation and sodium hypochlorite solution on Enterococcus faecalis biofilm. Photomed. Laser Surg. 2012, 30, 637–641. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Goodis, H.E.; Rose, C.M. Nd:YAG pulsed infrared laser for treatment of root surface. J. Calif. Dent. Assoc. 1991, 19, 55–58. [Google Scholar] [PubMed]
- de Souza, E.B.; Cai, S.; Simionato, M.R.; Lage-Marques, J.L. High-power diode laser in the disinfection in depth of the root canal dentin. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, e68–e72. [Google Scholar] [CrossRef]
- Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asnaashari, M.; Safavi, N. Disinfection of contaminated canals by different lasers: An in vitro study. J. Lasers Med. Sci. 2013, 4, 8–16. [Google Scholar] [PubMed]
- Dawasaz, A.A. In Vivo Efficacy of Diode Laser as a Monotherapy in Root Canal Disinfection: A Systematic Review and Meta-Analysis. Photobiomodul. Photomed. Laser Surg. 2022, 40, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Fransson, H.; Larsson, K.M.; Wolf, E. Efficacy of lasers as an adjunct to chemo-mechanical disinfection of infected root canals: A systematic review. Int. Endod. J. 2013, 46, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef]
- Asnaashari, M.; Sadeghian, A.; Hazrati, P. The Effect of High-Power Lasers on Root Canal Disinfection: A Systematic Review. J. Lasers Med. Sci. 2022, 13, e66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Fabbro, M.; Corbella, S.; Sequeira-Byron, P.; Tsesis, I.; Rosen, E.; Lolato, A.; Taschieri, S. Endodontic procedures for retreatment of periapical lesions. Cochrane Database Syst. Rev. 2016, 10, CD005511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noferesti, M.; Darmiani, S.; Rastegar, H. A 980 nm Diode Laser as an Adjunctive Therapy on the Healing of Apical Periodontitis Following Endodontic Retreatment: A Randomized Controlled Clinical Trial Study. J. Lasers Med. Sci. 2024, 15, e36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Source | Search Term | Number of Results |
|---|---|---|
| PubMed | (“Nd:YAG” [Title/Abstract] OR “neodymium:YAG” [Title/Abstract] OR “Nd:YAG laser” [Title/Abstract]) AND (“root canal disinfection” [Title/Abstract] OR “endodontic disinfection” [Title/Abstract] OR “root canal therapy” [Title/Abstract]) AND (disinfection [Title/Abstract] OR sterilization [Title/Abstract] OR “microbial reduction” [Title/Abstract]) | 14 |
| Embase | (‘nd:yag’:ab,ti OR ‘neodymium:yag’:ab,ti OR ‘nd:yag laser’:ab,ti) AND (‘root canal disinfection’:ab,ti OR ‘endodontic disinfection’:ab,ti OR ‘root canal therapy’:ab,ti) AND (disinfection:ab,ti OR sterilization:ab,ti OR ‘microbial reduction’:ab,ti) | 17 |
| Scopus | (TITLE-ABS(“Nd:YAG”) OR TITLE-ABS(“neodymium:YAG”) OR TITLE-ABS(“Nd:YAG laser”)) AND (TITLE-ABS(“root canal disinfection”) OR TITLE-ABS(“endodontic disinfection”) OR TITLE-ABS(“root canal therapy”)) AND (TITLE-ABS(disinfection) OR TITLE-ABS(sterilization) OR TITLE-ABS(“microbial reduction”)) | 17 |
| Cochrane | (“Er:YAG laser”:ti,ab OR “erbium:YAG laser”:ti,ab) AND (disinfection:ti,ab OR antibacterial:ti,ab OR bactericidal:ti,ab) AND (efficacy:ti,ab OR effectiveness:ti,ab) AND (bacteria:ti,ab OR microbial:ti,ab OR microbiological:ti,ab) | 0 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bergmans et al., 2006 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 | Low |
| Cheng et al., 2012 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Low |
| Jurić et al., 2016 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Low |
| Kasić et al., 2017 [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 | Low |
| Katalinić et al., 2014 [42] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Low |
| Meire et al., 2012 [43] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Low |
| Moshonov et al., 1995 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 | Low |
| Pirnat et al., 2011 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | Low |
| Schoop et al., 2004 [46] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 | Low |
| Vatkar et al., 2016 [47] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | Low |
| Wang et al., 2018 [48] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 7 | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiegler-Rudol, J.; Skaba, D.; Wiench, R. Antimicrobial Efficacy of Nd:YAG Laser in Polymicrobial Root Canal Infections: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 5631. https://doi.org/10.3390/ijms26125631
Fiegler-Rudol J, Skaba D, Wiench R. Antimicrobial Efficacy of Nd:YAG Laser in Polymicrobial Root Canal Infections: A Systematic Review of In Vitro Studies. International Journal of Molecular Sciences. 2025; 26(12):5631. https://doi.org/10.3390/ijms26125631
Chicago/Turabian StyleFiegler-Rudol, Jakub, Dariusz Skaba, and Rafał Wiench. 2025. "Antimicrobial Efficacy of Nd:YAG Laser in Polymicrobial Root Canal Infections: A Systematic Review of In Vitro Studies" International Journal of Molecular Sciences 26, no. 12: 5631. https://doi.org/10.3390/ijms26125631
APA StyleFiegler-Rudol, J., Skaba, D., & Wiench, R. (2025). Antimicrobial Efficacy of Nd:YAG Laser in Polymicrobial Root Canal Infections: A Systematic Review of In Vitro Studies. International Journal of Molecular Sciences, 26(12), 5631. https://doi.org/10.3390/ijms26125631





