Lactate-Mediated Crosstalk Between Tumor Cells and Cancer-Associated Fibroblasts: Mechanisms and Therapeutic Opportunities
Abstract
1. Introduction
2. Production and Biological Functions of Lactate
3. Lactylation Modification: Mechanisms, Regulation and Function
3.1. Mechanism of Lactylation Modification
3.2. Regulation of Lactylation
3.3. Functional Roles of Lactylation in Cancer
4. Lactate-Mediated Crosstalk Between CAFs and Tumors
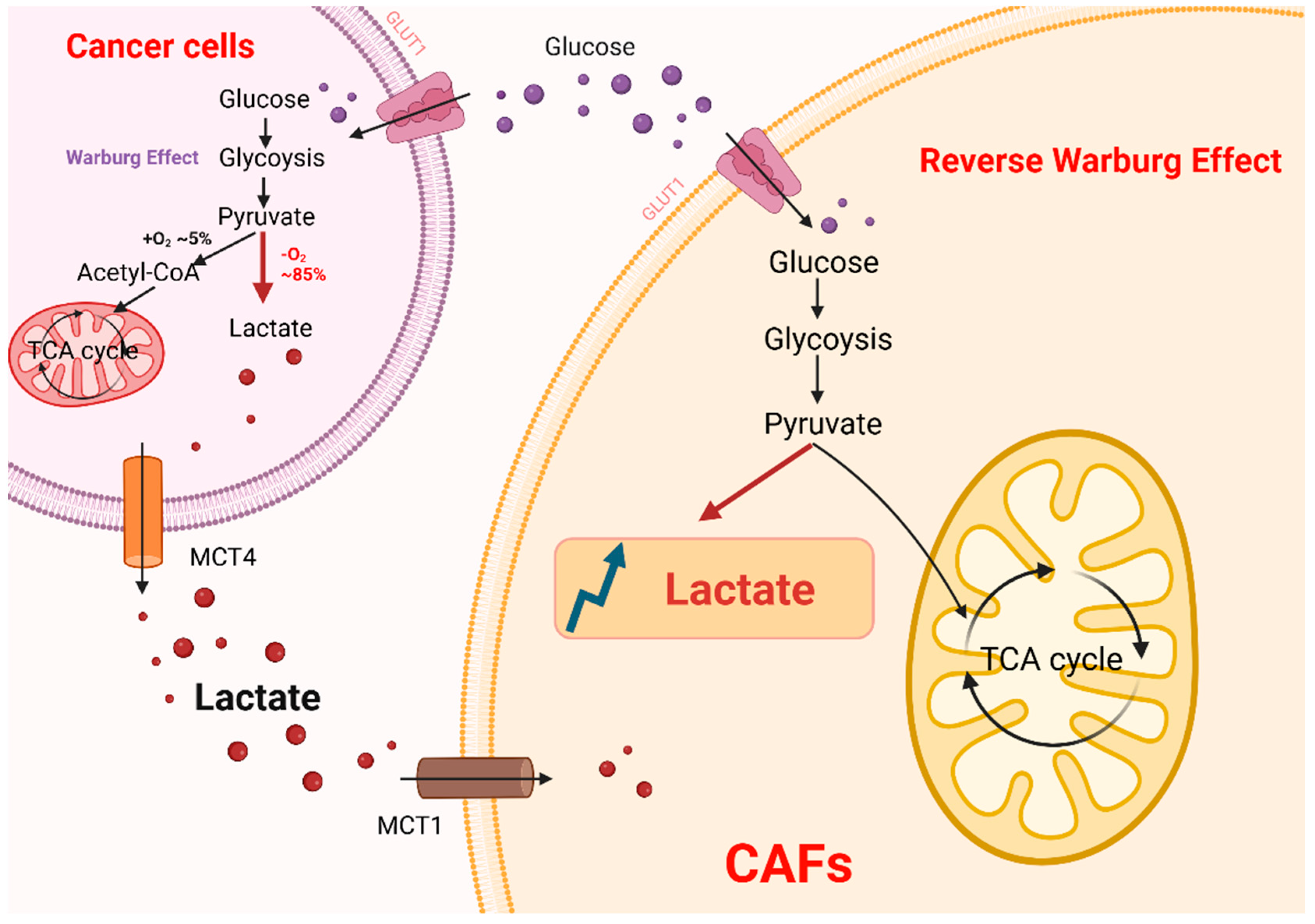
4.1. Lactate Secretion and Shuttling in CAFs
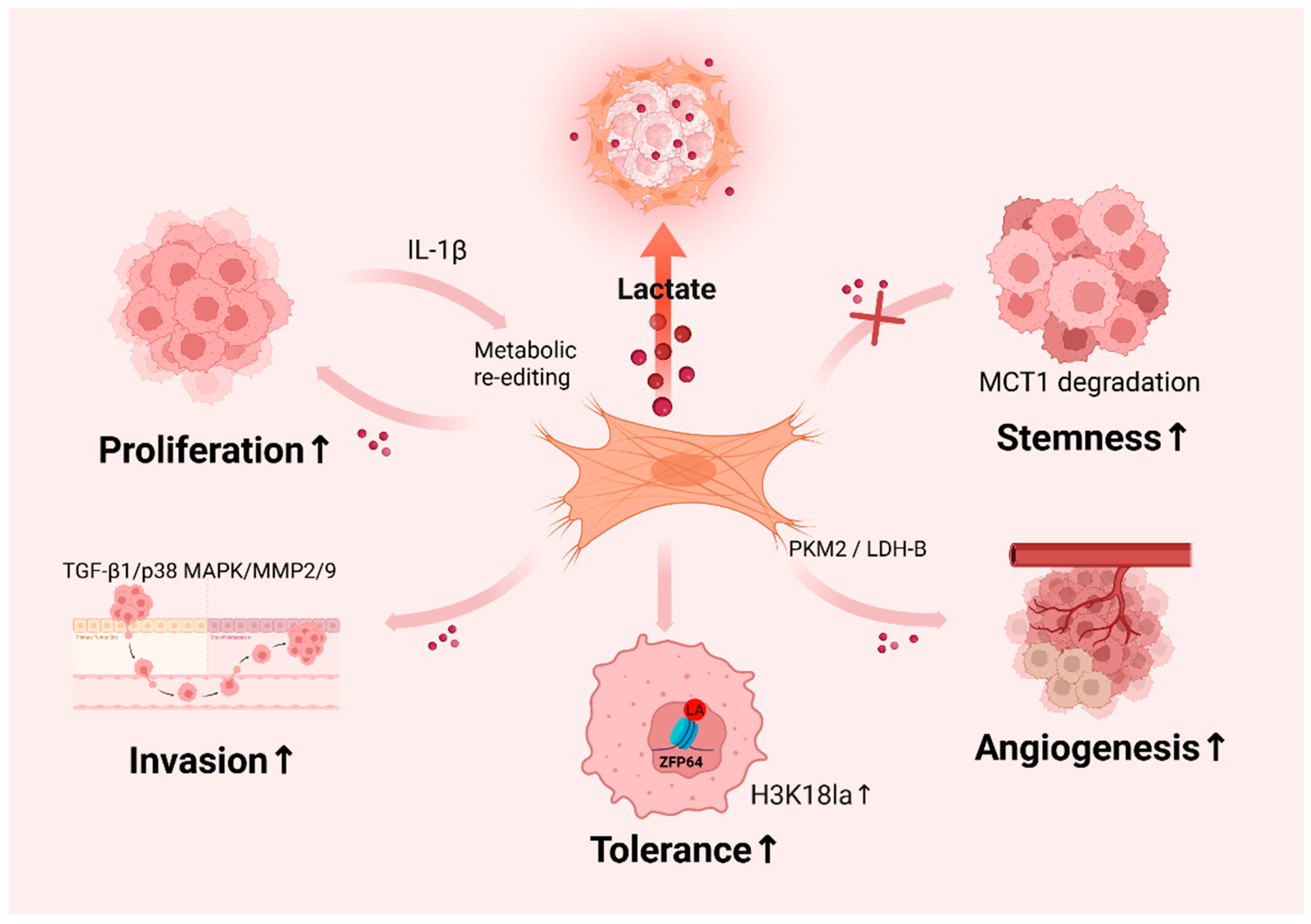
4.2. Tumor-Promoting Effects of CAF-Derived Lactate
4.3. Lactate-Induced CAF Activation
5. Therapeutic Strategies Targeting Lactate in Cancer
5.1. Targeting Monocarboxylate Transporters (MCTs)
5.2. Lactate Dehydrogenase (LDH) Inhibitors and Nanoparticle Delivery
6. Discussion
6.1. Lactylation: A Frontier in Epigenetic and Metabolic Regulation
6.2. Heterogeneity of CAFs and Lactate-Driven Phenotypes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| ACSS2 | Acetyl-CoA synthetase 2 |
| AK2 | Adenylate kinase 2 |
| α-SMA | Alpha smooth muscle actin |
| αKG | Alpha-ketoglutarate |
| ARG1 | Arginase 1 |
| AARS1 | Alanyl-tRNA synthetase 1 |
| ATP | Adenosine triphosphate |
| BMDMs | Bone-marrow-derived macrophages |
| BM | Basement membrane |
| CAFs | Cancer-associated fibroblasts |
| COL1A1 | Collagen type I alpha 1 chain |
| CXCL12/SDF1 | C-X-C motif chemokine ligand 12 |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| D-LA | D-lactic acid |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| FAP | Fibroblast activation protein |
| FSP | Fibroblast-specific protein |
| GLUT1 | Glucose transporter 1 |
| GPR81 | G protein-coupled receptor 81 |
| GTPSCS | GTP-specific succinyl-CoA synthetase |
| H3K9la | Histone H3 lysine 9 lactylation |
| H3K18la | Histone H3 lysine 18 lactylation |
| H4K8la | Histone H4 lysine 8 lactylation |
| HBO1 | Histone acetyltransferase binding to ORC1 |
| HCC | Hepatocellular carcinoma |
| HDAC1-3 | Histone deacetylase 1-3 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HSCs | Hepatic stellate cells |
| IL-1β | Interleukin-1 beta |
| IL-8 | Interleukin-8 |
| iCAF | Inflammatory cancer-associated fibroblast |
| ITGB4 | Integrin beta 4 |
| KAT2A | Lysine acetyltransferase 2A |
| KAT8 | Lysine acetyltransferase 8 |
| Kla | Lysine lactylation |
| L-LA | L-lactic acid |
| LDHA | Lactate dehydrogenase A |
| LDHB | Lactate dehydrogenase B |
| MCT1-4 | Monocarboxylate transporter 1-4 |
| MDSCs | Myeloid-derived suppressor cells |
| myoCAF | Myofibroblastic cancer-associated fibroblast |
| NAD+ | Nicotinamide adenine dinucleotide |
| NFs | Normal fibroblasts |
| OXPHOS | Oxidative phosphorylation |
| TCA | Tricarboxylic acid cycle |
| TME | Tumor microenvironment |
| YAP | Yes-associated protein |
| ZFP64 | Zinc-finger protein 64 |
References
- Li, W.; Zhou, C.; Yu, L.; Hou, Z.; Liu, H.; Kong, L.; Xu, Y.; He, J.; Lan, J.; Ou, Q.; et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 2024, 20, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Yao, D.; Wu, L.; Luo, C.; Wang, Z.; Zhang, J.; Liu, B. Targeting the warburg effect: A revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm. Sin. B 2024, 14, 953–1008. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Wang, X.; Mang, G.; Chen, J.; Yan, X.; Tong, Z.; Yang, Q.; Wang, M.; Chen, L.; et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ. Res. 2022, 131, 893–908. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, J.; Wang, P.; Wu, B.; Lu, S.; Lu, X.; You, S.; Huang, X.; Li, M.; et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023, 33, 679–698. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Gao, P.; Hu, H. Lactylation in cancer: Current understanding and challenges. Cancer Cell 2024, 42, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef]
- Watson, M.J.; Delgoffe, G.M. Fighting in a wasteland: Deleterious metabolites and antitumor immunity. J. Clin. Investig. 2022, 132, e148549. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic instruction of immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Caronni, N.; Simoncello, F.; Stafetta, F.; Guarnaccia, C.; Ruiz-Moreno, J.S.; Opitz, B.; Galli, T.; Proux-Gillardeaux, V.; Benvenuti, F. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 2018, 78, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Angelin, A.; Gil-de-Gómez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J.; Kopinski, P.K.; Wang, L.; et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017, 25, 1282–1293.e7. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Mao, X.; Wang, L.; Chen, Z.; Wang, W.; Zhao, C.; Li, G.; Guo, W.; Hu, Y. Lactate/GPR81 recruits regulatory T cells by modulating CX3CL1 to promote immune resistance in a highly glycolytic gastric cancer. Oncoimmunology 2024, 13, 2320951. [Google Scholar] [CrossRef]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018, 28, 848–865.e6. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Zhou, C.; Liu, L.; Huang, C. Epithelial-Mesenchymal Transition: The History, Regulatory Mechanism, and Cancer Therapeutic Opportunities. MedComm 2022, 3, e144. Available online: https://pubmed.ncbi.nlm.nih.gov/35601657/ (accessed on 1 May 2025). [CrossRef]
- Sonveaux, P.; Copetti, T.; De Saedeleer, C.J.; Végran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frérart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Caligiuri, G.; Tuveson, D.A. Activated fibroblasts in cancer: Perspectives and challenges. Cancer Cell 2023, 41, 434–449. [Google Scholar] [CrossRef]
- Ippolito, L.; Duatti, A.; Iozzo, M.; Comito, G.; Pardella, E.; Lorito, N.; Bacci, M.; Pranzini, E.; Santi, A.; Sandrini, G.; et al. Lactate supports cell-autonomous ECM production to sustain metastatic behavior in prostate cancer. EMBO Rep. 2024, 25, 3506–3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, S.; Lu, T.; Han, D.; Zhang, K.; Gan, L.; Wu, X.; Li, Y.; Zhao, X.; Li, Z.; et al. Single-cell analysis reveals the COL11A1+ fibroblasts are cancer-specific fibroblasts that promote tumor progression. Front. Pharmacol. 2023, 14, 1121586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Ji, J.; Liu, H.; Luo, H.; Li, J.; Han, X. Colorectal cancer cells establish metabolic reprogramming with cancer-associated fibroblasts (CAFs) through lactate shuttle to enhance invasion, migration, and angiogenesis. Int. Immunopharmacol. 2024, 143, 113470, Erratum in Int. Immunopharmacol. 2025, 146, 113958. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tang, S.; Hou, Y.; Xi, L.; Chen, Y.; Yin, J.; Peng, M.; Zhao, M.; Cui, X.; Liu, M. Corrigendum for “Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling” [eBioMedicine 41 (2019) 370-383]. eBioMedicine 2025, 112, 105542. [Google Scholar]
- Linares, J.F.; Cid-Diaz, T.; Duran, A.; Osrodek, M.; Martinez-Ordoñez, A.; Reina-Campos, M.; Kuo, H.-H.; Elemento, O.; Martin, M.L.; Cordes, T.; et al. The lactate-NAD+ axis activates cancer-associated fibroblasts by downregulating p62. Cell Rep. 2022, 39, 110792. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, Y.; Su, J.; Wang, S.; Su, X.; Ding, X.; Jiang, L.; Fei, X.; Zhang, W. Lactate-induced activation of tumor-associated fibroblasts and IL-8-mediated macrophage recruitment promote lung cancer progression. Redox Biol. 2024, 74, 103209. [Google Scholar] [CrossRef]
- Haas, R.; Cucchi, D.; Smith, J.; Pucino, V.; Macdougall, C.E.; Mauro, C. Intermediates of metabolism: From bystanders to signalling molecules. Trends Biochem. Sci. 2016, 41, 460–471. [Google Scholar]
- Lv, X.; Lv, Y.; Dai, X. Lactate, histone lactylation and cancer hallmarks. Expert Rev. Mol. Med. 2023, 25, e7. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Naylor, J.M.; Zello, G.A. D-lactate in human and ruminant metabolism. J. Nutr. 2005, 135, 1619–1625. [Google Scholar]
- Li, J.; Ma, P.; Liu, Z.; Xie, J. L- and D-lactate: Unveiling their hidden functions in disease and health. Cell Commun. Signal. 2025, 23, 134. [Google Scholar]
- Walenta, S.; Schroeder, T.; Mueller-Klieser, W. Lactate in solid malignant tumors: Potential basis of a metabolic classification in clinical oncology. Curr. Med. Chem. 2004, 11, 2195–2204. [Google Scholar] [PubMed]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfør, K.; Rofstad, E.K.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Schou Oxvig, A.-M.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem. Biol. 2020, 27, 206–213.e6. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar]
- Liu, R.; Ren, X.; Park, Y.E.; Feng, H.; Sheng, X.; Song, X.; AminiTabrizi, R.; Shah, H.; Li, L.; Zhang, Y.; et al. Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis. Cell Metab. 2025, 37, 377–394.e9. [Google Scholar] [CrossRef]
- Zhu, R.; Ye, X.; Lu, X.; Xiao, L.; Yuan, M.; Zhao, H.; Guo, D.; Meng, Y.; Han, H.; Luo, S.; et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 2025, 37, 361–376.e7. [Google Scholar] [PubMed]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Matthews, Q.L.; Liu, R.-M.; Liu, G. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, M.; Li, J.; Cui, J.; Zhang, P.; Liu, F.; Wu, Y.; Deng, W.; Ma, J.; Li, X.; et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2314128121. [Google Scholar] [PubMed]
- Niu, Z.; Chen, C.; Wang, S.; Lu, C.; Wu, Z.; Wang, A.; Mo, J.; Zhang, J.; Han, Y.; Yuan, Y.; et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 2024, 15, 3561. [Google Scholar]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef]
- Zu, H.; Li, C.; Dai, C.; Pan, Y.; Ding, C.; Sun, H.; Zhang, X.; Yao, X.; Zang, J.; Mo, X. SIRT2 Functions as a Histone Delactylase and Inhibits the Proliferation and Migration of Neuroblastoma Cells. Cell Discov. 2022, 8, 54. Available online: https://pubmed.ncbi.nlm.nih.gov/35672301/ (accessed on 1 May 2025). [CrossRef]
- Li, F.; Si, W.; Xia, L.; Yin, D.; Wei, T.; Tao, M.; Cui, X.; Yang, J.; Hong, T.; Wei, R. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer 2024, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.Q.; Ray, J.D.; Zerio, C.J.; Trujillo, M.N.; McDonald, D.M.; Chapman, E.; Spiegel, D.A.; Galligan, J.J. Sirtuin 2 regulates protein LactoylLys modifications. ChemBioChem 2021, 22, 2102–2106. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, R.; Niu, J.; Yang, S.; Ma, H.; Zhao, S.; Li, H. Molecular Basis for Hierarchical Histone De-β-Hydroxybutyrylation by SIRT3. Cell Discov. 2019, 5, 53. Available online: https://pubmed.ncbi.nlm.nih.gov/31636949/ (accessed on 1 May 2025).
- Peng, T.; Sun, F.; Yang, J.-C.; Cai, M.-H.; Huai, M.-X.; Pan, J.-X.; Zhang, F.-Y.; Xu, L.-M. Novel lactylation-related signature to predict prognosis for pancreatic adenocarcinoma. World J. Gastroenterol. 2024, 30, 2575–2602. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chai, P.; Xie, M.; Ge, S.; Ruan, J.; Fan, X.; Jia, R. Histone Lactylation Drives Oncogenesis by Facilitating m6A Reader Protein YTHDF2 Expression in Ocular Melanoma. Genome Biol. 2021, 22, 85. Available online: https://pubmed.ncbi.nlm.nih.gov/33726814/ (accessed on 1 May 2025). [CrossRef] [PubMed]
- Chai, P.; Zhao, F.; Jia, R.; Zhou, X.; Fan, X. Lactate/lactylation in ocular development and diseases. Trends Mol. Med. 2024. [Google Scholar] [CrossRef]
- Pan, L.; Feng, F.; Wu, J.; Fan, S.; Han, J.; Wang, S.; Yang, L.; Liu, W.; Wang, C.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar]
- Xiong, J.; He, J.; Zhu, J.; Pan, J.; Liao, W.; Ye, H.; Wang, H.; Song, Y.; Du, Y.; Cui, B.; et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 2022, 82, 1660–1677.e10. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Huang, Y.; Li, D.; Zheng, Z.; Xie, K.; Cao, C.; Wang, Q.; Zhao, X.; Huang, Z.; et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist. Updates 2024, 73, 101059. [Google Scholar]
- Zhang, K.; Guo, L.; Li, X.; Hu, Y.; Luo, N. Cancer-associated fibroblasts promote doxorubicin resistance in triple-negative breast cancer through enhancing ZFP64 histone lactylation to regulate ferroptosis. J. Transl. Med. 2025, 23, 247. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Bai, L.; Wang, D.; Ding, W.; Cao, Z.; Yan, P.; Li, Y.; Xi, L.; Wang, Y.; Zheng, X.; et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 2023, 24, e56052. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cen, K.; Song, Y.; Zhang, X.; Liou, Y.-C.; Liu, P.; Huang, J.; Ruan, J.; He, J.; Ye, W.; et al. NUSAP1-LDHA-glycolysis-lactate feedforward loop promotes warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 2023, 567, 216285. [Google Scholar] [CrossRef]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392.e33. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, H.; Lin, M.; Yan, Z.; An, L.; Cao, Z.; Geng, D.; Yue, J.; Tang, Y.; Tian, L.; et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Investig. 2024, 134, e174587. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Z.; Yu, Y.; Zhang, P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.; Zhai, L.; Zhang, T.; Yin, H.; Gao, H.; Zhao, F.; Wang, Z.; Yang, X.; Jin, M.; et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 2024, 187, 294–311.e21. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, H.; Chen, X.; Fu, H.; Mao, D.; Chen, W.; Lan, L.; Wang, C.; Hu, K.; et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 2024, 631, 663–669. [Google Scholar] [CrossRef]
- Akter, R.; Awais, M.; Boopathi, V.; Ahn, J.C.; Yang, D.C.; Kang, S.C.; Yang, D.U.; Jung, S.-K. Inversion of the warburg effect: Unraveling the metabolic nexus between obesity and cancer. ACS Pharmacol. Transl. Sci. 2024, 7, 560–569. [Google Scholar]
- Liang, L.; Li, W.; Li, X.; Jin, X.; Liao, Q.; Li, Y.; Zhou, Y. “reverse warburg effect” of cancer-associated fibroblasts (review). Int. J. Oncol. 2022, 60, 67. [Google Scholar] [CrossRef] [PubMed]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Metabolic coupling and the reverse warburg effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef]
- Benny, S.; Mishra, R.; Manojkumar, M.K.; Aneesh, T.P. From warburg effect to reverse warburg effect; the new horizons of anti-cancer therapy. Med. Hypotheses 2020, 144, 110216. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Nunes, A.; Simões-Sousa, S.; Pinheiro, C.; Miranda-Gonçalves, V.; Granja, S.; Baltazar, F. Targeting lactate production and efflux in prostate cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165894. [Google Scholar]
- Shi, Y.; Du, L.; Lin, L.; Wang, Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017, 16, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Marchiq, I.; Pouysségur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J. Mol. Med. Berl. Ger. 2016, 94, 155–171. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Li, Z.; Sun, C.; Qin, Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Park, S.-A.; Park, K.S.; Park, S.; Heo, K.; Seo, Y.-K.; Noh, D.-Y.; Ryu, S.H.; Suh, P.-G. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget 2016, 7, 70898–70911. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Böhme, I.; Bosserhoff, A.K. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016, 29, 508–523. [Google Scholar]
- Wu, J.; Hong, Y.; Wu, T.; Wang, J.; Chen, X.; Wang, Z.; Cheng, B.; Xia, J. Stromal-epithelial lactate shuttle induced by tumor-derived interleukin-1β promotes cell proliferation in oral squamous cell carcinoma. Int. J. Mol. Med. 2018, 41, 687–696. [Google Scholar] [CrossRef]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Whitaker-Menezes, D.; Castello-Cros, R.; Pavlides, S.; Pestell, R.G.; Fatatis, A.; Witkiewicz, A.K.; Vander Heiden, M.G.; Migneco, G.; Chiavarina, B.; et al. The reverse warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 2010, 9, 1960–1971. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Chen, Y.; Liang, W.; Liu, H.; Du, R.; Sun, Y.; Hu, C.; Shang, Z. CAFs-derived lactate enhances the cancer stemness through inhibiting the MST1 ubiquitination degradation in OSCC. Cell Biosci. 2024, 14, 144. [Google Scholar] [CrossRef]
- Li, T.; Hu, C.; Huang, T.; Zhou, Y.; Tian, Q.; Chen, H.; He, R.; Yuan, Y.; Jiang, Y.; Jiang, H.; et al. Cancer-associated fibroblasts foster a high-lactate microenvironment to drive perineural invasion in pancreatic cancer. Cancer Res. 2025, CAN-24-3173. [Google Scholar] [CrossRef]
- Martínez-Ordoñez, A.; Seoane, S.; Avila, L.; Eiro, N.; Macía, M.; Arias, E.; Pereira, F.; García-Caballero, T.; Gómez-Lado, N.; Aguiar, P.; et al. POU1F1 transcription factor induces metabolic reprogramming and breast cancer progression via LDHA regulation. Oncogene 2021, 40, 2725–2740. [Google Scholar] [CrossRef]
- Mai, R.-Y.; Ye, J.-Z.; Gao, X.; Wen, T.; Li, S.-Z.; Zeng, C.; Cen, W.-J.; Wu, G.-B.; Lin, Y.; Liang, R.; et al. Up-regulated ITGB4 promotes hepatocellular carcinoma metastasis by activating hypoxia-mediated glycolysis and cancer-associated fibroblasts. Eur. J. Pharmacol. 2025, 986, 177102. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, T.D.; Von Ahrens, D.; Dawlaty, M.; Zou, Y.; Baddour, J.; Achreja, A.; Zhao, H.; Yang, L.; Patel, B.; Kwak, C.; et al. Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. eLife 2019, 8, e50663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, W.; Xu, H.; Xu, J.; Li, J.; Liu, X.; Lu, X.; Dai, J.; Jiang, Y.; Wang, W.; et al. Cancer-associated fibroblasts promote EGFR-TKI resistance via the CTHRC1/glycolysis/H3K18la positive feedback loop. Oncogene 2025, 44, 1400–1414. [Google Scholar] [CrossRef]
- Kong, W.; He, J.; Zhou, Q.; Zhou, X.; Wei, X.; Yang, Y.; Mei, Y.; Wang, S.; Zhang, X.; Yao, B.; et al. Histone lactylation-related genes correlate with the molecular patterns and functions of cancer-associated fibroblasts and have significant clinical implications in clear cell renal cell carcinoma. Heliyon 2024, 10, e33554. [Google Scholar] [CrossRef]
- Liang, L.; Yang, X.; Yao, S.; Li, X.; Wang, F. Identification of lactylation-associated fibroblast subclusters predicting prognosis and cancer immunotherapy response in colon cancer. Gene 2025, 940, 149220. [Google Scholar] [PubMed]
- Kitamura, F.; Semba, T.; Yasuda-Yoshihara, N.; Yamada, K.; Nishimura, A.; Yamasaki, J.; Nagano, O.; Yasuda, T.; Yonemura, A.; Tong, Y.; et al. Cancer-associated fibroblasts reuse cancer-derived lactate to maintain a fibrotic and immunosuppressive microenvironment in pancreatic cancer. JCI Insight 2023, 8, e163022. [Google Scholar]
- Polański, R.; Hodgkinson, C.L.; Fusi, A.; Nonaka, D.; Priest, L.; Kelly, P.; Trapani, F.; Bishop, P.W.; White, A.; Critchlow, S.E.; et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 2014, 20, 926–937. [Google Scholar] [CrossRef]
- Guan, X.; Rodriguez-Cruz, V.; Morris, M.E. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 2019, 21, 13. [Google Scholar] [CrossRef]
- Noble, R.A.; Bell, N.; Blair, H.; Sikka, A.; Thomas, H.; Phillips, N.; Nakjang, S.; Miwa, S.; Crossland, R.; Rand, V.; et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and burkitt lymphoma. Haematologica 2017, 102, 1247–1257. [Google Scholar] [CrossRef]
- Babl, N.; Decking, S.-M.; Voll, F.; Althammer, M.; Sala-Hojman, A.; Ferretti, R.; Korf, C.; Schmidl, C.; Schmidleithner, L.; Nerb, B.; et al. MCT4 blockade increases the efficacy of immune checkpoint blockade. J. Immunother. Cancer 2023, 11, e007349. [Google Scholar] [CrossRef]
- Coronel-Hernández, J.; Salgado-García, R.; Cantú-De León, D.; Jacobo-Herrera, N.; Millan-Catalan, O.; Delgado-Waldo, I.; Campos-Parra, A.D.; Rodríguez-Morales, M.; Delgado-Buenrostro, N.L.; Pérez-Plasencia, C. Combination of metformin, sodium oxamate and doxorubicin induces apoptosis and autophagy in colorectal cancer cells via downregulation HIF-1α. Front. Oncol. 2021, 11, 594200. [Google Scholar]
- Zhai, X.; Yang, Y.; Wan, J.; Zhu, R.; Wu, Y. Inhibition of LDH-a by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol. Rep. 2013, 30, 2983–2991. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the akt-mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Jia, W.; Li, L.; Li, Y.; Hu, J.; Luo, W.; Li, R.; Ye, D.; Lan, P. Histone lactylation-driven B7-H3 expression promotes tumor immune evasion. Theranostics 2025, 15, 2338–2359. [Google Scholar] [CrossRef] [PubMed]
- Podsednik, A.; Jiang, J.; Jacob, A.; Li, L.Z.; Xu, H.N. Optical redox imaging of treatment responses to nampt inhibition and combination therapy in triple-negative breast cancer cells. Int. J. Mol. Sci. 2021, 22, 5563. [Google Scholar] [CrossRef]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef]
- Bayindir-Bilgic, M.; Duman, E.; Turgut, D.; Kadikoylu, A.N.; Ekimci-Gurcan, N.; Ozbey, U.; Kuskucu, A.; Bayrak, O.F. Investigation of the synergistic effect of metformin and FX11 on PANC-1 cell lines. Biol. Res. 2025, 58, 15. [Google Scholar]
- Shibata, S.; Sogabe, S.; Miwa, M.; Fujimoto, T.; Takakura, N.; Naotsuka, A.; Kitamura, S.; Kawamoto, T.; Soga, T. Identification of the first highly selective inhibitor of human lactate dehydrogenase B. Sci. Rep. 2021, 11, 21353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liu, H.; Chen, X.; Wang, Z.; Wang, X.; Gu, X.; Tong, Y.; Ba, X.; He, Y.; Wu, J.; et al. Reprogramming of glucose metabolism by nanocarriers to improve cancer immunotherapy: Recent advances and applications. Int. J. Nanomed. 2025, 20, 4201–4234. [Google Scholar] [CrossRef]
- Lecoultre, M.; Dutoit, V.; Walker, P.R. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: A review. J. Immunother. Cancer 2020, 8, e001408. [Google Scholar] [CrossRef]
- Shi, W.; Cassmann, T.J.; Bhagwate, A.V.; Hitosugi, T.; Ip, W.K.E. Lactic Acid Induces Transcriptional Repression of Macrophage Inflammatory Response via Histone Acetylation. Cell Rep. 2024, 43, 113746. Available online: https://pubmed.ncbi.nlm.nih.gov/38329873/ (accessed on 1 May 2025). [CrossRef]
- Altinoz, M.A.; Ozpinar, A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed. Pharmacother. 2022, 147, 112686. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, X.; Shi, J.; Huang, J.; Wang, S.; Li, X.; Lin, H.; Zhao, D.; Ye, M.; Zhang, S.; et al. Elevated protein lactylation promotes immunosuppressive microenvironment and therapeutic resistance in pancreatic ductal adenocarcinoma. J. Clin. Investig. 2025, 135, e187024. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, H.; Zhang, Y.; Wei, H.; Zhu, Z.; Zhu, B.; Yang, M.; Cao, W.; Wang, L.; Wu, Z. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 2017, 36, 5829–5839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Zhang, Y.; Zhou, W.; Zhang, X.; Peng, C.; Ji, T.; Zou, X.; Zhang, Z.; Ren, Z. Spatial transcriptomics reveals that metabolic characteristics define the tumor immunosuppression microenvironment via iCAF transformation in oral squamous cell carcinoma. Int. J. Oral Sci. 2024, 16, 9. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Sun, Y.; Wang, Z.; Qian, Y.; Duraisamy, V.; Antary, T.M.A. Zerumbone-induced reactive oxygen species-mediated oxidative stress re-sensitizes breast cancer cells to paclitaxel. Biotechnol. Appl. Biochem. 2023, 70, 28–37. [Google Scholar] [CrossRef]
- Blaszczak, W.; Williams, H.; Swietach, P. Autoregulation of H+/lactate efflux prevents monocarboxylate transport (MCT) inhibitors from reducing glycolytic lactic acid production. Br. J. Cancer 2022, 127, 1365–1377. [Google Scholar] [CrossRef]
- Guan, X.; Morris, M.E. In vitro and in vivo efficacy of AZD3965 and alpha-cyano-4-hydroxycinnamic acid in the murine 4T1 breast tumor model. AAPS J. 2020, 22, 84. [Google Scholar] [CrossRef]
- Beloueche-Babari, M.; Wantuch, S.; Casals Galobart, T.; Koniordou, M.; Parkes, H.G.; Arunan, V.; Chung, Y.-L.; Eykyn, T.R.; Smith, P.D.; Leach, M.O. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 2017, 77, 5913–5924. [Google Scholar] [CrossRef]
- Afonso, J.; Pinto, T.; Simões-Sousa, S.; Schmitt, F.; Longatto-Filho, A.; Pinheiro, C.; Marques, H.; Baltazar, F. Clinical significance of metabolism-related biomarkers in non-hodgkin lymphoma—MCT1 as potential target in diffuse large B cell lymphoma. Cell. Oncol. 2019, 42, 303–318. [Google Scholar] [CrossRef]
- Halford, S.; Veal, G.J.; Wedge, S.R.; Payne, G.S.; Bacon, C.M.; Sloan, P.; Dragoni, I.; Heinzmann, K.; Potter, S.; Salisbury, B.M.; et al. A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin. Cancer Res. 2023, 29, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.-M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef]
- Benjamin, D.; Hall, M.N. Combining metformin with lactate transport inhibitors as a treatment modality for cancer—Recommendation proposal. Front. Oncol. 2022, 12, 1034397. [Google Scholar] [CrossRef]
- Arrieta, O.; Barrón, F.; Padilla, M.-Á.S.; Avilés-Salas, A.; Ramírez-Tirado, L.A.; Arguelles Jiménez, M.J.; Vergara, E.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Cardona, A.F.; et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, e192553. [Google Scholar] [CrossRef]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Z.; Jin, P.; Yang, X.; Li, X.; Wei, X.; Wang, Y.; Long, S.; Zhang, T.; Chen, G.; et al. Metformin suppresses tumor progression by inactivating stromal fibroblasts in ovarian cancer. Mol. Cancer Ther. 2018, 17, 1291–1302. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, L.; An, G.; Zhang, L.; Jing, X.; Luo, M.; Li, W.; Meng, D.; Ning, Q.; Zhao, X.; et al. Metformin suppresses HIF-1α expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. FASEB J. 2020, 34, 10860–10870. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Budhu, S.; Serganova, I.; Dong, L.; Mangarin, L.M.; Khan, J.F.; Bah, M.A.; Assouvie, A.; Marouf, Y.; Schulze, I.; et al. Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models. J. Clin. Investig. 2024, 134, e177606. [Google Scholar] [CrossRef]
- Comandatore, A.; Franczak, M.; Smolenski, R.T.; Morelli, L.; Peters, G.J.; Giovannetti, E. Lactate dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin. Cancer Biol. 2022, 86, 93–100. [Google Scholar] [CrossRef]
- Urbańska, K.; Orzechowski, A. Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int. J. Mol. Sci. 2019, 20, 2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, L.; Wang, Z.; He, H.; Chen, H.; Duan, Z.; Li, Y.; Si, Q.; Chuang, T.-H.; Chen, C.; et al. Lactate Dehydrogenase-a (LDH-a) Preserves Cancer Stemness and Recruitment of Tumor-Associated Macrophages to Promote Breast Cancer Progression. Front. Oncol. 2021, 11, 654452. Available online: https://pubmed.ncbi.nlm.nih.gov/34178639/ (accessed on 1 May 2025). [CrossRef]
- Dai, M.; Wang, L.; Yang, J.; Chen, J.; Dou, X.; Chen, R.; Ge, Y.; Lin, Y. LDHA as a regulator of T cell fate and its mechanisms in disease. Biomed. Pharmacother. 2023, 158, 114164. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, S.; Zhu, L.; Wu, Y.; Zhang, W.; Zhao, C. LDHA: The obstacle to T cell responses against tumor. Front. Oncol. 2022, 12, 1036477. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, N.V.; Dutta, P.; Yabuuchi, S.; de Wilde, R.F.; Martinez, G.V.; Le, A.; Kamphorst, J.J.; Rabinowitz, J.D.; Jain, S.K.; Hidalgo, M.; et al. Therapeutic targeting of the warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res. 2015, 75, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Brisson, L.; Bański, P.; Sboarina, M.; Dethier, C.; Danhier, P.; Fontenille, M.-J.; Van Hée, V.F.; Vazeille, T.; Tardy, M.; Falces, J.; et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell 2016, 30, 418–431. [Google Scholar] [CrossRef]
- McCleland, M.L.; Adler, A.S.; Shang, Y.; Hunsaker, T.; Truong, T.; Peterson, D.; Torres, E.; Li, L.; Haley, B.; Stephan, J.-P.; et al. An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res. 2012, 72, 5812–5823. [Google Scholar] [CrossRef]

| Processes | Enzyme | Mechanism | References |
|---|---|---|---|
| Lactic acid accumulation | LDHA, LDHB | LDHA preferentially catalyzes the reduction of pyruvate to lactate while regenerating NAD+, and LDHB primarily facilitates the oxidation of lactate back to pyruvate. | [34] |
| The formation of lactyl coenzyme A | ACSS2 | The ACSS2/KAT2A complex emerges as a central epigenetic regulator in cancer progression, where EGFR activation induces ERK-dependent phosphorylation and the nuclear translocation of ACSS2. | [36] |
| GTPSCS | Nuclear GTPSCS functions as a lactyl-CoA synthetase that complexes with p300 to promote lactyl-CoA production and subsequent histone H3K18 lactylation (H3K18la). | [35] | |
| Lactyltransferase | p300 | P300 mediates lactate-induced histone lactylation on pro-fibrotic gene promoters in macrophages. | [37,42] |
| KAT8 | KAT8 identified as eEF1A2 K408 lactate transferase, promoting colorectal tumor growth. | [36,38] | |
| CGN5 | Interleukin-1β-dependent GCN5 (general control non-inhibitory 5) recruitment catalyzes histone H3K18 lactylation. | [4] | |
| HBO1 | HBO1-mediated H3K9la has been confirmed to be associated with tumorigenesis across multiple cancer cell lines, including HeLa (cervical cancer), HepG2 (hepatocellular carcinoma), U87MG (glioblastoma), KYSE-30 (esophageal squamous cell carcinoma), MDA-MB-231 (breast cancer), HCT116 (colon cancer), and H460 (non-small cell lung cancer). | [39] | |
| De-lactylation modification | HDAC1-3 | HDAC1-3 exhibits potent activity not only against K(L-la) but also against K(D-la) and various short-chain acyl modifications. HDAC1 and HDAC2: reversing H3K9la, H3K18la, and H4K8la. | [40] |
| SIRT | SIRT2 can remove the lactyl group from synthetic peptides related to pyruvate kinase M2 (PKM2), SIRT3 exhibits class-selective histone deacetylase activity, preferentially recognizing H3 K4, K9, K18, K23, K27, and H4K16. | [41,43,44] |
| The Main Targeted Drugs | Cancer Types and CAFs | The Study Outcomes | Ref | Limitation | |
|---|---|---|---|---|---|
| MCT inhibitors | AZD3965 | Small Cell Lung cancer | Patients who express MCT1 but not MCT4 have a better outcome. | [87] | The compensatory function of MCT4 |
| AZD3965 | Breast cancer | AZD3965 exerts slowly reversible inhibition of MCT1-mediated L-lactate uptake. | [88] | ||
| AZD3965 and OXPHOS | Lymphoma | Combining AZD3965 with an inhibitor of oxidative phosphorylation (OXPHOS) can induce significant tumor cell death. | [89] | ||
| AZD3965,a novel MCT4 inhibitor and immune checkpoint drugs NAC and AZD3965 | Colorectal cancer Colorectal cancer and CAFs | Improved leukocyte infiltration and T cell activation, delayed tumor growth, and prolonged survival in vivo. It inhibits the expression of NF-κB and HIF-1α, thereby exerting its anticancer function. | [90] [23] | ||
| LDH inhibitors and nanoparticle delivery systems | Oxamate Oxamate Oxamate Oxamate and PD-1 inhibitor FX11 and FK866 FX11 and metformin FX11 AXKO-0046 Nanoparticles editing the LDHA gene | Colorectal cancer Nasopharyngeal carcinoma Gastric cancer Hepatocellular Breast cancer Pancreatic cancer CAFs and PC - Cancers | Triple therapy abolishes proliferation and induces apoptosis and autophagy in CRC cells through the induction of ULK1 which is regulated by the mir-26a/HIF-1α axis Oxamate increased the radiosensitivity in NPC cells in vitro Oxamate-mediated inhibition of the Akt-mTOR signaling pathway activates autophagy and exhibits anticancer activity. Through inhibition of histone H3K18 lactylation, thereby enhancing antitumor immunity. Inhibits tumor growth through dual metabolic blockade. Activation of the AMPKα axis causes stress and apoptosis in tumor cells.Reduces the number of CAFs. A chemical probe that inhibits LDHB. Inhibition of glycolysis in multiple tumor cells. | [91] [92] [93] [94] [95,96] [97] [86] [98] [99,100] | Inhibit the normal function of LDH The compensatory mechanisms of metabolic pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Zhou, F.; Wu, X. Lactate-Mediated Crosstalk Between Tumor Cells and Cancer-Associated Fibroblasts: Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 5583. https://doi.org/10.3390/ijms26125583
Tan S, Zhou F, Wu X. Lactate-Mediated Crosstalk Between Tumor Cells and Cancer-Associated Fibroblasts: Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences. 2025; 26(12):5583. https://doi.org/10.3390/ijms26125583
Chicago/Turabian StyleTan, Siqi, Faxiao Zhou, and Xiaoming Wu. 2025. "Lactate-Mediated Crosstalk Between Tumor Cells and Cancer-Associated Fibroblasts: Mechanisms and Therapeutic Opportunities" International Journal of Molecular Sciences 26, no. 12: 5583. https://doi.org/10.3390/ijms26125583
APA StyleTan, S., Zhou, F., & Wu, X. (2025). Lactate-Mediated Crosstalk Between Tumor Cells and Cancer-Associated Fibroblasts: Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences, 26(12), 5583. https://doi.org/10.3390/ijms26125583







