Sugar-Linked Diethyldithiocarbamate Derivatives: A Novel Class of Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
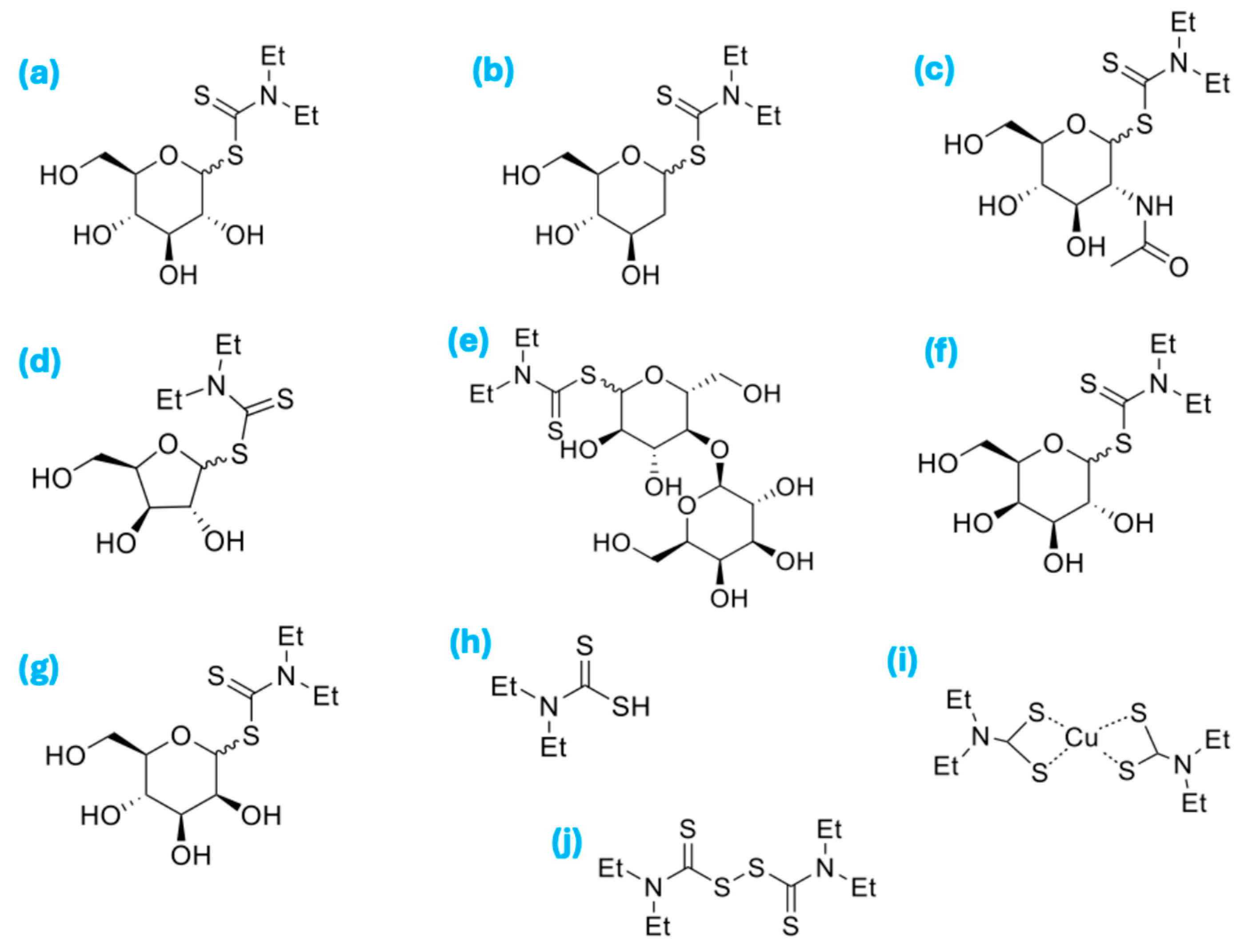
3.1. Methods for the Preparation of Saccharide-Diethyldithiocarbamate (Saccharide-DDC)
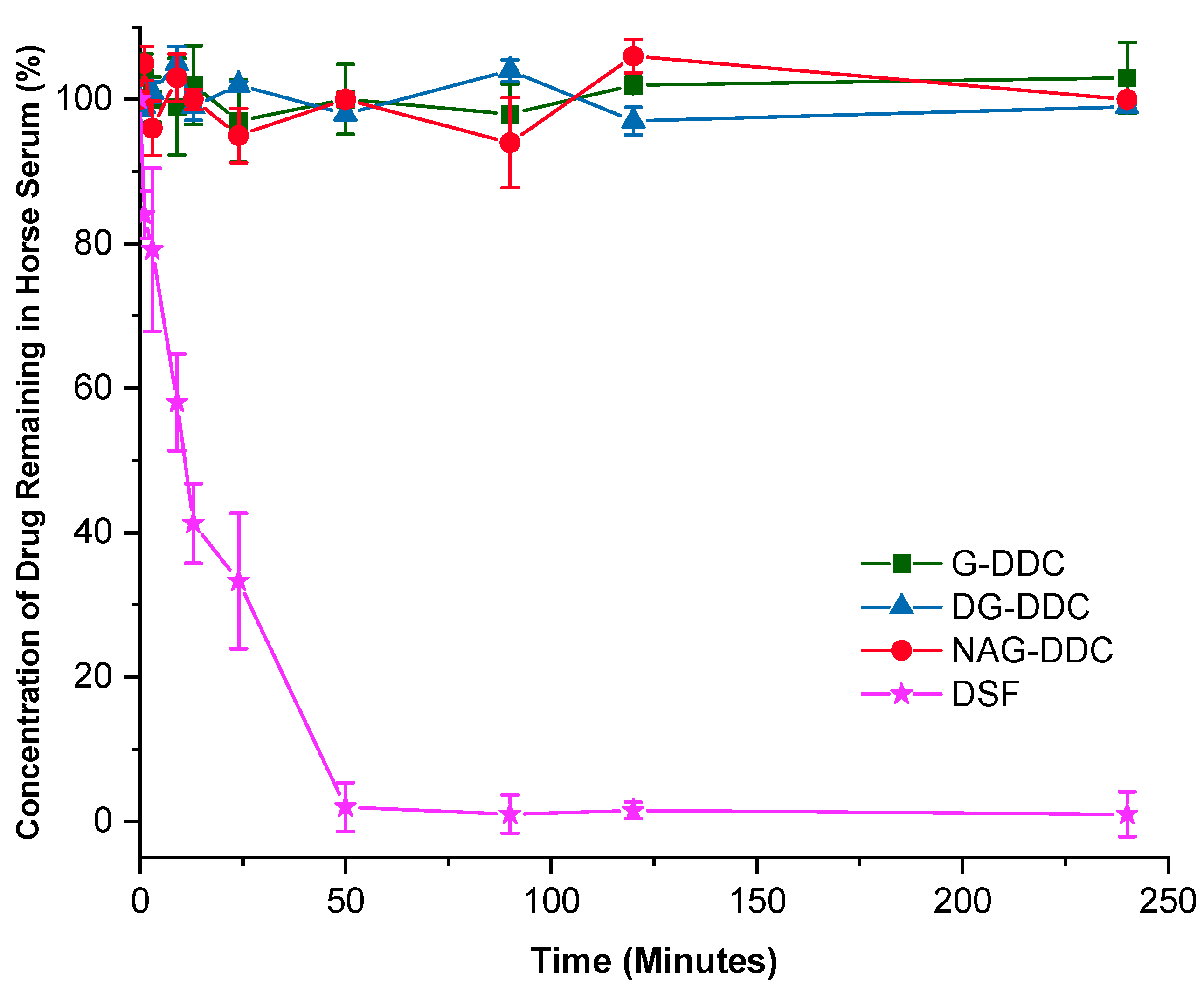
3.2. In Vitro Biostability
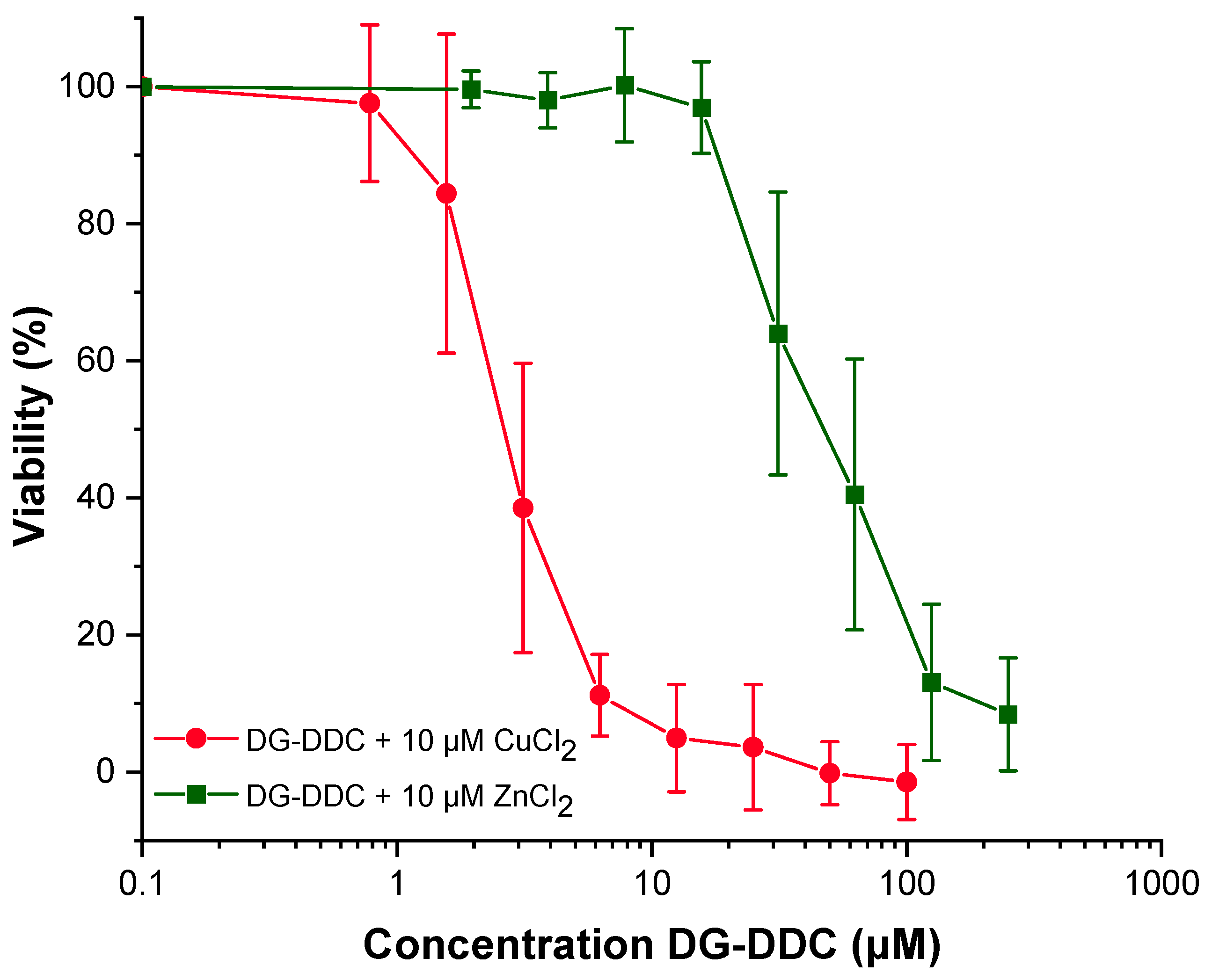
3.3. MTT Assay
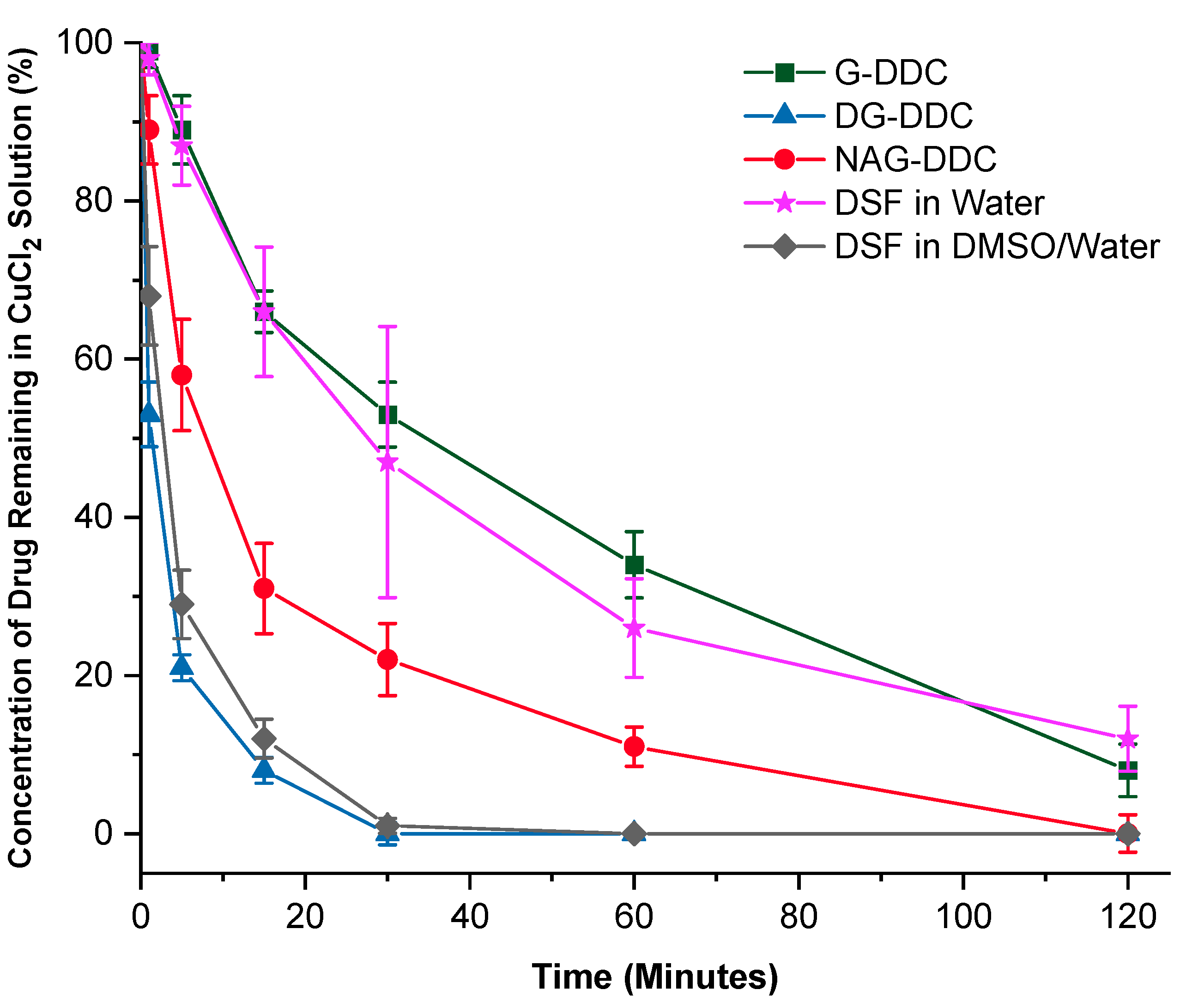
3.4. Reaction with Copper
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxy-D-glucose |
| ALDH | Aldehyde dehydrogenase |
| Cu(DDC)2 | Copper(II)bis(N,N-diethyldithiocarbamate) |
| DDC | Diethyldithiocarbamate |
| DMC | 2-chloro-1,3-dimethylimidazolinium chloride |
| DG-DDC | N,N-diethyl 2-deoxy-D-glucopyranosyl dithiocarbamate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DSF | Disulfiram |
| Et3N | Triethylamine |
| Ga-DDC | N,N-diethyl-D-galactopyranosyl dithiocarbamate |
| G-DDC | N,N-diethyl-D-glucopyranosyl dithiocarbamate |
| La-DDC | N,N-diethyl-lactosyl dithiocarbamate |
| Ma-DDC | N,N-diethyl-D-mannopyranosyl dithiocarbamate |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| NAG-DDC | N,N-diethyl 2-acetoamido-2-deoxy-D-glucopyranosyl dithiocarbamate |
| NF-κB | nuclear factor kappa B |
| XY-DDC | N,N-diethyl-D-xylopyranosyl dithiocarbamate |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M. The Drug Repurposing Hub: A next-generation drug library and information resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wang, Z.; Kurusamy, S.; Zhang, Z.; Morris, M.R.; Najlah, M.; McConville, C.; Kannappan, V.; Wang, W. PLGA-Nano-Encapsulated Disulfiram Inhibits Cancer Stem Cells and Targets Non-Small Cell Lung Cancer In Vitro and In Vivo. Biomolecules 2024, 14, 1651. [Google Scholar] [CrossRef] [PubMed]
- Iljin, K.; Ketola, K.; Vainio, P.; Halonen, P.; Kohonen, P.; Fey, V.; Grafström, R.C.; Perälä, M.; Kallioniemi, O. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin. Cancer Res. 2009, 15, 6070–6078. [Google Scholar] [CrossRef] [PubMed]
- Skrott, Z.; Mistrik, M.; Andersen, K.K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Deshmukh, P.; Tedstone, A.A.; Tuna, F.; O’Brien, P. On the interaction of copper(II) with disulfiram. Chem. Comm. 2014, 50, 13334–13337. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Anantha, M.; Shi, M.; Leung, A.W.-y.; Dragowska, W.H.; Sanche, L.; Bally, M.B. Development and optimization of an injectable formulation of copper diethyldithiocarbamate, an active anticancer agent. Int. J. Nanomed. 2017, 12, 4129–4146. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, V.; Ali, M.; Small, B.; Rajendran, G.; Elzhenni, S.; Taj, H.; Wang, W.; Dou, Q.P. Recent advances in repurposing disulfiram and disulfiram derivatives as copper-dependent anticancer agents. Front. Mol. Biosci. 2021, 8, 741316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Y.; Zhou, C.; Wan, R.; Li, Y. Anticancer effects of disulfiram: A systematic review of in vitro, animal, and human studies. Syst. Rev. 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Cvek, B.; Milacic, V.; Taraba, J.; Dou, Q.P. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem. 2008, 51, 6256–6258. [Google Scholar] [CrossRef] [PubMed]
- Horita, Y.; Takii, T.; Chiba, T.; Kuroishi, R.; Maeda, Y.; Kurono, Y.; Inagaki, E.; Nishimura, K.; Yamamoto, Y.; Abe, C. Synthesis of new sugar derivatives and evaluation of their antibacterial activities against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2009, 19, 6313–6316. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Noguchi, M.; Kashiwagura, H.; Tanaka, Y.; Serizawa, K.; Shoda, S.-i. Protection-free synthesis of glycosyl dithiocarbamates in aqueous media by using 2-chloroimidazolinium reagent. Tetrahedron. Lett. 2016, 57, 3529–3531. [Google Scholar] [CrossRef]
- Davis, B.G.; Robinson, M.A. Drug delivery systems based on sugar-macromolecule conjugates. Curr. Opin. Drug Discov. Dev. 2002, 5, 279–288. [Google Scholar]
- McCallum, N.; Najlah, M. The Anticancer Activity of Monosaccharides: Perspectives and Outlooks. Cancers 2024, 16, 2775. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using chitosan or chitosan derivatives in cancer therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Aft, R.L.; Zhang, F.; Gius, D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: Mechanism of cell death. Br. J. Cancer 2002, 87, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, U.; Frei, E.; Frank, N.; Kliem, H.; Wiessler, M.; Bertram, B.; Bhattacharya, R. Chemopreventive effects of dithiocarbamates on aflatoxin B1 metabolism and formation of AFB1 adducts with glutathione. Anticancer Res. 1998, 18, 1827–1832. [Google Scholar] [PubMed]
| Compound | H630 WT | H630 R10 | MDA-MB-231 | A549 |
|---|---|---|---|---|
| G-DDC (µM) | - * | - | 2178 ± 356.1 | - |
| G-DDC + Cu2+ (µM) | 127 ± 13.2 | - | 347.5 ± 44.7 | 95.9 ± 21.3 |
| DG-DDC (µM) | - | - | 2055 ± 54.70 | |
| DG-DDC + Cu2+ (µM) | 5.2 ± 1.7 | 5.3 ± 0.9 | 3.62 ± 0.22 | 2.79 ± 0.25 |
| DG-DDC + Zn2+ (µM) | - | - | - | 49.6 ± 18.6 |
| XY-DDC + Cu2+ (µM) | 6.9 ± 2.6 | - | - | - |
| NAG-DDC + Cu2+ (µM) | 5.8 ± 1.2 | - | - | - |
| La-DDC + Cu2+ (µM) | - | - | 89.7 ± 12.6 | - |
| Ga-DDC + Cu2+ (µM) | - | - | 17.7 ± 8.2 | - |
| Ma-DDC + Cu2+ (µM) | - | - | 78.6 ± 12.6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najlah, M.; McCallum, N.; Pereira, A.M.; Alves, D.; Ansari-Fard, N.; Rehmani, S.; Kaya, A. Sugar-Linked Diethyldithiocarbamate Derivatives: A Novel Class of Anticancer Agents. Int. J. Mol. Sci. 2025, 26, 5589. https://doi.org/10.3390/ijms26125589
Najlah M, McCallum N, Pereira AM, Alves D, Ansari-Fard N, Rehmani S, Kaya A. Sugar-Linked Diethyldithiocarbamate Derivatives: A Novel Class of Anticancer Agents. International Journal of Molecular Sciences. 2025; 26(12):5589. https://doi.org/10.3390/ijms26125589
Chicago/Turabian StyleNajlah, Mohammad, Niamh McCallum, Ana Maria Pereira, Dan Alves, Niusha Ansari-Fard, Sahrish Rehmani, and Ayşe Kaya. 2025. "Sugar-Linked Diethyldithiocarbamate Derivatives: A Novel Class of Anticancer Agents" International Journal of Molecular Sciences 26, no. 12: 5589. https://doi.org/10.3390/ijms26125589
APA StyleNajlah, M., McCallum, N., Pereira, A. M., Alves, D., Ansari-Fard, N., Rehmani, S., & Kaya, A. (2025). Sugar-Linked Diethyldithiocarbamate Derivatives: A Novel Class of Anticancer Agents. International Journal of Molecular Sciences, 26(12), 5589. https://doi.org/10.3390/ijms26125589







