Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola
Abstract
1. Introduction
2. Results
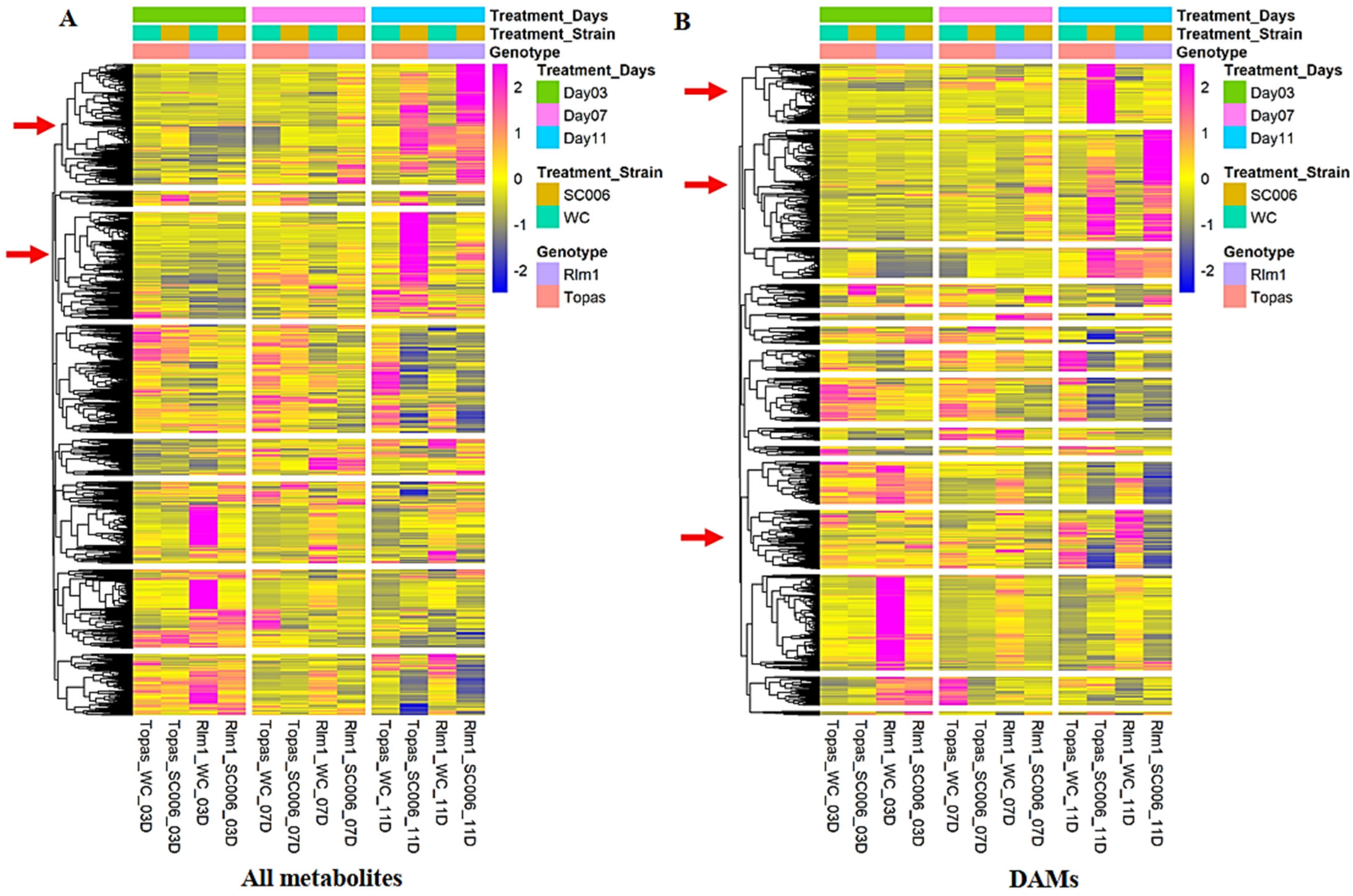
2.1. Multivariate Analysis of Metabolomic Data
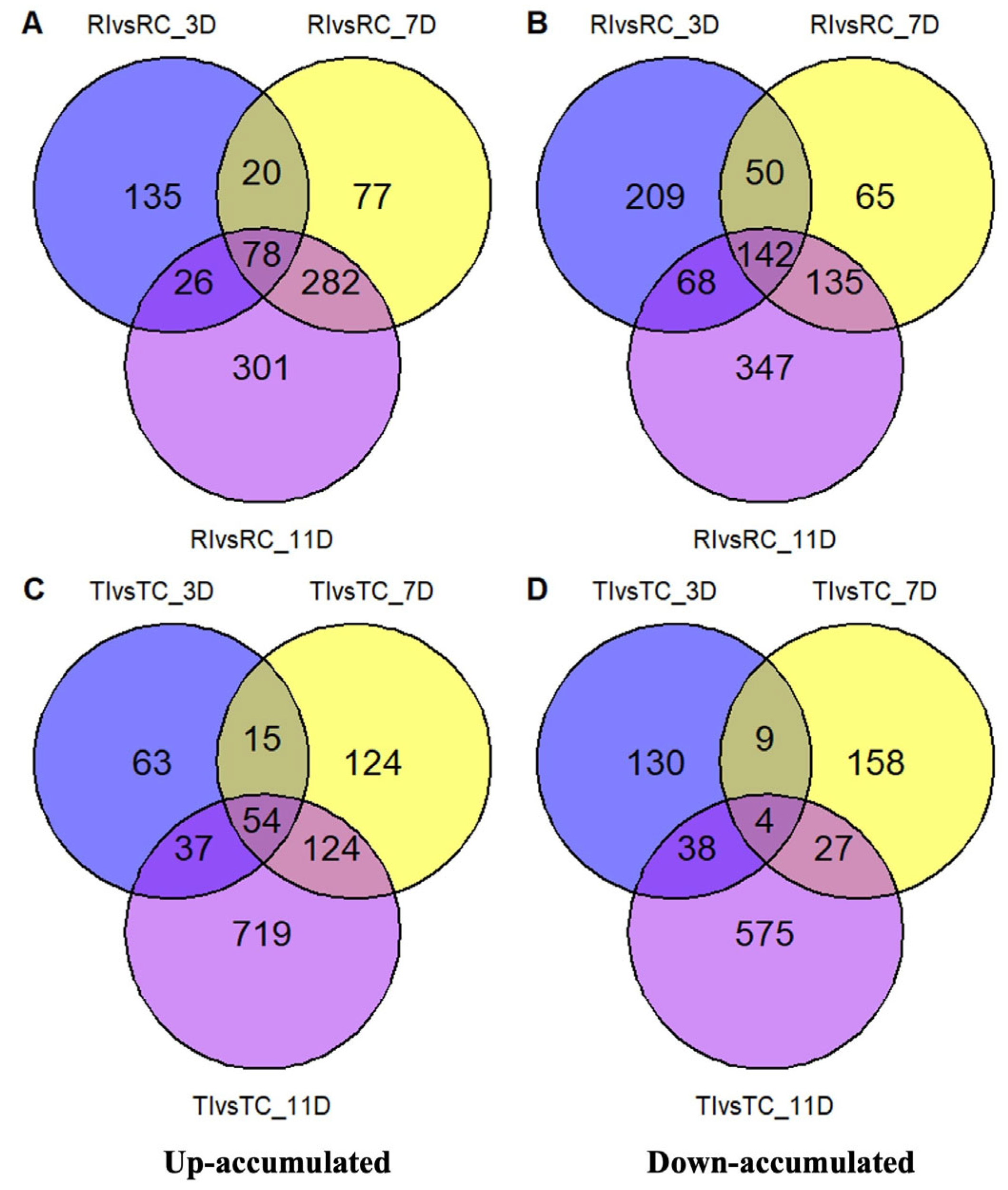
2.2. Univariate Analysis of Metabolomic Data
2.3. DAMs in Relation to Inoculation and Resistance
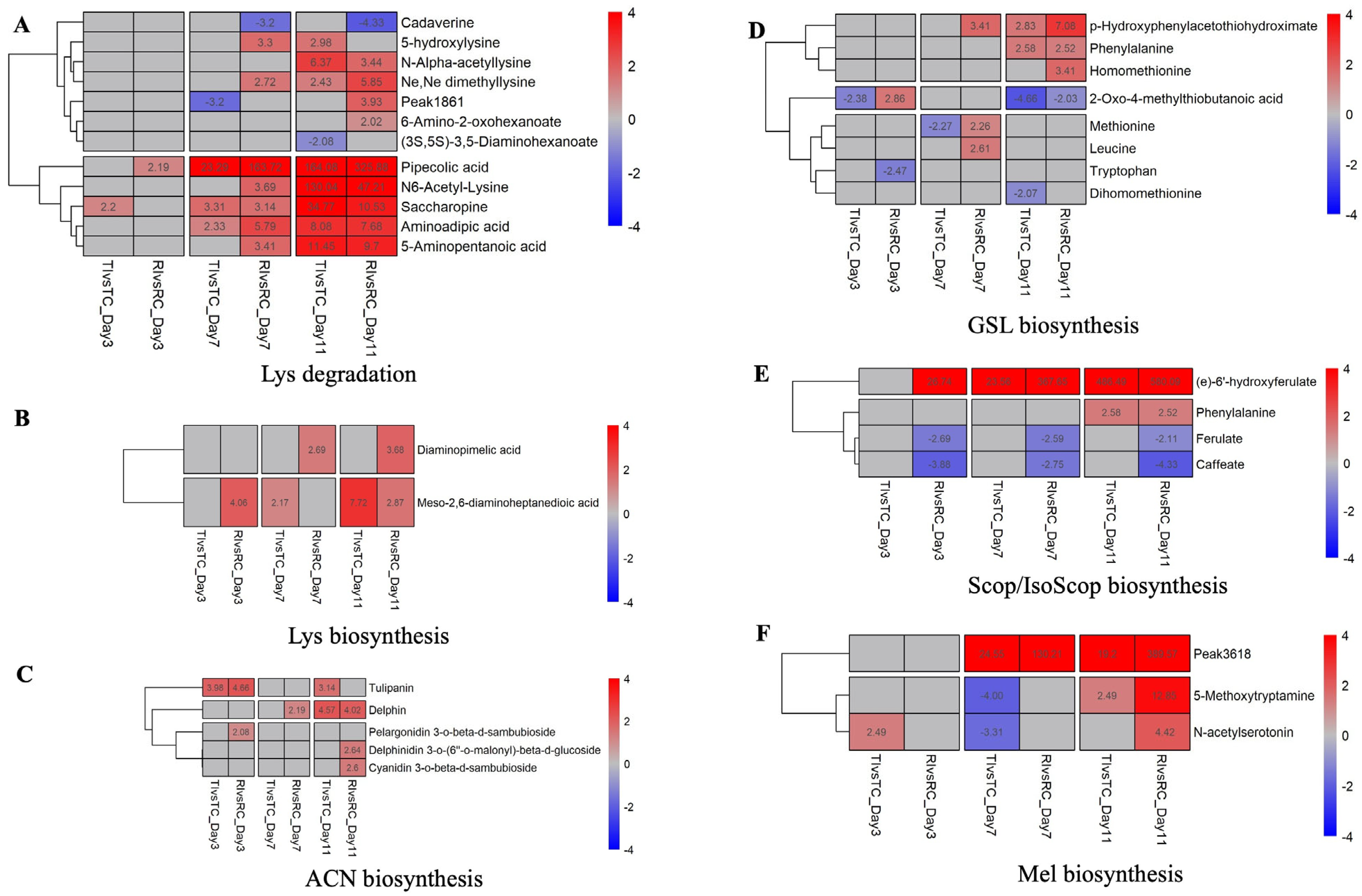
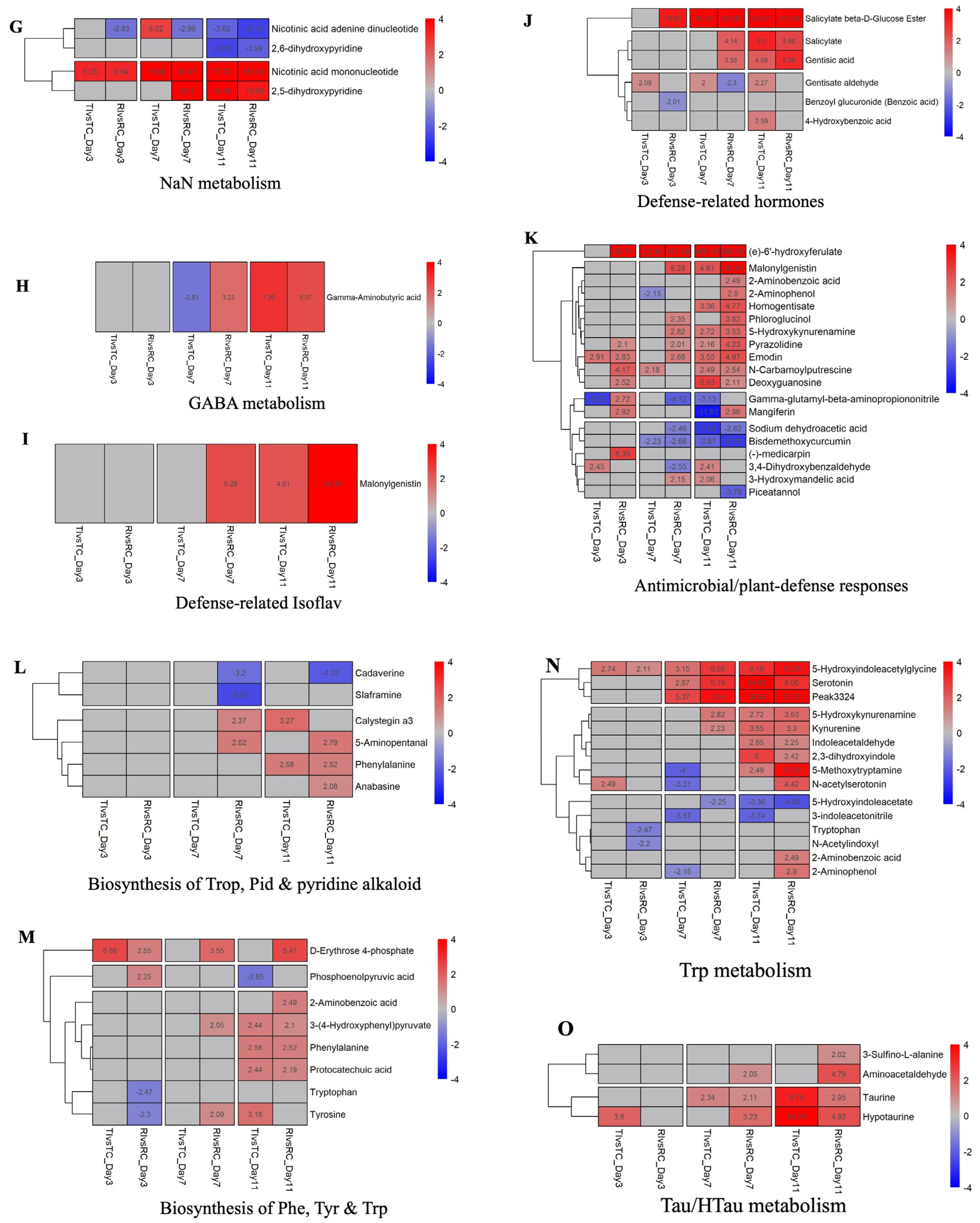
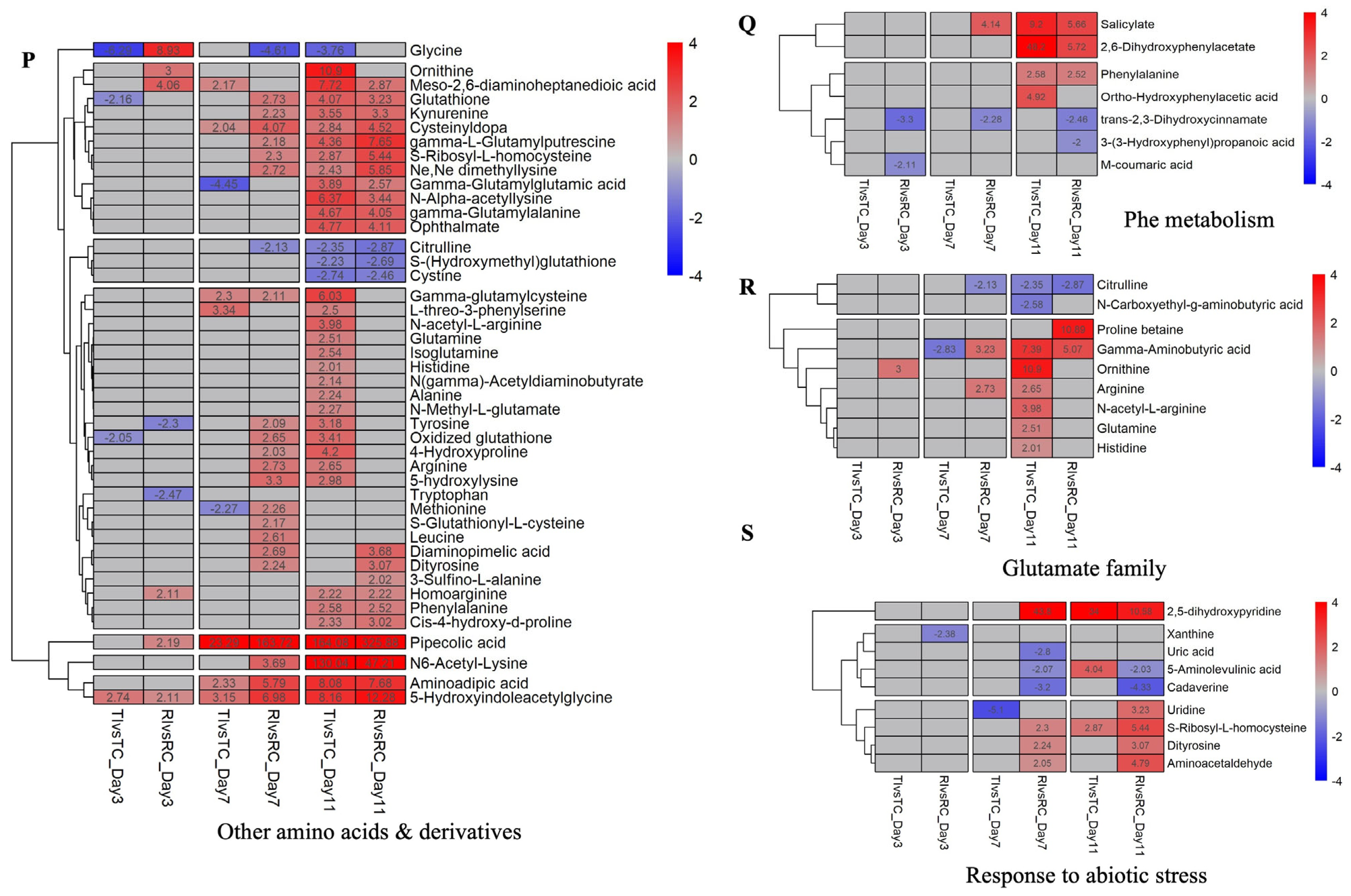
2.4. Prominent DAMs and Their Related Pathways
2.5. Metabolites/Pathways Potentially Related to Rlm1-Mediated Resistance
2.5.1. Lysine Metabolism and Degradation
2.5.2. Defense Signaling Molecules
2.5.3. Antimicrobial Metabolites
2.5.4. Amino Acid and Secondary Metabolite Pathways
2.5.5. Scopoletin/Isoscopoletin Biosynthesis
2.5.6. Redox Metabolism (GSH/GSSG)
2.5.7. Flavonoid-Related Pathways
2.6. Validating DAM Candidates for Their Potential Roles in Rlm1-Mediated Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Pathogen Isolates
4.2. Plant Inoculation, Infection Assessment, and Leaf-Tissue Sampling
4.3. Sample Preparation for Metabolomic Analysis Using CIL LC–MS
4.4. Metabolome Quantification
4.5. LC−MS Analysis
4.6. LC–MS Raw Data Extraction and Processing
4.7. Validating the Potential Involvement of Selected Metabolites in Resistance
4.7.1. Chemical (Metabolite) Preparation
4.7.2. Application of Metabolites
4.7.3. Inoculation and Infection Assessment
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistics Canada. Production of Principal Field Crops, November 2024. The Daily, 5 December 2024. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/241205/dq241205b-eng.htm (accessed on 31 March 2025).
- Chen, G.; Wu, C.; Li, B.; Su, H.; Zhen, S.; An, Y. Detection of Leptosphaeria maculans from imported Canola seeds/Nachweis von Leptosphaeria maculons in importiertem Rapssaatgut. J. Plant Dis. Prot. 2010, 117, 173–176. [Google Scholar] [CrossRef]
- Fitt, B.D.; Hu, B.; Li, Z.; Liu, S.; Lange, R.; Kharbanda, P.; Butterworth, M.; White, R. Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 2008, 57, 652–664. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, G.; Kutcher, H.R.; Balesdent, M.-H.; Delourme, R.; Fernando, W.D. Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada. Eur. J. Plant Pathol. 2016, 145, 659–674. [Google Scholar] [CrossRef]
- Hubbard, M.; Zhai, C.; Peng, G. Exploring mechanisms of quantitative resistance to Leptosphaeria maculans (Blackleg) in the cotyledons of canola (Brassica napus) based on transcriptomic and microscopic analyses. Plants 2020, 9, 864. [Google Scholar] [CrossRef]
- Hubbard, M.; Peng, G. Quantitative resistance against an isolate of Leptosphaeria maculans (blackleg) in selected Canadian canola cultivars remains effective under increased temperatures. Plant Pathol. 2018, 67, 1329–1338. [Google Scholar] [CrossRef]
- Balesdent, M.-H.; Gautier, A.; Plissonneau, C.; Le Meur, L.; Loiseau, A.; Leflon, M.; Carpezat, J.; Pinochet, X.; Rouxel, T. Twenty years of Leptosphaeria maculans population survey in France suggests pyramiding Rlm3 and Rlm7 in rapeseed is a risky resistance management strategy. Phytopathology® 2022, 112, 2126–2137. [Google Scholar] [CrossRef]
- Liban, S.; Cross, D.; Kutcher, H.; Peng, G.; Fernando, W. Race structure and frequency of avirulence genes in the western Canadian Leptosphaeria maculans pathogen population, the causal agent of blackleg in brassica species. Plant Pathol. 2016, 65, 1161–1169. [Google Scholar] [CrossRef]
- Soomro, W.; Kutcher, R.; Yu, F.; Hwang, S.-F.; Fernando, D.; Strelkov, S.E.; Peng, G. The race structure of Leptosphaeria maculans in western Canada between 2012 and 2014 and its influence on blackleg of canola. Can. J. Plant Pathol. 2021, 43, 480–493. [Google Scholar] [CrossRef]
- Liu, F.; Zou, Z.; Peng, G.; Dilantha Fernando, W. Leptosphaeria maculans isolates reveal their allele frequency in Western Canada. Plant Dis. 2021, 105, 1440–1447. [Google Scholar] [CrossRef]
- Rouxel, T.; Balesdent, M. The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Mol. Plant Pathol. 2005, 6, 225–241. [Google Scholar] [CrossRef]
- Rouxel, T.; Penaud, A.; Pinochet, X.; Brun, H.; Gout, L.; Delourme, R.; Schmit, J.; Balesdent, M.-H. A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur. J. Plant Pathol. 2003, 109, 871–881. [Google Scholar] [CrossRef]
- Borhan, M.H.; Van de Wouw, A.P.; Larkan, N.J. Molecular interactions between Leptosphaeria maculans and Brassica species. Annu. Rev. Phytopathol. 2022, 60, 237–257. [Google Scholar] [CrossRef]
- Chu, M.; Song, T.; Falk, K.C.; Zhang, X.; Liu, X.; Chang, A.; Lahlali, R.; McGregor, L.; Gossen, B.D.; Yu, F. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophora brassicae. BMC Genom. 2014, 15, 1166. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chu, M.; Lahlali, R.; Yu, F.; Peng, G. Shotgun label-free proteomic analysis of clubroot (Plasmodiophora brassicae) resistance conferred by the gene Rcr1 in Brassica rapa. Front. Plant Sci. 2016, 7, 1013. [Google Scholar] [CrossRef] [PubMed]
- Fudal, I.; Ross, S.; Gout, L.; Blaise, F.; Kuhn, M.; Eckert, M.; Cattolico, L.; Bernard-Samain, S.; Balesdent, M.; Rouxel, T. Heterochromatin-like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: Map-based cloning of AvrLm6. Mol. Plant-Microbe Interact. 2007, 20, 459–470. [Google Scholar] [CrossRef]
- Zhai, C.; Liu, X.; Song, T.; Yu, F.; Peng, G. Genome-wide transcriptome reveals mechanisms underlying Rlm1-mediated blackleg resistance on canola. Sci. Rep. 2021, 11, 4407. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- AbuQamar, S.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; Araújo, F.C.; Ferreira-Neto, J.R.; Silva, R.L.; Borges, A.N.; Matos, M.K.; Silva, J.B.; Silva, M.D.; Kido, E.A.; Benko-Iseppon, A.M. NBS-LRR genes—Plant health sentinels: Structure, roles, evolution and biotechnological applications. In Applied Plant Biotechnology for Improving Resistance to Biotic Stress; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–120. [Google Scholar]
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In Biotechnology of Hairy Root Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 55–89. [Google Scholar]
- Lobo, M.; Hounsome, N.; Hounsome, B. Biochemistry of vegetables: Secondary metabolites in vegetables—Terpenoids, phenolics, alkaloids, and sulfur-containing compounds. In Handbook of Vegetables and Vegetable Processing; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 47–82. [Google Scholar]
- Kumar, A.; Irchhaiya, R.; Yadav, A.; Gupta, N.; Kumar, S.; Gupta, N.; Kumar, S.; Yadav, V.; Prakash, A.; Gurjar, H. Metabolites in plants and its classification. World J. Pharm. Pharm. Sci. 2015, 4, 287–305. [Google Scholar]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I. Metabolomics in food science. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 67, pp. 1–24. [Google Scholar]
- Luo, X.; Gu, X.; Li, L. Development of a simple and efficient method of harvesting and lysing adherent mammalian cells for chemical isotope labeling LC-MS-based cellular metabolomics. Anal. Chim. Acta 2018, 1037, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, L. Differential 12C-/13C-isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome. Anal. Chem. 2009, 81, 3919–3932. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, S.; Huan, T.; Sun, D.; Friis, R.M.N.; Schultz, M.C.; Li, L. High-performance chemical isotope labeling liquid chromatography–mass spectrometry for profiling the metabolomic reprogramming elicited by ammonium limitation in yeast. J. Proteome Res. 2016, 15, 1602–1612. [Google Scholar] [CrossRef]
- Shen, W.; Han, W.; Li, Y.; Meng, Z.; Cai, L.; Li, L. Development of chemical isotope labeling liquid chromatography mass spectrometry for silkworm hemolymph metabolomics. Anal. Chim. Acta 2016, 942, 1–11. [Google Scholar] [CrossRef]
- Tunsagool, P.; Wang, X.; Leelasuphakul, W.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Chen, G.; Li, L. Metabolomic study of stress responses leading to plant resistance in mandarin fruit mediated by preventive applications of Bacillus subtilis cyclic lipopeptides. Postharvest Biol. Technol. 2019, 156, 110946. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Bi, Y.; Jiang, Q.; Mao, R.; Liu, Z.; Huang, Y.; Zhang, M.; Prusky, D.B. Induction of defense response against Alternaria rot in Zaosu pear fruit by exogenous L-lysine through regulating ROS metabolism and activating defense-related proteins. Postharvest Biol. Technol. 2021, 179, 111567. [Google Scholar] [CrossRef]
- Yang, H.; Ludewig, U. Lysine catabolism, amino acid transport, and systemic acquired resistance: What is the link? Plant Signal. Behav. 2014, 9, e28933. [Google Scholar] [CrossRef]
- Shan, L.; He, P. Pipped at the post: Pipecolic acid derivative identified as SAR regulator. Cell 2018, 173, 286–287. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Wang, C.; Shine, M.; Yu, K.; Kachroo, A.; Kachroo, P. Nitric oxide and reactive oxygen species are required for systemic acquired resistance in plants. Plant Signal. Behav. 2015, 10, e998544. [Google Scholar] [CrossRef]
- Ghosh, S.; Kanwar, P.; Jha, G. Alterations in rice chloroplast integrity, photosynthesis and metabolome associated with pathogenesis of Rhizoctonia solani. Sci. Rep. 2017, 7, 41610. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, X.; Liu, Y.; Zhang, Y.; Yang, L.; Zhang, S.; Xu, J. Induction of γ-aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae. J. Integr. Plant Biol. 2020, 62, 1797–1812. [Google Scholar] [CrossRef]
- Rani, M.; Jha, G. Host gamma-aminobutyric acid metabolic pathway is involved in resistance against Rhizoctonia solani. Phytopathol® 2021, 111, 1207–1218. [Google Scholar] [CrossRef]
- Mäkinen, K.; De, S. The significance of methionine cycle enzymes in plant virus infections. Curr. Opin. Plant Biol. 2019, 50, 67–75. [Google Scholar] [CrossRef]
- Bellés, J.M.; Garro, R.; Pallás, V.; Fayos, J.; Rodrigo, I.; Conejero, V. Accumulation of gentisic acid as associated with systemic infections but not with the hypersensitive response in plant-pathogen interactions. Planta 2006, 223, 500–511. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, L. Development of Chemical Isotope Labeling Liquid Chromatography Orbitrap Mass Spectrometry for Comprehensive Analysis of Dipeptides. Anal. Chem. 2023, 95, 6629–6636. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Han, W.; Chan, W.; Li, L. Metabolomic coverage of chemical-group-submetabolome analysis: Group classification and four-channel chemical isotope labeling LC-MS. Anal. Chem. 2019, 91, 12108–12115. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Zhou, J.; Zuniga, A.; Stanislaus, A.E.; Wu, Y.; Huan, T.; Zheng, J.; Shi, Y.; Wishart, D.S. MyCompoundID: Using an evidence-based metabolome library for metabolite identification. Anal. Chem. 2013, 85, 3401–3408. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, gkae253. [Google Scholar] [CrossRef]
- Stevenson, P.; Turner, H.; Haware, M. Phytoalexin accumulation in the roots of chickpea (Cicer arietinum L.) seedlings associated with resistance to fusarium wilt (Fusarium oxysporum f. sp. ciceri). Physiol. Mol. Plant Pathol. 1997, 50, 167–178. [Google Scholar] [CrossRef]
- Gupta, A.; Awasthi, P.; Sharma, N.; Parveen, S.; Vats, R.P.; Singh, N.; Kumar, Y.; Goel, A.; Chandran, D. Medicarpin confers powdery mildew resistance in Medicago truncatula and activates the salicylic acid signalling pathway. Mol. Plant Pathol. 2022, 23, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Moydeen, M.; Al-Deyab, S.S.; Manilal, A.; Idhayadhulla, A. Synthesis of new morpholine-connected pyrazolidine derivatives and their antimicrobial, antioxidant, and cytotoxic activities. Bioorg. Med. Chem. Lett. 2017, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Jyotshna; Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. Biofactors 2016, 42, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Biswas, K.; Chakrabarti, D.K.; Basu Chaudhary, K. Control of Fusarium wilt of safflower by mangiferin. Phytopathology 1977, 67, 548–550. [Google Scholar] [CrossRef]
- Gao, X.; Li, K.; Ma, Z.; Zou, H.; Jin, H.; Wang, J. Cucumber Fusarium wilt resistance induced by intercropping with celery differs from that induced by the cucumber genotype and is related to sulfur-containing allelochemicals. Sci. Hortic. 2020, 271, 109475. [Google Scholar] [CrossRef]
- Godard, S.; Slacanin, I.; Viret, O.; Gindro, K. Induction of defence mechanisms in grapevine leaves by emodin-and anthraquinone-rich plant extracts and their conferred resistance to downy mildew. Plant Physiol. Biochem. 2009, 47, 827–837. [Google Scholar] [CrossRef]
- da Graça, J.P.; Ueda, T.E.; Janegitz, T.; Vieira, S.S.; Salvador, M.C.; de Oliveira, M.C.; Zingaretti, S.M.; Powers, S.J.; Pickett, J.A.; Birkett, M.A. The natural plant stress elicitor cis-jasmone causes cultivar-dependent reduction in growth of the stink bug, Euschistus heros and associated changes in flavonoid concentrations in soybean, Glycine max. Phytochemistry 2016, 131, 84–91. [Google Scholar] [CrossRef]
- Schwenen, L.; Komoßa, D.; Barz, W. Metabolism and degradation of nicotinic acid in parsley (Petroselinum hortense) cell suspension cultures and seedlings. Z. Naturforschung C 1986, 41, 148–157. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Zhang, Y.; Yan, X.; Jin, X.; Yao, X.; Chen, P.; Zheng, B. PtKTI12 genes influence wobble uridine modifications and drought stress tolerance in hybrid poplar. Tree Physiol. 2020, 40, 1778–1791. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Shorina, M.; Aronova, E.; Stetsenko, L.; Rakitin, V.; Shevyakova, N. NaCl-and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant Sci. 2007, 172, 363–370. [Google Scholar] [CrossRef]
- Sun, T.; Pei, T.; Yang, L.; Zhang, Z.; Li, M.; Liu, Y.; Ma, F.; Liu, C. Exogenous application of xanthine and uric acid and nucleobase-ascorbate transporter MdNAT7 expression regulate salinity tolerance in apple. BMC Plant Biol. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Lee, J.; Ahn, Y.; Hwang, Y.S.; Park, J.; Lee, J.; Choi, J. Metabolome and transcriptome analyses of plants grown in naturally attenuated soil after hydrogen fluoride exposure. J. Hazard. Mater. 2022, 437, 129323. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Lalehloo, B.S.; Bayat, M.; Sharafi, S.; Habibi, F. Using physiological traits to evaluating resistance of different barley promising lines to water deficit stress. Int. J. Sci. Res. Environ. Sci. 2014, 2, 209. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Chamoun, R.; Aliferis, K.A.; Jabaji, S. Identification of signatory secondary metabolites during mycoparasitism of Rhizoctonia solani by Stachybotrys elegans. Front. Microbiol. 2015, 6, 138210. [Google Scholar] [CrossRef]
- Moulin, M.; Deleu, C.; Larher, F.; Bouchereau, A. The lysine-ketoglutarate reductase–saccharopine dehydrogenase is involved in the osmo-induced synthesis of pipecolic acid in rapeseed leaf tissues. Plant Physiol. Biochem. 2006, 44, 474–482. [Google Scholar] [CrossRef]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, H.; Jiao, P.; Wei, X.; Liu, S.; Guan, S.; Ma, Y. The Integration of Metabolomics and Transcriptomics Provides New Insights for the Identification of Genes Key to Auxin Synthesis at Different Growth Stages of Maize. Int. J. Mol. Sci. 2022, 23, 13195. [Google Scholar] [CrossRef]
- Bayoumi, S.A.; Rowan, M.G.; Beeching, J.R.; Blagbrough, I.S. Investigation of biosynthetic pathways to hydroxycoumarins during post-harvest physiological deterioration in cassava roots by using stable isotope labelling. ChemBioChem 2008, 9, 3013–3022. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Sui, Z.; Huang, C.; Zeng, Z.; Kong, L. The molecular and structural basis of O-methylation reaction in coumarin biosynthesis in Peucedanum praeruptorum Dunn. Int. J. Mol. Sci. 2019, 20, 1533. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Whitworth, R.J.; Stuart, J.J.; Chen, M.-S. Unbalanced activation of glutathione metabolic pathways suggests potential involvement in plant defense against the gall midge Mayetiola destructor in wheat. Sci. Rep. 2015, 5, 8092. [Google Scholar] [CrossRef]
- Youssef, S.A.; Tartoura, K.A. Compost enhances plant resistance against the bacterial wilt pathogen Ralstonia solanacearum via up-regulation of ascorbate-glutathione redox cycle. Eur. J. Plant Pathol. 2013, 137, 821–834. [Google Scholar] [CrossRef]
- Schalk, M.; Cabello-Hurtado, F.; Pierrel, M.-A.s.; Atanassova, R.; Saindrenan, P.; Werck-Reichhart, D.l. Piperonylic acid, a selective, mechanism-based inactivator of the trans-cinnamate 4-hydroxylase: A new tool to control the flux of metabolites in the phenylpropanoid pathway. Plant Physiol. 1998, 118, 209–218. [Google Scholar] [CrossRef]
- da Costa, T.P.S.; Hall, C.J.; Panjikar, S.; Wyllie, J.A.; Christoff, R.M.; Bayat, S.; Hulett, M.D.; Abbott, B.M.; Gendall, A.R.; Perugini, M.A. Towards novel herbicide modes of action by inhibiting lysine biosynthesis in plants. Elife 2021, 10, e69444. [Google Scholar]
- Dzierzbicka, K. Synthesis of 2, 6-diaminopimelic acid (DAP) and its analogues. Pol. J. Chem. 2007, 81, 455–473. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Klessig, D.F. SOS–too many signals for systemic acquired resistance? Trends Plant Sci. 2012, 17, 538–545. [Google Scholar] [CrossRef]
- Návarová, H.; Bernsdorff, F.; Döring, A.-C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef]
- Vranova, V.; Lojkova, L.; Rejsek, K.; Formanek, P. Significance of the natural occurrence of L-versus D-pipecolic acid: A review. Chirality 2013, 25, 823–831. [Google Scholar] [CrossRef]
- Prabhu, B.R.; Mulchandani, N.B. Biosynthesis of piperlongumine. Phytochemistry 1985, 24, 2589–2591. [Google Scholar] [CrossRef]
- Vogel-Adghough, D.; Stahl, E.; Návarová, H.; Zeier, J. Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal. Behav. 2013, 8, e26366. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Su, J.; Tian, B.; Fang, A.; Yu, Y.; Bi, C.; Yang, Y. Integrated metabolo-transcriptomics reveals the defense response of homogentisic acid in wheat against Puccinia striiformis f. sp. tritici. J. Agric. Food Chem. 2022, 70, 3719–3729. [Google Scholar] [CrossRef]
- Arruda, P.; Barreto, P. Lysine catabolism through the saccharopine pathway: Enzymes and intermediates involved in plant responses to abiotic and biotic stress. Front. Plant Sci. 2020, 11, 535796. [Google Scholar] [CrossRef]
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E. A central role of salicylic acid in plant disease resistance. Science 1994, 266, 1247–1250. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Grant, J.J.; Chini, A.; Basu, D.; Loake, G.J. Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol. Plant-Microbe Interact. 2003, 16, 669–680. [Google Scholar] [CrossRef]
- Zhu, X.; Soliman, A.; Islam, M.R.; Adam, L.R.; Daayf, F. Verticillium dahliae’s isochorismatase hydrolase is a virulence factor that contributes to interference with potato’s salicylate and jasmonate defense signaling. Front. Plant Sci. 2017, 8, 399. [Google Scholar] [CrossRef]
- Rochon, A.; Boyle, P.; Wignes, T.; Fobert, P.R.; Després, C. The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell 2006, 18, 3670–3685. [Google Scholar] [CrossRef]
- Cameron, R.K.; Paiva, N.L.; Lamb, C.J.; Dixon, R.A. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol. Mol. Plant Pathol. 1999, 55, 121–130. [Google Scholar] [CrossRef]
- Lincoln, J.E.; Sanchez, J.P.; Zumstein, K.; Gilchrist, D.G. Plant and animal PR1 family members inhibit programmed cell death and suppress bacterial pathogens in plant tissues. Mol. Plant Pathol. 2018, 19, 2111–2123. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Singapore, 2018; pp. 265–281. [Google Scholar]
- Yi, S.Y.; Shirasu, K.; Moon, J.S.; Lee, S.-G.; Kwon, S.-Y. The activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition. PLoS ONE 2014, 9, e88951. [Google Scholar] [CrossRef]
- Neuenschwander, U.; Vernooij, B.; Friedrich, L.; Uknes, S.; Kessmann, H.; Ryals, J. Is hydrogen peroxide a second messenger of salicylic acid in systemic acquired resistance? Plant J. 1995, 8, 227–233. [Google Scholar] [CrossRef]
- Kovács, J.; Poór, P.; Szepesi, Á.; Tari, I. Salicylic acid induced cysteine protease activity during programmed cell death in tomato plants. Acta Biol. Hung. 2016, 67, 148–158. [Google Scholar] [CrossRef]
- Greenberg, J.T.; Guo, A.; Klessig, D.F.; Ausubel, F.M. Programmed cell death in plants: A pathogen-triggered response activated coordinately with multiple defense functions. Cell 1994, 77, 551–563. [Google Scholar] [CrossRef]
- Mittler, R.; Del Pozo, O.; Meisel, L.; Lam, E. Pathogen-induced programmed cell death in plants, a possible defense mechanism. Dev. Genet. 1997, 21, 279–289. [Google Scholar] [CrossRef]
- Garattini, E.; Mendel, R.; Romão, M.J.; Wright, R.; Terao, M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: Structure, genetics, regulation, function and pathophysiology. Biochem. J. 2003, 372, 15–32. [Google Scholar] [CrossRef]
- Bellés, J.M.; Garro, R.; Fayos, J.; Navarro, P.; Primo, J.; Conejero, V. Gentisic acid as a pathogen-inducible signal, additional to salicylic acid for activation of plant defenses in tomato. Mol. Plant-Microbe Interact. 1999, 12, 227–235. [Google Scholar] [CrossRef]
- Campos, L.; Granell, P.; Tárraga, S.; López-Gresa, P.; Conejero, V.; Bellés, J.M.; Rodrigo, I.; Lisón, P. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 2014, 77, 35–43. [Google Scholar] [CrossRef]
- Yalpani, N.; León, J.; Lawton, M.A.; Raskin, I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993, 103, 315–321. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate hydrolysis products in the crucifer Barbarea vulgaris include a thiazolidine-2-one from a specific phenolic isomer as well as oxazolidine-2-thiones. Phytochemistry 2015, 115, 143–151. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Sanchez-Vallet, A.; Ramos, B.; Bednarek, P.; López, G.; Piślewska-Bednarek, M.; Schulze-Lefert, P.; Molina, A. Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant J. 2010, 63, 115–127. [Google Scholar] [CrossRef]
- Smolinska, U.; Knudsen, G.; Morra, M.; Borek, V. Inhibition of Aphanomyces euteiches f. sp. pisi by volatiles produced by hydrolysis of Brassica napus seed meal. Plant Dis. 1997, 81, 288–292. [Google Scholar] [CrossRef]
- Van Eylen, D.; Bellostas, N.; Strobel, B.W.; Oey, I.; Hendrickx, M.; Van Loey, A.; Sørensen, H.; Sørensen, J.C. Influence of pressure/temperature treatments on glucosinolate conversion in broccoli (Brassica oleraceae L. cv Italica) heads. Food Chem. 2009, 112, 646–653. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Singh, A.; Guest, D.; Copeland, L. Associations Between Glucosinolates, White Rust, and Plant Defense Activators in Brassica Plants: A Review. Int. J. Veg. Sci. 2014, 21, 297–313. [Google Scholar] [CrossRef]
- Zhai, K.; Liang, D.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X. NLRs guard metabolism to coordinate pattern-and effector-triggered immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef]
- Escaray, F.; Felipo-Benavent, A.; Vera, P. Linking plant metabolism and immunity through methionine biosynthesis. Mol. Plant 2022, 15, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, L.; Pang, H.; Wang, P.; Liu, L.; Cheng, Y.; Cheng, J.; Guo, Y.; Li, Q. METHIONINE SYNTHASE1 is involved in chromatin silencing by maintaining DNA and histone methylation. Plant Physiol. 2019, 181, 249–261. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal. Res. 2016, 60, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Choi, G.H.; Lee, H.Y.; Back, K. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J. Exp. Bot. 2015, 66, 6917–6925. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, G.; Bai, Y.; Xia, F.; He, C.; Shi, H.; Foyer, C. Two transcriptional activators of N-acetylserotonin O-methyltransferase 2 and melatonin biosynthesis in cassava. J. Exp. Bot. 2017, 68, 4997–5006. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal. Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Kong, M.; Sheng, T.; Liang, J.; Ali, Q.; Gu, Q.; Wu, H.; Chen, J.; Liu, J.; Gao, X. Melatonin and Its Homologs Induce Immune Responses via Receptors trP47363-trP13076 in Nicotiana benthamiana. Front. Plant Sci. 2021, 12, 691835. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Li, J.; Ali, M.; Wang, Y.; Liu, X.; Li, F.; Li, X. GABA primes defense responses against Botrytis cinerea in tomato fruit by modulating ethylene and JA signaling pathways. Postharvest Biol. Technol. 2024, 208, 112665. [Google Scholar] [CrossRef]
- Xuan Phong, H.; Le Viet, Q.; Minh Chau, L.; Long, D.; Bui, H.; Thanh, N.N.; Tan Phat, D.; Truong, L.D. Isolation and selection of lactic acid bacteria with the capacity of producing γ-aminobutyric acid (GABA) and antimicrobial activity: Its application in fermented meat product. Curr. Nutr. Food Sci. 2023, 19, 831–837. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, J.; Dong, X.; Du, N.; Piao, F. Gamma-aminobutyric acid improves phenanthrene phytotoxicity tolerance in cucumber through the glutathione-dependent system of antioxidant defense. Ecotoxicol. Environ. Saf. 2021, 217, 112254. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta 2018, 248, 909–918. [Google Scholar] [CrossRef]
- Meher, H.C.; Gajbhiye, V.T.; Singh, G. Salicylic acid-induced glutathione status in tomato crop and resistance to root-knot nematode, Meloidogyne incognita (Kofoid & White) Chitwood. J. Xenobiotics 2011, 1, e5. [Google Scholar]
- Meher, H.C.; Gajbhiye, V.T.; Singh, G.; Chawla, G. Altered metabolomic profile of selected metabolites and improved resistance of Cicer arietinum (L.) against Meloidogyne incognita (Kofoid & White) Chitwood following seed soaking with salicylic acid, benzothiadiazole or nicotinic acid. Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar]
- Hiruma, K.; Fukunaga, S.; Bednarek, P.; Piślewska-Bednarek, M.; Watanabe, S.; Narusaka, Y.; Shirasu, K.; Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. USA 2013, 110, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Xing, L.-P.; Wu, G.-J.; Wang, H.-Z.; Wang, X.-E.; Cao, A.-Z.; Chen, P.-D. Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum). Plant Cell Physiol. 2007, 48, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Muroi, A.; Ishihara, A.; Tanaka, C.; Ishizuka, A.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana. Planta 2009, 230, 517–527. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Huang, R.; Qu, L.; Liu, J.; Zhang, C.; Ge, Y. Ferulic acid enhanced resistance against blue mold of Malus domestica by regulating reactive oxygen species and phenylpropanoid metabolism. Postharvest Biol. Technol. 2023, 202, 112378. [Google Scholar] [CrossRef]
- Gozzo, F. Systemic acquired resistance in crop protection: From nature to a chemical approach. J. Agric. Food Chem. 2003, 51, 4487–4503. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Liu, X.; Xu, T.; Zhang, Y.; Meng, H.; Xia, T. High temperature increased lignin contents of poplar (Populus spp.) stem via inducing the synthesis caffeate and coniferaldehyde. Front. Genet. 2022, 13, 1007513. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Lei, Y.; Hu, G.; Liu, J.; Hao, M.; Chen, A.; Peng, Q.; Wu, J. Cotton WATs modulate SA biosynthesis and local lignin deposition participating in plant resistance against Verticillium dahliae. Front. Plant Sci. 2019, 10, 526. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Yan, C.-H.; Zhang, X.-R.; Tu, Q.-B.; Xu, Y.; Sheng, S.; Wu, F.-A.; Wang, J. A novel nanoparticle loaded with methyl caffeate and caffeic acid phenethyl ester against Ralstonia solanacearum—A plant pathogenic bacteria. RSC Adv. 2020, 10, 3978–3990. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- McCalla, D.; Neish, A. Metabolism of phenylpropanoid compounds in Salvia: II. Biosynthesis of phenolic cinnamic acids. Can. J. Biochem. Physiol. 1959, 37, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Wiklund, P.; Bergman, J. The chemistry of anthranilic acid. Curr. Org. Synth. 2006, 3, 379–402. [Google Scholar] [CrossRef]
- Winter, A. A hypothetical route for the biogenisis of IAA. Planta 1966, 71, 229–239. [Google Scholar] [CrossRef]
- Doyle, S.M.; Rigal, A.; Grones, P.; Karady, M.; Barange, D.K.; Majda, M.; Parizkova, B.; Karampelias, M.; Zwiewka, M.; Pencik, A.; et al. A role for the auxin precursor anthranilic acid in root gravitropism via regulation of PIN-FORMED protein polarity and relocalisation in Arabidopsis. New Phytol. 2019, 223, 1420–1432. [Google Scholar] [CrossRef]
- Yang, S.Y.; Park, M.R.; Kim, I.S.; Kim, Y.C.; Yang, J.W.; Ryu, C.-M. 2-Aminobenzoic acid of Bacillus sp. BS107 as an ISR determinant against Pectobacterium carotovorum subsp. carotovotrum SCC1 in tobacco. Eur. J. Plant Pathol. 2011, 129, 371–378. [Google Scholar] [CrossRef]
- Hossain, M.; Hossain, M.; Islam, R.; Alam, A.; Zahan, K.; Sarkar, S.; Farooque, M. Antimicrobial and cytotoxic activities of 2-aminobenzoic acid and 2-aminophenol and their coordination complexes with Magnesium (Mg-II). Pak. J. Biol. Sci. 2004, 7, 25–27. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Bi, X.; Du, X.; Liu, H.; An, T.; Zhao, Y.; Yu, H.; Chen, Y.; Wen, J. Comparative metabolomics reveal the participation of soybean unique rhizosphere metabolites in susceptibility and resistance of host soybean to Phytophthora sojae. Plant Soil 2022, 480, 185–199. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef]
- Takahashi, Y. The role of polyamines in plant disease resistance. Environ. Control Biol. 2016, 54, 17–21. [Google Scholar] [CrossRef]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. Advances in polyamine research in 2007. J. Plant Res. 2007, 120, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Berberich, T.; Yamashita, K.; Uehara, Y.; Miyazaki, A.; Kusano, T. Identification of tobacco HIN1 and two closely related genes as spermine-responsive genes and their differential expression during the Tobacco mosaic virus-induced hypersensitive response and during leaf-and flower-senescence. Plant Mol. Biol. 2004, 54, 613–622. [Google Scholar] [CrossRef]
- Yamakawa, H.; Kamada, H.; Satoh, M.; Ohashi, Y. Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 1998, 118, 1213–1222. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, G.; Li, Y.; Lv, H. Changes in quality characteristics and metabolites composition of wheat under different storage temperatures. J. Stored Prod. Res. 2024, 105, 102229. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Lin, S.; Xing, Y.; Zhang, X.; Ye, M.; Chang, Y.; Guo, H.; Sun, X. (+)-Catechin, epicatechin and epigallocatechin gallate are important inducible defensive compounds against Ectropis grisescens in tea plants. Plant Cell Environ. 2022, 45, 496–511. [Google Scholar] [CrossRef]
- Piispanen, J.; Bergmann, U.; Karhu, J.; Kauppila, T.; Kaitera, J. Variation of compounds in leaves of susceptible and resistant alternate hosts of Cronartium pini and C. ribicola. Eur. J. Plant Pathol. 2023, 165, 677–692. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Wang, J.; Zhu, Q.; Lin, S.; Xu, J.; Zheng, C.; Chen, J.; Qin, X.; Fang, C. Mixed phenolic acids mediated proliferation of pathogens Talaromyces helicus and Kosakonia sacchari in continuously monocultured Radix pseudostellariae rhizosphere soil. Front. Microbiol. 2016, 7, 335. [Google Scholar] [CrossRef]
- Schoch, G.A.; Nikov, G.N.; Alworth, W.L.; Werck-Reichhart, D. Chemical inactivation of the cinnamate 4-hydroxylase allows for the accumulation of salicylic acid in elicited cells. Plant Physiol. 2002, 130, 1022–1031. [Google Scholar] [CrossRef]
- Desmedt, W.; Jonckheere, W.; Nguyen, V.H.; Ameye, M.; De Zutter, N.; De Kock, K.; Debode, J.; Van Leeuwen, T.; Audenaert, K.; Vanholme, B. The phenylpropanoid pathway inhibitor piperonylic acid induces broad-spectrum pest and disease resistance in plants. Plant Cell Environ. 2021, 44, 3122–3139. [Google Scholar] [CrossRef]
- Nawrath, C.; Métraux, J.-P. Salicylic acid induction–deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar] [PubMed]
- Larkan, N.J.; Yu, F.; Lydiate, D.J.; Rimmer, S.R.; Borhan, M.H. Single R gene introgression lines for accurate dissection of the Brassica-Leptosphaeria pathosystem. Front. Plant Sci. 2016, 7, 1771. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Liu, X.; Wang, R.; Zhai, C.; Peng, G.; Yu, F.; Fernando, W.D. Fine mapping of Brassica napus blackleg resistance gene Rlm1 through bulked segregant RNA sequencing. Sci. Rep. 2019, 9, 14600. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.; Parkin, I.; Keith, D.; Lydiate, D. Frequent nonreciprocal translocations in the amphidiploid genome of oilseed rape (Brassica napus). Genome 1995, 38, 1112–1121. [Google Scholar] [CrossRef]
- Yu, F.; Lydiate, D.J.; Gugel, R.; Sharpe, A.; Rimmer, S. Introgression of Brassica rapa subsp. sylvestris blackleg resistance into B. napus. Mol. Breed. 2012, 30, 1495–1506. [Google Scholar] [CrossRef]
- Chen, Y.; Fernando, W. Prevalence of pathogenicity groups of Leptosphaeria maculans in western Canada and North Dakota, USA. Can. J. Plant Pathol. 2006, 28, 533–539. [Google Scholar] [CrossRef]
- Koch, E.; Badawy, H.; Hoppe, H. Differences between aggressive and non-aggressive single spore lines of Leptosphaeria maculans in cultural characteristics and phytotoxin production. J. Phytopathol. 1989, 124, 52–62. [Google Scholar] [CrossRef]
- Li, H.; Sivasithamparam, K.; Barbetti, M.J.; Kuo, J. Germination and invasion by ascospores and pycnidiospores of Leptosphaeria maculans on spring-type Brassica napus canola varieties with varying susceptibility to blackleg. J. Gen. Plant Pathol. 2004, 70, 261–269. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, X.; Li, L. Chemical isotope labeling LC-MS for high coverage and quantitative profiling of the hydroxyl submetabolome in metabolomics. Anal. Chem. 2016, 88, 10617–10623. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Zhou, R.; Tseng, C.-L.; Huan, T.; Li, L. IsoMS: Automated processing of LC-MS data generated by a chemical isotope labeling metabolomics platform. Anal. Chem. 2014, 86, 4675–4679. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A 2016, 1430, 80–95. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2024. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. Create Elegant Data Visualisations Using the Grammar of Graphics. Version 3.5.1. CRAN. 2024. Available online: https://ggplot2.tidyverse.org (accessed on 30 May 2025).
- Yan, L. ggvenn: Draw Venn Diagram by ‘ggplot2’. R Package Version 0.1.10. CRAN. 2023. Available online: https://CRAN.R-project.org/package=ggvenn (accessed on 30 May 2025).
- Kolde, R. Pretty Heatmaps. R Package Version 1.0.12. CRAN. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 30 May 2025).
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.2. CRAN. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 30 May 2025).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-7. CRAN. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 30 May 2025).
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The aligned rank transform for nonparametric factorial analyses using only anova procedures. In Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI 2011), Vancouver, BC, Canada, 7–12 May 2011; ACM Press: New York, NY, USA, 2011; pp. 143–146. [Google Scholar]
- Mangiafico, S.S. Aligned Ranks Transformation ANOVA. Summary and Analysis of Extension Program Evaluation in R; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2016; pp. 315–327. [Google Scholar]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. In Proceedings for The 34th annual ACM Symposium on User Interface Software and Technology; Nichols, J., Kumar, R., Eds.; Association for Computing Machinery: New York, NY, USA, 2021; pp. 754–768. [Google Scholar]
- Kay, M.; Elkin, L.A.; Higgins, J.J.; Wobbrock, J.O. ARTool: Aligned Rank Transform. Version 0.10.7. CRAN. 2020. Available online: https://CRAN.R-project.org/package=ARTool (accessed on 30 May 2025).
- Lenth, R.V. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.4. CRAN. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 30 May 2025).
- Mangiafico, S. rcompanion: Functions to Support Extension Education Program Evaluation; Version 2.4.36; CRAN; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2024; Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 30 May 2025).
- Yurekten, O.; Payne, T.; Tejera, N.; Amaladoss, F.X.; Martin, C.; Williams, M.; O’Donovan, C. MetaboLights: Open data repository for metabolomics. Nucleic Acids Res. 2024, 52, D640–D646. [Google Scholar] [CrossRef]




| Pathways | Total Compounds | Hits | Raw p | Impact |
|---|---|---|---|---|
| Flavone and flavonol biosynthesis | 10 | 4 | 5.2649 × 10−5 | 0.5 |
| Isoquinoline alkaloid biosynthesis | 6 | 1 | 0.013614 | 0.5 |
| Arginine and proline metabolism | 32 | 3 | 0.11938 | 0.32738 |
| Biosynthesis of various plant secondary metabolites | 29 | 1 | 0.24485 | 0.24 |
| Glycine, serine and threonine metabolism | 33 | 2 | 0.018356 | 0.22375 |
| Lysine biosynthesis | 9 | 1 | 0.000103 | 0.16216 |
| Arginine biosynthesis | 18 | 1 | 0.17605 | 0.13981 |
| Tryptophan metabolism | 29 | 1 | 0.010177 | 0.10687 |
| Glyoxylate and dicarboxylate metabolism | 29 | 1 | 0.11936 | 0.10147 |
| Tyrosine metabolism | 17 | 1 | 0.013614 | 0.10056 |
| Phenylpropanoid biosynthesis | 43 | 2 | 0.00128 | 0.09634 |
| Glutathione metabolism | 26 | 1 | 0.11936 | 0.07114 |
| Pyrimidine metabolism | 41 | 1 | 0.021007 | 0.02929 |
| Purine metabolism | 73 | 3 | 0.000316 | 0.02344 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 22 | 2 | 0.011081 | 0.02002 |
| Flavonoid biosynthesis | 47 | 2 | 4.2424 × 10−5 | 0.00338 |
| Cysteine and methionine metabolism | 47 | 1 | 0.000562 | 0.00265 |
| Lipoic acid metabolism | 24 | 1 | 0.11936 | 0.0016 |
| D-Amino acid metabolism | 7 | 1 | 0.000103 | 0 |
| Indole alkaloid biosynthesis | 4 | 1 | 0.010177 | 0 |
| Glucosinolate biosynthesis | 65 | 1 | 0.010177 | 0 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 47 | 1 | 0.013614 | 0 |
| Anthocyanin biosynthesis | 11 | 1 | 0.014562 | 0 |
| Lysine degradation | 20 | 1 | 0.038832 | 0 |
| Thiamine metabolism | 22 | 1 | 0.11936 | 0 |
| Cyanoamino acid metabolism | 29 | 2 | 0.16748 | 0 |
| Pathways | Total Compounds | Hits | Raw p | Impact |
|---|---|---|---|---|
| Taurine and hypotaurine metabolism | 5 | 2 | 0.004092 | 1 |
| Glutathione metabolism | 26 | 5 | 0.000843 | 0.51637 |
| Isoquinoline alkaloid biosynthesis | 6 | 3 | 0.000207 | 0.5 |
| Tyrosine metabolism | 17 | 4 | 0.001364 | 0.32961 |
| Glycine, serine and threonine metabolism | 33 | 1 | 7.41 × 10−5 | 0.22375 |
| Arginine biosynthesis | 18 | 2 | 0.020249 | 0.17088 |
| Lysine degradation | 20 | 3 | 5.38 × 10−5 | 0.16667 |
| Lysine biosynthesis | 9 | 1 | 0.000818 | 0.16216 |
| Flavone and flavonol biosynthesis | 10 | 2 | 0.00029 | 0.15 |
| Arginine and proline metabolism | 32 | 3 | 0.040159 | 0.14584 |
| Butanoate metabolism | 17 | 1 | 0.13621 | 0.13636 |
| Cysteine and methionine metabolism | 47 | 2 | 0.000311 | 0.13181 |
| Alanine, aspartate, and glutamate metabolism | 22 | 1 | 0.13621 | 0.1295 |
| Purine metabolism | 73 | 3 | 0.001022 | 0.10374 |
| Glyoxylate and dicarboxylate metabolism | 29 | 1 | 7.41 × 10−5 | 0.10147 |
| Phenylpropanoid biosynthesis | 43 | 1 | 0.017894 | 0.05935 |
| Pyrimidine metabolism | 41 | 1 | 0.000913 | 0.02929 |
| Folate biosynthesis | 31 | 1 | 0.39026 | 0.02624 |
| Porphyrin metabolism | 48 | 1 | 0.001198 | 0.02261 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 47 | 2 | 4.84 × 10−5 | 0.02209 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 22 | 2 | 4.84 × 10−5 | 0.02002 |
| Tryptophan metabolism | 29 | 3 | 0.000722 | 0.01527 |
| Lipoic acid metabolism | 24 | 1 | 7.41 × 10−5 | 0.0016 |
| Thiamine metabolism | 22 | 1 | 7.41 × 10−5 | 0 |
| Cyanoamino acid metabolism | 29 | 2 | 8.89 × 10−5 | 0 |
| Glucosinolate biosynthesis | 65 | 3 | 0.000128 | 0 |
| Flavonoid biosynthesis | 47 | 1 | 0.000193 | 0 |
| Biosynthesis of various plant secondary metabolites | 29 | 1 | 0.000248 | 0 |
| Valine, leucine, and isoleucine degradation | 37 | 1 | 0.000662 | 0 |
| Valine, leucine, and isoleucine biosynthesis | 22 | 1 | 0.000662 | 0 |
| D-Amino acid metabolism | 7 | 1 | 0.000818 | 0 |
| Tropane, piperidine, and pyridine alkaloid biosynthesis | 9 | 2 | 0.003453 | 0 |
| Anthocyanin biosynthesis | 11 | 1 | 0.012109 | 0 |
| Zeatin biosynthesis | 21 | 1 | 0.044967 | 0 |
| Pathways | Total Compounds | Hits | Raw p | Impact |
|---|---|---|---|---|
| Taurine and hypotaurine metabolism | 5 | 3 | 0.003645 | 1 |
| Phenylalanine metabolism | 12 | 1 | 0.000113 | 0.42308 |
| Glutathione metabolism | 26 | 3 | 9.13 × 10−5 | 0.40276 |
| Tyrosine metabolism | 17 | 5 | 0.000141 | 0.39665 |
| Phenylpropanoid biosynthesis | 43 | 8 | 9.23 × 10−5 | 0.28583 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 47 | 2 | 0.000443 | 0.1998 |
| beta-Alanine metabolism | 18 | 2 | 0.022568 | 0.19444 |
| Lysine degradation | 20 | 3 | 0.000743 | 0.16667 |
| Lysine biosynthesis | 9 | 1 | 0.000192 | 0.16216 |
| Arginine and proline metabolism | 32 | 3 | 3.65 × 10−6 | 0.15774 |
| Butanoate metabolism | 17 | 1 | 0.000299 | 0.13636 |
| Alanine, aspartate and glutamate metabolism | 22 | 1 | 0.000299 | 0.1295 |
| Purine metabolism | 73 | 3 | 0.003473 | 0.09255 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 22 | 3 | 1.11 × 10−5 | 0.09159 |
| Arginine biosynthesis | 18 | 1 | 0.004743 | 0.08641 |
| Pyrimidine metabolism | 41 | 2 | 0.002791 | 0.07198 |
| Flavonoid biosynthesis | 47 | 5 | 0.00363 | 0.06956 |
| Cysteine and methionine metabolism | 47 | 5 | 0.000575 | 0.05644 |
| Glucosinolate biosynthesis | 65 | 3 | 1.36 × 10−6 | 0.04236 |
| Tryptophan metabolism | 29 | 4 | 4.93 × 10−5 | 0.03054 |
| Pantothenate and CoA biosynthesis | 25 | 1 | 0.16968 | 0.02796 |
| Porphyrin metabolism | 48 | 1 | 0.013372 | 0.02261 |
| Flavone and flavonol biosynthesis | 10 | 2 | 6.24 × 10−5 | 0 |
| Cyanoamino acid metabolism | 29 | 1 | 0.000113 | 0 |
| Tropane, piperidine and pyridine alkaloid biosynthesis | 9 | 3 | 0.000161 | 0 |
| D-Amino acid metabolism | 7 | 1 | 0.000192 | 0 |
| Anthocyanin biosynthesis | 11 | 2 | 0.00027 | 0 |
| Glycine, serine and threonine metabolism | 33 | 1 | 0.002717 | 0 |
| Zeatin biosynthesis | 21 | 1 | 0.005646 | 0 |
| Isoquinoline alkaloid biosynthesis | 6 | 2 | 0.006486 | 0 |
| Common Name | Abbreviation | Chemical Name | Mol. Formula | Concentration 2 | Supplier |
|---|---|---|---|---|---|
| Pipecolic acid | PA | Piperidine-2-carboxylic acid | C6H11NO2 | 40 mM | Tokyo Chemical Industry (TCI) (Portland, OR, USA) |
| Salicylic acid (sodium salt) | SA | Sodium 2-hydroxybenzoate | C7H5NaO3 | 1 mM | Thermo Fisher (Ottawa, ON, Canada) |
| Gentisic acid (sodium salt hydrate) | GA | 2,5-Dihydroxybenzoic acid sodium salt | C7H5O4Na | 10 mM | Sigma Aldrich (MilliporeSigma Canada Ltd., Oakville, ON, Canada) |
| Glutathione | GSH | γ-L-glutamyl-L-cysteinylglycine | C6H11NO2 | 20 mM | Thermo Fisher |
| Lysine | Lys | (S)-2,6-Diaminocaproic acid | C6H14N2O2 | 10 mM | Sigma Aldrich |
| Diaminopimelic acid | DAP | 2,6-Diaminopimelic acid | C7H14N2O4 | 30 mM | Sigma Aldrich |
| Ferulic acid | FA | Trans-ferulic acid | C10H10O4 | 1 mM 3 | Sigma Aldrich |
| Caffeic acid | CFA | (E)-3-(3,4-dihydroxyphenyl) prop-2-enoic acid | C9H8O4 | 10 mM 4 | Sigma Aldrich |
| Benzoic acid | BA | Benzoic acid | C7H6O2 | 10 mM 5 | Thermo Fisher |
| Piperonylic acid | PipA | 1,3-benzodioxole-5-carboxylic acid | C8H6O4 | 3 mM 6 | Sigma Aldrich |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Gao, P.; Zhao, S.; Luo, X.; Li, L.; Peng, G. Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola. Int. J. Mol. Sci. 2025, 26, 5627. https://doi.org/10.3390/ijms26125627
Zhu X, Gao P, Zhao S, Luo X, Li L, Peng G. Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola. International Journal of Molecular Sciences. 2025; 26(12):5627. https://doi.org/10.3390/ijms26125627
Chicago/Turabian StyleZhu, Xiaohan, Peng Gao, Shuang Zhao, Xian Luo, Liang Li, and Gary Peng. 2025. "Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola" International Journal of Molecular Sciences 26, no. 12: 5627. https://doi.org/10.3390/ijms26125627
APA StyleZhu, X., Gao, P., Zhao, S., Luo, X., Li, L., & Peng, G. (2025). Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola. International Journal of Molecular Sciences, 26(12), 5627. https://doi.org/10.3390/ijms26125627








